Open Access Article
Open Access ArticleProbing the separation mechanism of solid–liquid components in oily sludge and sludge containing polymers for a harmless treatment process of sludge
Yang Songab,
Hongbao Liang *a,
Jun Mac,
Xiaoyu Wanga,
Zihao Lia,
Jianmeng Youa and
Shuzhan Wanga
*a,
Jun Mac,
Xiaoyu Wanga,
Zihao Lia,
Jianmeng Youa and
Shuzhan Wanga
aSchool of Mechanical Science and Engineering, Northeast Petroleum University, Daqing, Heilongjiang 163318, PR China. E-mail: lianghbwenzhang@163.com
bThe No. 1 Oil Production Plant of Daqing Oilfield Co., Ltd, ., Daqing, Heilongjiang 163000, PR China
cDaqing Oilfield Design Institute Co., Ltd., Daqing, Heilongjiang 163712, PR China
First published on 17th October 2022
Abstract
In this study, the compositions of oily sludge and sludge containing polymers were analyzed. Sludge was separated preliminarily by conditioning centrifugal technology, whereby the temperature of the centrifugal process and the amount of oil/water separating agent were optimized. Thermal decomposition technology was combined in order to achieve the efficient treatment of sludge. The results showed that the compositions of oily sludge and sludge containing polymers were complex. Therefore, they could not meet the requirements of environmental protection only by conditioning centrifugal technology. After optimization, the best conditioning centrifugal temperature was 70 °C and the content of the oil/water separating agent was 2%. The high efficiency treatment of oily sludge and sludge containing polymers could be realized by combining conditioning centrifugal technology with the thermal decomposition technology. It is found that the sludge could meet the requirements of environmental protection after this combined treatment. This technology has strong adaptability to different types of oilfield sludge. This work is of great significance for the efficient treatment of oily sludge and sludge containing polymers and for environmental protection.
1 Introduction
Oily sludge is the waste produced in the process of oilfield development. It mainly comes from the sewage discharge of oilfield combined station systems, the sewage discharge of underground operations, pipeline leakage, and tank-cleaning sludge. When the liquid produced by an oil well is dehydrated, oily sludge will settle and accumulate in the electric dehydration device. Oily sludge is usually transported by vehicles to environmental protection units for treatment. The transportation is long and can easily lead to secondary pollution. The existing treatment technology is difficult to use and expensive, which puts great pressure on environmental protection. If the oily sludge is discharged improperly, it can cause serious environmental pollution. Aiming at the treatment of oily sludge, Zhong et al.1 summarized the treatment process of oily sludge by a hydrothermal method, and put forward a treatment method involving the sub/supercritical water treatment of oily sludge. Barskaya et al.2 obtained three organic components in oily sludge by centrifugation, namely trap oil, reversible adsorption oil, and irreversible adsorption oil. It was considered that the irreversible adsorption oil was mainly composed of weak carbonization structures. The thermal oxidation degradation temperature of irreversible adsorption oil could reach 300 °C. Que et al.3 analyzed the pyrolysis behavior and volatile products of oily cold rolling mill sludge by thermogravimetric analysis, infrared spectroscopy, gas chromatography/mass spectrometry and so on. Zhang et al.4 studied the mechanism of the degradation of oily sludge waste by an iron oxide catalyst. The migration pathway of the heteroatoms was investigated by the molecular dynamics method. It was believed that under the action of supercritical water, the O heteroatom would be removed. The iron heavy metal particles in oily sludge combined with the Fe2O3 catalyst. This combination was conducive to accelerating the degradation of asphaltene. Qi et al.5 analyzed the main pyrolysis stages of oily sludge by thermogravimetric experiments. The mechanism of magnetic nanoparticles for enhancing the effect of the microwave pyrolysis of oily sludge was studied, and it was found that magnetic ZnFe2O4 particles could improve the pyrolysis efficiency. Xu et al.6 treated oily sludge samples from Jidong Oilfield by a microemulsion system with sodium dodecyl benzene sulfonate (SDBS) as a surfactant. The formula system of the oily sludge treatment agent was determined, and the optimal oil-removal rate was 82.5%. Hui et al.7 calculated the thermal decomposition kinetics parameters of oil sludge by three isoconversional methods, and considered that a high heating rate could promote the pyrolysis of oil sludge. In the thermal decomposition stage, the apparent activation energy increased with the increased conversion. Shi et al.8 prepared a hydrogel flocculating agent by the graft polymerization of acrylamide on a metal tube filter. A technology for recovering crude oil from oily wastewater was designed based on the hydrogel flocculating agent. Su et al.9 devised a new oily sludge treatment process combining a subcritical hydrothermal treatment (SHT) and biodegradation process (BP), which could improve the oil-removal rate of sludge to 96.73%. Peng et al.10 conducted supercritical hydration experiments for the special bonding structure of oily sludge, and proved that this method could effectively improve the treatment capacity of polymer-containing oily sludge.The composition of oily sludge is complex, and the emulsification problem of oily sludge is also serious. It is difficult for conventional treatment methods to achieve effective treatment. The combination of the use of chemical agents and a heating process can obviously realize the separation of oily sludge. However, for oily sludge and sludge containing polymers, the treatment result is limited. Therefore, in order to analyze the separation mechanism in the heating method for oily sludge and sludge containing polymers, further research needs to be carried out. In this work, the components of oily sludge and sludge containing polymers were analyzed first, and the sludge was separated by the use of the conditioning centrifugal technology. Then, the separation mechanism of the oily sludge and sludge containing polymers was analyzed by thermogravimetric experiments. Finally, the sludge was decomposed by a drum-type oil sludge thermal decomposer. The theory and technology for the thermal decomposition of oily sludge and sludge containing polymers were further improved. This work is of great significance for the efficient treatment of oily sludge and sludge containing polymers and for environmental protection.
