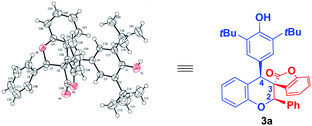Open Access Article
Open Access ArticleDiastereoselective synthesis of chroman bearing spirobenzofuranone scaffolds via oxa-Michael/1,6-conjugated addition of para-quinone methides with benzofuranone-type olefins†
Hongmei Qina,
Qimei Xieb and
Long He *ab
*ab
aCollege of Chemistry and Materials Engineering, Guiyang University, Guiyang, 550005, P. R. China. E-mail: longhe@cwnu.edu.cn
bCollege of Chemistry and Chemical Engineering, China West Normal University, Nanchong, 637002, P. R. China
First published on 6th June 2022
Abstract
A simple and convenient cyclization of ortho-hydroxyphenyl-substituted para-quinone methides with benzofuran-2-one type active olefins via oxa-Michael/1,6-conjugated addition has been developed, which afforded an easy access to enriched functionalized chroman-spirobenzofuran-2-one scaffolds with good to excellent yields (up to 90%) and diastereoselectivities (up to >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). This reaction provided an efficient method for constructing desired spirocyclic compounds combining both well-known heterocyclic pharmacophores chroman and benzofuran-2-one.
1 dr). This reaction provided an efficient method for constructing desired spirocyclic compounds combining both well-known heterocyclic pharmacophores chroman and benzofuran-2-one.
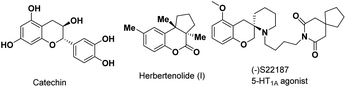
The chroman framework represents a privileged heterocyclic core commonly found within a wide variety of biologically active natural products1 and synthetic compounds of medicinal interest (Fig. 1).2 Owing to the wide application of these heterocyclic molecules, over the past few decades, numerous efforts have been devoted to the efficient synthesis of chroman nucleus motifs.3,4 In particular, incorporating chroman into spiro-bridged and spiro-fused heterocyclic systems is appealing due to its fascinating molecular architecture and proven biological activity.5 Among the existing methods, the [4 + m] cycloaddition of para-quinone methides (p-QMs) is found to be an efficient pathway to access these valuable spirocyclic skeletons.6 For instance, the Enders group synthesized functionalized chromans with an oxindole motif by the asymmetric organocatalytic domino oxa-Michael/1,6-addition reaction.7 After that, the Hao,8a Peng,8b Shi,8c,d Zhou,8e Liang8f and Wang8g groups developed convenient methods to construct chromans bearing spirocyclic skeletons from p-QMs, respectively. Despite all these shining achievements, however, it is still very challenging to simply and conveniently construct chromans bearing quaternary carbon spirals for organic chemical or drug discovery among these [4 + m] cycloaddition reactions.
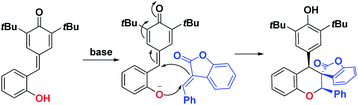
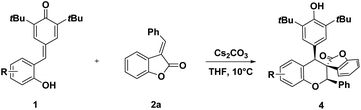
Benzofuran-2-(3H)-ones as one of the important oxygen-containing heterocycles that exist in a broad array of natural products9 and potential medicines.10 The streamlined synthesis of benzofuran-2-ones pose considerable challenge due to their quaternary carbon centers at the C-3 position,11 especially those featuring relatively congested spirocyclic motifs represent challenging synthetic targets.12,13 In our continuous interests in developing efficient method for the synthesis of spirocyclic compounds based on cyclization reaction,14 we wish to report a cycloaddition of para-quinone methides with benzofuranone derived olefins, affording the spiro-cycloadducts in good to excellent yields and diastereoselectivities. This cyclization features the simultaneous formation of chroman and spirobenzofuran-2-one skeletons in a single step (Scheme 1), which may be potentially applied as pharmaceutical agents.
