Open Access Article
Open Access ArticleDevelopment of nanoemulsions for the delivery of hydrophobic and hydrophilic compounds against carbapenem-resistant Klebsiella pneumoniae†
Hossam H. Tayeb *ab,
Shahd A. Moqaddamabc,
Nojod H. Hasaballaha and
Raed I. Felimbanbd
*ab,
Shahd A. Moqaddamabc,
Nojod H. Hasaballaha and
Raed I. Felimbanbd
aA Nanomedicine Unit, Centre of Innovations in Personalized Medicine (CIPM), King Abdulaziz University, 21589, Jeddah, Saudi Arabia. E-mail: hhtayeb@kau.edu.sa
bDepartment of Medical Laboratory Technology, Faculty of Applied Medical Sciences, King Abdulaziz University, 21589, Jeddah, Saudi Arabia
cClinical and Molecular Microbiology Laboratories, King Abdulaziz University Hospital, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
d3D Bioprinting Unit, Centre of Innovations in Personalized Medicine (CIPM), King Abdulaziz University, 21589, Jeddah, Saudi Arabia
First published on 16th September 2022
Abstract
Antimicrobial resistance (AR), particularly the limited antimicrobial activities of antibiotics and natural compounds, has prompted research into new antimicrobials. Nanoemulsions (NEs) have been found to improve the activity of antimicrobial compounds. This study developed clove essential oil-in-water NEs (CEO-NEs) and water-in-oil-in-water NEs co-encapsulating CEO and meropenem (CEO–MEM-NEs) to investigate the antibacterial activity of these loaded NEs against carbapenem-resistant Klebsiella pneumoniae. Ultrasonication was used to prepare CEO-NEs and CEO–MEM-NEs. Tween 80 and Imwitor 375 surfactants were used to produce CEO-NEs while Tween 80, Imwitor 375, and PGPR were used to produce CEO–MEM-NEs. Droplets' sizes were 138 ± 1.769 and 183.600 ± 0.889 for CEO-NEs and CEO–MEM-NEs, respectively. The resultant NEs were monodispersed, negatively charged, and physically stable. The antibacterial activities of NEs were investigated using broth microdilution, checkerboard, and time-kill assays to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). CEO-NEs (0.16% CEO MIC) and CEO–MEM-NEs (0.08% CEO and 1 μg mL−1 MEM MICs) completely inactivated K. pneumoniae, and showed functional stability after two weeks of storage at 4 °C. In conclusion, the formulated NEs significantly enhanced the antibacterial activity of CEO and MEM and have great potential as delivery systems of antimicrobial compounds.
Introduction
Antimicrobial resistance (AR) was recently declared by the World Health Organization (WHO) as one of the top global threats to public health and development. AR contributes to high mortality rates annually and occurs when bacteria acquire resistance genes that interfere with drug action by either preventing drug uptake, modifying the drug target, inactivating the drug, or causing active efflux of the drug. The improper use of broad-spectrum antibiotics, especially in hospitals, is one of the leading factors of AR. The rapid spread of antibiotic resistant pathogens and ineffective antimicrobial drugs exacerbates the challenges of treating bacterial infections, resulting in financial burdens and high mortality rates.1 Despite huge research efforts into producing novel antimicrobials, effective antibacterial therapeutics are limited.2Carbapenem-resistant Klebsiella pneumoniae (K. pneumoniae) is one of the most clinically concerning multi-drug resistant (MDR) pathogens, causing a wide range of hospital-acquired infections and outbreaks including pneumonia, septicaemia, and urinary tract infections. Development of carbapenem resistance in K. pneumoniae is attributed to the acquisition of specific resistant mechanisms. For instance, carbapenem-resistant K. pneumoniae can hydrolyse various broad-spectrum β-lactam antibiotics due to their secretion of β-lactamase enzymes, including extended-spectrum β-lactamases (ESBLs) and carbapenemases.3 In addition, the involvement of plasmids in the biomanufacturing of β-lactamases by carbapenem-resistant K. pneumoniae facilitates a high dynamic exchange rate of resistance genes, that complicates the control and treatment of K. pneumoniae.4 These challenges highlight the need for novel, safe, and effective antibacterial drug formulations.5
Plants and their extracts, including essential oils (EOs), have gained considerable attention as antimicrobials. EOs are volatile, hydrophobic compounds known to exhibit antimicrobial activity against several microorganisms.6 Particularly, clove essential oil (CEO) has broad antioxidant, antibacterial, and antifungal properties that contribute to its potential in food, cosmetics, and medical applications. For example, the antimicrobial activities of CEO have been demonstrated in oral hygiene and applied to extend the shelf-life of various food products.7 However, the applications of CEO as an antimicrobial agent are limited by volatility, water insolubility, and light and oxidation sensitivity,8 therefore, these limitations must be overcome before CEO can be developed into new and effective antimicrobial formulations.
