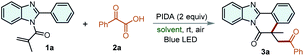Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visible-light-promoted decarboxylative radical cascade cyclization to acylated benzimidazo/indolo[2,1-a]isoquinolin-6(5H)-ones in water†
Lili Tanga,
Yuejun Ouyang*a,
Kai Sun*ab and
Bing Yu *b
*b
aHunan Engineering Research Center for Recycled Aluminum, College of Chemistry & Materials Engineering, Huaihua University, Huaihua 418008, China. E-mail: oyyj0816@163.com; sunkchem@163.com
bCollege of Chemistry, Zhengzhou University, Zhengzhou 450001, China. E-mail: bingyu@zzu.edu.cn
First published on 7th July 2022
Abstract
A metal-free visible-light-induced decarboxylative radical addition/cyclization procedure at room temperature was described for the synthesis of acylated benzimidazo/indolo[2,1-a]isoquinolines. The procedure was prepared in water via a reaction of functionalized 2-arylbenzoimidazoles or 2,3-diarylindoles and α-oxocarboxylic acids in the presence of phenyliodine(III) diacetate (PIDA) in one step under mild reaction conditions. In this procedure, traditional heating and metal reagents could be effectively avoided to access 1,4-dicarbonyl-containing benzimidazo/indolo[2,1-a]isoquinoline-6(5H)-ones in satisfactory yields.
In recent years, photocatalysis has proven to be a sustainable and green synthetic platform which can efficiently convert light energy into chemical energy to form new chemical bonds.1 However, most photocatalytic organic conversions are performed in toxic and volatile organic solvents.2 Compared with traditional organic solvents, water has unique features to be a promising alternative to volatile solvents with the advantages of being a safe, non-toxic, abundant, and eco-friendly natural source.3 Undoubtedly, the development of novel visible-light promoted synthetic methods in water is more in line with the principles of green chemistry, which is highly desired.
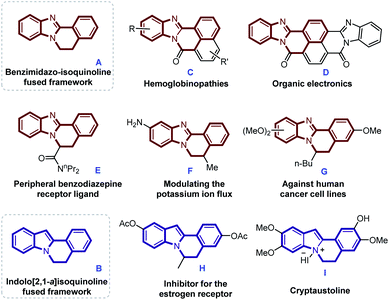
Nitrogen-containing heterocyclic compounds play extremely important roles in medicinal chemistry, functional materials, and natural products.4 Especially, benzimidazole-isoquinoline fused framework A and indole[2,1-a]isoquinoline containing tetracyclic core structure B are not only widely present in some biologically active molecules, but also as important components of synthetic intermediates and functional materials.5 Several representative polyheterocycles containing skeletons A and B with promising applications in the field of treating hemoglobinopathies and cancer, peripheral benzodiazepine receptor ligand, modulating the potassium ion flux or organic electronics are shown in Scheme 1.6 Benefit from their significant importance, the developments of novel synthetic method for benzimidazo/indolo[2,1-a]isoquinolin-6(5H)-ones have attracted considerable attention in the organic synthetic field.
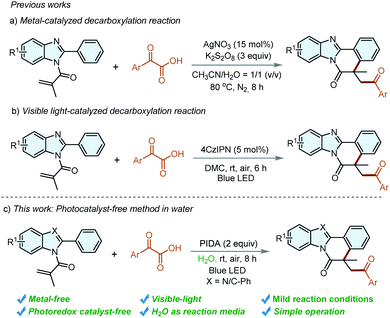
Recently, α-oxocarboxylic acids have gained much prominence to be acylation reagents owing to their easy preparation, stability, and high reactivity.7 However, most transformations that employed α-oxocarboxylic acids as the acyl radical precursor require high-cost noble-metal-based catalysts8 or some metal-free organocatalysts.9 Radical-initiated cyclizations reaction has developed to be efficient processes for the construction of benzimidazo/indolo-isoquinoline-6(5H)-ones,10 and the introduction of an acyl group to these meaningful frameworks has been realized using α-oxocarboxylic acids as acyl radical precursors. For instance, our group reported a decarboxylative cascade cyclization of N-methacryloyl-2-phenylbenzoimidazole with α-oxocarboxylic acids for the construction of acylated benzimidazo[2,1-a]isoquinolin-6(5H)-ones in the presence of AgNO3/K2S2O8 in 2019 (Scheme 2a).11 However, the requirement of a high-cost metal catalyst, high reaction temperature and strong oxidant is unavoidable. Very recently, our group disclosed a metal-free visible-light-induced decarboxylative arylation procedure for the construction of acylated benzimidazo/indolo[2,1-a]isoquinolines by using 2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile (4CzIPN) as a photocatalyst in organic solvents (Scheme 2b).12 However, the increased manufacturing cost and inconvenient operation of eliminating the photocatalyst impede the applications of these methods in practical application, especially in the pharmaceutical industry. It's still highly desirable to explore a photocatalyst-free approach for the synthesis of acylated benzimidazo/indolo[2,1-a]isoquinolines in a sustainable reaction media.
