Open Access Article
Open Access ArticleI2-mediated Csp2–P bond formation via tandem cyclization of o-alkynylphenyl isothiocyanates with organophosphorus esters†
Yang Liu,
Wenjin Wu,
Xiaoyan Sang,
Yu Xia,
Guojian Fang and
Wenyan Hao *
*
College of Chemistry and Chemical Engineering, Jiangxi Normal University, Nanchang 330022, People's Republic of China. E-mail: wenyanhao@jxnu.edu.cn
First published on 20th June 2022
Abstract
A novel, I2-mediated tandem cyclization of o-alkynylphenyl isothiocyanates with organophosphorus esters has been developed under mild conditions. Different kinds of 4H-benzo[d][1,3]thiazin-2-ylphosphonate could be synthesized in moderate to excellent yields. This method has the advantages of easy access to raw materials, free-metal catalyst, simple operation, high yield and high functional group tolerance.
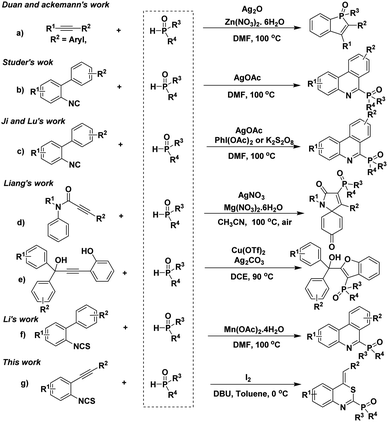
As an important class of organic products, organophosphorus compounds have received considerable attention because they have broad application in the field of materials science,1 medicinal chemistry,2 organic synthesis,3 natural products,4 and ligand chemistry.5 Phosphorus-containing compounds are valuable precursors of many biologically active molecules which can act as antibiotics,6 anti-tumor agents7 and enzyme inhibitors.8 Traditionally, the preparation of organophosphorus compounds relies on a transition-metal-catalyzed cross-coupling of phosphine reagents with electrophilic aryl halides (Ar-X),9 aryl boronic acids (Ar-B),10 aryl diazonium salts (Ar-N),11 and so on.12 Recently, the construction of a Csp2–P bond on heterocycles is another powerful method to synthesize the organophosphorus compounds.13 For instance, the Duan group and the Ackermann group reported a Ag-mediated C–H/P–H functionalization method to construct a Csp2–P bond by using arylphosphine oxides and internal alkynes as the substrates13a,b (Scheme 1a). In 2014, Studer and co-workers reported a pioneering radical cascade reaction for the synthesis of 6-phosphorylated phenanthridines from 2-isocyanobiphenyls and diphenylphosphine oxides (Scheme 1b).13c Before long, Ji and Lu's group described two similar radical process with excess of PhI(OAc)2 or K2S2O8 as the oxidant (Scheme 1c).13d,e Recently, Liang and co-works developed two cases of cascade functionalization to construct phosphorylated heterocycles via the ionic pathway (Scheme 1d and 1e).13f,g Meanwhile, Li and coworkers reported a Mn(II)-promoted tandem cyclization reaction of 2-biaryl isothiocyanates with phosphine oxides which went through the same mechanism(Scheme 1f).13h Despite the usefulness of the above methods, common problems, such as complex reaction substrates, relatively high temperature, excess amounts of oxidants, limited their applications. Furthermore, transition metals are required in these reactions, thereby resulting in limitations in reactants. Therefore, the development of a simple and transition-metal-free method for the formation of the Csp2–P bond from easily prepared starting materials is highly desirable.
o-Alkynylphenyl isothiocyanates are easily prepared organic synthons with versatile chemical reactivity,14 and they could be used as electrophiles,15 nucleophiles,16 and radical receptors17 due to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S moiety in the structure. Recently, the rapid development of the transition-metal-catalyzed cascade cycloaddition of o-alkynylphenyl isothiocyanates with various nucleophiles provides a new and powerful synthetic strategy to synthesize different heterocycles. Very recently, we have developed a tandem cyclization process for the synthesis of 4H-benzo[d][1,3]thiazin-2-yl)phosphonates by using this strategy.18 As part of our continuing interest in the transformation of o-alkynylphenyl isothiocyanates,19 we describe herein a novel I2-promotedtandem reaction to construct Csp2–P bond from o-alkynylphenyl isothiocyanates and phosphine oxides(Scheme 1g).