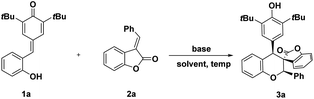
We initiated our investigations with the readily available ortho-hydroxyphenyl-substituted para-quinone methides 1a and 3-benzylidenebenzofuran-2-one 2a in toluene at room temperature in the presence of base. Unfortunately, no desired chroman derivatives bearing spirobenzofuranone scaffolds 3a was isolated in the presence of 2 eq. Na2CO3 after stirring at room temperature for 48 h (entry 1, Table 1). A base survey showed that K2CO3 and CsF led to desired cycloaddcuts 3a even if with a disappointing yield (entries 2–4, Table 1). Gratifyingly, Cs2CO3 furnished the desired product in 70% yield and with a generally acceptable 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr value (entry 5, Table 1). A solvent screening indicated that the yield and diastereoselectivity are both dependent on the solvent (Table 1). Thus, the oxygenated solvents such as THF, diethyl ether and 1,4-dioxane gave comparably high yields and diastereoselectivities than polar solvents as exemplified by CH3CN or DMF (entries 5–10). To our delight, performing the reaction in THF led to desired chroman-spirobenzofuranone 3a with an excellent diastereoselectivity, albeit with a very subtle erosion of the yield (entry 6). Further optimization of the reaction conditions by varying the temperature was also investigated. When higher temperature was used, no improvement in the final yield was observed (entry 11). Performing the reaction at lower 10 °C resulted in a increase of the yield accompanied with no erosion in diastereoselectivity (entry 12). However, the yield decreased to 64% when the reaction performed at lower 0 °C (entry 13).
1 dr value (entry 5, Table 1). A solvent screening indicated that the yield and diastereoselectivity are both dependent on the solvent (Table 1). Thus, the oxygenated solvents such as THF, diethyl ether and 1,4-dioxane gave comparably high yields and diastereoselectivities than polar solvents as exemplified by CH3CN or DMF (entries 5–10). To our delight, performing the reaction in THF led to desired chroman-spirobenzofuranone 3a with an excellent diastereoselectivity, albeit with a very subtle erosion of the yield (entry 6). Further optimization of the reaction conditions by varying the temperature was also investigated. When higher temperature was used, no improvement in the final yield was observed (entry 11). Performing the reaction at lower 10 °C resulted in a increase of the yield accompanied with no erosion in diastereoselectivity (entry 12). However, the yield decreased to 64% when the reaction performed at lower 0 °C (entry 13).
| Entry | Base | Solvent | Yieldb (%) | Drc |
|---|---|---|---|---|
| a Reaction conditions: p-QMs 1a (0.1 mmol), benzofuranones 2a (0.12 mmol) and base (0.2 mmol) in 2 mL of solvent for 6–48 h.b Isolated yields.c Determined by crude 1H NMR analysis.d Performed at 30 °C.e Performed at 10 °C.f Performed at 0 °C. | ||||
| 1 | Na2CO3 | Toluene | — | — |
| 2 | DMAP | Toluene | — | — |
| 3 | K2CO3 | Toluene | 25 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | CsF | Toluene | 52 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | Cs2CO3 | Toluene | 70 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | Cs2CO3 | THF | 68 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | Cs2CO3 | Et2O | 60 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | Cs2CO3 | Dioxane | 53 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | Cs2CO3 | CH3CN | 50 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | Cs2CO3 | DMF | 31 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11d | Cs2CO3 | THF | 65 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12e | Cs2CO3 | THF | 75 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13f | Cs2CO3 | THF | 64 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
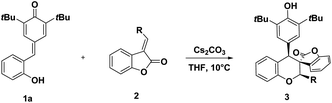
With the optimized reaction conditions in hand, we explored the substrate scope of this cyclization reaction with a selection of benzofuranones. The results are shown in Table 2. To our pleasure, a wide range of benzofuran-2-ones derived olefins were compatible affording the corresponding chromans-spirobenzofuranone scaffolds 3 in good results. In detail, the steric hindrance of substituents had a significant impact on the cyclization reaction. The substrates with substituents on para- or meta-position on phenyl ring were tolerable affording the desired cycloadducts in good yields regardless of the electronic nature of substituents (entries 5–14). However, good diastereoselectivities were also observed in ortho-substituted substrates (entries 2–4). Furthermore, 2-naphthyl derived substrate gave also good yield and sole diastereoselectivity (entry 15). Multi-substituted substrate was also able to participate in this cyclization, for example, 3,4,5-trimethoxylphenyl substituted benzofuranone delivered cycloadduct 3p in 88% yield and with 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr value (entry 16). Interestingly, the extension of the reaction conditions to heteroaromatic substrates including 3-pyridyl and 3-thiophenyl benzofuranones were proceeded smoothly, giving rise to cyclization products 3q and 3r in 79% and 80% yield, respectively (entries 17–18).
