Open Access Article
Open Access ArticleHighly sensitive detection of polyborosiloxane (PBS) hydrolysis with mannitol using electrochemical methodology†
Baoliang Liu a,
Xiaoyang Zhangb,
Qikun Zhangb,
Yucheng Suna and
Zaijun Lu*a
a,
Xiaoyang Zhangb,
Qikun Zhangb,
Yucheng Suna and
Zaijun Lu*a
aKey Laboratory for Special Functional Aggregated Materials of Ministry of Education, School of Chemistry and Chemical Engineering, Shandong University, Jinan, 250100, P. R. China. E-mail: sdulab601@163.com; Fax: +86 531 88564464; Tel: +86 531 88361599
bCollege of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University, Jinan 250014, P. R. China
First published on 1st November 2022
Abstract
The complexation of polyhydric alcohols, such as mannitol, with boric acid ion promotes the ionization of boric acid. The hydrolysis performance of PBSs was determined using an electrochemical approach for the first time. Compared with the traditional methods, this approach includes the advantages of high sensitivity, continuity, and digitization.
Polyborosiloxanes (PBSs) have significant and varied applications. The PBS material that was studied as an alternative to natural rubber was previously called supramolecular elastomer1,2 or bouncing putty.3 It is a well-known solid–liquid material.4 Because of the excellent performance of PBSs, researchers worldwide are studying them. Owing to the intrinsic flame retardancy of boron, PBSs can be used as green flame retardants.5,6 In addition, they are applied in numerous fields, which include self-healing materials7–9 and advanced electronics industry10–12 (chemical sensors,13 thermal sensors,14 super capacitors,15 and lithium ion batteries).16 Electronic skin17 has also been extensively studied.
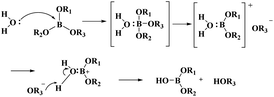
PBSs have excellent properties but are easy to hydrolyse.18 Leaving an empty p-orbital, the boron atoms in most boron compounds are sp2 hybridized. This empty p-orbital is vulnerable to the lone pair electron attack of water molecules and shows the property of easy hydrolysis. The lone pair of water molecules attacks the B atom to form a four-coordinated intermediate of the B atom (Fig. 1). The four-coordinated intermediate tends to form a three-coordinated intermediate19 and then one molecule of alcohol is removed to form a hydroxyl-borate. Finally, PBSs are hydrolysed to boric acid.
The current detection methods for PBSs mainly include the following four methods: weighing, half-life, open observation, and saturated steam. Most of the hydrolysis detection methods have revolved around the above-mentioned methods. The weighing method involves placing PBSs in water and weighing the remaining mass after a certain period of time. This method is too simple to determine accurate hydrolysis performance. Half life method is a titration method, which records PBSs hydrolysis titration time from the beginning of to 50% progress. However, one can only measure hydrolysis at a certain moment, and there should be no organic impurities or nitrogen as they will affect the titration results. The open observation20 method make PBSs into a certain concentration of paraffin oil solution and determines hydrolysis based on the time when turbidity appears. Because of the presence of other organic impurities in PBSs, there are several limitations that affect detection, and the subjectivity of human observation also causes measurement errors. The saturated steam method21 involves filling a dryer with water vapour in its natural state. This condition should be maintained, and the sample should be placed in the dryer. In this case, there will be subjective errors similar to the exposure observation method. Puneet et al.22 explored the coverage of PBSs for the detection of fluoride ions. If the hydrolysis characterization of PBSs is added to the study, the test results would be more convincing. In addition to subjective and pollutant errors, all the above-mentioned methods have common limitations with which they cannot obtain a curve of hydrolysis over time and only ∼10−1 mol L−1 can be detected.
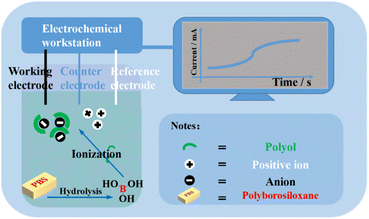
Based on the above-mentioned reasons, this study shows the detection of the hydrolysis of PBSs using electrochemical methods. Polyols can promote the ionization of boric acid that is produced by PBS hydrolysis (Fig. 2). The change in ion concentration in the solution is continuously output as an electrical signal with a constant voltage.23 The constant voltage test uses 3.0 V as the set value because the borate we prepared is used in the electrolyte system of 3.0 V button batteries. This method combines PBS hydrolysis and electrochemistry for the first time to obtain a method for detecting PBS hydrolysis with the advantages of continuity, selectivity (for boric acid lipid polymers), and digitality.
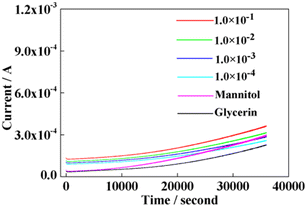
In order to study the influence of polyols on the detection error of boric acid by PBS hydrolysis, we used electrochemical constant voltage to detect the current change of mannitol and glycerin under the same conditions. The current change of mannitol at various concentrations at a constant voltage was tested and compared with that in secondary water (Fig. 3(a)). As can be observed from the figure, the current of mannitol with a concentration of four orders of magnitude difference was almost the same. Further, compared with secondary water, it was found that there was almost no difference in the current. This shows that the concentration of mannitol during the experiment did not cause false positive errors in the test results.
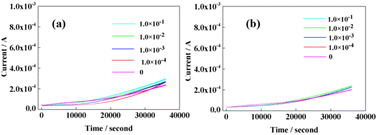 | ||
| Fig. 3 (a) Mannitol of different concentrations, in mol. (b) Glycerol of different concentrations, in mol. | ||
The same principle is shown in Fig. 3(b). We tested current of glycerol at different concentrations, which illustrated that glycerol concentration did not cause false positive errors in the test results during the experiment. The current signal exhibited by glycerol concentration is more difficult to distinguish from that by mannitol. One of the reasons is that glycerol has fewer hydroxyl groups compared to mannitol. Additionally, currents with pure boric acid solutions at different concentrations were investigated using the above constant voltage method (Fig. 4). The current signals with various concentrations of pure boric acid with pure glycerol (0.1 mol L−1) and pure mannitol (0.1 mol L−1) are compared in this figure. It can be observed that the changes in the current with four orders of magnitude of boric acid concentration are that the electrical signal of polyol is weaker than that of boric acid. This is similar to the current of glycerol and mannitol solutions. There is almost no obvious difference in the current change shown by the pure boric acid solution at all concentrations. Moreover, currents of glycerol and mannitol at the same concentration were compared, as shown in Fig. S1 in ESI. It is observed that there is almost no difference in the current magnitude between glycerol and mannitol at the same concentration. Hence, it also indicates that this will not cause a false positive test result in the detection of PBS hydrolysis with polyols.
From the above discussion, we understand that if the PBS hydrolysis solution is directly electrochemically detected, it is difficult to be applied when the concentration of the hydrolysed boric acid is high. Therefore, we introduced the complex mechanism of polyols and boric acid to detect PBS hydrolysis. As we all know, generated by the hydrolysis of PBSs, boric acid can be ionized by hydrogen ions and borate ions. There are three hydrogen atoms in boric acid that can only generate one hydrogen molecule, so boric acid is a weaker acid than carbonic acid and hypochlorous acid. Its ionization constant pK is 9.25, which is difficult to detect even under adequately concentrated conditions. Fortunately, researchers have conducted studies on the ionization of boric acid.24 Polyols can promote the ionization of boric acid, which is generally represented by mannitol and glycerol. Owing to the complexation of polyols, the ionization constant, pK, of boric acid changed from 9.25 to 5.15, which is almost equivalent to the acidity of acetic acid (pK = 4.74). The degree of ionization increased by at least four orders of magnitude, which enhanced the detection precision.