With our continuing interests in green synthesis,13 we herein describe an environmentally-friendly strategy to access benzimidazo/indolo[2,1-a]isoquinolin-6(5H)-ones under the irradiation of blue light. To the best of our knowledge, this is the first example of the synthesis of acylated indolo/benzimidazo[2,1-a]isoquinolin-6(5H)-ones under irradiation of visible light in water, in which additional photocatalyst and volatile organic solvents could be effectively avoided.
For our initial explorations, we started our study by using 2-methyl-1-(2-phenyl-1H-benzo[d]imidazol-1-yl)prop-2-en-1-one (1a) and 2-oxo-2-phenylacetic acid (2a) as the model substrates for the optimization of the reaction conditions in various solvents under irradiation of 5 W blue LED (Table 1). To our delight, this transformation reacted smoothly in presence of PIDA in MeCN or DCE for 8 h to access the desired product in 90% and 61% yield, respectively (entries 1–2). To reduce the impact of solvents on the environment, some green solvents including DMC, PC, H2O, 2-CH3-THF, EG, TBME, PEG-200, and PEG-400 (entries 2–10) were investigated. Most of the tested solvents could serve as reaction medium, and 80% yield of desired product 3a could be obtained when the reaction was performed in water. Considering that water has the advantages of excellent biocompatibility, non-combustibility and high specific heat capacity, the employment of water as solvents for organic transformation is desirable and attractive.14 Therefore, we finally chose H2O as the best reaction solvent. To further improve the reaction efficiency, mixed solvents, such as MeOH/water, EtOH/water, THF/water in different ratios were examined (entries 11–16). However, no positive results could be obtained. Next, several control experiments were conducted, which showed that PIDA and light were essential for the transformation (entries 17 and 18). Moreover, the reaction that happened under the N2 atmosphere provided slightly lower yields than that in the air (entry 19). Ultimately, the optimal reaction conditions were established as follows: 1a (0.2 mmol), 2a (0.4 mmol), and PIDA (0.4 mmol) in H2O at room temperature for 8 h under air atmosphere with the irradiation of 5 W blue LED.
| Entry | Solvent | Yield (%) |
|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.4 mmol), PIDA (0.4 mmol), solvent (2 mL) under air for 8 h, rt, 430 nm Blue LED (5 W). N.D. = Not detected. Isolated yields were given. DCE = 1,2-dichloroethane, DMC = dimethyl carbonate. TBME = tert-butyl methyl ether, EG = ethylene glycol, PC = propylene carbonate.b Without PIDA.c Experiment performed in the dark.d Reaction under N2 atmosphere. | ||
| 1 | MeCN | 90 |
| 2 | DCE | 61 |
| 3 | DMC | 86 |
| 4 | PC | Trace |
| 5 | H2O | 80 |
| 6 | 2-CH3-THF | 42 |
| 7 | EG | 19 |
| 8 | TBME | 57 |
| 9 | PEG-200 | Trace |
| 10 | PEG-400 | Trace |
| 11 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (v/v = 1 EtOH (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
77 |
| 12 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (v/v = 2 EtOH (v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
70 |
| 13 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (v/v = 1 MeOH (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
78 |
| 14 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (v/v = 2 MeOH (v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
72 |
| 15 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF (v/v = 1 THF (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