S moiety in the structure. Recently, the rapid development of the transition-metal-catalyzed cascade cycloaddition of o-alkynylphenyl isothiocyanates with various nucleophiles provides a new and powerful synthetic strategy to synthesize different heterocycles. Very recently, we have developed a tandem cyclization process for the synthesis of 4H-benzo[d][1,3]thiazin-2-yl)phosphonates by using this strategy.18 As part of our continuing interest in the transformation of o-alkynylphenyl isothiocyanates,19 we describe herein a novel I2-promotedtandem reaction to construct Csp2–P bond from o-alkynylphenyl isothiocyanates and phosphine oxides(Scheme 1g).
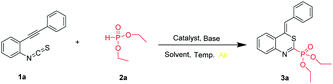
The starting o-alkynylphenyl isothiocyanates were prepared via the Sonogashira coupling of 2-iodoanilines with terminal alkynes,20 followed by the treatment with thiophosgene according to the literature procedure.21 We commenced our studies with the reaction of o-phenylethynylphenyl isothiocyanate (1a, 0.2 mmol) and diethyl phosphonate (2a, 0.6 mmol) in the presence of I2 (0.5 equiv.) as the catalyst, 8-diazabicyclo[5,4,0]undec-7-ene (DBU, 3.0 equiv.) as the base, in dichloromethane (DCM, 2 mL) at 0 °C for 12 h in air atmosphere. Gratifyingly, the desired product diethyl (Z)-(4-benzylidene-4H-benzo[d][1,3] thiazin-2-yl)phosphonate 3a was obtained in 75% yield (Table 1, entry 1). Next, different iodized salts, no better results can be obtained (Table 1, entries 2–4). It is worth noting that no product was obtained in the absence of base (Table 1, entry 5). This result indicated that a base is indispensable to afford the target product. Subsequently, we examined the base effect on the reaction (Table 1, entries 6–11). The reaction can hardly proceed when other bases such as DABCO, KOAc, NaOAc, K2HPO4, Cs2CO3 and NaOH were employed. We next examined the solvent effect (Table 1, entries 12–17). When toluene was employed as the solvent, the highest yield of 84% was obtained. Then, we examined the effect of temperature on the reaction. When the reaction temperature was increased to room temperature, the reaction was completed with a yield of 70% (Table 1, entry 18). Increasing the reaction temperature to 80 °C or reducing the reaction temperature to −10 °C resulted in a diminished yield (Table 1, entries 19–21) (Schemes 1–4)
| Entry | Catalyst | Base | Solvent | Temp. | Yield (%)b |
|---|---|---|---|---|---|
| a Reaction was performed with 1a (0.2 mmol), 2a (0.6 mmol), catalyst (0.1 mmol), base (0.6 mmol), in solvent (2 mL) for 12 h.b Isolated yield based on o-phenylethynylphenyl isothiocyanate 1a. | |||||
| 1 | I2 | DBU | DCM | 0 °C | 75 |
| 2 | KI | DBU | DCM | 0 °C | 64 |
| 3 | NaI | DBU | DCM | 0 °C | 50 |
| 4 | ZnI2 | DBU | DCM | 0 °C | 55 |
| 5 | I2 | — | DCM | 0 °C | NR |
| 6 | I2 | DABCO | DCM | 0 °C | Trace |
| 7 | I2 | KOAc | DCM | 0 °C | NR |
| 8 | I2 | NaOAc | DCM | 0 °C | NR |
| 9 | I2 | K2HPO4 | DCM | 0 °C | NR |
| 10 | I2 | Cs2CO3 | DCM | 0 °C | Trace |
| 11 | I2 | NaOH | DCM | 0 °C | Trace |
| 12 | I2 | DBU | DCE | 0 °C | 28 |
| 13 | I2 | DBU | CHCl3 | 0 °C | 32 |
| 14 | I2 | DBU | DMF | 0 °C | Trace |
| 15 | I2 | DBU | 1,4-Dioxane | 0 °C | 50 |
| 16 | I2 | DBU | MeCN | 0 °C | 35 |
| 17 | I2 | DBU | Toluene | 0 °C | 84 |
| 18 | I2 | DBU | Toluene | 25 °C | 70 |
| 19 | I2 | DBU | Toluene | 40 °C | 52 |
| 20 | I2 | DBU | Toluene | 80 °C | 42 |
| 21 | I2 | DBU | Toluene | −10 °C | 66 |
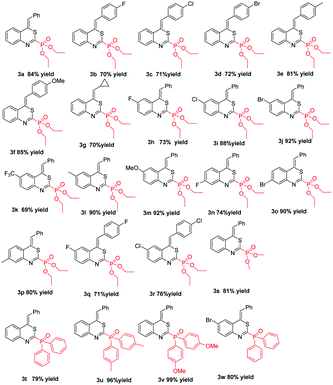
In order to further demonstrate the substrate scope, different o-alkynyl phenylisothiocyanates were then explored and the results are summarized in Table 2. All reactions proceeded smoothly, leading to the desired 4H-benzo[d][1,3]thiazin-2-yl phosphonates in moderate to good yields. For example, the reactions of o-alkynyl phenylisothiocyanates 1b–1f completed at 0 °C in 12 h to give corresponding products 3b–3f in 70–85% yields. Among them, substrates 1 with an electron-rich aryl group such as p-MeOC6H4 and p-MeC6H4 at the R2 position, showed good results (81% and 85%, 3e and 3f). As reported in our other articles,19 no desired products were obtained when the R2 group in the substrate o-alkynylphenyl isothiocyanates 1 was an alkyl group, such as n-butyl, t-butyl, and n-hexyl. Similarly, when the R2 group in the substrate o-alkynylphenyl isothiocyanates 1 was the cyclopropyl group, the desired desired 4H-benzo[d][1,3]thiazin-2-yl phosphonate 3g was obtained in 70% yield. On the other hand, the reactions of o-alkynylphenyl isothiocyanates bearing various substituents such as fluoro, chloro, bromo, trifluoromethyl, methyl and methoxy groups on the aryl rings at the R1 position, regardless of their electronic properties and substitution positions, gave the desired products 3h–3r in moderate to good yields. Particularly, p-Br substituted 1j and 1o appeared excellent reactivity and the corresponding products 3j and 3o were obtained in 92% and 90% yield, respectively. In order to further expand the substrate scope, we moved on to examine the P-reagents under the optimal conditions. The reaction of dimethyl phosphate and diphenylphosphine oxide with 1a under the standard conditions, the corresponding products dimethyl (Z)-(4-benzylidene-4H-benzo[d][1,3]thiazin-2-yl)phosphonate and (Z)-(4-benzylidene-4H-benzo[d][1,3]thiazin-2-yl)diphenyl phosphine oxide (3s and 3t) were obtained in 81% yield and 79% yield, respectively. It is noteworthy that the corresponding target products (3u and 3v) with excellent yield (96% and 99% yield) are obtained when we replace diphenylphosphine oxide with di-p-tolylphosphine oxide and bis(4-methoxyphenyl)phosphine oxide. Similarly, all products were uniformly formed as the Z-isomer, which might be due to a kinetic effect according to Baldwin's rules and a smaller steric effect compared to the E-isomer.22
Next, we examined the reaction of 2-isothiocyanato-3-(phenylethynyl)pyridine 1x with 2a under the standard conditions (Scheme 2). Not surprisingly, the corresponding product 3x diethyl (Z)-(4-benzylidene-4H-pyrido[2,3-d][1,3]thiazin-2-yl) phosphonate was obtained in 47% yield.