2 Experimental
2.1 Experimental materials
Polyoxyethylene polyoxypropylene octadecyl ether (molecular formula: C12H25O(EO)m(PO)nH, m = 3, n = 6) was provided by Daqing Oilfield Exploration and Development Institute and used as an oil/water separation agent. The chemical agent for the oily sludge (sodium dodecyl sulfate, analytically pure) was provided by sludge station transportation manufacturers. Beakers, a measuring cylinder, mixer, medicine spoon, mixing rod, constant temperature water bath equipment, petroleum ether (analytical purity), UV1801 spectrophotometer, STA449C simultaneous thermogravimetric analyzer, AE200 electronic balance, laser particle size-distribution instrument, and centrifuge were used to carry out the experiments.Two kinds of representative oily sludge were selected, which were from the oily sludge treatment station of No. 1 Oil Production Plant and the sludge produced by chemical dredging reduction in No. 2 Oil Production Plant of Daqing Oilfield. The sludge in the sludge pool of the treatment station in No. 1 Oil Production Plant was mainly the oil sludge produced by the dredging of the base station (with less bulk impurities). The sludge produced by chemical flooding desilting reduction in the No. 1 Oil Production Plant contained a high content of polymers, which could not be treated in the constructed sludge station at present (Fig. 1).
2.2 Experiments for composition characterization of the oily sludge and sludge containing polymers
The oily sludge and sludge containing polymers used in the experiment had long been accumulated in the treatment field. The light components in the crude oil had already volatilized at ambient temperature (20–40 °C). Therefore, the moisture content in the oily sludge and sludge containing polymers could be analyzed by drying at room temperature, according to the following procedure:(1) 100 g oily sludge and 100 g polymer sludge were selected and put into evaporating pans, respectively, and then the pans containing the sludge samples were put into the oven. The experimental temperature was set at 35 °C to avoid the thermal decomposition of the sludge. The samples were taken out from the oven every 2 h to measure the quality in the evaporating pans. When the quality of the evaporating pans no longer changed, the quality of the sludge after drying was measured. The quality of the sludge before and after drying was recorded, and the difference between them was calculated. This difference was regarded as the quality of the water in the sludge.
(2) The dried sludge was repeatedly washed by petroleum ether and centrifuged until the color of the petroleum ether did not change. Then, the secondary drying was carried out. The quality of the sludge before and after washing was recorded, and the difference between them was calculated. This difference was regarded as the quality of the oil in the sludge.
(3) Finally, the sludge was put into the muffle furnace for further decomposition. The muffle furnace temperature was set to 800 °C, and the decomposition time was 5 h. The sludge samples were removed after decomposition. The quality of the sludge before and after decomposition was recorded, and the difference between them was calculated. This difference was regarded as the quality of the non-oil organic matter in the sludge. The quality of the residual sludge was the quality of the inorganic minerals.
2.3 Experiments for optimization of the conditioning parameters of the oily sludge and sludge containing polymers
First, 20 g oily sludge and 20 g sludge containing polymers were mixed with 80 ml warm water respectively. The mixtures were stirred in a heated magnetic agitator by constant temperature water bath until fluidization. The experiment was carried out according to the conventional conditioning process of the oily sludge treatment station. After the conditioning process, the mixtures were centrifuged for 20 min at a centrifuge speed of 3000 rpm.2.4 Experiments on the thermal decomposition of the oily sludge and sludge containing polymers
(1) Thermogravimetric experiments for the oily sludge and sludge containing polymersA STA449C simultaneous thermogravimetric analyzer was used in the experiments, which could not only measure the mass change of the samples during the experiment, but also analyze the heat absorption and release processes. The main purpose of this experiment was to study the different heating rates of the oily sludge under a nitrogen atmosphere, so as to draw TG (thermal gravity) and DTG (differential thermal gravity) curves of the thermal decomposition of the oily sludge and sludge containing polymers. The DTG curve clearly showed the intensity degree of the reaction. By analyzing the TG and DTG curves, the kinetic parameters and reaction mechanism in the thermal decomposition process of the oily sludge and sludge containing polymers could be obtained. The influencing factors for the thermal decomposition were analyzed. The mechanism function and kinetic parameters (activation energy E and preexponential factors A) in the thermal decomposition process of the oily sludge and sludge containing polymer were solved by different mathematical methods.
TG curve: thermal gravity analysis refers to the quality change of the samples with temperature or time in an applied temperature program (heating/cooling/constant temperature or a combination).
DTG curve: the differential thermal gravity curve can be obtained by differential calculation of the TG curve, and provides more information, such as the weight change rate.