1 dr value (entry 16). Interestingly, the extension of the reaction conditions to heteroaromatic substrates including 3-pyridyl and 3-thiophenyl benzofuranones were proceeded smoothly, giving rise to cyclization products 3q and 3r in 79% and 80% yield, respectively (entries 17–18).
| Entry | R | 3 | Yieldb (%) | Drc |
|---|---|---|---|---|
| a Reaction conditions: p-QMs 1a (0.1 mmol), benzofuranones 2 (0.12 mmol) and Cs2CO3 (0.2 mmol) in 2 mL of THF.b Isolated yields for 4–48 h.c Determined by crude 1H NMR analysis. | ||||
| 1 | C6H5 | 3a | 75 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 2-ClC6H4 | 3b | 69 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 2-BrC6H4 | 3c | 67 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 2-CH3C6H4 | 3d | 64 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 3-CH3C6H4 | 3e | 75 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 3-MeOC6H4 | 3f | 72 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 3-NO2C6H4 | 3g | 71 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 3-BrC6H4 | 3h | 75 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 4-CH3C6H4 | 3i | 73 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 4-MeOC6H4 | 3j | 86 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 4-CF3C6H4 | 3k | 82 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | 4-NO2C6H4 | 3l | 85 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | 4-ClC6H4 | 3m | 83 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | 4-BrC6H4 | 3n | 81 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | 2-Naphthyl | 3o | 74 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 16 | 3,4,5-(OMe)3C6H2 | 3p | 88 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 17 | 3-Pyridinyl | 3q | 79 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 18 | 3-Thiophenyl | 3r | 80 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Subsequently, the generality of this cyclization reaction was further evaluated through varying p-QMs 1. As shown in Table 3. It turned out that various p-QMs 1 can be employed to the reaction, which delivered functionalized chroman-spiro-benzofuran-2-ones scaffolds 4 in high yields (up to 90%) and with good diastereoselectivities. It seems that the position of the substituents had some delicate influence on the reaction. The C5-methoxyl- or methyl-substituted substrates p-QMs 1 generated the products with a higher yield than those of the C4- substituted counterparts (entries 3–6). Moreover, the C5-chloro- and bromo-substituted p-QMs 1 afforded the products 4a and 4b in moderate yield (entries 1–2).
| Entry | R | 4 | Yieldb (%) | Drc |
|---|---|---|---|---|
| a Reaction conditions: p-QMs 1 (0.1 mmol), benzofuranones 2a (0.12 mmol) and Cs2CO3 (0.2 mmol) in 2 mL of THF for 6–48 h.b Isolated yields.c Determined by crude 1H NMR analysis. | ||||
| 1 | 5-Cl | 4a | 73 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 5-Br | 4b | 65 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 5-CH3 | 4c | 90 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 5-OCH3 | 4d | 80 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 4-CH3 | 4e | 72 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 4-OCH3 | 4f | 66 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
The structure and relative configuration of 3a were determined by HRMS, NMR spectroscopy and single-crystal X-ray analysis.15 The relative configuration of other cycloadducts were tentatively assigned by analogy (Fig. 2 and see ESI†).