61 |
| 16 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF (v/v = 2 THF (v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
68 |
| 17b | H2O | N.D. |
| 18c | H2O | N.D. |
| 19d | H2O | 74 |
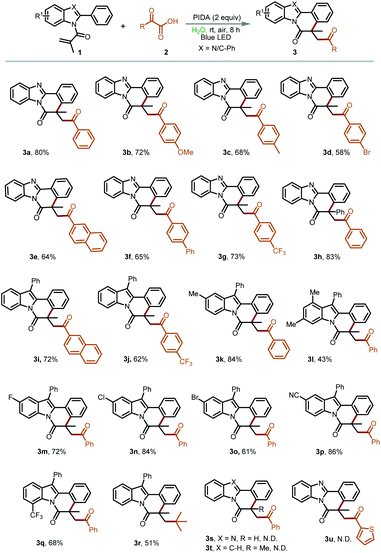
With the above-optimized reaction conditions established, we subsequently started to explore the substrate scope and limitations of this transformation (Table 2). Initially, a series of α-oxocarboxylic acids 2 were employed as the acyl radical precursors to react with 2-methyl-1-(2-phenyl-1H-benzo[d]imidazol-1-yl)prop-2-en-1-one (1a) under the standard reaction conditions. It was pleasing to see that the α-keto acids with both electron-donating and electron-withdrawing substituents were all suitable substrates to be converted into the desired annulation products 3a–3g in good yields (58–80%). Then, N-phenylacryloyl-2-phenylbenzoimidazole (1h) was utilized to react with 2a under the standard conditions. The target product 3h was obtained in an 83% yield, demonstrating negligible steric hindrance effects. Afterward, the reactivity of the 2-aryl indoles was evaluated. To our delight, indole derivatives bearing –Me, –F, –Cl, –Br, –CF3, and –CN on the indole ring were all compatible to deliver the annulation reaction products 3i–3q in moderate to good yields (43–86%). Notably, an unexpected product 3r was observed when 3,3-dimethyl-2-oxobutyric acid was used as the acyl radical precursor. We assumed that the in situ generated 3,3-dimethyl-2-oxobutyric radical could be converted into the more stable tert-butyl radical in this process. Regrettably, 1-(2-phenyl-1H-benzo[d]imidazol-1-yl)prop-2-en-1-one, 2-methyl-1-(2-phenyl-1H-indol-1-yl)prop-2-en-1-one and 2-thiopheneglyoxylic acid were not suitable substrates in this transformation (3s–u).
| a Reaction conditions: 1 (0.2 mmol), 2 (0.4 mmol) and PIDA (0.4 mmol) in H2O (2 mL) with the irradiation of 5 W blue LED under air at room temperature for 8 h. Isolated yields. |
|---|
 |
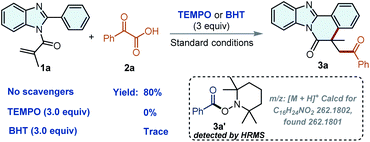
Subsequently, we conducted some control experiments to explore the mechanism process in depth. When 3.0 equiv. of 2,2,6,6-tetramethylpiperidin-1-yl-oxidanyl (TEMPO) or 2,6-di-tert-butyl-4-methylphenol (BHT) as radical scavengers were subjected to the reaction, the reaction could be completely suppressed, respectively (Scheme 3). Additionally, the complex of TEMPO-benzoyl radical was observed by high-resolution mass spectrometry (HRMS), indicating that a radical process might be involved in this transformation (Fig. S2†).
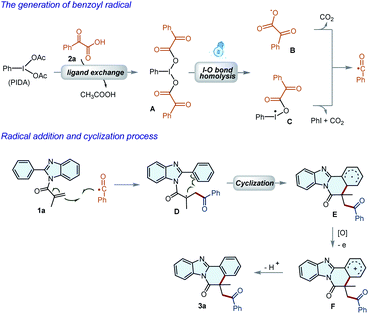
Based on the experimental results and previous reports,15 a plausible mechanism for the reaction was thus proposed as illustrated in Scheme 4. First, a ligand exchange interaction between PIDA and 2a led to the formation of hypervalent iodine(III) reagent A, which underwent I–O bond cleavage under the irradiation of visible light to deliver radicals B and C. Subsequently, the benzoyl radical could be generated via the fragmentation of B and C. The benzoyl radical was then added to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of 1a to afford a radical species D, which underwent an intramolecular cyclization to give radical E. Radical E was then converted into carbocation F by PIDA or oxygen through a single-electron oxidation process. Finally, product 3a could be formed by rapid deprotonation of the carbon cation F.