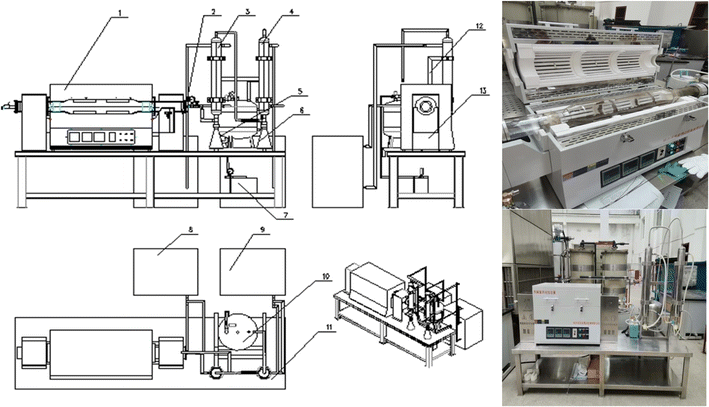
(2) Sludge treatment experiments based on a drum-type oil sludge thermal decomposer and the equipment used for treating the oily sludge and sludge containing polymers
The operation mode of an intermittent pyrolysis furnace was simulated by a laboratory pyrolysis experimental device. By studying the influence of the thermal decomposition temperature and time on the thermal decomposition effect of the oily sludge and sludge containing polymers, the main parameters of the sludge for reaching the required standard after thermal decomposition were finally determined.
The drum-type pyrolysis furnace commonly used to treat oily sludge and sludge containing polymers was simulated by a small laboratory thermal decomposition device. The device needed to achieve the functions of oxygen-insulation pyrolysis of the oily sludge, the condensation recovery of pyrolysis gas, tail gas treatment, and so on. The processing capacity was 5 L, and the total power was set to 9 kW. The size of the equipment was 3000 × 2000 × 1700 mm, and the thermal decomposition temperature went from room temperature to 900 °C.
Pyrolysis furnace: this was used for oxygen-insulation pyrolysis of the oily sludge, and allowed accurately controlling the pyrolysis temperature, heating rate, and pyrolysis time.
Operating platform and collector: these played the role of collecting the gaseous substances produced by the pyrolysis and heat insulation.
Condenser: the mixture gas produced during pyrolysis was condensed by a condenser.
Tail gas purifier: this was used for purifying the tail gas generated by pyrolysis of the oily sludge to avoid secondary pollution (Fig. 2).
Oily sludge and sludge containing polymers obtained after centrifugation from the constructed sludge treatment station were used as the raw sludge for the experiments. The quality of each loading was 1000 g. The pyrolysis temperature for the indoor pyrolysis test device was set as 300 °C, 400 °C, 500 °C, 600 °C, 700 °C, 800 °C, 900 °C, and the pyrolysis time was set as 30 min, 60 min, and 90 min for the indoor orthogonal tests. After stopping heating, the sludge was cooled naturally and the oil content was analyzed.
The oily sludge and sludge containing polymers were loaded into the pyrolysis furnace. After sealing and isolating the oxygen, nitrogen was passed into the furnace to replace the oxygen. Then the pyrolysis furnace was started, and the sludge was heated according to the set temperatures. During the pyrolysis process, the sludge in the pyrolysis furnace was continuously turned over to ensure that the sludge was uniformly heated. The volatile gas generated during pyrolysis was drawn to the condenser tube through the guide tube. The condensed volatile matter was collected after a two-stage condensation. The residual gas collected by condensation entered the exhaust gas purifier for purification and was then discharged. The residue after pyrolysis was discharged from the pyrolysis furnace, and the condensate oil after condensation was discharged into the collector for analysis.
3 Results
3.1 Composition and characterization of the oily sludge and sludge containing polymers
First, the composition of the oily sludge and sludge containing polymers was analyzed according to the experimental process outlined in Section 2.2. The results for the properties analysis are shown in Table 1.| Samples | Oily sludge | Sludge containing polymers | Remark |
|---|---|---|---|
| Water content/% | 20.88 | 57.45 | Oven drying |
| Oil content/% | 47.71 | 24.81 | Extraction-gravimetric method |
| Content of inorganic minerals/% | 44.12 | 61.42 | High temperature ashing of the muffle furnace |
| Content of non-oil organic matter/% | 8.17 | 13.77 | Material balance calculation |
Non-oil organic matter in the data referred to the residual organic components of the dry oily sludge extracted by non-polar organic solvents, including polymers, surfactants, and some possible wax components. The reference values were calculated by material balance. It can be seen from Table 1 that the non-oil organic compounds accounted for 8.17% and 13.77% of the dry sludge. In contrast, the content of non-oil organic compounds in the sludge containing polymers were higher. These non-oil organic matter were preliminarily identified as polymers in composite flooding. The water content and inorganic mineral content of the sludge containing polymers were also higher. This indicated that the polymers not only had a stronger ability to store water, but also could carry inorganic minerals in the formation. In addition, the polymers could carry them from the formation to the surface of the oil–water treatment system, so that the inorganic mineral components in the oily sludge increased.