Conclusions
In conclusion, we described a cyclization reaction of ortho-hydroxyphenyl-substituted para-quinone methides with benzofuran-2-one derived olefins via oxa-Michael/1,6-conjugated addition, which efficiently constructed enriched functionalized spirocyclic compounds combining both well-known heterocyclic pharmacophores chroman and benzofuran-2-one in good to excellent yields (up to 90%) and diastereoselectivities (up to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the National Natural Science Foundation of China (21772158), Scientific Research Funds of Guiyang University [GYU-KY-2022] and Guizhou Education Department Youth Science and Technology Talents Growth Project KY[2019]094.Notes and references
- (a) H. Hasler, F. Kaufmann, W. Pirson and F. Schneider, Eur. J. Med. Chem., 1987, 22, 559 CrossRef CAS; (b) R. Hiessbock, C. Wolf, E. Richter, M. Hitzler, P. Chiba, M. Kratzel and G. Ecker, J. Med. Chem., 1999, 42, 1921 CrossRef CAS PubMed; (c) E. Middleton, C. Kandaswami and T. C. Theoharides, Pharmacol. Rev., 2000, 52, 673 CAS; (d) H. C. Shen, Tetrahedron, 2009, 65, 3931 CrossRef CAS; (e) R. K. Kushwaha, K. Singh, P. Kumar, D. Chandra and J. Research, Pharm. and Tech., 2019, 12, 5566 Search PubMed.
- (a) M. Uroos and C. J. Hayes, Org. Lett., 2010, 12, 5294 CrossRef CAS PubMed; (b) M. Uroos, W. Lewis, A. J. Blake and C. J. Hayes, J. Org. Chem., 2010, 75, 8465 CrossRef CAS PubMed; (c) C. Y. Ding, L. L. Wang, H. J. Chen, C. Wild, N. Ye, Y. Ding, T. Z. Wang, M. A. White, Q. Shen and J. Zhou, Org. Biomol. Chem., 2014, 12, 8442 RSC.
- Selected examples: (a) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef CAS PubMed; (b) Y. Yamamoto and K. Itonaga, Org. Lett., 2009, 11, 717 CrossRef CAS PubMed; (c) X. T. Meng, Y. Huang, H. X. Zhao, P. Z. Xie, J. Z. Ma and R. Chen, Org. Lett., 2009, 11, 991 CrossRef CAS PubMed; (d) A. F. Ward, Y. Xu and J. P. Wolfe, Chem. Commun., 2012, 48, 609 RSC; (e) R. R. R. Taylor and R. A. Batey, J. Org. Chem., 2013, 78, 1404 CrossRef CAS; (f) P. S. Wang, P. Liu, Y. J. Zhai, H. C. Lin, Z. Y. Han and L. Z. Gong, J. Am. Chem. Soc., 2015, 137, 12732 CrossRef CAS PubMed; (g) S. S. Wang, J. He and Z. An, Chem. Commun., 2017, 53, 8882 RSC; (h) H. Ren, X. Y. Song, S. R. Wang, L. J. Wang and Y. Tang, Org. Lett., 2018, 20, 3858 CrossRef CAS PubMed.
- Selected examples for the synthesis of chroman via p-QMs, see: (a) K. Chen, S. Liu, D. Wang, W. J. Hao, P. Zhou, S. J. Tu and B. Jiang, J. Org. Chem., 2017, 82, 11524 CrossRef CAS PubMed; (b) J. Y. Liao, Q. Ni and Y. Zhao, Org. Lett., 2017, 19, 4074 CrossRef CAS PubMed; (c) X.-Z. Zhang, K. J. Gan, X.-X. Liu, Y. H. Deng, F. X. Wang, K. Y. Yu, J. Zhang and C.-A. Fan, Org. Lett., 2017, 19, 3207 CrossRef CAS PubMed; (d) G. J. Mei, S. L. Xu, W. Q. Zheng, C. Y. Bian and F. Shi, J. Org. Chem., 2018, 83, 1414 CrossRef CAS PubMed; (e) Z. P. Zhang, K. X. Xie, C. Yang, M. Li and X. Li, J. Org. Chem., 2018, 83, 364 CrossRef CAS PubMed.
- (a) C. C. Lindsey, K. L. Wu and T. R. R. Pettus, Org. Lett., 2006, 8, 2365 CrossRef CAS PubMed; (b) F. Wang, M. Qu, X. Lu, F. Chen and M. Shi, Chem. Commun., 2012, 48, 6259 RSC; (c) D. B. Ramachary, M. Shiva Prasad, S. Vijaya Laxmi and R. Madhavachary, Org. Biomol. Chem., 2014, 12, 574 RSC; (d) B. V. Subba Reddy, V. Hanuman Reddy, D. Medaboina, B. Sridhar and Y. V. Rami Reddy, Org. Biomol. Chem., 2016, 14, 3234 RSC; (e) Z. Cao, G.-X. Zhou, C. Ma, K. Jiang and G.-J. Mei, Synthesis, 2018, 50, 1307 CrossRef CAS; (f) K. Yoshida, H. Inoue, Y. Oji, H. Suzuki and F.- I Takao, J. Org. Chem., 2020, 85, 10189 CrossRef CAS PubMed; (g) A. G. K. Reddy, P. Niharika, S. Zhou, S. K. Jia, T. D. Shi, X. F. Xu, Y. Qian and W. H. Hu, Org. Lett., 2020, 22, 2925 CrossRef PubMed; (h) N. Liu, W. J. Zhu, J. Yao, L. Yin, T. Lu and X. W. Dou, ACS Catal., 2020, 10, 2596 CrossRef CAS; (i) V. B. Gudise, P. C. Settipalli, Y. P. Reddy and S. D. Anwar, ChemistrySelect, 2021, 6, 13589 CrossRef CAS.
- (a) W. Li, X. Xu, P. Zhang and P.-F. Li, Chem.–Asian J., 2018, 13, 2350 CrossRef CAS PubMed; (b) M. Xiang, C.-Y. Li, X.-J. Song, Y. Zou, Z.-C. Huang, X. Li, F. Tian and L.-X. Wang, Chem. Commun., 2020, 56, 14825 RSC; (c) W. Si, F. Xu, Z. Liu, R. Song and J. Lv, J. Tetrahedron Lett., 2020, 61, 152171 CrossRef CAS; (d) W. Mao, S. Lin, L. Zhang, H. Lu, J. Jia and Z. Xu, Org. Chem. Front., 2020, 7, 856 RSC; (e) C. G. S. Lima, F. P. Pauli, D. C. S. Costa, A. S. Souza, L. S. M. Forezi, V. F. Ferreira and F. C. Silva, Eur. J. Org. Chem., 2020, 18, 2650 CrossRef; (f) T. Varlet, M. Matišić, E. Elslande, L. Neuville, V. Gandon and G. Masson, J. Am. Chem. Soc., 2021, 143, 11611 CrossRef CAS PubMed.
- K. Zhao, Y. Zhi, T. Shu, A. Valkonen, K. Rissanen and D. Enders, Angew. Chem., Int. Ed., 2016, 55, 12104 CrossRef CAS PubMed.
- (a) S. L. Liu, X. C. Lan, K. Chen, W. J. Hao, G. Li, S. J. Tu and B. Jiang, Org. Lett., 2017, 19, 3831 CrossRef CAS PubMed; (b) Y. Z. Han, Y. Z. Zhu, P. M. M. Zhang, W. J. Li and P. F. Li, ChemistrySelect, 2017, 2, 11380 CrossRef CAS; (c) C. S. Wang, Y. C. Cheng, J. Zhou, G. J. Mei, W. L. Wang and F. Shi, J. Org. Chem., 2018, 83, 13861 CrossRef CAS PubMed; (d) Y.-X. Wang, Y.-N. Lu, L.-L. Xu, F. T. Sheng, J. P. Zhang, W. Tan and F. Shi, Synthesis, 2020, 52, 2979 CAS; (e) Z. Ye, L. Bai, Y. Bai, Z. Gan, H. Zhou, T. Pan, Y. Yu and J. Zhou, Tetrahedron, 2019, 75, 682 CrossRef CAS; (f) M. Huo, J. Zhou, L. Bai, Q. Xu, Z. Zhou, H. Zhou and G. Liang, Tetrahedron, 2019, 75, 130752 CrossRef; (g) J. P. Tan, H. K. Zhang, Z. Y. Jiang, Y. Chen, X. Y. Ren, C. H. Jiang and T. L. Wang, Adv. Synth. Catal., 2020, 362, 1058 CrossRef CAS.
- Selected examples, see: (a) N. Nakatani and R. Inatani, Agric. Biol. Chem., 1983, 47, 353 CAS; (b) Y.-J. Kwon, M.-J. Sohn, C.-J. Zheng and W.-G. Kim, Org. Lett., 2007, 9, 2449 CrossRef CAS PubMed; (c) S. I. Wada, T. Hitomi, H. Tokuda and R. Tanaka, Chem. Biodiv., 2010, 7, 2303 CrossRef CAS; (d) M. W. Pertino, C. J. Theoduloz, A. Rodriguez and V. J. Lazo, Nat. Prod., 2010, 73, 639 CrossRef CAS PubMed; (e) S. S. Soman and T. H. Thaker, Med. Chem. Res., 2013, 22, 4223 CrossRef CAS; (f) P. P. Kaishap, G. Duarah, B. Sarma, D. Chetia and S. Gogoi, Angew. Chem., Int. Ed., 2018, 57, 456 CrossRef CAS PubMed; (g) Z. Y. Wang, F.-M. Shen, T. Yang, J. K. Zhang, R.-X. Chen, K. K. Wang and H. X. Liu, Asian. J. Org. Chem., 2021, 10, 3293 CrossRef CAS.
- (a) S. A. Adediran, D. Cabaret, B. Drouillat, R. F. Pratt and M. Wakselman, Bioorg. Med. Chem., 2001, 9, 1175 CrossRef CAS PubMed; (b) C. Balestrieri, F. Felice, S. Piacente, C. Pizza, P. Montoro, W. Oleszek, V. Visciano and M. L. Balestrieri, Biochem. Pharmacol., 2006, 71, 1479 CrossRef CAS PubMed; (c) K. C. Nicolaou, T. R. Wu, Q. Kang and D. Y. K. Chen, Angew. Chem., Int. Ed., 2009, 48, 3440 CrossRef CAS PubMed; (d) K. C. Nicolaou, Q. Kang, T. R. Wu, C. S. Lim and D. Y.-K. Chen, J. Am. Chem. Soc., 2010, 132, 7540 CrossRef CAS PubMed.
- Selected examples for the construction of 3,3-disubstitued benzofuran-2-ones: (a) I. D. Hills and G. C. Fu, Angew. Chem., Int. Ed., 2003, 42, 3921 CrossRef CAS PubMed; (b) S. A. Shaw, P. Aleman, J. Christy, J. W. Kampf, P. Va and E. Vedejs, J. Am. Chem. Soc., 2006, 128, 925 CrossRef CAS PubMed; (c) X. Li, Z. G. Xi, S. Z. Luo and J. P. Cheng, Adv. Synth. Catal., 2010, 352, 1097 CrossRef CAS; (d) X. Li, S. S. Hu, Z. G. Xi, L. Zhang, S. Z. Luo and J.-P. Cheng, J. Org. Chem., 2010, 75, 8697 CrossRef CAS PubMed; (e) C.-L. Zhu, F.-G. Zhang, W. Meng, J. Nie, D. Cahard and J.-A. Ma, Angew. Chem., Int. Ed., 2011, 50, 5869 CrossRef CAS PubMed; (f) X.-F. Cheng, Y. Li, Y.-M. Su, F. Yin, J.-Y. Wang, J. Sheng, H. U. Vora, X.-S. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 1236 CrossRef CAS PubMed; (g) K. Ohmatsu, M. Ito, T. Kunieda and T. Ooi, J. Am. Chem. Soc., 2013, 135, 590 CrossRef CAS PubMed; (h) Y. Zhu, E.-G. Zhang, C. Luo, X. Li and J.-P. Cheng, Tetrahedron, 2015, 71, 4090 CrossRef CAS; (i) Y. Liu, C. Zhou, M. Xiong, J. Jiang and J. Wang, Org. Lett., 2018, 20, 5889 CrossRef CAS PubMed; (j) Z. Huang, X. Yang, F. Yang, T. Lu and Q. Zhou, Org. Lett., 2017, 19, 3524 CrossRef CAS PubMed; (k) T. Cruchter, M. G. Medvedev, X. Shen, T. Mietke, K. Harms, M. Marsch and E. Meggers, ACS Catal., 2017, 7, 5151 CrossRef CAS; (l) M. Santi, D. M. C. Ould, J. Wenz, Y. Soltani, R. L. Melen and T. Wirth, Angew. Chem., Int. Ed., 2019, 58, 7861 CrossRef CAS PubMed.
- Selected examples for enantioselective construction of C3-spiro quaternary center of benzofuran-2-ones: (a) M. Zhang, J. X. Wang, S. Q. Chang, X. L. Liu, X. Zuo and Y. Zhou, Chin. Chem. Lett., 2020, 31, 381 CrossRef CAS; (b) D. Wang, G. G. P. Wang, Y. L. Sun, S. F. Zhu, Y. Wei, Q. L. Zhou and M. Shi, Chem. Sci., 2015, 6, 7319 RSC; (c) X. Li, C. Yang, J. L. Jin, X. S. Xue and J. P. Cheng, Chem. - Asian J., 2013, 8, 997 CrossRef CAS PubMed; (d) C. Cassani, X. Tian, E. C. Escudero-Adan and P. Melchiorre, Chem. Commun., 2011, 47, 233 RSC; (e) X. Companyó, A. Zea, A. N. R. ACPa, A. Mazzanti, A. Moyano and R. Rios, Chem. Commun., 2010, 46, 6953 RSC.
- Selected diastereoselective synthesis of C3-spirocyclic benzo- furan-2-ones: (a) X. Li, F. Wang, N. Dong and J.-P. Cheng, Org. Biomol. Chem., 2013, 11, 1451 RSC; (b) C. J. Yang, J. J. Li, R. Zhou, X. Y. Chen, Y. P. Gao and Z. J. He, Org. Biomol. Chem., 2015, 13, 4869 RSC; (c) C. B. Zhang, P. H. Dou, J. Zhang, Q. Q. Wei, Y. B. Wang, J. Y. Zhu, J. Y. Fu and T. Ding, ChemistrySelect, 2016, 1, 4403 CrossRef CAS; (d) R. Li, L. Yao, Y. B. Wang, J. J. Zhu, L. X. Zhang, J. Y. Fu, C. B. Zhang and L. L. Zhao, Org. Lett., 2021, 23, 5611 CrossRef CAS PubMed.
- (a) Z. S. Liu, W. K. Li, T. R. Kang, L. He and Q.-Z. Liu, Org. Lett., 2015, 17, 150 CrossRef CAS PubMed; (b) X. B. Huang, X. J. Li, T. T. Li, B. Chen, W. D. Chu, L. He and Q.-Z. Liu, Org. Lett., 2019, 21, 1713 CrossRef CAS PubMed; (c) F. Cao, F. Hu, Q. M. Xie, G. Y. Luo, W. D. Chu, L. He and Q.-Z. Liu, Asian J. Org. Chem., 2018, 7, 36 CrossRef.
- The relative configuration of 3a was confirmed by X-ray crystallography experiments: CCDC 2128983 contains the supplementary crystallographic data for this paper.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2128983. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2ra03031d |
| This journal is © The Royal Society of Chemistry 2022 |