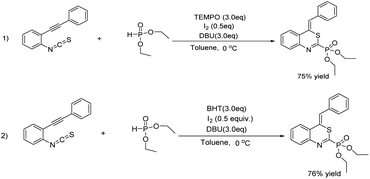
Two control experiments were carried out to obtain some mechanism insight into the reaction. Firstly, 3.0 equiv. of 2,2,6,6 tetramethylpiperidine N-oxide (TEMPO) was added in the reaction of o-alkynylphenyl isothiocyanate 1a with diethyl phosphonate 2a, and product 3a could be isolated in 75% yield (Scheme 3, 1). Similarly, the yield of 3a was not influenced when we added 3.0 equiv. of 2,6-di-tert-butyl-4-methylphenol (BHT) in the reaction (Scheme 3, 2). These results probably suggested that the reaction may not follow a radical pathway.
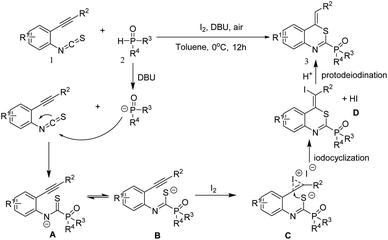
Base on the above results and previous reports,23 a possible mechanism was proposed for this reaction (Scheme 4). Firstly, in the presence of DBU as the base, the nucleophilic addition of P-anion to an isothiocyanate moiety in compound 1 would occur to produce the intermediate A. Then, Intermediate A could undergo isomerization to afford intermediate B. Next, molecular iodine serves as a π-acid to react with triple bond, giving iodocyclized intermediates C which followed by an intramolecular nucleophilic addition to give the intermediate D. Finally, intermediate D underwent the protodeiodination to give the target product 3.
Conclusions
In summary, we have developed molecular-iodine-catalyzed cyclization reactions of o-alkynylphenyl isothiocyanates and organophosphorus esters as a mild synthetic method of organophosphorus compounds. Different kinds of 4H-benzo[d][1,3]thiazin-2-ylphosphonate could be synthesized in moderate to good yields. Avoiding the use of metal catalysts and the availability of raw materials are the advantages of this approach which provided a simple and direct pathway to construct organophosphorus compounds.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Natural Science Foundation of China (NSFC) (grant number 22061021, 21762023) and the Scientific Research Fund of Jiangxi Provincial Education Department are gratefully acknowledged.Notes and references
- (a) V. Spampinato, N. Tuccitto, S. Quici, V. Calabrese, G. Marletta, A. Torrisi and A. Licciardello, Langmuir, 2010, 26, 8400 CrossRef CAS PubMed; (b) S. Kirumakki, J. Huang, A. Subbiah, J. Y. Yao, A. Rowland, B. Smith, A. Mukherjee, S. Samarajeewa and A. Clearfield, J. Mater. Chem., 2009, 19, 2593 RSC; (c) A. Georgeand and A. Veis, Chem. Rev., 2008, 108, 4670 CrossRef PubMed; (d) E.-Q. Li and Y. Huang, Chem. Commun., 2020, 56, 680 RSC.
- (a) X. Q. Chen, D. J. Kopecky, J. Mihalic, S. Jeffries, X. S. Min, J. Heath, J. Deignan, S. J. Lai, Z. Fu, C. Guimaraes, S. Shen, L. Li, S. S. Johnstone, S. Thibault, H. D. Xu, M. Cardozo, W. Shen, N. Walker, F. Kayser and Z. L. Wang, J. Med. Chem., 2012, 55, 3837 CrossRef CAS PubMed; (b) Q. Dang, Y. Liu, D. K. Cashion, S. R. Kasibhatla, T. Jiang, F. Taplin, J. D. Jacintho, H. Q. Li, Z. L. Sun, Y. Fan, J. DaRe, F. Tian, W. Y. Li, T. Gibson, R. Lemus, P. D. van Poelje, S. C. Potter and M. D. Erion, J. Med. Chem., 2011, 54, 153 CrossRef CAS PubMed; (c) T. S. Kumar, S.-Y. Zhou, B. V. Joshi, R. Balasubramanian, T. H. Yang, B. T. Liang and K. A. Jacobson, J. Med. Chem., 2010, 53, 2562 CrossRef CAS PubMed; (d) L. Bialy and H. Waldmann, Angew. Chem., Int. Ed., 2005, 44, 3814 CrossRef CAS PubMed.
- (a) A. R. Choudhury and S. Mukherjee, Adv. Synth. Catal., 2013, 355, 1989 CrossRef CAS; (b) D. P. Zhao and R. Wang, Chem. Soc. Rev., 2012, 41, 2095 RSC; (c) T. Baumgartner and R. Reau, Chem. Rev., 2006, 106, 4681 CrossRef CAS PubMed; (d) W. J. Tang and X. M. Zhang, Chem. Rev., 2003, 103, 3029 CrossRef CAS PubMed; (e) G. Chelucci, G. Orru and G. A. Pinna, Tetrahedron, 2003, 59, 9471 CrossRef CAS; (f) A. K. Bhattacharya and G. Thyagarajan, Chem. Rev., 1981, 81, 415 CrossRef CAS.
- S.-J. Tan, J.-L. Lim, Y.-Y. Low, K.-S. Sim, S.-H. Lim and T.-S. Kam, J. Nat. Prod., 2014, 77, 2068 CrossRef CAS PubMed.
- (a) H. Fernandez-Pérez, P. Etayo, A. Panossian and A. Vidal-Ferran, Chem. Rev., 2011, 111, 2119 CrossRef PubMed; (b) M. N. Birkholz, Z. Freix and P. W. N. M. van Leeuwen, Chem. Soc. Rev., 2009, 38, 1099 RSC; (c) Z. Wang, L. Wei, Z. Cheng, J. Xia and Z. Chen, Chin. Chem. Lett., 2021, 32, 2756–2760 CrossRef CAS.
- F. R. Atherton, C. H. Hassall and R. W. Lambert, J. Med. Chem., 1986, 29, 29 CrossRef CAS PubMed.
- G. Lavielle, P. Hautefaye, C. Schaeffer, J. A. Boutin, C. A. Cudennec and A. J. Pierre, Med. Chem., 1991, 34, 1998 CrossRef CAS PubMed.
- R. Hirschmann, A. B. Smith III, C. M. Taylor, P. A. Benkovic, S. D. Taylor, K. M. Yager, P. A. Sprengler and S. J. Benkovic, Science, 1994, 265, 234 CrossRef CAS PubMed.
- (a) T. Hirao, T. Masunaga, Y. Ohshiro and T. Agawa, Synthesis, 1981, 56 CrossRef CAS; (b) T. Hirao, T. Masunaga, N. Yamada, Y. Ohshiro and T. Agawa, Bull. Chem. Soc. Jpn., 1982, 55, 909 CrossRef CAS; (c) M. Kalek, M. Jezowska and J. Stawinski, Adv. Synth. Catal., 2009, 351, 3207 CrossRef CAS; (d) M. C. Kohler, J. G. Sokol and R. A. Stockland Jr, Tetrahedron Lett., 2009, 50, 457 CrossRef CAS; (e) J. Xu, P. Zhang, Y. Gao, Y. Chen, G. Tang and Y. Zhao, J. Org. Chem., 2013, 78, 8176 CrossRef CAS PubMed; (f) C. Huang, X. Tang, H. Fu, Y. Jiang and Y. Zhao, J. Org. Chem., 2006, 71, 5020 CrossRef CAS PubMed; (g) J. Ballester, J. Gatignol, G. Schmidt, C. Alayrac, A.-C. Gaumont and M. Taillefer, ChemCatChem, 2014, 6, 1549 CrossRef CAS; (h) Y. L. Zhao, G. J. Wu, Y. Li, L. X. Gao and F. S. Han, Chem.–Eur. J., 2012, 18, 9622 CrossRef CAS PubMed; (i) A. Kinbara, M. Ito, T. Abe and T. Yamagishi, Tetrahedron, 2015, 71, 7614 CrossRef CAS; (j) M. Kalek, A. Ziadi and J. Stawinski, Org. Lett., 2008, 10, 4637 CrossRef CAS PubMed; (k) E. Jablonkai and G. Keglevich, Tetrahedron Lett., 2014, 44, 4185 Search PubMed; (l) R. S. Shaikh, S. J. S. Düsel and B. König, ACS Catal., 2016, 6, 8410 CrossRef CAS; (m) I. Ghosh, R. S. Shaikh and B. König, Angew. Chem., Int. Ed., 2017, 129, 8664 CrossRef; (n) W. Lecroq, P. Bazille, F. Morlet-Savary, M. Breugst, J. Lalevée, A.-C. Gaumont and S. Lakhdar, Org. Lett., 2018, 20, 4164 CrossRef CAS PubMed; (o) H. Zeng, Q. Dou and C.-J. Li, Org. Lett., 2019, 21, 1301 CrossRef CAS PubMed; (p) S. Sengmany, A. Ollivier, E. Le Gall and E. Léonel, Org. Biomol. Chem., 2018, 16, 4495 RSC; (q) S. Wang, C. Yang, S. Sun and J. Wang, Chem. Commun., 2019, 55, 14035 RSC; (r) Y. Bai, N. Liu, S. Wang, S. Wang, S. Ning, L. Shi, L. Cui, Z. Zhang and J. Xiang, Org. Lett., 2019, 21, 6835 CrossRef CAS PubMed.
- (a) M. Andaloussi, J. Lindh, J. Savmarker, P. J. R. Sjoberg and M. Larhed, Chem.–Eur. J., 2009, 15, 13069 CrossRef CAS PubMed; (b) R. Zhuang, J. Xu, Z. Cai, G. Tang, M. Fang and Y. Zhao, Org. Lett., 2011, 13, 2110 CrossRef CAS PubMed; (c) G. Hu, W. Chen, T. Fu, Z. Peng, H. Qiao, Y. Gao and Y. Zhao, Org. Lett., 2013, 15, 5362 CrossRef CAS PubMed; (d) I. Hicks, J. McTague, T. Hapatsha, R. Teriak and P. Kaur, Molecules, 2020, 25, 290 CrossRef CAS PubMed; (e) T.-H. Chen, D. M. Reddy and C.-F. Lee, RSC Adv., 2017, 7, 30214 RSC.
- (a) R. Berrino, S. Cacchi, G. Fabrizi, A. Goggiamani and P. Stabile, Org. Biomol. Chem., 2010, 8, 4518 RSC; (b) Y. He, H. Wu and F. D. Toste, Chem. Sci., 2015, 46, 1194 RSC; (c) S. Wang, D. Qiu, F. Mo, Y. Zhang and J. Wang, J. Org. Chem., 2016, 81, 11603 CrossRef CAS PubMed; (d) W. Xu, G. Hu, P. Xu, Y. Gao, Y. Yin and Y. Zhao, Adv. Synth. Catal., 2014, 14, 2948 CrossRef; (e) S.-Y. Chen, R.-S. Zeng, J.-P. Zou and O. T. Asekun, J. Org. Chem., 2014, 79, 1449 CrossRef CAS PubMed; (f) R. Li, X. Chen, S. Wei, K. Sun, L.-L. Fan, Y. Liu, L. Qu, Y. Zhao and B. Yu, Adv. Synth. Catal., 2018, 360, 4807 CrossRef; (g) D. Qiu, C. Lian, J. Mao, Y. Ding, Z. Liu, L. Wei, M. Fagnoni and S. Protti, Adv. Synth. Catal., 2019, 361, 5239 CrossRef CAS; (h) Y. Jian, M. Chen, B. Huang, W. Jia, C. Yang and W. Xia, Org. Lett., 2018, 20, 5370 CrossRef CAS PubMed.
- (a) R. A. Dhokale and S. B. Mhaske, Org. Lett., 2013, 15, 2218 CrossRef CAS PubMed; (b) Q. Chen, X. Yan, Z. Du, K. Zhang and C. Wen, J. Org. Chem., 2016, 81, 276 CrossRef CAS PubMed; (c) G. Yang, C. Shen, M. Quan and W. Zhang, Tetrahedron, 2016, 72, 333 CrossRef CAS; (d) T. Wang, S. Sang, L. Liu, H. Qiao, Y. Gao and Y. Zhao, J. Org. Chem., 2014, 79, 608 CrossRef CAS PubMed; (e) H. Luo, H. Liu, X. Chen, K. Wang, X. Luo and K. Wang, Chem. Commun., 2017, 53, 956 RSC; (f) M. Matsumura, Y. Dong, N. Kakusawa and S. Yasuike, Chem. Pharm. Bull., 2015, 63, 130 CrossRef CAS PubMed; (g) Y. Yu, S. Yi, C. Zhu, W. Hu, B. Gao, Y. Chen, W. Wu and H. Jiang, Org. Lett., 2016, 18, 400 CrossRef CAS PubMed.
- (a) R. Chen and W.-L. Duan, J. Am. Chem. Soc., 2013, 135, 16754 CrossRef PubMed; (b) W. B. Ma and L. Ackermann, Synthesis, 2014, 46, 2297 CrossRef CAS; (c) B. Zhang, C. G. Daniliuc and A. Studer, Org. Lett., 2014, 16, 250 CrossRef CAS PubMed; (d) J. J. Cao, T. H. Zhu, Z. Y. Gu, W. J. Hao, S. Y. Wang and S. J. Ji, Tetrahedron, 2014, 70, 6985 CrossRef CAS; (e) C. X. Li, D. S. Tu, R. Yao, H. Yan and C. S. Lu, Org. Lett., 2016, 18, 4928 CrossRef CAS PubMed; (f) L.-J. Wang, A.-Q. Wang, Y. Xia, X.-X. Wu, X.-Y. Liu and Y.-M. Liang, Chem. Commun., 2014, 50, 13998 RSC; (g) X.-S. Li, Y.-P. Han, X.-Y. Zhu, M. Li, W.-X. Wei and Y.-M. Liang, J. Org. Chem., 2017, 82, 11636 CrossRef CAS PubMed; (h) W.-S. Guo, Q. Dou, J. Hou, L.-R. Wen and M. Li, J. Org. Chem., 2017, 82, 7015 CrossRef CAS PubMed; (i) T. Q. Chen, J.-S. Zhang and L.-B. Han, Dalton Trans., 2016, 45, 1843 RSC; (j) Y. Z. Gao, G. Tang and Y. F. Zhao, Phosphorus, Sulfur, Silicon Relat. Elem., 2017, 192, 589 CrossRef CAS; (k) Z. W. Luo, J. H. Ding, D. Y. Huang, X. M. Wu and Y. C. Bi, Tetrahedron Lett., 2022, 96, 153757 CrossRef CAS.
- A. K. Mukerjee and R. Ashare, Chem. Rev., 1991, 91, 1 CrossRef CAS.
- (a) J. J. Chu, B. L. Hu, Z. Y. Liao and X. G. Zhang, J. Org. Chem., 2016, 81, 8647 CrossRef CAS PubMed; (b) T. Xie, Y. Xiao, S. Zhao, X. Q. Hu and P. F. Xu, J. Org. Chem., 2016, 81, 10499 CrossRef CAS PubMed; (c) W. Hao, J. Zeng and M. Cai, Chem. Commun., 2014, 50, 11686 RSC.
- (a) L. R. Wen, Q. Y. Shen, W. S. Guo and M. Li, Org. Chem. Front., 2016, 3, 870 RSC; (b) P. Zhao, Y. Liu and C. Xi, Org. Lett., 2015, 17, 4388 CrossRef CAS PubMed; (c) P. Zhao, X. Yan, H. Yin and C. Xi, Org. Lett., 2014, 16, 1120 CrossRef CAS PubMed; (d) A. F. G. Goldberg, N. R. O'Connor, R. A. II. Craig and B. M. Stoltz, Org. Lett., 2012, 14, 5314 CrossRef CAS PubMed.
- (a) Y. He, J. Li, S. Luo, J. Huang and Q. Zhu, Chem. Commun., 2016, 52, 8444 RSC; (b) X. Tang, Z. Zhu, C. Qi, W. Wu and H. Jiang, Org. Lett., 2016, 18, 180 CrossRef CAS PubMed; (c) L. Benati, G. Calestani, R. Leardini, M. Minozzi, D. Nanni, P. Spagnolo, S. Strazzari and G. Zanardi, J. Org. Chem., 2003, 68, 3454 CrossRef CAS PubMed.
- Y. Liu, S. J Yao, C. L. Wang, Y. H. Zhang and W. Y. Hao, RSC Adv., 2020, 10, 32211 RSC.
- (a) W. Y. Hao, J. Tian, W. Li, R. Y. Shi, Z. L. Huang and A. W. Lei, Chem.–Asian J., 2016, 11, 1664 CrossRef CAS PubMed; (b) W. Y. Hao, Y. Y. Jiang and M. Z. Cai, J. Org. Chem., 2014, 79, 3634 CrossRef CAS PubMed; (c) W. Y. Hao, J. B. Zeng and M. Z. Cai, Chem. Commun., 2014, 50, 11686 RSC; (d) W. Y. Hao, J. Huang, S. S. Jie and M. Z. Cai, Eur. J. Org. Chem., 2015, 6655 CrossRef CAS; (e) W. Y. Hao, T. L. Zhang and M. Z. Cai, Tetrahedron, 2013, 69, 9219 CrossRef CAS; (f) W. Y. Hao, X. Y. Sang, J. Jiang and M. Z. Cai, Tetrahedron Lett., 2016, 57, 1511 CrossRef CAS; (g) W. Y. Hao, X. Y. Sang, J. Jiang and M. Z. Cai, Tetrahedron Lett., 2016, 57, 4207 CrossRef CAS; (h) W. Y. Hao, Y. C. Sha, Y. Deng, Y. Luo, L. Zeng, S. Tang, Y. Weng, C.-W. Chiang and A. W. Lei, Chem.–Eur. J., 2019, 25, 4931 CrossRef CAS PubMed; (i) F. H. Zou, X. W. Chen and W. Y. Hao, Tetrahedron, 2017, 73, 758 CrossRef CAS; (j) J. K. Miao, Y. H. Zhang, X. Y. Sang and W. Y. Hao, Org. Biomol. Chem., 2019, 17, 2336 RSC.
- L. Benati, G. Calestani, R. Leadini, M. Minozzi, D. Nanni, P. Spagnolo, S. S. Stazzari and G. Zanardi, J. Org. Chem., 2003, 68, 3454 CrossRef CAS PubMed.
- (a) K. Sonogashira, Y. Tohda and N. Hagiwara, Tetrahedron Lett., 1975, 16, 4467 CrossRef; (b) K. J. Sonogashira, Organomet. Chem., 2002, 653, 46 CrossRef CAS.
- (a) M. Uchiyama, H. Ozawa, K. Takuma, Y. Matsumoto, M. Yonehara, K. Hiroya and T. Sakamoto, Org. Lett., 2006, 8, 5517 CrossRef CAS PubMed; (b) J. H. Park, S. V. Bhilare and S. W. Youn, Org. Lett., 2011, 13, 2228 CrossRef CAS PubMed; (c) K. Gilmore and I. V. Alabugin, Chem. Rev., 2011, 111, 6513 CrossRef CAS PubMed.
- (a) Y. Takeda, R. Kajihara, N. Kobayashi, K. Noguchi and A. Akio Saito, Org. Lett., 2017, 19, 6744 CrossRef CAS PubMed; (b) Y. Tang and C. Z. Li, Org. Lett., 2004, 6, 3229 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03072a |
| This journal is © The Royal Society of Chemistry 2022 |