The organic composition in the oily sludge was qualitatively analyzed by gas chromatography-mass spectrometry (GC-MS), and the measurement results are shown in Table 2.
| No. | Oily sludge | Sludge containing polymers | No. | Oily sludge | Sludge containing polymers |
|---|---|---|---|---|---|
| 1 | 1,2,4-Trimethyl-cyclopentane | Butyrolactone | 16 | 2,6,10-Trimethyl-dodecane | 4-Methyl-dodecane |
| 2 | 2,3-Dimethyl-hexane | Octane | 17 | 3-Methyl-5-propyl-nonane | 2,6,11-Trimethyl-dodecane |
| 3 | 2-Methyl-heptane | 2-Methyl-3-heptene | 18 | Octadecane | Hexadecane |
| 4 | 3-Methyl-heptane | 1,2,3-Trimethyl-cyclopentane | 19 | 3,8-Dimethyl-undecane | 2,6,10-Trimethyl-pentadecane |
| 5 | Trans-1,3-dimethyl-cyclohexane | 2,3-Dimethyl-hexane | 20 | 2,6,11-Trimethyl-dodecane | 2,7,10-Trimethyl-dodecane |
| 6 | 1,1-Dimethyl-cyclohexane | 2-Methyl-heptane | 21 | Nonadecane | Tridecane |
| 7 | 1-Ethyl-2-methyl-cyclopentane | 3-Methyl-heptane | 22 | 2,6,10-Trimethyl-dodecane | 5-(1-Methylpropyl)-nonane |
| 8 | Trans-4-octene | Cis-1,3-dimethyl-cyclohexane | 23 | 3,8-Dimethyl-undecane | Nonadecane |
| 9 | Octane | 1-Ethyl-1-methyl-cyclopentane | 24 | — | Hexadecane |
| 10 | 1,3-Dimethyl-cyclohexane | Trans-1,2-dimethyl-cyclohexane | 25 | — | 2,6,11-Trimethyl-dodecane |
| 11 | 2-Methyl-2-phenol butenoate | Octane | 26 | — | 3-Methyl-5-propyl-nonane |
| 12 | 4,7-Dimethyl-undecane | 1,4-Dimethyl-cyclohexane | 27 | — | 3,8-Dimethyl-undecane |
| 13 | Hexadecane | Tridecane | 28 | — | 2-Methyl-5-propyl-nonane |
| 14 | Hexadecane | 2,3,3-Trimethyl-octane | 29 | — | Nonadecane |
| 15 | Heptadecane | Tetradecane | 30 | — | Decyl-2-ethylhexyl sulfonic acid |
It can be seen from Table 2 that hydrocarbon organic matter in sludge existed from C8 to C19 hydrocarbons, including saturated hydrocarbons, olefins, straight chains, branched chains, cyclohydrocarbons, and a few heteroatoms. There were 20 kinds of hydrocarbons. Hydrocarbons below 7 carbons were not detected. This may be related to the long-term storage of the oily sludge in the environment and the volatilization of the volatile components into the atmosphere. This indicated that the main factors affecting the difficult treatment of oily sludge and sludge containing polymers may be the residual heavy hydrocarbon components. These components can easily interact with inorganic minerals and adsorb on the surface of solid particles. It is difficult for conventional treatment methods to decompose oily sludge and sludge containing polymers. In addition, organic components are more complex in the sludge containing polymers. This result can be explained by the polymers having a strong carrying capacity, which make them more capable of wrapping crude oil and thus avoiding the volatilization of some organic components in the crude oil.
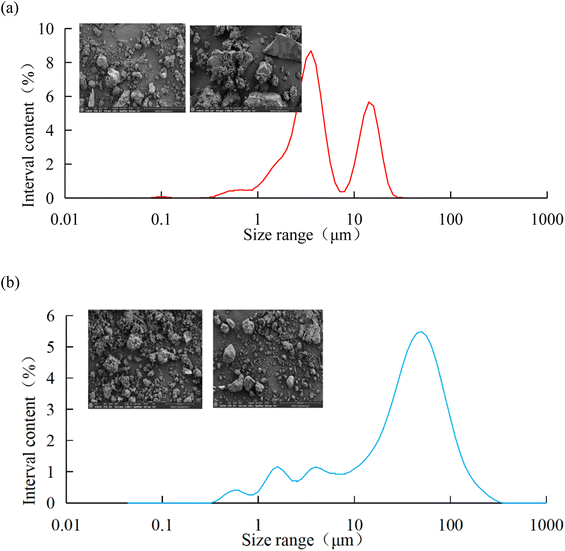
In order to further analyze the composition of the solid particles in the oily sludge and sludge containing polymers, the solid particles after removing water and oil were observed by scanning electron microscopy (SEM). The median particle size of the solid particles in the oily sludge and polymer-containing sludge was measured by a laser particle size-distribution instrument. The composition of the solid particles was analyzed by energy dispersive X-ray spectroscopy (EDS). The results are shown in Fig. 3 and Table 3.
| Elements | Oily sludge | Sludge containing polymers | ||
|---|---|---|---|---|
| Percentage by weight (%) | Atomic percentage (%) | Percentage by weight (%) | Atomic percentage (%) | |
| C | 9.14 | 19.48 | 21.33 | 37.79 |
| O | 33.26 | 53.24 | 23.92 | 31.81 |
| Al | 0.89 | 0.84 | 3.88 | 3.06 |
| Si | 0.93 | 0.85 | 20.78 | 15.75 |
| K | — | — | 3.12 | 1.70 |
| Ca | — | — | 4.53 | 2.40 |
| Ba | — | — | 4.66 | 0.72 |
| Fe | 55.78 | 25.58 | 17.78 | 6.77 |
From the SEM photographs in Fig. 3a, it can be seen that the solid particles in the pure oily sludge were irregular and the particle size was about 50 μm. The particles in the sludge containing polymers were dispersed, multi-angular, and finely broken. The particle sizes were thus significantly different, of which the largest particle size was around 200 μm, and the smallest particle size was about 5 μm. It can be seen from Table 3 that the contents of C, O, Si, and Fe in the pure oily sludge were higher, and it also contained K, Al, Ca, and Ba. The contents of O, C, Si, Fe, and Ba in the sludge containing polymers were higher, and it also contained Al. The composition of the solid particles in oily sludge was relatively less, while the composition of the solid particles in the sludge containing polymers was more complex. This was mainly due to the existence of more polymers in the sludge containing polymers, which could very easily carry other minerals in the formation. As a result, there were complex components in the sludge containing polymers.