C bond of 1a to afford a radical species D, which underwent an intramolecular cyclization to give radical E. Radical E was then converted into carbocation F by PIDA or oxygen through a single-electron oxidation process. Finally, product 3a could be formed by rapid deprotonation of the carbon cation F.
Conclusions
In summary, we have successfully disclosed a metal-free visible-light-induced decarboxylative radical cyclization process to construct acylated benzimidazo/indolo[2,1-a]isoquinolines in water under photoredox catalyst-free conditions. The remarkable advantages of this strategy include photoredox catalyst and metal-free reaction conditions, sustainable reaction medium, room reaction temperature, and high efficiency. Further explorations on the applications of visible-light-induced organic synthetic transformations are currently ongoing in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Hunan Provincial Natural Science Foundation of China (2021JJ40432, 2020JJ4073).Notes and references
-
(a) Q. Yan, W. Cui, J. Li, G. Xu, X. Song, J. Lv and D. Yang, Org. Chem. Front., 2022, 9, 2653 RSC
; (b) P. Xiang, K. Sun, S. Wang, X. Chen, L. Qu and B. Yu, Chin. Chem. Lett., 2022 DOI:10.1016/j.cclet.2022.03.096
; (c) D. Yang, Q. Yan, E. Zhu, J. Lv and W.-M. He, Chin. Chem. Lett., 2022, 33, 1798 CrossRef CAS
; (d) X.-Y. Yu, J.-R. Chen and W.-J. Xiao, Chem. Rev., 2021, 121, 506 CrossRef CAS PubMed
; (e) I. D. Lemir, W. D. Castro-Godoy, A. A. Heredia, L. C. Schmidt and J. E. Argüello, RSC Adv., 2019, 9, 22685 RSC
; (f) Y. Zhang, C. Ye, S. Li, A. Ding, G. Gu and H. Guo, RSC Adv., 2017, 7, 13240 RSC
; (g) Q.-W. Gui, F. Teng, Z.-C. Li, Z.-Y. Xiong, X.-F. Jin, Y.-W. Lin, Z. Cao and W.-M. He, Chin. Chem. Lett., 2021, 32, 1907 CrossRef CAS
; (h) B. Wang, L. Zou, L. Wang, M. Sun and P. Li, Chin. Chem. Lett., 2021, 32, 1229 CrossRef CAS
; (i) Z. Wang, L. Wang, Z. Wang, P. Li and Y. Zhang, Chin. Chem. Lett., 2021, 32, 429 CrossRef CAS
.
-
(a) L. Wang, M. Zhang, Y. Zhang, Q. Liu, X. Zhao, J.-S. Li, Z. Luo and W. Wei, Chin. Chem. Lett., 2020, 31, 67 CrossRef CAS
; (b) L.-P. Tan, D. Liang, Y. Cheng, W.-J. Xiao and J.-R. Chen, Org. Chem. Front., 2021, 8, 5052 RSC
; (c) X. Li, M. Jiang, X. Zhu, X. Song, Q. Deng, J. Lv and D. Yang, Org. Chem. Front., 2022, 9, 386 RSC
; (d) M. Ji, L. Xu, X. Luo, M. Jiang, S. Wang, J.-Q. Chen and J. Wu, Org. Chem. Front., 2021, 8, 6704 RSC
; (e) B. Cai and J. Xuan, Chin. J. Org. Chem., 2021, 41, 4565 CrossRef
; (f) Y. Sun, J. Song, Q. Qin, E. Zhang, Q. Han, S. Yang, Z. Wang, S. Yue and D. Dong, Chin. J. Org. Chem., 2021, 41, 4651 CrossRef
; (g) S. He, H. Li, X. Chen, I. B. Krylov, A. O. Terent'ev, L. Qu and B. Yu, Chin. J. Org. Chem., 2021, 41, 4661 CrossRef
.
-
(a) K. Sun, Q.-Y. Lv, X.-L. Chen, L.-B. Qu and B. Yu, Green Chem., 2021, 23, 232 RSC
; (b) X.-Y. Yuan, F.-L. Zeng, H.-L. Zhu, Y. Liu, Q.-Y. Lv, X.-L. Chen, L. Peng and B. Yu, Org. Chem. Front., 2020, 7, 1884 RSC
.
-
(a) T. Shi, Y.-T. Liu, S.-S. Wang, Q.-Y. Lv and B. Yu, Chin. J. Chem., 2022, 40, 97 CrossRef CAS
; (b) N. Neerathilingam and R. Anandhan, RSC Adv., 2022, 12, 8368 RSC
; (c) D.-Y. Liu, X. Liu, Y. Gao, C.-Q. Wang, J.-S. Tian and T.-P. Loh, Org. Lett., 2020, 22, 8978 CrossRef CAS PubMed
; (d) T. Xiao, L. Li, Y. Xie, Z.-W. Mao and L. Zhou, Org. Lett., 2016, 18, 1004 CrossRef CAS PubMed
; (e) Z.-L. Wu, J.-Y. Chen, X.-Z. Tian, W.-T. Ouyang, Z.-T. Zhang and W.-M. He, Chin. Chem. Lett., 2022, 33, 1501 CrossRef CAS
; (f) Z. Wang, Q. Liu, R. Liu, Z. Ji, Y. Li, X. Zhao and W. Wei, Chin. Chem. Lett., 2022, 33, 1479 CrossRef CAS
.
-
(a) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed
; (b) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS PubMed
; (c) M. Somei and F. Yamada, Nat. Prod. Rep., 2004, 21, 278 RSC
; (d) C. Bressy, D. Alberico and M. Lautens, J. Am. Chem. Soc., 2005, 127, 13148 CrossRef CAS PubMed
; (e) N. Fuentes, W. Kong, L. Fernández-Sánchez, E. Merino and C. Nevado, J. Am. Chem. Soc., 2015, 137, 964 CrossRef CAS PubMed
; (f) W.-t. Wei, X.-j. Dong, S.-z. Nie, Y.-y. Chen, X.-j. Zhang and M. Yan, Org. Lett., 2013, 15, 6018 CrossRef CAS PubMed
.
-
(a) K. Alam, S. W. Hong, K. H. Oh and J. K. Park, Angew. Chem., Int. Ed., 2017, 56, 13387 CrossRef CAS PubMed
; (b) J. Cai, B. Wu, G. Rong, C. Zhang, L. Qiu and X. Xu, Org. Lett., 2018, 20, 2733 CrossRef CAS PubMed
; (c) Y.-C. Chou, R.-L. Chen, Z.-S. Lai, J.-S. Song, Y.-S. Chao and C.-K. J. Shen, Mol. Cell. Biol., 2015, 35, 2541 CrossRef CAS PubMed
; (d) B. Liu, F. Hu and B.-F. Shi, Adv. Synth. Catal., 2014, 356, 2688 CrossRef CAS
; (e) J. Liu, Z. Xue, Z. Zeng, Y.-x. Chen and G. Chen, Adv. Synth. Catal., 2016, 358, 3694 CrossRef CAS
; (f) S. Mai, Y. Luo, X. Huang, Z. Shu, B. Li, Y. Lan and Q. Song, Chem. Commun., 2018, 54, 10240 RSC
; (g) C. C. McAtee, P. S. Riehl and C. S. Schindler, J. Am. Chem. Soc., 2017, 139, 2960 CrossRef CAS PubMed
; (h) M. J. Taublaender, F. Glöcklhofer, M. Marchetti-Deschmann and M. M. Unterlass, Angew. Chem., Int. Ed., 2018, 57, 12270 CrossRef CAS PubMed
; (i) J. Yin, F. Zhou, L. Zhu, M. Yang, Y. Lan and J. You, Chem. Sci., 2018, 9, 5488 RSC
; (j) Z. Zeng, H. Jin, K. Sekine, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2018, 57, 6935 CrossRef CAS PubMed
; (k) Y. Li, J. Zhu, H. Xie, S. Li, D. Peng, Z. Li, Y. Wu and Y. Gong, Chem. Commun., 2012, 48, 3136 RSC
; (l) S. J. Mahoney and E. Fillion, Chem.–Eur. J., 2012, 18, 68 CrossRef CAS PubMed
; (m) K. Morimoto, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2010, 12, 2068 CrossRef CAS PubMed
; (n) M.-H. Shen, Y.-P. Pan, Z.-H. Jia, X.-T. Ren, P. Zhang and H.-D. Xu, Org. Biomol. Chem., 2015, 13, 4851 RSC
.