Lipid-based nanocarriers, like nanoemulsions (NEs), have shown great potential for improving the physiochemical and antimicrobial properties of both hydrophilic and hydrophobic compounds.9,10 NEs are heterogeneous systems composed of two immiscible liquids where one liquid is dispersed (i.e., the dispersed phase), into other liquid, continuous phase. Amphiphilic surfactants are integrated at the liquid–liquid interface to stabilize NE droplets and impart surface functionalities.11,12 NEs are kinetically stable, 20 to 500 nm droplets, and categorized based on the nature of the dispersed phase (core) as either single (oil-in-water (O/W) and water-in-oil (W/O)) or double (water-in-oil-in-water (W/O/W) and oil-in-water-in-oil (O/W/O)) emulsions. Single NEs allow for the encapsulation of one cargo type, either water or oil-soluble compounds, while the multi-compartment double NEs accommodate and co-deliver two cargos simultaneously to the intended site of action.13
NEs have been reported as effective platforms to improve the antibacterial activities of EOs in food and medical fields.14–16 For instance, NEs were recently developed to enhance peppermint oil physical and antimicrobial properties and showed physiochemical stability and potent antimicrobial against Escherichia coli.17 The use of generally recognized as safe (GRAS) surfactants and EOs provides NEs with biocompatible profiles for medical applications. Other advantages of NEs include long-term stability, small droplet size, high surface-to-volume ratio, high encapsulation efficiency, and enhanced water solubility of lipophilic compounds.18
NEs long-term stability offer protection of encapsulated EOs from oxidation and volatility. NEs can also enhance the antimicrobial activity of encapsulated compounds by mass transfer, promoting passive cellular absorption and binding to and penetrating cell membranes lipid bilayers via electrostatic interactions and ligand-based targeting.19,20 The advantages and possible delivery mechanisms suggest that NEs could be promising carriers for natural and synthetic antimicrobials.
In this study, novel W/O/W NEs were developed as an antibacterial formulation for the co-delivery of natural (CEO) and synthetic (meropenem, (MEM)) compounds, CEO–MEM-NE. Aqueous solution of CEO and CEO-loaded O/W NE (CEO-NE) were also prepared. Physical stabilities of fresh samples and in different storage conditions of the CEO–MEM-NE and CEO-NE formulations were also investigated. Additionally, the synergistic antimicrobial effect of the encapsulated active natural and synthetic compounds within the W/O/W NEs on carbapenem resistant K. pneumoniae was evaluated and compared to the CEO hydro-soluble solution and CEO-loaded O/W NEs through the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), checkerboard, and time-killing assays. Finally, functional stabilities of the developed NE formulations were also similarly investigated using the same antimicrobial activity tests.
Experimental section
Materials
A 100% pure CEO (Syzygium aromaticum) was purchased from Abazeer (Jeddah, Saudi Arabia). Mueller–Hinton broth (MHB) and Mueller–Hinton agar (MHA) media were purchased from Saudi Prepared Media Laboratory Company Ltd. (SPML) (Riyadh, Saudi Arabia). Tween 80 (polysorbate 80) (Fig. S1†), 2,3,5-triphenyltetrazolium chloride (TTC) solution, and pure MEM were purchased from Sigma-Aldrich Corporation (Burlington, United States). A 10 mg mL−1 stock solution of MEM was prepared by dissolving MEM powder in sterile distilled water. 100% pure surfactants or emulsifiers, including Imwitor® 600 (polyglyceryl-3 polyricinoleate, PGPR), and Imwitor® 375 (glyceryl citrate/lactate/linoleate/oleate), and Miglyol® 812 oil (medium-chain triglyceride) were kindly provided by IOI Oleochemicals (Hamburg, Germany) (Fig. S1†). HLB values for Tween 80, Imwitor® 600 (PGPR) and Imwitor® 375 are 15, 4, and 10–12, respectively. Carbapenem resistant K. pneumoniae strain (KPC-2, ATCC BAA-1705) and sensitive strain, E. coli (ATCC 25922), were kindly provided by the Clinical and Molecular Microbiology Laboratories at King Abdulaziz University Hospital, King Abdulaziz University (Jeddah, Saudi Arabia).Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio), and an aqueous phase (with a final concentration of 4% v/v Tween 80 dissolved in sterile distilled water). The aqueous and oil phases were emulsified using a 125 W ultrasonic processor (Qsonica, Q125 system) with a total of six bursts, 45 seconds each, at 40% amplitude. During preparation, the CEO-NEs were placed in an ice bath for one minute following each burst to allow for cooling.
1 ratio), and an aqueous phase (with a final concentration of 4% v/v Tween 80 dissolved in sterile distilled water). The aqueous and oil phases were emulsified using a 125 W ultrasonic processor (Qsonica, Q125 system) with a total of six bursts, 45 seconds each, at 40% amplitude. During preparation, the CEO-NEs were placed in an ice bath for one minute following each burst to allow for cooling.The CEO–MEM-NEs were formulated in two stages. First, W/O NEs were prepared by dispersing the aqueous phase that contained MEM at a final concentration of 705.5 μg mL−1 into the hydrophobic phase composed of 6% v/v PGPR dissolved in Miglyol 812 at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, and 55% v/v CEO as final concentrations. The prepared W/O NEs were then used to prepare double W/O/W NEs with final CEO and MEM concentrations of 10% v/v and 128 μg mL−1, respectively. W/O/W NEs were prepared by dispersing the primary W/O NE, hydrophobic phase, into the external aqueous phase using an ultrasonicator in the presence of 6% v/v Imwitor 375 and 4% v/v Tween 80. The ultrasonication parameters used to synthesize the CEO–MEM-NEs were identical to those used for the preparation of CEO-NEs.
1 ratio, and 55% v/v CEO as final concentrations. The prepared W/O NEs were then used to prepare double W/O/W NEs with final CEO and MEM concentrations of 10% v/v and 128 μg mL−1, respectively. W/O/W NEs were prepared by dispersing the primary W/O NE, hydrophobic phase, into the external aqueous phase using an ultrasonicator in the presence of 6% v/v Imwitor 375 and 4% v/v Tween 80. The ultrasonication parameters used to synthesize the CEO–MEM-NEs were identical to those used for the preparation of CEO-NEs.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in sterile distilled water before measurements were performed. Final measurements were expressed as the average of three readings at 25 °C.
100 in sterile distilled water before measurements were performed. Final measurements were expressed as the average of three readings at 25 °C.To determine the MBCs, 100 μL of each test well that exhibited no bacterial growth in the MIC tests was subcultured on MHA plates. After overnight incubation at 37 °C, MBCs were determined by observing the lowest concentrations that showed no bacterial growth. To confirm that the selected TTC concentration for the MIC and MBC tests was not contributing to toxic effects on bacterial cells, MBC results were determined without and with TTC solution. The MIC and MBC results were subsequently used for the calculation of MBC/MIC ratios. The antibacterial effects of NE surfactants (Tween 80, Imwitor 375, and PGPR), at the same final concentrations used to prepare CEO-NEs and CEO–MEM-NEs, were also explored against K. pneumoniae using the same MIC values of CEO-NE and CEO–MEM-NE from the microdilution method. Assays were performed using at least three independent replicates.
| FICI of CEO-NE = MIC of CEO in CEO–MEM-NE/MIC of CEO-NE |
| FICI of aqueous solution = MIC of CEO in CEO–MEM-NE/MIC of CEO aqueous solution |
| FICI of MEM = MIC of MEM in CEO–MEM-NE/MIC of MEM solution |
| FICIc = FICI of CEO-NE + FICI of MEM |
| FICIc = FICI of CEO aqueous solution + FICI of MEM |
FICIc values ≤ 0.5 indicate synergism, FICIc values between 0.5 and 4.0 indicate an additive effect, and FICIc values > 4 indicate antagonism. Assays were performed using at least three independent replicates.
Results and discussion
Characterization of CEO-NEs and CEO–MEM-NEs
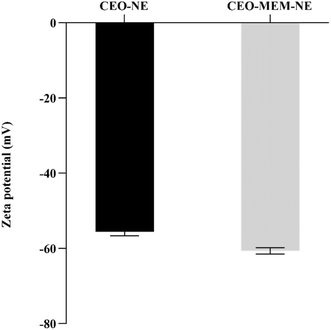
Single CEO-NEs and double CEO–MEM-NEs were prepared using the ultrasonication method and characterized by average droplet size, ZP (surface charge), and PDI. CEO-NEs and double CEO–MEM-NEs were prepared to enhance the antimicrobial activity of the encapsulated compounds (Fig. 1). The average droplet sizes of CEO-NEs and CEO–MEM-NEs were 138 ± 1.769 nm and 183.600 ± 0.889 nm, respectively (Table S1†). Investigations into the prepared CEO-NEs and CEO–MEM-NEs revealed average droplets' sizes below 200 nm, representing an appropriate size range for the biointeraction with bacterial cells and biofilms due to the high surface-to-volume ratio and porins penetration capabilities. A slight difference in droplet size was observed between the two formulations; however, this variation can be explained by the presence of two different interfaces within CEO–MEM-NE. PDI values of CEO-NEs and CEO–MEM-NEs were 0.177 ± 0.008 and 0.160 ± 0.014, respectively (Table S1†). The PDI values of CEO-NEs and CEO–MEM-NEs were lower than 0.2, indicating that both formulations are composed of homogeneous, monodispersed oil droplets (Fig. S2†). CEO-NEs and CEO–MEM-NEs exhibited negatively charged surfaces at −55.60 ± 1.852 mV and −60.67 ± 1.436 mV, respectively, (Fig. 2), demonstrating sufficient electrostatic repulsive forces between oil droplets.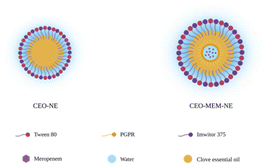 | ||
| Fig. 1 The structure of CEO-NE and CEO–MEM-NE. It depicts the surface and core materials used to formulate CEO-NE and CEO–MEM-NE. | ||
Physical stability of CEO-NEs and CEO–MEM-NEs
Physical stability of pharmaceutical formulations, particularly, drug delivery systems, is an important aspect that determines downstream applications. In this study, CEO-NEs and CEO–MEM-NEs displayed physical stability in high salt concentration conditions and commonly used media in antimicrobial activity studies (i.e., PBS and MHB) (Table S1†).This can be attributed to the selection of appropriate surfactants to stabilize the CEO droplets.23,24 Stability of NEs is also dependent on surface charge and electrostatic repulsive forces between nanodroplets, that delay or prevent droplet aggregation and extend formulation shelf-life. The highly negative surface charges of CEO-NEs (−55.6 mV) and CEO–MEM-NEs (−60.67 mV) are attributed to the semi-synthetic, non-ionic surfactants, Tween 80, and the natural, anionic surfactant Imwitor 375 (Fig. 2). CEO-NEs and CEO–MEM-NEs stability in the high salt medium indicate that the NEs functionalities are retained during the biointeraction with K. pneumoniae.
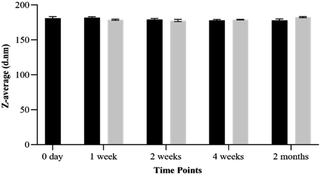
Shelf-life and stability of NEs in different storage conditions are important factors for ensuring effectiveness in pharmaceutical applications. The stability of CEO-NEs and CEO–MEM-NEs was tested at room temperature and 4 °C over two months. Both formulations remained stable at room temperature and 4 °C following two months in storage. No significant changes to droplet size were observed in CEO-NEs and CEO–MEM-NEs during storage at room temperature or 4 °C over two months (Fig. 3 and 4). Selecting low aqueous solubility oil core, and surfactants with appropriate chemical characteristics can effectively minimize or delay destabilization mechanisms in NEs, like Ostwald ripening.
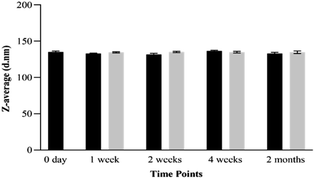 | ||
| Fig. 3 Stability of CEO-NE based on droplets' average size for two months storage. Room temperature (black), 4 °C (grey). | ||
 | ||
| Fig. 4 Stability of CEO–MEM-NE based on droplets' average size for two months storage. Room temperature (black), 4 °C (grey). | ||
Antimicrobial activity of CEO aqueous solution and NEs
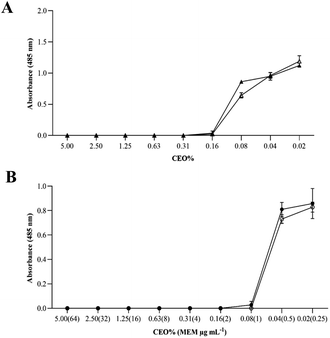
The antimicrobial activities of CEO-NEs and CEO–MEM-NEs were evaluated qualitatively and quantitatively by the MIC and MBC assays using TTC – a widely used indicator of bacterial growth that is reduced during active bacterial metabolism to produce red-coloured formazan. A recent study reported that high concentrations of TTC can exert cytotoxic effects that interfere with the growth of bacterial cells.25 Therefore, optimum assay conditions are essential for producing valid results that most accurately reflect actual antimicrobial activity of test samples.To determine the appropriate concentration of TTC for the MIC assays, two TTC concentrations (0.05% and 0.005%) were compared to TTC-free conditions during the microdilution assay. Findings from the MIC assay revealed that 0.05% v/v TTC demonstrated two-fold higher antimicrobial activities for MEM and CEO aqueous solution when compared to MEM and CEO aqueous solution assayed in the presence of 0.005% TTC, expanding cytotoxicity on bacterial cells (Fig. 5 and S3–S5, and Table S2†). These results indicated that 0.05% TTC had a slight toxic effect on K. pneumoniae exacerbating the antimicrobial activity of the different test samples and interfering with the final MIC results (Fig. 5 and S3–S7 and Table S2†).
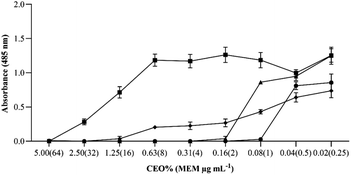 | ||
| Fig. 5 MICs of CEO aqueous solution and NEs against K. pneumoniae BAA-1705 with addition of 0.005% TTC. (■) CEO aqueous solution, (▲) CEO-NE, (●) CEO–MEM-NE, (♦) meropenem. | ||
To further confirm that 0.005% TTC has no contribution to the antimicrobial activity of the test samples, MBC assays were performed with and without 0.005% TTC. No significant differences were observed between MBCs with and without 0.005% TTC, indicating that 0.005% TTC had no cytotoxic effect on bacterial cells (Table 1). 0.005% TTC was found to be a suitable concentration for measuring bacterial growth of CEO-NE and CEO–MEM-NEs. Similar results were also reported in a previous study showing that 0.005% TTC can be used as a non-toxic indicator for the antibacterial activity testing against various genera of the Enterobacterales.26
| 0.005% TTC | No TTC | |||
|---|---|---|---|---|
| MIC | MBC | MBC/MIC | MBC | |
| MEM (μg mL−1) | 16 | 16 | 1 | 16 |
| CEO aqueous solution (%) | 5 | 5 | 1 | 5 |
| CEO-NE (%) | 0.16 | 0.16 | 1 | 0.16 |
| CEO–MEM-NE CEO% (MEM μg mL−1) | 0.08 (1) | 0.08 (1) | 1 | 0.08 (1) |
Preparation of NEs with GRAS components ensures safety for human application. The antimicrobial activities of the starting components (CEO aqueous solution, MEM, and surfactants) used to formulate CEO-NE and CEO–MEM-NE were evaluated and shown in Fig. 5. The MICs of CEO aqueous solution and MEM were 5% v/v and 16 μg mL−1, respectively. The incubation of K. pneumoniae with the GRAS surfactants used to prepare the NEs, showed absorbance levels similar to the positive control (i.e., no bactericidal effect), indicating that Imwitor 375, PGPR, and Tween 80 have no cytotoxic effect on bacterial cells at the MIC concentrations (as shown in Fig. S8†).
The antimicrobial activities of CEO-NEs, and CEO–MEM-NEs were tested using MIC and MBC assays. Encapsulation of CEO within the core of O/W NEs significantly enhanced CEO antibacterial activity against K. pneumoniae, from 5% to 0.16%, when compared to pure CEO (Fig. 5, S5 and S6†). These findings indicate that NEs facilitated CEO delivery and interaction with K. pneumoniae, significantly reducing the required CEO concentration to exert a bactericidal effect.
Co-encapsulation of natural and synthetic drugs in nanocarriers is a promising approach for tackling antimicrobial resistance. In this study, the encapsulation of CEO and MEM in surfactant-stabilized W/O/W NE significantly (p-value < 0.0001) reduced the minimum concentration required to inhibit growth of K. pneumoniae from 5% to 0.08% and 16 μg mL−1 to 1 μg mL−1, respectively (Fig. 5 and S7† and Table 1). CEO–MEM-NEs have demonstrated significantly higher bactericidal activity against carbapenem-resistant K. pneumoniae when compared to CEO aqueous solution and CEO-NEs (p-value 0.0072) (Fig. 5 and S7,† and Table 1). These promising results are attributed to the co-encapsulation of MEM and CEO into double NE compartments, producing synergism and potent bactericidal effect on K. pneumoniae. The resulting antimicrobial activity of CEO–MEM-NEs reduced the required MICs of MEM and CEO to completely kill K. pneumoniae by 16 and 64 folds, respectively.
Droplet sizes of the prepared NEs were less than 200 nm and at least 10 times smaller than the size of bacterial cells, facilitating cargo delivery via cell membrane through hydrophilic porin channels and/or passive cellular absorption. Subsequent release of NE contents by various mechanisms such as mass transport, adhesion, and simple diffusion, disrupts bacterial cellular membranes leading to cell death.24,27–29 Similarly, previous studies have shown that NEs can enhance the antimicrobial activity of CEO and other EOs, such as cinnamon, tea tree, and thyme.28,30,31 However, deeper understanding of NEs biointeraction with bacterial cells and release of payload is still needed.
Previous research suggests that the cationic surfaces of nanoparticles facilitate biointeractions with the negatively charged bacterial cells while anionic surfaces may interfere with cell binding. However, charge-dependent drug delivery can also contribute to non-specific binding to normal mammalian cells and undesired cytotoxicity.32 In this study, the anionic surfaces of CEO-NEs and CEO–MEM-NEs did not interfere with the delivery of cargos and antibacterial activity against K. pneumoniae.
Antimicrobials are classified either as bactericidal or bacteriostatic compounds. Antibacterial compound is classified as bactericidal if the MBC/MIC ratio is ≤4 and bacteriostatic if >4.33 In this study, the MBC/MIC ratios indicate that CEO aqueous solution and NEs have bactericidal activity against carbapenem-resistant K. pneumoniae BAA-1705 (Table 1).
Checkerboard assay
To study synergism between combination of antimicrobials, checkerboard assay was applied. The FICIc (0.079) between the CEO aqueous solution and MEM and CEO-loaded NEs indicated a synergistic effect. The FICIc (0.563) between CEO-NEs and CEO–MEM-NEs also indicated a synergistic effect (Table 2). These results suggest the co-delivery of encapsulated compounds within CEO–MEM-NEs compartments, CEO and MEM, were successfully delivered to the targeted microorganism.| MICO | MICC | FICI | FICIc | Type of interaction | |
|---|---|---|---|---|---|
| a MICO: MIC of one component alone, MICC: MIC of one component in the most effective combination, FICI: fractional inhibitory concentration index of one component in the most effective combination, FICIc: total FICI of the combination of both components. | |||||
| MEM (μg mL−1) | 16 | 1 | 0.063 | — | — |
| CEO aqueous solution (%) | 5 | 0.08 | 0.016 | 0.079 | Synergistic |
| CEO-NE (%) | 0.16 | 0.08 | 0.5 | 0.563 | Synergistic |
Time kill analysis
Bactericidal activity of antimicrobials can be concentration or time dependent. In other words, agents that kill microorganisms faster at concentrations higher than the MIC are concentration-dependent while antimicrobial agents that exhibit a slow killing rate at concentrations higher than or equal to the MIC are known as time-dependent antimicrobials.33 The time-kill points for CEO-NEs and CEO–MEM-NEs against K. pneumoniae were determined in response to the MIC, ((0.16% CEO for CEO-NEs) and (0.08% CEO and 1 μg mL−1 MEM for CEO–MEM-NEs)), and two-fold the MIC, (0.31% CEO for CEO-NEs) and (0.16% CEO and 2 μg mL−1 MEM for CEO–MEM-NEs). Measurements were taken at 10 minute-intervals over an hour and showed that 60 and 20 minutes of incubation with K. pneumoniae were sufficient for the MIC (0.16%) and two-fold the MIC (0.31%) of CEO-NE to completely kill the cells, respectively (Fig. S9†). However, six and one hours of K. pneumoniae incubation with the MIC (0.08% CEO and 1 μg mL−1 MEM) and two-fold the MIC (0.16% CEO and 2 μg mL−1 MEM) of CEO–MEM-NEs were needed to completely kill the K. pneumoniae cells, respectively. Time-kill assays showed that CEO–MEM-NEs have a slower killing rate but at 2 folds lower concentration of the encapsulated CEO when compared to CEO-NEs (MICs of CEO–MEM-NEs and CEO-NEs).CEO–MEM-NEs can achieve similar killing rate of CEO-NE at two-fold higher CEO concentration of CEO-NE. This can be explained by the antibacterial synergistic effect of the encapsulated compounds, CEO and MEM, loaded within the multicompartment CEO–MEM-NEs. K. pneumoniae treatment with one-half the MIC (0.08% CEO for CEO-NEs, and 0.04% CEO and 0.5 μg mL−1 MEM for CEO–MEM-NEs) did not affect cells viability with CFU results were similar to those of untreated K. pneumoniae cells (Fig. S9†). These results demonstrate that both NEs exhibited concentration-dependent killing activity.
Functional stability
Antibacterial activities of two weeks stored CEO-NEs and CEO–MEM-NE were evaluated against K. pneumoniae BAA-1705. Results are presented in Fig. 6 and show that the MICs of freshly prepared NEs are similar to MICs of NEs stored for two weeks at 4 °C. These results suggest that long term storage at 4 °C did not impact the antimicrobial activity of NEs (Fig. S10†). These findings demonstrate that the antimicrobial properties of the encapsulated CEO and MEM are preserved by NEs, facilitating possible future clinical translation and commercialization.Conclusions
Carbapenem-resistant Enterobacterales have become a major global problem in healthcare settings where drug resistance and the limited emergence of new and effective antibiotics necessitate research into alternative antimicrobial agents. Natural compounds, such as EOs, have demonstrated antimicrobial activities; however, the efficacies of natural products are often hindered by the poor aqueous solubility, volatility, and instability against light and oxidative agents. NEs are promising drug delivery systems that could enhance the activities of natural and synthetic hydrophobic and hydrophilic compounds through effective surface interactions with bacterial cells.CEO-NEs and CEO–MEM-NEs were successfully prepared using the ultrasonic emulsification technique yielding small (<200 nm) monodispersed droplets with highly negative surface charges. Stability studies revealed that these characteristics prevented droplet aggregation and gravitational precipitation and improved stability of the resultant NEs.
Bacterial growth conditions are important factors for studying the activity of antimicrobial compounds. In this study, a non-toxic concentration of the bacterial growth indicator, TTC, was determined to conduct reliable microdilution assays. The antibacterial studies showed significant improvement in the efficacy of the encapsulated antimicrobial compounds, CEO and MEM. CEO–MEM-NEs reduced the MIC of CEO and MEM by 64 and 16 folds, respectively. CEO–MEM-NEs demonstrated potent antimicrobial effect against K. pneumoniae, that is attributed to the achieved synergism between CEO and MEM.
Preparation of NEs with GRAS materials ensures safety for human application. The raw materials used for NE preparation, including CEO, demonstrated no or minimal antimicrobial properties when applied individually on bacterial cells. By contrast, the developed single and multiple compartment NEs significantly enhanced the delivery of both lipophilic and hydrophilic compounds, creating a potent antibacterial effect on K. pneumoniae. CEO-NEs and CEO–MEM-NEs demonstrated a concentration-dependent antibacterial killing activity. These results support previous studies that describe how NEs can improve the delivery and therapeutic efficiency of encapsulated antimicrobial compounds with acceptable safety profiles.
Long-term functional stability of the NEs as a drug delivery system could pave the way for further downstream applications, particularly for enhancing the antimicrobial activity of different EOs and antibiotics in the treatment of human pathogens. Mechanistic understanding of surface interactions of the developed NEs with K. pneumoniae and other clinically targeted microorganisms is urged in future studies.
Author contributions
The manuscript was written through contributions of all authors (H. T., Hossam H. Tayeb; S. M., Shahd A. Moqaddam; N. H., Nojod H. Hasaballah; and R. F. and Raed I. Felimban). All authors have given approval to the final version of the manuscript. The manuscript was proposed and supervised by H. T., who have significantly contributed to structure, context, revision, editing, reference formatting and experimental work. Authors have discussed the figures design, which were produced by S. M. and N. H. and reviewed by H. T. S. M, N. H, H. T, and R. F. have contributed to writing the following manuscript sections; Introduction, Materials and Methods. All authors have participated in tables design, which were reviewed by H. T. H. T. and S. M. have written both Results and discussion sections, ESI† were prepared by S. M. and H. T., and reviewed by N. H. All authors have contributed to experimental work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia under grant code no. G: 1387-142-1440. The authors also acknowledge technical support provided by DSR.Notes and references
- D. Larsson and C.-F. Flach, Nat. Rev. Microbiol., 2021, 1–13 Search PubMed.
- B. Aslam, W. Wang, M. I. Arshad, M. Khurshid, S. Muzammil, M. H. Rasool, M. A. Nisar, R. F. Alvi, M. A. Aslam, M. U. Qamar, M. K. F. Salamat and Z. Baloch, Infect. Drug Resist., 2018, 11, 1645–1658 CrossRef CAS PubMed.
- M. K. Paczosa and J. Mecsas, Microbiol. Mol. Biol. Rev., 2016, 80, 629–661 CrossRef CAS PubMed.
- X. Yang, N. Dong, E. W.-C. Chan, R. Zhang and S. Chen, Trends Microbiol., 2021, 29, 65–83 CrossRef CAS PubMed.
- I. Karaiskos, I. Galani, V. Papoutsaki, L. Galani and H. Giamarellou, Expert Rev. Anti-Infect. Ther., 2021, 3, 1–17 Search PubMed.
- S. Chouhan, K. Sharma and S. Guleria, Medicines, 2017, 4, 58–62 CrossRef PubMed.
- M. Radünz, M. L. M. da Trindade, T. M. Camargo, A. L. Radünz, C. D. Borges, E. A. Gandra and E. Helbig, Food Chem., 2019, 276, 180–186 CrossRef.
- N. Hasheminejad, F. Khodaiyan and M. Safari, Food Chem., 2019, 275, 113–122 CrossRef CAS.
- S. M. Jafari, P. Paximada, I. Mandala, E. Assadpour and M. A. Mehrnia, Nanoencapsulation technologies for the food and nutraceutical industries, Elsevier, 2017, pp. 36–73 Search PubMed.
- L. Pavoni, D. R. Perinelli, G. Bonacucina, M. Cespi and G. F. Palmieri, Nanomaterials, 2020, 10, 135–158 CrossRef CAS PubMed.
- H. H. Tayeb, M. Stienecker, A. P. J. Middelberg and F. Sainsbury, Langmuir, 2019, 35, 13588–13594 CrossRef CAS PubMed.
- L. Bai and D. J. McClements, J. Colloid Interface Sci., 2016, 479, 71–79 CrossRef CAS.
- T. Sheth, S. Seshadri, T. Prileszky and M. E. Helgeson, Nat. Rev. Mater., 2020, 5, 214–228 CrossRef.
- C. R. Garcia, M. H. Malik, S. Biswas, V. H. Tam, K. P. Rumbaugh, W. Li and X. Liu, Biomater. Sci., 2022, 633–653 RSC.
- B. D. da Silva, D. K. A. do Rosário, D. A. Weitz and C. A. Conte-Junior, Trends Food Sci. Technol., 2022, 1–13 CrossRef.
- V. Ghosh, A. Mukherjee and N. Chandrasekaran, Colloids Surf., B, 2014, 114, 392–397 CrossRef CAS PubMed.
- Q. Liu, Y. Gao, X. Fu, W. Chen, J. Yang, Z. Chen, Z. Wang, X. Zhuansun, J. Feng and Y. Chen, Colloids Surf., B, 2021, 201, 111626–111635 CrossRef CAS.
- H. H. Tayeb and F. Sainsbury, Nanomedicine, 2018, 13, 2507–2525 CrossRef CAS PubMed.
- J. M. V. Makabenta, A. Nabawy, C.-H. Li, S. Schmidt-Malan, R. Patel and V. M. Rotello, Nat. Rev. Microbiol., 2021, 19, 23–36 CrossRef CAS.
- V. Ryu, M. G. Corradini, D. J. McClements and L. McLandsborough, J. Colloid Interface Sci., 2019, 556, 568–576 CrossRef CAS PubMed.
- A. Man, L. Santacroce, R. Iacob, A. Mare and L. Man, Pathogens, 2019, 8, 1–11 Search PubMed.
- S.-K. Yang, K. Yusoff, W. Thomas, R. Akseer, M. S. Alhosani, A. Abushelaibi and K.-S. Lai, Sci. Rep., 2020, 10, 1–14 CrossRef.
- P.-H. Li and W.-C. Lu, Food Hydrocolloids, 2016, 53, 218–224 CrossRef CAS.
- L. Ghaderi, R. Moghimi, A. Aliahmadi, D. McClements and H. Rafati, J. Appl. Microbiol., 2017, 123, 832–840 CrossRef CAS.
- A. Veiga, T. T. Maria da Graça, L. S. Rossa, M. Mengarda, N. C. Stofella, L. J. Oliveira, A. G. Gonçalves and F. S. Murakami, J. Microbiol. Methods, 2019, 162, 50–61 CrossRef CAS PubMed.
- M. Rahman, I. Kühn, M. Rahman, B. Olsson-Liljequist and R. Möllby, Appl. Environ. Microbiol., 2004, 70, 2398–2403 CrossRef CAS PubMed.
- W.-C. Lu, D.-W. Huang, C.-C. Wang, C.-H. Yeh, J.-C. Tsai, Y.-T. Huang and P.-H. Li, J. Food Drug Anal., 2018, 26, 82–89 CrossRef CAS.
- H. Falleh, M. B. Jemaa, M. A. Neves, H. Isoda, M. Nakajima and R. Ksouri, Food Chem., 2021, 359, 129963 CrossRef CAS PubMed.
- D. J. McClements, A. K. Das, P. Dhar, P. K. Nanda and N. Chatterjee, Front. Sustain. Food Syst., 2021, 5, 35 Search PubMed.
- J. Franklyne, A. Mukherjee and N. Chandrasekaran, Lett. Appl. Microbiol., 2016, 63, 322–334 CrossRef CAS PubMed.
- Y. Shahbazi, Nanomed. Res. J., 2019, 4, 204–208 CAS.
- H. Majeed, F. Liu, J. Hategekimana, H. R. Sharif, J. Qi, B. Ali, Y.-Y. Bian, J. Ma, W. Yokoyama and F. Zhong, Food Chem., 2016, 197, 75–83 CrossRef CAS PubMed.
- A. L. Leber, Clinical Microbiology Procedures Handbook, John Wiley & Sons, 2016 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03925g |
| This journal is © The Royal Society of Chemistry 2022 |