3.2 Optimization of the conditioning parameters of the oily sludge and sludge containing polymers
According to the experimental process for optimizing the conditioning parameters outline in Section 2.3, the oil contents of the oily sludge and sludge containing polymers after centrifugation were investigated. Then the appropriate conditioning temperatures for sludge treatment were determined. The results of the hot washing-centrifugation indoor experiments are shown in Table 4.| No. | Conditioning temperature (°C) | Oil content of the sludge after conditioning (%) | Oil content of dry sludge by commutation (%) | Oil-removal rate by commutation (%) | Remarks |
|---|---|---|---|---|---|
| 1 | 50 | 3.66 | 4.43 | 84.07 | Oily sludge |
| 2 | 60 | 2.98 | 3.73 | 86.58 | |
| 3 | 70 | 2.29 | 3.66 | 87.84 | |
| 4 | 80 | 2.16 | 3.43 | 88.66 | |
| 5 | 50 | 3.42 | 4.12 | 86.03 | Sludge containing polymers |
| 6 | 60 | 2.67 | 2.88 | 87.62 | |
| 7 | 70 | 2.25 | 2.46 | 88.31 | |
| 8 | 80 | 1.98 | 2.03 | 90.01 |
It can be seen from Table 4 that there was a certain similarity in the variation laws between the conditioning temperature and the oil-removal rate for the oily sludge and sludge containing polymers. When the temperature of hot washing was 50–80 °C, the oil-removal rate of the oily sludge fluctuated slightly. The oil-removal rate of hot washing at 50 °C was the lowest, and that of the oily sludge at 80 °C was the highest. Thus, in the process of hot washing at different temperatures, the oil-removal rate increased with the increase in the hot washing temperature. However, it must be considered that it is relatively difficult to reach a temperature above 70 °C in the actual conditioning process of an oilfield. In addition, when the hot washing temperature reached 70 °C, the oil content of the oily sludge was 2.29%. The oil content of the sludge after 70 °C and 80 °C hot washing showed little difference. Therefore, the following indoor experiments were conducted at 70 °C for hot washing, and the sludge after conditioning is shown in Fig. 4.
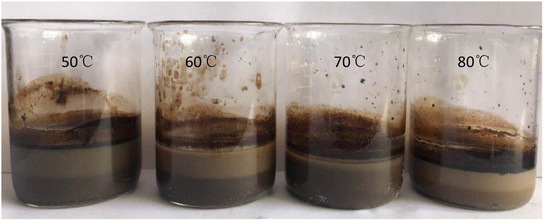 | ||
| Fig. 4 Comparison of the oily sludge from Daqing Oilfield No. 1 Oil Production Plant after different conditioning temperatures. | ||
The oil content in the oily sludge after centrifugation was investigated to determine the appropriate dosage for oily sludge treatment. The results of the hot washing-centrifugal indoor experiments are shown in Table 5.
| No. | Oil/water separating agent (%) | Oil content of the sludge after conditioning (%) | Oil content of dry sludge by commutation (%) | Oil-removal rate by commutation (%) | Remarks |
|---|---|---|---|---|---|
| 1 | 0 | 4.47 | 7.51 | 67.23 | Oily sludge |
| 2 | 0.5 | 3.39 | 5.96 | 74.01 | |
| 3 | 2 | 2.80 | 5.58 | 75.64 | |
| 4 | 3 | 2.50 | 3.34 | 85.41 | |
| 5 | 0 | 8.54 | 10.96 | 52.17 | Sludge containing polymer |
| 6 | 0.5 | 5.31 | 8.03 | 64.99 | |
| 7 | 2 | 1.13 | 3.30 | 85.61 | |
| 8 | 3 | 0.99 | 1.69 | 92.62 |
It can be seen from Table 5 that in the comparison experiments for the oily sludge and sludge containing polymers, with the increase in dosage of the oil/water separating agent, the oil content gradually decreased after hot washing, and the oil-removal rate gradually increased. When the dosage reached 3%, the lowest oil content of the oily sludge after treatment was 2.5%, and the lowest oil content of the sludge containing polymer was 0.99%. It can be concluded that when the oil/water separating agent dosage was more, the conditioning effect for the oily sludge was better. However, considering the cost factor and the subsequent corresponding treatment process, the concentration of the oil/water separating agent was finally optimized at 2%.