-
(a) S. Manna and K. R. Prabhu, J. Org. Chem., 2019, 84, 5067 CrossRef CAS PubMed
; (b) F. Penteado, E. F. Lopes, D. Alves, G. Perin, R. G. Jacob and E. J. Lenardão, Chem. Rev., 2019, 119, 7113 CrossRef CAS PubMed
; (c) K. Xie, M. Jiang, X. Chen, Q. Lv and B. Yu, Chin. J. Org. Chem., 2021, 41, 4575 CrossRef
; (d) X. Zhang, P. Zhu, R. Zhang, X. Li and T. Yao, J. Org. Chem., 2020, 85, 9503 CrossRef CAS PubMed
.
-
(a) J. Liu, Q. Liu, H. Yi, C. Qin, R. Bai, X. Qi, Y. Lan and A. Lei, Angew. Chem., Int. Ed., 2014, 53, 502 CrossRef CAS PubMed
; (b) A. B. Rolka and B. Koenig, Org. Lett., 2020, 22, 5035 CrossRef CAS PubMed
; (c) L. Chu, J. M. Lipshultz and D. W. C. MacMillan, Angew. Chem., Int. Ed., 2015, 54, 7929 CrossRef CAS PubMed
; (d) N. Xu, P. Li, Z. Xie and L. Wang, Chem.–Eur. J., 2016, 22, 2236 CrossRef CAS PubMed
.
-
(a) Q. Shi, P. Li, X. Zhu and L. Wang, Green Chem., 2016, 18, 4916 RSC
; (b) Y. Su, R. Zhang, W. Xue, X. Liu, Y. Zhao, K.-H. Wang, D. Huang, C. Huo and Y. Hu, Org. Biomol. Chem., 2020, 18, 1940 RSC
; (c) D.-L. Zhu, Q. Wu, D. J. Young, H. Wang, Z.-G. Ren and H.-X. Li, Org. Lett., 2020, 22, 6832 CrossRef CAS
.
-
(a) R. Boora, G. R. Kumar and B. V. Subba Reddy, Org. Biomol. Chem., 2019, 17, 9627 RSC
; (b) Y.-L. Wei, J.-Q. Chen, B. Sun and P.-F. Xu, Chem. Commun., 2019, 55, 5922 RSC
.
- K. Sun, S.-J. Li, X.-L. Chen, Y. Liu, X.-Q. Huang, D.-H. Wei, L.-B. Qu, Y.-F. Zhao and B. Yu, Chem. Commun., 2019, 55, 2861 RSC
.
- H.-L. Zhu, F.-L. Zeng, X.-L. Chen, K. Sun, H.-C. Li, X.-Y. Yuan, L.-B. Qu and B. Yu, Org. Lett., 2021, 23, 2976 CrossRef CAS
.
-
(a) K. Sun, A. Shi, Y. Liu, X. Chen, P. Xiang, X. Wang, L. Qu and B. Yu, Chem. Sci., 2022, 13, 5659 RSC
; (b) A. Shi, K. Sun, X. Chen, L. Qu, Y. Zhao and B. Yu, Org. Lett., 2022, 24, 299 CrossRef CAS
.
-
(a) J. Li, W. Tang, D. Ren, J. Xu and Z. Yang, Green Chem., 2019, 21, 2088 RSC
; (b) X. C. Liu, X. L. Chen, Y. Liu, K. Sun, Y. Y. Peng, L. B. Qu and B. Yu, ChemSusChem, 2019, 13, 298 CrossRef PubMed
; (c) X.-Y. Li, Y. Liu, X.-L. Chen, X.-Y. Lu, X.-X. Liang, S.-S. Zhu, C.-W. Wei, L.-B. Qu and B. Yu, Green Chem., 2020, 22, 4445 RSC
.
-
(a) J. Lu, X. K. He, X. Cheng, A. J. Zhang, G. Y. Xu and J. Xuan, Adv. Synth. Catal., 2020, 362, 2178 CrossRef
; (b) H. Tan, H. Li, W. Ji and L. Wang, Angew. Chem., Int. Ed., 2015, 54, 8374 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03467k |
| This journal is © The Royal Society of Chemistry 2022 |