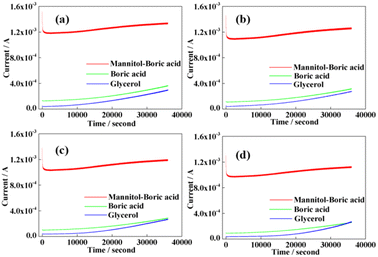
We conducted electrochemical detection for the same concentration of mannitol and boric acid using different gradients, as shown in Fig. S2(a).† Moreover, the electrochemical detection of the same concentration of glycerol and boric acid with different gradients is shown in Fig. S2(b).† The results showed that the two polyols increased the sensitivity for the detection of boric acid. Moreover, it can be observed that the difference in currents between the four solutions of mannitol with different concentrations is more obvious than the current signals presented by the four different concentrations of glycerol. The above illustration shows that the complexing ability of mannitol and boric acid is better than that of glycerol and boric acid, and the difference in the concentration gradient is also more evident. The current signal presented by the pure boric acid solution and that presented by the pure mannitol are significantly smaller than the current signal of the complex of boric acid and mannitol, as shown in Fig. 5. For the combined solution, the higher the concentration, the stronger is the current signal, which is considerably more evident than that of pure boric acid. The cumulative value of the current signal presented by mannitol and boric acid is much smaller than the current signal after mannitol and boric acid are complexed. The reason for the above phenomenon is that mannitol can promote the ionization of boric acid. Hence, the concentration of ions in the solution increases such that the current in the mixed system is higher than that in the pure boric acid and mannitol solution.
The same situation, as shown in Fig. S3,† also demonstrates an enhanced detection sensitivity of boric acid using polyols. This shows that mannitol can greatly improve the sensitivity of the detection of boric acid in the solution. Sudden changes in the concentration of boric acid result in changes in the electrical signal, which also confirms the potential of this method for detecting the hydrolytic stability of PBSs.
In view of the above analysis, we prepared three kinds of PBSs to verify the effectiveness of this method for detecting PBSs from an experimental point of view. The preparation methods for the three PBS materials are illustrated in ESI.† It can be observed from Fig. 6(a) that the FT-IR spectrum shows that PBSs have been successfully prepared. Then, we tested the hydrolysis performance of PBS. In Fig. 6(b), the blue line is the current signal of pure mannitol, the other three lines (red, green and black) are the three types of PBSs placed in the mannitol solution, and the three PBSs hydrolysis performance of current under constant voltage. It can be observed from the figure that the initial current of PBSs in the solution is larger than that of pure mannitol, and the slope of the time period of 0–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s is steeper than that after 10
000 s is steeper than that after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s, which shows that the current changes faster because of the total ion concentration in the solution. The increase is relatively fast; the hydrolysed boric acid rapidly ionizes positive and negative ions under the complexation of mannitol, and the concentration change is relatively large. Further, changes in the current are relatively large, which illustrates that the three PBS powders are placed in the mannitol aqueous solution. Further, PBSs hydrolyse quickly at the beginning. The slope after 10
000 s, which shows that the current changes faster because of the total ion concentration in the solution. The increase is relatively fast; the hydrolysed boric acid rapidly ionizes positive and negative ions under the complexation of mannitol, and the concentration change is relatively large. Further, changes in the current are relatively large, which illustrates that the three PBS powders are placed in the mannitol aqueous solution. Further, PBSs hydrolyse quickly at the beginning. The slope after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s was milder than before 0–10
000 s was milder than before 0–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s, predominantly because the concentration of ions remained unchanged. The same explanation applies to the hydrolysis rate, which is slower than that of 0–10
000 s, predominantly because the concentration of ions remained unchanged. The same explanation applies to the hydrolysis rate, which is slower than that of 0–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s. Moreover, it can be clearly seen from Fig. 6(b) that the hydrolysis of the PBS-C is faster than the other two PBSs. This is because the content of Si–O–B bonds in PBS-C is high, as shown in Fig. S8,† and there are obvious changes that are evident when compared with the other PBSs.
000 s. Moreover, it can be clearly seen from Fig. 6(b) that the hydrolysis of the PBS-C is faster than the other two PBSs. This is because the content of Si–O–B bonds in PBS-C is high, as shown in Fig. S8,† and there are obvious changes that are evident when compared with the other PBSs.
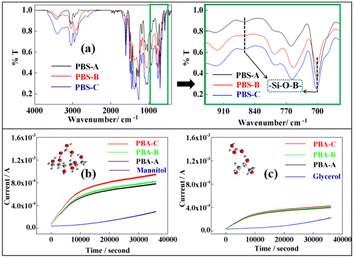 | ||
| Fig. 6 (a) FT-IR spectrum of three PBSs. (b) Detection of PBS hydrolysis with mannitol. (c) Detection of PBS hydrolysis with glycerol. | ||
Moreover, to detect the three kinds of PBS hydrolysis, we further prepared a complex of glycerol and boric acid, as shown in Fig. 6(c). It can be observed from the figure that glycerol can enhance the sensitivity of detection, and the three lines of electrical signal changes exhibited by the three hydrolysis conditions of PBSs are almost folded together, making it difficult to distinguish.
A comparison of Fig. 6(b) and (c) shows that mannitol can promote the detection of PBS hydrolysis to a great extent and that the effect is more evident. The details of the weighing method are shown in Table S1 and Fig. S10–S12 in the ESI.†
From an experimental point of view, it is not only proved that polyols have the characteristics of improving the sensitivity of PBS hydrolysis detection but also illustrated that the effect of mannitol is better than that of glycerol. The density functional theory (DFT) with Gaussian software was used to calculate the binding energy of mannitol and glycerol to borate ions. All calculations were performed using the Gaussian 09 program. The B3LYP/6-31G basis set was used to optimize the configuration of mannitol, glycerin, and boric acid ions. It is known that the complexing ability of mannitol with borate ions is stronger than that of glycerol with borate ions. The DFT results show that the binding capacity of mannitol for borate ions is stronger than that of glycerol for borate ions. It can be observed from Table 1 that the binding energy of mannitol to borate ions is 134.6881 kJ mol−1, and the binding energy of glycerol to borate ions is 71.6762 kJ mol−1. From this perspective, the binding energy of mannitol to borate ions is greater than that of glycerol to borate ions, ensuring that mannitol is more stable than the binding particles of borate ions. This indicates, from the perspective of calculation, that the binding ability of mannitol to boric acid is stronger than that of glycerol to boric acid. Herein, the calculated results are consistent with the experimental results, which show that mannitol can enhance the detection sensitivity of detecting PBS hydrolysis.
| Species | Energy/a.u. |
|---|---|
| Boric acid ion | −252.0022 |
| Mannitol | −688.3986 |
| Mannitol–boric acid ion | −940.4521 |
| Glycerol | −344.7923 |
| Glycerol–boric acid ion | −596.8218 |
| Mannitol–boric acid ion: ER = 134.6881 kJ mol−1 | |
| Glycerol–boric acid ion: ER = 71.6762 kJ mol−1 | |
Based on the above analysis, the proposed formula for PBS hydrolysis rate is obtained as follows:
| ω = I/(Imax − I0) × 100% |
In summary, mannitol can promote the ionization of boric acid produced by the hydrolysis of PBSs. Electrochemical methods are used to convert a highly sensitive ion concentration signal of a solution into an electrical signal that can be 1000 times more sensitive. Moreover, DFT analysis proved that mannitol is superior to glycerol and is suitable for the detection of PBS hydrolysis. Further, the detection aid used in this method is mannitol, which is employed as a sugar-free sweetener in the food industry; thus it is a non-toxic and green detection method. This study introduced electrochemistry into PBS hydrolysis detection for the first time. This method, which turned out to be more than just a method for the hydrolysis detection of PBSs, provides an exploration idea for hydrolysis detection methods for other materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the National Nature Science Foundation of China (51973104), the Program (Joint Laboratory Project of Electromagnetic Structure Technology) (ESTL202204) funded by AVIC Research Institute for Special Structures of Aeronautical Composites (China, RISAC), and the basic research Program (YJG202102) funded by Shandong University-Xin'an Group Silicon Based High-end New Materials Research Institute for their financial support.References
- P. Cordier, F. Tournilhac, C. Soulié-Ziakovic and L. Leibler, Nature, 2008, 451, 977–980 CrossRef PubMed.
- R. J. Wojtecki, M. A. Meador and S. J. Rowan, Nat. Mater., 2011, 10, 14–27 CrossRef PubMed.
- A. Juhász, P. Tasnadi and L. Fabry, Phys. Educ., 1984, 19, 302–304 CrossRef.
- T. F. Wu and B. Q. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 24071–24078 CrossRef PubMed.
- J. D. Qiu, X. J. Lai, H. Q. Li, J. F. Gao, X. R. Zeng and X. H. Liao, Composites, Part B, 2019, 175, 107068 CrossRef.
- Y. L. Zhang, C. G. Zeng, Q. J. Jiao and Y. F. She-li, J. Mater. Sci., 2020, 55, 14264–14279 CrossRef CAS.
- Q. Wu, H. Xiong, Y. Peng, Y. Yang, J. Kang, G. S. Huang, X. C. Ren and J. R. Wu, ACS Appl. Mater. Interfaces, 2019, 11, 19534–19540 CrossRef CAS.
- M. Tang, P. Zheng, K. Q. Wang, Y. J. Qin, Y. Z. Jiang, Y. R. Cheng, Z. Li and L. M. Wu, J. Mater. Chem. A, 2019, 7, 27278–27288 RSC.
- Y. H. Chen, X. Pu, M. M. Liu, S. Y. Kuang, P. P. Zhang, Q. L. Hua, Z. F. Cong, W. B. Guo, W. G. Hu and Z. L. Wang, ACS Nano, 2019, 13, 8936–8945 CrossRef CAS.
- H. Sun, X. You, Y. S. Jiang, G. Z. Guan, X. Fang, J. Deng, P. N. Chen, Y. F. Luo and H. S. Peng, Angew. Chem., Int. Ed., 2014, 53, 9526–9531 CrossRef CAS PubMed.
- C. S. Boland, U. Khan, G. Ryan, S. Barwich, R. Charifou, A. Harvey, C. Backes, Z. L. Li, M. Ferreira, M. Mobius, R. Young and J. Coleman, Science, 2016, 354, 1257–1260 CrossRef CAS PubMed.
- D. P. Qi, K. Y. Zhang, G. W. Tian, B. Jiang and Y. D. Huang, Adv. Mater., 2020, 2003155 Search PubMed.
- W. G. Huang, K. Besar, Y. Zhang, S. Yang, G. Wiedman, Y. Liu, W. M. Gao, J. Song, K. Hemker, K. Hristova, I. J. Kymissis and H. E. Katz, Adv. Funct. Mater., 2015, 25, 3745–3755 CrossRef CAS.
- Y. L. He, S. L. Liao, H. Y. Jia, Y. Y. Cao, Z. N. Wang and Y. P. Wang, Adv. Mater., 2015, 27, 4622–4627 CrossRef PubMed.
- Y. Huang, Y. Huang, M. S. Zhu, W. J. Meng, Z. X. Pei, C. Liu, H. Hu and C. Y. Zhi, ACS Nano, 2015, 9, 6242–6251 CrossRef.
- Y. Huang, M. Zhong, Y. Huang, M. S. Zhu, Z. X. Pei, Z. F. Wang, Q. Xue, X. Xie and C. Y. Zhi, Nat. Commun., 2015, 6, 10310 CrossRef PubMed.
- E. D. Elia, S. Barg, N. Ni, V. G. Rocha and E. Saiz, Adv. Mater., _2015, 27, 4788–4794 Search PubMed.
- Z. Liu, S. J. Pichen and N. A. M. Besseling, Macromolecules, 2014, 47, 4531–4537 CrossRef.
- J. Lam, L. L. Cao, J. M. Farrell and D. W. Stephan, Dalton Trans., 2020, 49, 1839–1846 RSC.
- G. B. Yang, J. H. Zhao, L. Cui, S. Y. Song, S. M. Zhang, L. G. Yu and P. Y. Zhang, RSC Adv., 2017, 7, 7944–7953 RSC.
- X. L. Zhang, Q. K. Ma and X. M. Jia, Die & Mould Industry, 2009, 35, 67–70 Search PubMed.
- P. Puneet, R. Vedarajan and N. Matsumi, ACS Sens., 2016, 1, 1198–1202 CrossRef.
- Q. K. Zhang, B. L. Liu, L. P. Yu, Y. L. Bei and B. Tang, ChemCatChem, 2020, 12, 334–341 CrossRef.
- M. E. Meyerhoff, Anal. Chem., 1980, 52, 1534–1535 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra04514a |
| This journal is © The Royal Society of Chemistry 2022 |