3.3 Effect of the thermal decomposition of the oily sludge and sludge containing polymers
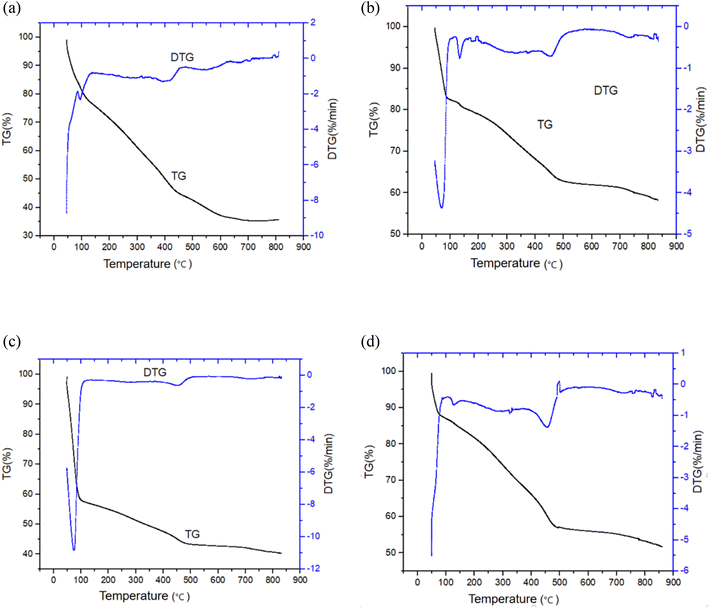
The thermal decomposition status of the oily sludge and sludge containing polymers was obtained by thermogravimetric experiments. The variations for the oily sludge and sludge containing polymers during pyrolysis was studied by thermogravimetric curves measured at a constant heating rate of 20 °C min−1. Thermal gravity (TG) curves and differential thermal gravity (DTG) curves were plotted and are shown in Fig. 5.It can be seen from Fig. 5 that there was no significant difference between the TG/DTG curves of the oily sludge and sludge containing polymers during pyrolysis. This result could be explained by the polymer retaining more water at indoor temperature. However, at high temperature, the stability of the polymer itself became worse, resulting in water not being effectively retained. As the water content of the oily sludge after centrifugation was low, the representative oily sludge was selected for analysis. Here, the water content had no effect on the residual oil content, pyrolysis reaction time, and pyrolysis process. Nonetheless, the higher the water content of the oily sludge, the more heat was needed for water evaporation and thus the more fuel consumed. It is also necessary to reduce the water content of oily sludge raw materials in industry, so the samples after centrifugation were selected for analysis.
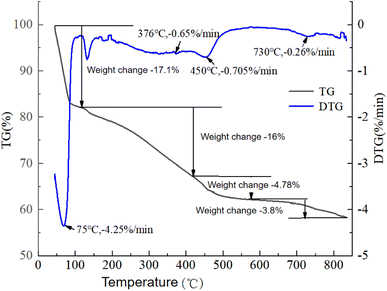
It can be seen from Fig. 6 that the pyrolysis process of oily sludge can be roughly divided into the four stages.
The first stage was water evaporation, when heating from an initial temperature to 115 °C. This stage was a slow process of moisture evaporation on the surface of the sludge by heat absorption. This process only involved a heated evaporation of the water vapor, and no other chemical reactions occurred. The TG curve showed a sharp downward trend from the beginning of heating, and the weight loss rate was 17.1%. The mass-loss rate at this stage was large, and there was an obvious weight-loss peak shown on the DTG curve. The fastest weight loss was 4.25% min−1 at 75 °C, and the peak ended at 115 °C. This was because a large amount of water in the sludge during this stage was continuously heated and volatilized, which consumed a lot of heat in the heat absorption state.
The second stage was the fast (light oil) pyrolysis stage, which occurred at 115–410 °C. This stage involved the volatilization of the light oil components after heating the sludge, and also included the process of water evaporation in some water-in-oil emulsions. Due to the lower temperature not meeting the requirements for in-depth reactions, this stage of volatilization occurred only on the surface of the sludge. The components with a lower boiling point in the sludge were heated and volatilized. With the increase in temperature, a small part of the unstable organic matter decomposed into small molecular gases, such as CH4 and H2. The TG curve at this temperature stage was smoother than that at the first stage, indicating that the weight loss at this stage was relatively slow. The corresponding DTG curve also proved that there was a less obvious small peak at the fastest weight loss at about 376 °C, with a peak of 0.65% min−1, which was much smaller than that in the first stage. Due to the large amount of light oil with a low boiling point in the sludge, the weight loss at this stage was more, and the total weight loss was 16%. This stage had less heat absorption, which was due to the volatilization process of a small part of the bound water and the low-boiling-point components, and was the main stage of the oil sludge pyrolysis.
The third stage was the slow (heavy oil) pyrolysis stage, which occurred at 410–575 °C. This stage was the pyrolysis reaction of the heavy components in the oil sludge. The TG curve at this temperature stage was much smoother than that at the second stage, indicating that the weight loss at this stage was slower. The peak value of the corresponding DTG curve was not obvious, and the weight loss was the fastest at about 450 °C, where the peak value was 0.705% min−1, and the mass loss was 4.78%. At this stage, the heavy oil was pyrolyzed at high temperature to generate small molecular organic matter and non-condensable gas, and there were also some polymerization processes that occurred. The reaction at this stage was complex, and it was an endothermic process.
The fourth stage was the final pyrolysis stage, which occurred at 575–850 °C. The pyrolysis reaction ended basically, the weight loss was 3.8%, and the TG curve decreased slowly. The DTG curve had a less obvious peak at 730 °C, with a peak of 0.26% min−1. This stage involved the precipitation of a small amount of residual heavy oil and inorganic matter in the oil sludge.
The percentage mass change and the temperature of the oily sludge and sludge containing polymers at different stages were compared under different experimental schemes, as shown in Table 6.
| Experiment number | Samples | Water evaporation stage | Light oil pyrolysis stage | Heavy oil pyrolysis stage | Final pyrolysis stage | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mass variation (%) | Temperature (°C) | Mass variation (%) | Temperature (°C) | Mass variation (%) | Temperature (°C) | Mass variation (%) | Temperature (°C) | ||
| a | Oily sludge | 19.8 | 125 | 34 | 440 | 8.8 | 650 | 7.4 | 810 |
| b | Oily sludge | 17.1 | 115 | 16 | 410 | 4.78 | 575 | 3.8 | 850 |
| c | Sludge containing polymers | 41.8 | 102 | 14.4 | 475 | 1.3 | 660 | 2.5 | 825 |
| d | Sludge containing polymers | 16.6 | 85 | 27.9 | 480 | 0.7 | 670 | 3.4 | 852 |
It can be seen from Table 6 that there were obvious differences in the quality changes of the oily sludge and sludge containing polymers samples in the different stages. The mass-loss rate of the oily sludge in the water evaporation stage was between 17.1–19.8%, while the mass-loss rate of the sludge containing polymers in the water evaporation stage was between 16.6–41.8%. This indicated that the sludge containing polymers could retain more water in the system, and the water in the system evaporated at a lower temperature. Since the sludge sample had been kept in the treatment plant for a long time, the light components, such as dissolved methane, ethane (short-chain saturated hydrocarbons), and other dissolved gases, had been volatilized at room temperature. After the remaining sludge sample entered the treatment system, the water evaporated first, while the light oil did not evaporate immediately. When the temperature was further raised to 400–480 °C, the light oil (long chain saturated hydrocarbon, aromatic hydrocarbon, etc.) in the sludge could be volatilized. The temperature required for the volatilization stage of light oil in the sludge containing polymers was higher, which was 475 °C and 480 °C, respectively. This was because the polymer system enhanced the stability of the oily sludge system, which increased the microscopic force between the oil phase and the solid particles, thereby increasing the pyrolysis temperature. When the temperature of the system was further increased, it entered the pyrolysis stage of heavy oil. The mass change in this stage was significantly reduced, indicating that the content of heavy oil (gum asphaltene) in the sludge accounted for less of the overall mass. Due to the large moisture content in the sludge containing polymers, the proportion of heavy matter was less, and the range of mass change was small. When the temperature of the system reached above 800 °C, it entered the final decomposition stage, and the mass change was also small. Some heavy oil and inorganic substances decomposed, so that the quality of the system changed, but the polymers did not have a great impact on the quality of the system.
The pyrolysis experiments were carried out using an indoor pyrolysis experimental device. The pyrolysis temperatures were 300 °C, 400 °C, 500 °C, 600 °C, 700 °C, 800 °C, and 900 °C, and the pyrolysis times were 30 min, 60 min, and 90 min to carry out the indoor orthogonal experiments. After stopping heating, the sludge was cooled naturally and the oil content was analyzed. The pyrolysis products and data are shown in Fig. 7 and Table 7.
 | ||
| Fig. 7 Photographs of sludge and oil–water mixtures produced after pyrolysis. (a) Photograph of oily sludge samples after pyrolysis. (b) Photograph of oil–water mixtures after pyrolysis. | ||
| Oil content (%) | Pyrolysis temperatures (°C) | |||||||
|---|---|---|---|---|---|---|---|---|
| 300 | 400 | 500 | 600 | 700 | 800 | 900 | ||
| Pyrolysis time (min) | 30 | 1.4795 | 1.7505 | 0.0579 | 0.0340 | 0.0224 | 0.0291 | 0.0431 |
| 60 | 1.9912 | 2.5916 | 0.0435 | 0.0245 | 0.0223 | 0.0101 | 0.0226 | |
| 90 | 1.6783 | 1.7847 | 0.0348 | 0.0222 | 0.0243 | 0.0196 | 0.0198 | |
As can be seen from Fig. 7a, with the increase in the pyrolysis temperature, the color of the sludge after pyrolysis gradually deepened, from taupe to black. It can be seen from Fig. 7b that the appearance of the oil–water mixtures after pyrolysis did not change significantly.
It can be seen from Table 7 that the pyrolysis temperature had a great influence on the oil content of the oily sludge. When the pyrolysis temperature was raised from 400 °C to 500 °C, the oil content decreased greatly. When the pyrolysis temperature reached 500 °C or even above 500 °C, the oil content of the sludge after pyrolysis was below 0.3%. At the same pyrolysis temperature but pyrolysis times of 30, 60, or 90 min, the oil content did not change significantly after pyrolysis. The existence of a little fluctuation was noted due to the difference in oil content of the sludges before pyrolysis. Therefore, the pyrolysis time was not the key factor affecting the final oil content of sludge pyrolysis, and the main influencing factor for the pyrolysis effect was the pyrolysis reaction temperature. Through the indoor experimental data, it could be preliminarily determined that when the pyrolysis temperature was greater than or equal to 500 °C and the pyrolysis time was 30 min, the oil content of the oily sludge could reach below 0.3%, so that it could reach the standard parameters for the pyrolysis process.
In order to obtain more economical and reasonable pyrolysis parameters, more accurate indoor pyrolysis temperature experiments were carried out. The experimental results are shown in Table 8.
| Oil content (%) | Pyrolysis temperatures (°C) | |||||
|---|---|---|---|---|---|---|
| 410 | 430 | 450 | 470 | 490 | ||
| Pyrolysis time (min) | 30 | 1.6948 | 1.6137 | 0.0873 | 0.0713 | 0.0612 |
When the pyrolysis temperature was above 450 °C and the pyrolysis time was 30 min, the oil content of the sludge after pyrolysis reached the standard. The different components of the sludge samples before and after pyrolysis were further measured to analyze whether they could meet the sludge treatment standards. The measurement results are shown in Table 9.
| No. | Item | Oily sludge mg kg−1 | Sludge containing polymers mg kg−1 | Standard category | Remarks | ||
|---|---|---|---|---|---|---|---|
| Provincial standard (well pad site) DB23/T 1413-2010 | Provincial standard (agricultural use) DB23/T 1413-2010 | National standard (B level) GB 4284-2018 | |||||
| 1 | Petroleum | 1022 | 1368 | ≤20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
≤3000 | <3000 | Qualification after pyrolysis |
| 2 | As | 3.6 | 7.7 | — | ≤75 | <75 | Qualification |
| 3 | Hg | 0.145 | 0.563 | ≤0.8 | ≤15 | <15 | Qualification |
| 4 | Cr | 142 | 176 | — | ≤1000 | <1000 | Qualification |
| 5 | Cu | 541 | 724 | ≤150 | ≤500 | <1500 | Accords with national standard B level |
| 6 | Zn | 216 | 368 | ≤600 | ≤1000 | <3000 | Qualification |
| 7 | Ni | 66 | 76.1 | ≤150 | ≤200 | <200 | Qualification |
| 8 | Pb | 73.7 | 142 | ≤375 | ≤1000 | <1000 | Qualification |
| 9 | Cd | 0.7 | 1.9 | ≤3 | ≤20 | <15 | Qualification |
| 10 | pH | 8 | 7.07 | ≥6 | Soil pH ≥ 6.5 | — | Qualification |
| 11 | Water content% | 1.88 | 0.724 | ≤40% | — | — | Qualification |
The content of pollutants in the oily sludge before and after pyrolysis was analyzed. It can be seen from Table 9 that due to the high content of Cu in the oily sludge from Daqing Oilfield, the Cu content of the oily sludge before and after pyrolysis did not meet the pollution control indexes of the well pad site and agricultural use according to the local standards of Heilongjiang for “Pollution Control Standard for Comprehensive Utilization of Oily Sludge in an Oilfield” (DB23/T 1413-2010), although it was in accordance with the B level sludge product pollution limit requirements of the national standard “Control standards of pollutants in sludge for agricultural use” (GB 4284-2018). This showed that the oily sludge and sludge containing polymers after conditioning and centrifugation could meet the treatment requirements after thermal decomposition. This technology has a strong adaptability to different types of oilfield sludge.
4 Conclusions
The compositions of oily sludge and sludge containing polymer are highly complex, so they cannot meet the requirements of environmental protection through using simple conditioning centrifugal technology. The high efficiency treatment of oily sludge and sludge containing polymers can be realized by combining conditioning centrifugal technology with thermal decomposition technology. After testing, the oily sludge and sludge containing polymers after undergoing conditioning and centrifugation could meet the treatment requirements after thermal decomposition. This technology has strong adaptability to different types of oilfield sludge.Conflicts of interest
There are no conflicts to declare.References
- C. Zhong, Z. Zheng and C. He, et al., Oily Sludge Treatment In Subcritical and Supercritical Water: A Review, J. Hazard. Mater., 2022, 433, 128761 CrossRef PubMed.
- E. E. Barskaya, E. S. Okhotnikova and Y. M. Ganeeva, et al., Distribution and Composition of High-Molecular-Mass Components in Oily Sludge, Pet. Chem., 2022, 62(2), 151–160 CrossRef CAS.
- Z. Que, Y. Fu, J. Shi, X. Ai and C. Xu, Pyrolysis and Volatile Evolution Behaviors of Cold-Rolling Oily Sludge, Processes, 2022, 10, 543, DOI:10.3390/pr10030543.
- H. Zhang, F. Chen and J. Xu, et al., Chemical reactions of oily sludge catalyzed by iron oxide under supercritical water gasification condition, Front. Chem. Sci. Eng., 2022, 6, 886–896 CrossRef.
- H. Qi, H. Jiang, Y. You, J. Hu, Y. Wang, Z. Wu and H. Qi, Mechanism of Magnetic Nanoparticle Enhanced Microwave Pyrolysis for Oily Sludge, Energies, 2022, 15, 1254, DOI:10.3390/en15041254.
- H. Xu, L. Qiang and X. Wu, et al., Oil Sludge Treatment by a Microemulsion System Containing Sodium Dodecyl Benzene Sulfonate, Chem. Technol. Fuels Oils, 2022, 57(6), 1000–1004 CrossRef CAS.
- H. Liu, C. Xiang and J. Mu, et al., Thermodynamic model and kinetic compensation effect of oil sludge pyrolysis based on thermogravimetric analysis, Therm. Sci., 2020, 350 CAS.
- S. A. Peng, A. Yg and L. A. Jiao, et al., Recycling of crude oil from oily wastewater via a novel hydrogel coalescer, Fuel, 2022, 313(1), 123040 Search PubMed.
- B. Hfsa, A. Jfl and A. Qyw, A clean production process on oily sludge with a novel collaborative process via integrating multiple approaches, J. Cleaner Prod., 2021, 322, 128983 CrossRef.
- P. Peng, S. Guo and L. Li, et al., Supercritical water gasification mechanism of polymer containing oily sludge, Int. J. Hydrogen Energy, 2021, 46(53), 26834–26847 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |