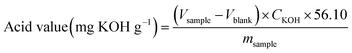Open Access Article
Open Access ArticlePreparation and research of epoxy modified by carboxyl-terminated polybutylene adipate at room temperature†
Xu Luo ,
Yu Li*,
Zhaoyi Sun,
Guorong Wang and
Jie Xin
,
Yu Li*,
Zhaoyi Sun,
Guorong Wang and
Jie Xin
Department of Basic, Naval University of Engineering, Wuhan 430033, China. E-mail: luoxu19980504@163.com
First published on 15th July 2022
Abstract
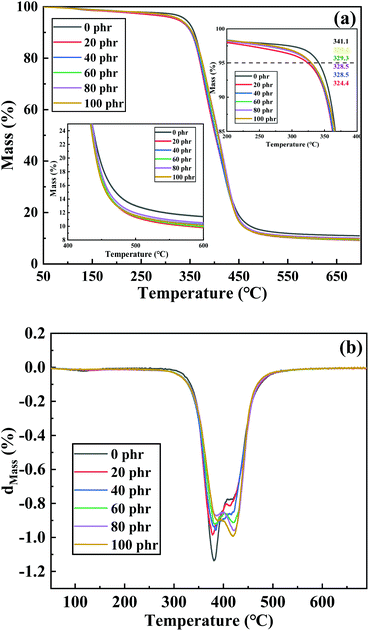
In this study, carboxyl-terminated polybutylene adipate (CTPBA) was used to modify epoxy resin, and the modified epoxy resin was cured by a room temperature rapid curing agent (T-31). The effects of CTPBA modification on bonding properties and mechanical properties of epoxy resin adhesive at room temperature were carefully studied. Epoxy-terminated prepolymer was synthesized by pre-polymerization and its structure was characterized. Compared with the addition method of direct blending, the bonding properties and mechanical properties of pre-polymerized epoxy resin adhesive were significantly better. Compared with unmodified epoxy resin, CTPBA modification significantly improved the bonding strength. Furthermore, with the increase of CTPBA content, the shear strength of the material increased first and then decreased, and reached the maximum when the addition amount was 40 phr. This shows that the tensile strength of the material decreased with the increase of CTPBA content, and the elongation at break increased with the increase of CTPBA content. Dynamic mechanical analyzer (DMA) test results showed that the addition of CTPBA reduced the glass transition temperature, but broadened the damping temperature range. TG analysis showed that the thermal stability of the modified epoxy resin was good, and compared with pure epoxy resin, the initial temperature of thermal weight loss and the maximum thermal decomposition rate decreased, but the overall thermal stability was not significantly different. In summary, CTPBA modification of epoxy resin is expected to improve the comprehensive mechanical properties at room temperature.
1 Introduction
Epoxy adhesive has high bonding strength and versatility, was once called the “universal adhesive”, and has been widely used in aerospace engineering, shipbuilding, the chemical industry, electronics and other fields.1–4 But the cured epoxy resin has the disadvantages of high brittleness, low fracture energy and poor impact resistance,5–7 which has limited its application in engineering. It is therefore of great interest to improve the fracture toughness of epoxy.8–11 Some new heterocyclic polymers (such as aziridine-based polymers) are similar to traditional epoxides in structure, but there are great differences in physical and chemical properties. These new heterocyclic polymers can also be used for molecular design, so as to develop functional materials with greater potential. However, due to the cost problem, epoxides are still widely used in industry.12,13 And with the improvement of people's health awareness, environmentally friendly epoxy adhesives with good quality and no pollution are becoming the mainstream in the field of adhesives.14,15A large number of studies have shown that the addition of toughening agent can effectively improve the toughness of epoxy resin, and the active end groups (such as carboxyl, hydroxyl, isocyanate, amino, etc.) can react with epoxy resin to improve the compatibility with resin matrix and improve the mechanical properties.16,17 Up to now, the active end-group toughening agents mainly include carboxyl-terminated liquid nitrile rubber (CTBN), amino-terminated liquid nitrile rubber (ATBN), polysulfide rubber and so on.18–21 In general, rubber toughened epoxy resin is achieved by modulus loss and glass transition temperature reduction, but the glass transition temperature can be increased by adding other fillers.22,23 The mechanism of action is generally that it reacts with the active groups in epoxy resin such as epoxy group and hydroxyl group to form block polymers. During the curing process, the rubber chain is separated from the system to form a two-phase structure. When the resin matrix is subjected to external force, the rubber disperse the force, so as to achieve the purpose of toughening.24 Substances with similar solubility parameters can dissolve each other, and rubber functionalization can improve the cohesive energy of substances and increase the compatibility between rubber and epoxy resin. Macroscopically, the impact strength or tensile shear strength increases, and the comprehensive performance is effectively improved.25
However, the double bonds in nitrile rubber chains may be oxidized during curing, and the application of nitrile rubber chains is limited due to the presence of a trace carcinogen acrylonitrile.26 Compared with CTBN, the linear carboxyl-terminated polyester has better oxidation stability because it does not contain unsaturated structure, and the polyester material is generally nontoxic, which is in line with the concept of green development. The reports on polyester modified epoxy resin mainly focus on unsaturated polyester and hyperbranched polyester,27–29 while the reports on linear saturated polyester are less. Achary et al.30 modified epoxy resin by carboxyl-terminated poly (propylene adipate) glycol ester (CTPPGA), found that the modified epoxy resin adhesive strength depends on the molecular weight and content, and the epoxy resin adhesive can be used at 120 °C. Ratna et al.26 used carboxyl-terminated polyethylene glycol adipate (CTPEGA) as toughening agent, reacted with epoxy resin to form epoxy-terminated prepolymer. It was found that the addition of CTPEGA greatly improved the impact strength and toughness of epoxy resin. However, these studies did not consider the effects of modification methods and pre-reaction ratio on the properties of epoxy resin modified by carboxyl-terminated polyester.
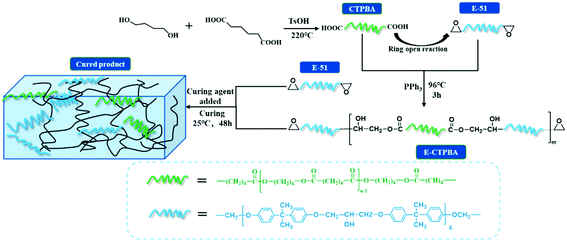
In this work, carboxyl-terminated poly (butylene adipate) (CTPBA) modified epoxy resin was mainly studied. The epoxy-terminated polybutylene adipate (E-CTPBA) was synthesized by controlling the molar ratio of CTPBA to epoxy resin, and then added to E-51 epoxy resin to improve the bonding performance and mechanical properties of epoxy resin. The effects of molecular weight, modification method and pre-reaction ratio of CTPBA on the properties of epoxy resin adhesive were studied in detail. The chemical structures of CTPBA and E-CTPBA were characterized and analyzed by Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR) and matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF MS). The thermal stability of modified epoxy resin was studied by thermogravimetric analyzer. The glass transition temperature of modified epoxy resin was studied by DMA. The impact section of cured epoxy resin was observed by scanning electron microscope (SEM).
2 Experimental
2.1. Materials
Bisphenol A epoxy resin (E-51) with epoxy value of 0.48–0.52, industrial grade, was purchased from Nantong Xingchen Synthetic Materials Co., Ltd, Jiangsu, China; adipic acid (≥99.5%), analytical reagent, was purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. The 1,4-butanediol (BDO, ≥99.0%), analytical reagent, was purchased from Xilong Scientific Co., Ltd, Guangdong, China. Triphenylphosphine (PPh3, ≥99.0%), analytical reagent, was purchased from Aladdin Reagent Co., Ltd, Shanghai, China. T-31 curing agent with amine value of 520 mg KOH g−1, industrial grade, was purchased from Guangzhou Yueqiang Chemical Co., Ltd, Guangdong, China. Anhydrous ethanol (EtOH, ≥99.7%) was purchased from Sinopharm Chemical Reagent Co., Ltd, Beijing, China. p-Toluenesulfonic acid (TsOH, 99%), analytical reagent, was purchased from Tianjin Damao Chemical Reagent Factory, Tianjin, China.2.2. Preparation of carboxyl-terminated polyester (CTPBA)
A certain proportion of adipic acid and 1,4-butanediol were weighed and added into a three-port flask containing a thermometer, a stirring device, an oil–water separator and a condensing reflux device. The catalyst p-toluene sulfonic acid (0.1% of the total mass) and a certain amount of toluene were added, and the air in the flask was discharged by nitrogen for about 10 minutes. Using oil bath heating, the temperature was slowly increased to 150 °C, and the top temperature reached 100 °C. The effluent rate in the oil–water separator was controlled, and the effluent was significantly reduced after 60 min of stable temperature. At this time, the temperature was slowly increased to 220 °C. After controlling the temperature of 220 °C for about 4 h, the acid value was measured. When the acid value reached the theoretical value, the reaction stopped. When the temperature was cooled to 70 °C, the toluene was removed by vacuum distillation device. The reaction formula of CTPBA is shown in Fig. 1.2.3. Preparation and curing of CTPBA modified epoxy resin
Metric carboxyl-terminated polyester (CTPBA) and epoxy resin (E-51) were added to a three-neck flask containing a stirrer and a thermometer for pre-reaction. Triphenylphosphine with mass fraction of 0.3% was used as a catalyst, and the temperature began to rise after passing through nitrogen for 10 minutes. The acid value of epoxy-terminated polyester (E-CTPBA) was determined after 6 hours of continuous reaction under N2 protection by controlling the oil bath temperature of 96 °C. The reaction process was tracked by acid titration method. When the acid value reached below 1 and did not change with time, the reaction stopped, and the epoxy-terminated polyester (E-CTPBA) was obtained. Fig. 1 shows the synthetic E-CTPBA schematic. E-CTPBA was added to epoxy resin E-51 as toughening agent, and all formulations were cured with 25 phr T-31 (relative to 100 g epoxy), mixed evenly and defoamed in vacuum, then cured at room temperature (25 °C) for 2 days.2.4. Characterization and performance testing
Fourier Transform infrared spectrometer with ATR attachment (FTIR, Thermo Scientific, Nicolet iS20) was used to carry out infrared characterization of the synthesized CTPBA and E-CTPBA, with scanning range of 4000 cm−1 to 400 cm−1 and resolution of 4 cm−1. The number of scans was 32. CTPBA and E-CTPBA were characterized by AB 5800 MALDI-TOF/TOF ultra-high resolution time-of-flight mass spectrometer (MALDI-TOF MS). Sample (1 mg) was dissolved in acetone (100 μl), and the sample solution was mixed with 1 μl of 2,5-dihydroxybenzoic acid (DHB) matrix solution. The laser wavelength was 355 nm, laser pulse was 3 ns, and the high resolution was used. The reflection mode was accelerated voltage of 20 kV. The CTPBA and E-CTPBA were tested by NMR using JNM-ECZS. The test was carried out at 600 MHz with deuterated chloroform (CDCl3) as the solvent. The internal standard was tetramethylsilane (TMS). GPC test was carried out on Waters 1525 & Agilent PL-GPC220 with chloroform as mobile phase and polystyrene as standard sample at 35 °C. HT/1600 thermo gravimetric analyzer was used to test the thermal stability of the cured epoxy resin. The samples of 5–10 mg were weighed in the crucible, the heating range was 25–700 °C, the heating rate was 10 °C min−1, and the gas atmosphere was N2. Dynamic thermomechanical analyzer (DMA1, Mettler Toledo) was used to test the glass transition temperature of the sample. The measurement mode was single cantilever, the heating rate was 3 °C min−1, the temperature range was 25–150 °C, and the test frequency was 1 Hz. The Czech TESCAN MIRA LMS scanning electron microscope was used to test the impact fracture of the modified epoxy resin. After gold plating on the fracture surface, the accelerating voltage is 15 keV.The tensile properties were measured at 25 °C according to GB/T 2567-2008 standard and the experimental speed was 10 mm min−1. According to GB/T 2567-2008 standard, the impact performance test was carried out by using the plastic pendulum impact tester (PTM1000) without notch impact. And the sample size was 120 mm × 15 mm × 10 mm.
According to GB/T 7124-2008 standard, the tensile shear strength of 100 mm × 25 mm × 1.6 mm SUS321 stainless steel sheet was selected. After grinding, the surface was cleaned with acetone, cleaned and dried for backup use. The length of the bonding surface was 12.5 ± 0.25 mm, the width was 25 ± 0.25 mm, and the thickness of the adhesive layer was about 0.2 mm. The overflow was cleaned in time. After complete curing, the universal tensile testing machine was used to test at 25 °C, and the test speed was 2 mm min−1. T-stripping strength reference GB/T 2791-1995, flexible materials are selected stainless steel, length 200 mm, width 25 mm, thickness 0.15 mm, coating length 150 mm, and running at 100 mm min−1. The gel time test was referred to GB/T 16995-1997. The modified epoxy resin and curing agent were added to the plastic cup to quickly stir and start timing. When the viscosity is increasing, stop stirring and start drawing. When the drawing becomes brittle to fracture and cannot be drawn into silk, stop timing.
According to HG/T 2708-95 chemical industry standard, acid value was measured by end-base titration method. Toluene–ethanol solution (volume ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 10 g L−1 phenolphthalein solution was used as indicator, and 0.1 mol L−1 potassium hydroxide ethanol standard titration solution was used to titrate to the solution. The solution was peach red and did not fade within 30 s. The calculation formula was as follows:
1), 10 g L−1 phenolphthalein solution was used as indicator, and 0.1 mol L−1 potassium hydroxide ethanol standard titration solution was used to titrate to the solution. The solution was peach red and did not fade within 30 s. The calculation formula was as follows:
3 Results and discussion
3.1. Structure characterization
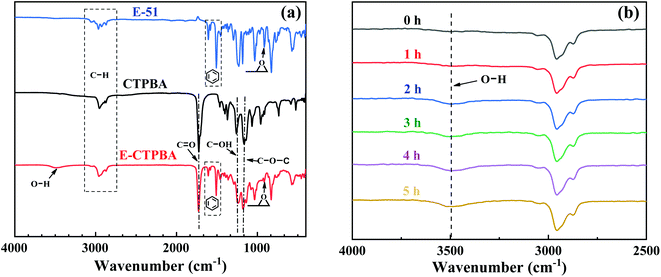
FTIR is a means to characterize the functional groups contained in the polymer, providing evidence for the structure of CTPBA and E-CTPBA. Fig. 2(a) shows the infrared spectra of CTPBA, E-51 and E-CTPBA. It can be seen from the figure that in the infrared spectrum of E-51, the characteristic absorption peak of epoxy group is at 914 cm−1, the characteristic absorption peaks of benzene skeleton are at 1508.51 cm−1 and 1606.59 cm−1, and the weak steamed bread peak is O–H stretching vibration absorption peak at 3489.16 cm−1. There are C–H stretching vibration absorption peaks near 2953.79 cm−1 and 2875 cm−1. In the infrared spectra of CTPBA, the stretching vibration absorption peaks of carboxylic acid C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and ester carbonyl were at 1723.98 cm−1, the stretching vibration peak of C–O–C in ester bond was at 1163.66 cm−1, and the stretching vibration absorption peak of C–OH in carboxyl was at 1257.19 cm−1, which proved that the carboxyl terminated polyester was successfully synthesized. Comparing the infrared spectra of CTPBA and E-CTPBA, it was found that a weak epoxy group absorption peak appeared at 914 cm−1, which proved the existence of epoxy groups. Similarly, the broad peak of steamed bread at 3489.16 cm−1 was the stretching vibration absorption peak of O–H, but the absorption peak was stronger. Therefore, epoxy resin E-51 was successfully sealed on CTPBA.
O and ester carbonyl were at 1723.98 cm−1, the stretching vibration peak of C–O–C in ester bond was at 1163.66 cm−1, and the stretching vibration absorption peak of C–OH in carboxyl was at 1257.19 cm−1, which proved that the carboxyl terminated polyester was successfully synthesized. Comparing the infrared spectra of CTPBA and E-CTPBA, it was found that a weak epoxy group absorption peak appeared at 914 cm−1, which proved the existence of epoxy groups. Similarly, the broad peak of steamed bread at 3489.16 cm−1 was the stretching vibration absorption peak of O–H, but the absorption peak was stronger. Therefore, epoxy resin E-51 was successfully sealed on CTPBA.
 | ||
| Fig. 2 (a) FTIR spectra of E-51, CTPBA and E-CTPBA; (b) synthetic infrared spectra of E-CTPBA varying with time. | ||
As shown in Fig. 2(b), the infrared spectrum of synthetic E-CTPBA changes with time. It can be seen from the figure that there was no obvious O–H stretching vibration absorption peak at 3489 cm−1 in the initial stage. With the progress of the reaction, the absorption peak gradually appeared, and the intensity of the absorption peak was increasing. This is because CTPBA reacts with E-51 to generate hydroxyl. With the progress of the reaction, the reaction degree is increasing, and the hydroxyl content is increasing, resulting in the increase of the intensity of the absorption peak.
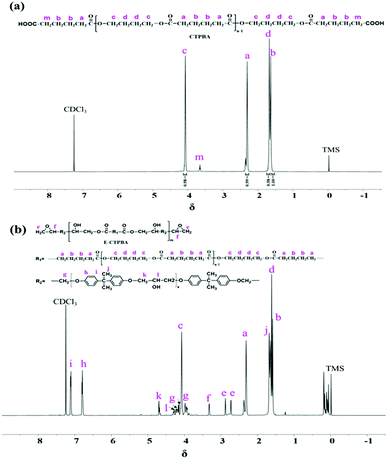
In order to further confirm the chemical structures of the synthesized CTPBA and E-CTPBA, they were characterized by 1H-NMR. Fig. 3(a) and (b) were the 1H-NMR spectra of CTPBA and E-CTPBA, respectively. In the spectrum (a), δ = 4.07 (c) and 1.67(d) belong to the characteristic absorption peaks of H on α carbon and β carbon in the unit structure of 1,4-butanediol, respectively. δ = 2.31(a) and 1.57(b) belong to the characteristic absorption peaks of H on α carbon and β carbon in the unit structure of adipic acid, respectively. A single peak appears at δ = 3.66(m), which belongs to the characteristic absorption peak of methylene connected with –COOH. Together with the infrared spectrum, CTPBA is successfully synthesized.
Fig. 3(b) was the nuclear magnetic resonance hydrogen spectrum of E-CTPBA. It can be seen that the characteristic absorption peaks of epoxy resin appear, δ = 2.73(e), 2.88(e) and 3.32(f) belong to the absorption peaks of protons on epoxy groups, δ = 6.80(h) and 7.11(i) belong to the absorption peaks of protons on benzene rings, δ = 3.98(g) and 4.17(g) belong to the absorption peaks of methylene protons (–CH2O). Most of the absorption peaks in CTPBA appeared in the spectrum, while the peaks at δ = 3.66(m) disappeared, and δ = 4.28(l) and 4.69(k) were attributed to the absorption peaks of methylene and methylene connected with hydroxyl, respectively. This indicated that –COOH reacted with epoxy group to generate –OH, which was proved by infrared spectroscopy to synthesize E-CTPBA. Since the hydrogen proton in –COOH and the hydroxyl generated by the reaction were active hydrogen, there was no absorption peak in the 1H-NMR spectrum.
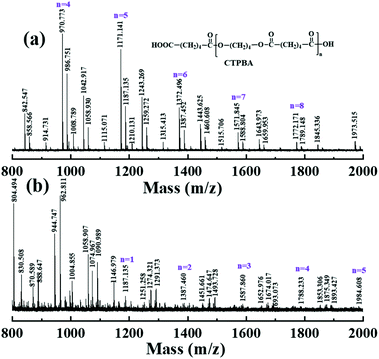
The composition and structure of the synthesized products were evaluated by 1H-NMR and MALDI-TOF MS. Fig. 4(a) was the MALDI-TOF MS spectra of CTPBA1000 samples under the reflected light mode. In order to identify the structure of CTPBA1000, the repeat unit is an important ‘judgment’ basis. According to the structural formula, the composition of the repeat unit is C10H16O4, and the adjacent peak spacing of each peak is 200, which is consistent with the mass number of the repeat unit of 200.12. All the peaks in this spectrum were consistent with n repeat units (200.12 × n) in CTPBA plus two terminal groups (–C6O3H9, M = 129.07 and –OH, M = 17.01) and Na+ and K+ (Na+ and K+ often appear in the MALDI spectrum of the sample). For example, the mass of the macromolecular chain containing four repeat units (n = 4) was 200.12 × 4 + 129.07 + 17.01 + 22.99 = 969.55, which was close to the peak of 970.77 Da in the spectrum. Together with the previous 1H-NMR and IR spectra, CTPBA was successfully synthesized.
Fig. 4(b) is the MALDI-TOF MS mass spectrum of E-CTPBA1000, its structure and synthesis process can be obtained from Fig. 1. We found that all peak shifts were consistent with the reaction. For example, when n = 4, the peak value of 1788.233 Da in E-CTPBA1000 can be expressed as 200.12 × 4 + 129.07 + 17.01 + 22.99 + 2 × 409 = 1787.55 Da, which was obtained by the peak value of 970.77 Da in CTPBA1000 plus the sum of two epoxy resins (E-51, M = 370–420).
3.2. Effect of different molecular weight CTPBA on properties of epoxy resin
Molecular weight has a great influence on the performance of epoxy resin, and finding out the optimal molecular weight is of great help for research. Calculation of molecular weight by acid titration, CTPBA with molecular weights of about 1000, 2000 and 3000 were synthesized by controlling the feed ratios of adipic acid to 1,4-butanediol, and named as CTPBA1000, CTPBA2000 and CTPBA3000, respectively, the GPC test results are shown in Table 1. The polyester end was blocked by the molar ratio of CTPBA to E-51 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and the corresponding epoxy-terminated polyesters were named as E-CTPBA1000-2-1, E-CTPBA2000-2-1 and E-CTPBA3000-2-1. The effect of different molecular weight carboxyl-terminated polyester modified epoxy resin on the properties of the adhesive was studied by controlling the addition of E-CTPBA added 20 phr. The results were shown in Table 2.
2, and the corresponding epoxy-terminated polyesters were named as E-CTPBA1000-2-1, E-CTPBA2000-2-1 and E-CTPBA3000-2-1. The effect of different molecular weight carboxyl-terminated polyester modified epoxy resin on the properties of the adhesive was studied by controlling the addition of E-CTPBA added 20 phr. The results were shown in Table 2.
| Sample | Adipic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-butanediol (molar ratio) 1,4-butanediol (molar ratio) |
Acid value (mg KOH g−1) | Hydroxyl value (mg KOH g−1) | Molecular weight measured by end-group titration method | Mn | Mw | Mz | Mw/Mn |
|---|---|---|---|---|---|---|---|---|
| CTPBA1000 | 1.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
122 | Nil | 919 | 2445 | 3943 | 6156 | 1.61 |
| CTPBA2000 | 1.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
51 | Nil | 2200 | 3838 | 8741 | 15850 | 2.28 |
| CTPBA3000 | 1.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
41 | Nil | 2737 | 4294 | 10200 | 19645 | 2.38 |
| Sample | Tensile shear strength (MPa) | T peel strength (kN m−1) | Tensile strength (MPa) | Elongation at break (%) | Impact strength (kJ m−2) |
|---|---|---|---|---|---|
| E-51 | 9.61 | 0.02 | 35.85 | 4.49 | 2.92 |
| E-CTPBA1000-2-1 | 11.35 | 0.08 | 30.66 | 22.21 | 3.71 |
| E-CTPBA2000-2-1 | 11.44 | 0.12 | 20.48 | 23.92 | 9.18 |
| E-CTPBA3000-2-1 | 4.38 | 0.24 | 20.20 | 24.02 | 3.42 |
As shown in Table 2, when the addition amount was 20 phr, the tensile shear strength of E-CTPBA2000-2-1 was the largest, and the peel strength increased with the increase of molecular weight. The tensile strength decreased with the increase of molecular weight, but the elongation at break increased with the increase of molecular weight. This may be due to the linear polyester was a flexible chain segment, and the compatibility between epoxy resin and E-51 was relatively good after epoxy end sealing. With the curing reaction, the chain extension of epoxy resin and the increase of molecular weight, the compatibility between the two gradually deteriorates, and gradually formed the island structure, which produced obvious toughening effect. Macroscopically, the elongation at break increased gradually. However, with the increase of molecular weight, the distance between cross-linking points in the cross-linking network structure increased, resulting in the decrease of cross-linking density, the decrease of intermolecular chain force, and the decrease of tensile strength macroscopically.31
The impact strength of E-CTPBA was significantly improved compared with that of pure epoxy resin, and reached the maximum when the molecular weight was 2000. This may be because CTPBA had good compatibility with the resin matrix after being terminated by epoxy resin, and the epoxy group was involved in the curing reaction, which improved the crosslinking density and increased the stress point. When subjected to instantaneous force, the impact strength increased due to the increase of the stress point and the good dispersion effect of the flexible segment.
3.3. Effect of different modification methods on properties of epoxy resin
In the process of modification of epoxy resin, the selection of methods is particularly important, and the modification effect of direct blending is often poor. Relatively speaking, the pre-reaction method can better improve the performance of epoxy resin. CTPBA1000 was selected as modifier to study the effect of three different modification methods on the performance of epoxy resin. In the previous studies, it was found that the adhesion and mechanical properties of the molecular weight of 1000 were good, so it was selected as the modifier for subsequent studies. The dosage of E-CTPBA1000-2-1 was controlled to be 20 phr, 40 phr and 60 phr, respectively. The dosage of other addition methods was the same, but the epoxy end was not sealed according to the ratio. The curing methods were all cured at room temperature (25 °C). The test was carried out after 2 days. And the test results were shown in Fig. 5.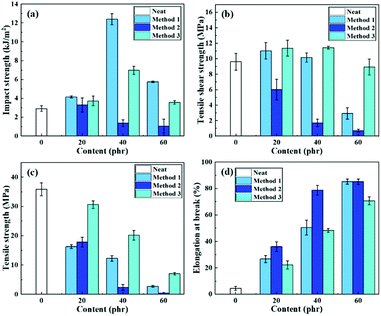 | ||
| Fig. 5 The curves of (a) impact strength (b) tensile shear strength (c) tensile strength and (d) elongation at break under different modification methods. | ||
Method 1: metric CTPBA was reacted with E-51 at 96 °C for 3 h, and 0.3 wt% catalyst triphenylphosphine was added.
Method 2: the measured CTPBA was directly blended with E-51.
Method 3: the CTPBA with molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was reacted with E-51 at 96 °C to obtain the epoxy-terminated E-CTPBA-2-1, which was then added into the epoxy resin in proportion.
2 was reacted with E-51 at 96 °C to obtain the epoxy-terminated E-CTPBA-2-1, which was then added into the epoxy resin in proportion.
It can be seen from Fig. 5 that different modification methods had a great influence on the adhesive properties of the system. When CTPBA was directly blended with E-51, it showed the characteristics of ordinary liquid rubber toughening, and the peeling strength was enhanced, but it was significantly worse than that of the other two addition methods. The tensile shear strength was lower than that of pure epoxy resin system, and decreased with the increase of addition amount. Relatively speaking, the bonding performance of controlled molecular weight synthesis was the best, which may be due to the poor compatibility between CTPBA and E-51 by direct blending, showing the toughening characteristics of ordinary liquid rubber, while the compatibility between E-CTPBA and E-51 formed by epoxy end sealing was better, and it was easy to form island structure in the curing process.
The tensile strength decreased with the increase of CTPBA, and the tensile strength after modification according to molecular weight design was greater than that of the other two methods. The elongation at break increased with the increase of CTPBA, which was greatly improved compared with pure epoxy resin. The impact strength of molecular weight design modification and pre-reaction both increased first and then decreased. The impact strength reached the maximum when the addition amount was 40 phr, which was 324.7% higher than that of pure epoxy. Relatively speaking, the way of controlling molecular weight design and modification is method 3, which has the best comprehensive performance.
3.4. Effect of CTPBA and E-51 ratio on properties of epoxy resin
In the synthesis process of E-CTPBA, the different ratio of CTPBA and E-51 will form block copolymers with different structures, but there are few studies on this aspect. The epoxy-terminated block copolymer was synthesized by CTPBA1000 and E-51 according to different molar ratios. The copolymer dosage was 20 phr (accounting for 20 wt% of the epoxy resin), and the T-31 curing agent was added (25 phr). The mixture was mixed and defoamed in vacuum for about 10 min. The test was carried out after two days at room temperature (25 °C).It can be seen from Table 3 that the peel strength, tensile shear strength and gel time increase with the increase of the feed ratio, which may be due to the larger the feed ratio, the lower the epoxy group content, and the increase of gel time. With the increase of the feed ratio, the tensile strength decreased gradually, but the elongation at break increased. This may be due to the rigid structure of the benzene ring in bisphenol A epoxy resin, which can improve the tensile strength. With the increase of the feed ratio, the content of the rigid structure of the benzene ring in the same mass of the copolymer decreased, which reduced the tensile strength and increased the elongation at break. Overall, the tensile strength of modified epoxy resin was lower than that of pure epoxy resin, and the elongation at break increased. The impact strength had little change, but it was significantly higher than that of pure epoxy resin.
| Sample | Molar ratio | Tensile shear strength (MPa) | T peel strength (kN m−1) | Gel time (min) | Tensile strength (MPa) | Elongation at break (%) | Impact strength (kJ m−2) |
|---|---|---|---|---|---|---|---|
| E-CTPBA1000-2-1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
11.35 | 0.08 | 75 | 30.66 | 18.22 | 3.71 |
| E-CTPBA1000-3-2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
12.18 | 0.11 | 80 | 22.81 | 19.38 | 3.15 |
| E-CTPBA1000-4-3 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
12.50 | 0.12 | 93 | 18.82 | 28.96 | 3.58 |
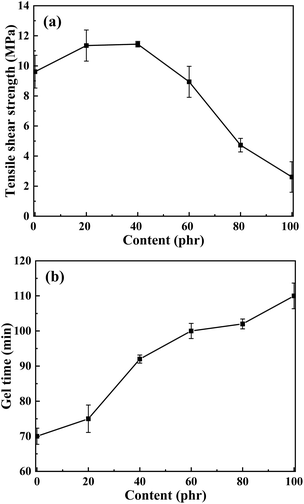
3.5. Effect of E-CTPBA addition on properties of epoxy resin adhesive
Considering the mechanical properties such as tensile shear strength and tensile strength, it can be obtained from the previous experimental results that the comprehensive mechanical properties of E-CTPBA1000-2-1 were the best. Therefore, it was selected as the raw material for the following study to study the effect of addition amount on the properties of modified epoxy resin adhesive.The failure mode of stainless steel sheet after tensile shear test of carboxyl-terminated polyester modified epoxy resin adhesive was analyzed. When the amount of carboxyl-terminated polyester modified epoxy resin adhesive was 80 phr and 100 phr, the adhesive on the bonding interface was peeled off from the whole fracture, one bonding surface was smooth, and the other bonding surface was attached to the whole adhesive, which belonged to the form of interface failure. When the addition amount was less than 80 phr, the bonding surface was rough, and adhesive was distributed on both steel sheets. At this time, interface failure and cohesion failure occurred simultaneously. The main reason for the interfacial failure may be that the content of polyester as a flexible segment is too high, resulting in low strength, which cannot be well bonded with the steel sheet, and it is easy to fall off from the steel sheet as a whole. When the polyester content is low, the epoxy resin modified by carboxyl terminated polyester and the epoxy resin E-51 form a close winding and crosslinking, forming a network structure, and the stress can be better dispersed. At this time, it is easy to cause the damage of the resin matrix itself, namely, the cohesive failure. Compared with the interfacial failure, more energy was needed to absorb, so that the tensile shear strength was increased.1,32
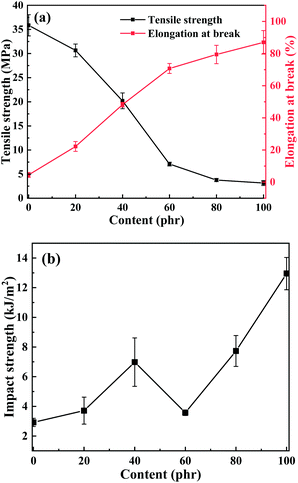 | ||
| Fig. 7 Effect curves of the E-CTPBA1000 addition on (a) tensile strength and elongation at break; (b) impact strength. | ||
The effect of E-CTPBA1000-2-1 addition on the impact strength of the system was shown in Fig. 7(b). It can be seen that with the increase of the addition amount, the impact strength increased first, then decreased and then increased. When the addition amount reached 100%, the impact strength reached the maximum, which was 343.5% higher than that of pure epoxy resin system. In similar studies on toughening, Shiai Xu used liquid carboxyl terminated nitrile rubber (CTBN) as a toughening agent. When the addition amount of CTBN was 15 phr, the toughness was the largest, and the impact strength was 1.95 kJ m−2. The toughening effect was not obvious.25 Akbari used CTBN as toughening agent, and its impact strength of pure epoxy resin was 7.19 kJ m−2. When the addition amount of CTBN was 15 phr, the impact strength reached 19.20 kJ m−2, increasing by 167.04%.24 As reported by D. Ratna, when the addition amount of CTPEGA was 20 phr, the maximum value was 32.0 J m−1, which was 73.0% higher than that of pure matrix, and the toughening effect was poor. The impact strength of the material mainly depended on the toughness of the material. The linear polyester was a kind of flexible material with great elasticity, while the epoxy resin E-51 contained phenyl functional groups and had great brittleness. As a flexible chain, polyester was interspersed in the cross-linked network structure. When subjected to impact, it can well disperse the stress and increase the fracture energy of the material, and increase the impact strength after adding an appropriate amount of CTPBA.
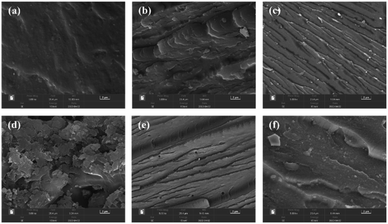
Fig. 8 was the SEM images of the impact section of epoxy resin cured with different amounts of E-CTPBA1000-2-1. It can be clearly seen from the figure that the impact fracture surface of pure epoxy resin was smooth without obvious defects, showing typical brittle fracture characteristics. Compared with epoxy resin, the fracture surface of carboxyl-terminated polyester modified epoxy resin was rough, showing ductile fracture characteristics. When the addition amount was 40 phr, the branching pattern appears, the surface was smoother, and the corresponding impact strength was relatively large. When the addition amount was 60 phr, there was a more serious agglomeration phenomenon, which reduced the impact strength, when the addition amount continued to increase, the polyester became a continuous phase, and the epoxy resin was a dispersed phase. The dimples increased, showing typical ductile fracture characteristics.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curve of cured products with different CTPBA content of 0, 20 phr, 40 phr, 60 phr, 80 phr and 100 phr. From the diagram, it can be seen that in the test temperature range of 25–150 °C, the cured products with different CTPBA content had only one tan
δ curve of cured products with different CTPBA content of 0, 20 phr, 40 phr, 60 phr, 80 phr and 100 phr. From the diagram, it can be seen that in the test temperature range of 25–150 °C, the cured products with different CTPBA content had only one tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak, corresponding to only one glass transition temperature, indicating that the cured epoxy resin cured with E-CTPBA1000-2-1 was a single component cured with complete thermodynamic compatibility. When the addition amount was 0–80 phr, the glass transition temperature decreased with the increase of E-CTPBA1000-2-1 addition amount, and the peak value of tan
δ peak, corresponding to only one glass transition temperature, indicating that the cured epoxy resin cured with E-CTPBA1000-2-1 was a single component cured with complete thermodynamic compatibility. When the addition amount was 0–80 phr, the glass transition temperature decreased with the increase of E-CTPBA1000-2-1 addition amount, and the peak value of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ increased with the increase of E-CTPBA addition amount. This may be due to the decrease of crosslinking degree and curing degree of epoxy resin with the increase of E-CTPBA addition amount, resulting in the decrease of glass transition temperature.34 The storage modulus of pure epoxy resin and cured epoxy resin modified with different amounts of E-CTPBA1000-2-1 was shown in Fig. 9(b). The storage modulus of all samples decreased with the increase of temperature. After adding E-CTPBA, the storage modulus of epoxy resin decreased significantly, which may be due to the low modulus of linear polyester. When the addition amount was 0–80 phr, the storage modulus E′ decreased with the increase of E-CTPBA1000-2-1 addition amount. When the addition amount was 100 phr, the storage modulus increased instead.
δ increased with the increase of E-CTPBA addition amount. This may be due to the decrease of crosslinking degree and curing degree of epoxy resin with the increase of E-CTPBA addition amount, resulting in the decrease of glass transition temperature.34 The storage modulus of pure epoxy resin and cured epoxy resin modified with different amounts of E-CTPBA1000-2-1 was shown in Fig. 9(b). The storage modulus of all samples decreased with the increase of temperature. After adding E-CTPBA, the storage modulus of epoxy resin decreased significantly, which may be due to the low modulus of linear polyester. When the addition amount was 0–80 phr, the storage modulus E′ decreased with the increase of E-CTPBA1000-2-1 addition amount. When the addition amount was 100 phr, the storage modulus increased instead.
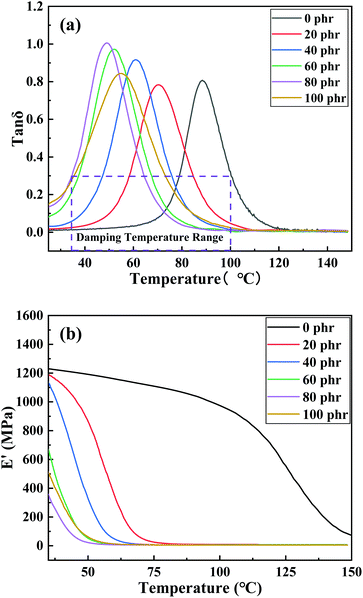 | ||
Fig. 9 Curve of E-CTPBA1000 addition to modified epoxy resin (a) tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ–T curve, (b) storage modulus E′. δ–T curve, (b) storage modulus E′. | ||
In China, the temperature range corresponding to the loss factor greater than or equal to 0.3 is called the effective damping temperature range DTR (Damping Temperature Range) of the damping material. The damping performance data were shown in Table 4. It can be seen that the damping temperature range gradually increased with the increase of the addition amount. When the addition was 100 phr, the glass transition temperature increased and the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak decreased.
δ peak decreased.
| Content | 0 phr | 20 phr | 40 phr | 60 phr | 80 phr | 100 phr |
|---|---|---|---|---|---|---|
| Tg/°C | 88.4 | 70.3 | 60.8 | 51.9 | 49.1 | 54.9 |
DTR/(tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ ≥ 0.3) δ ≥ 0.3) |
21 (79–100) | 26 (58–84) | 29 (47–76) | 30 (37–67) | 31 (34–65) | 37 (36–73) |
tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δmax δmax |
0.81 | 0.78 | 0.92 | 0.97 | 1.01 | 0.84 |
4 Conclusions
The carboxyl-terminated polybutylene adipate (CTPBA) with different molecular weight was synthesized. The effects of CTPBA content on the adhesive properties and mechanical properties of modified epoxy resin were studied. The effects of CTPBA on the glass transition temperature, thermal stability and impact fracture morphology of modified epoxy resin was analyzed. The epoxy resin curing system does not use toxic and volatile components, which is in line with the concept of green development. Its rapid gel time, good bonding properties and mechanical properties have certain application value for some areas requiring rapid curing (such as repair).The effects of different modification methods and molecular weight on the properties of epoxy resin were studied. When CTPBA with molecular weight of 1000 was used and the epoxy terminated polymer (E-CTPBA) was synthesized by controlling the molar ratio, the comprehensive mechanical properties of the modified epoxy resin were the best, which provided a screening method for other linear carboxyl-terminated polyesters (such as carboxyl-terminated poly adipic acid adipate, carboxyl-terminated polybutadiene glycol ester, etc.) to modify epoxy resin. In order to improve the adhesion and mechanical properties of epoxy resin, the modifier E-CTPBA was added to the E-51 resin matrix. The addition of E-CTPBA can improve the tensile shear strength, peel strength, elongation at break and impact strength, while the tensile strength decreases. When the addition amount was 40 phr, the adhesive property and toughness of the system were the best. Compared with pure epoxy resin, the tensile shear strength and toughness increased by 19.0% and 139.0%, respectively, but the tensile strength decreased by 43.6%. The thermal stability of the system was good. Compared with pure epoxy resin, the initial temperature and maximum thermal decomposition rate of the system were reduced, but the overall thermal stability is similar. Therefore, it is a promising method for modifying epoxy resin to control the end of epoxy resin by modification.
Author contributions
Yu Li supervised the projects. Xu Luo carried out the experiments. Zhaoyi Sun, Guorong Wang and Jie Xin analyzed the mechanism and thermal properties. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Notes and references
- G.-S. Chae, H.-W. Park, J.-H. Lee and S. Shin, Polymers, 2020, 12, 1549 CrossRef CAS.
- Y. Feng, J. Hu, F. Wang, Q. Huang, C. Peng and Z. Xu, Mater. Res. Express, 2018, 5, 065321 CrossRef.
- G. C. Papanicolaou and D. E. Anastasiou, J. Adhes. Sci. Technol., 2021, 35, 1138–1153 CrossRef CAS.
- J. Yeon, Y. Song, K. K. Kim and J. Kang, Materials, 2019, 12, 2396 CrossRef CAS.
- P. Mohan, Polym.-Plast. Technol. Eng., 2013, 52, 107–125 CrossRef CAS.
- S. R. Mousavi, S. Estaji, M. R. Javidi, A. Paydayesh, H. A. Khonakdar, M. Arjmand, E. Rostami and S. H. Jafari, J. Mater. Sci., 2021, 56, 18345–18367 CrossRef CAS.
- M. Peerzada, S. Abbasi, K. T. Lau and N. Hameed, Ind. Eng. Chem. Res., 2020, 59, 6375–6390 CrossRef CAS.
- U. Farooq, J. Teuwen and C. Dransfeld, Polymers, 2020, 12, 1908 CrossRef CAS.
- S. R. Mousavi, S. Estaji, A. Paydayesh, M. Arjmand, S. H. Jafari, S. Nouranian and H. A. Khonakdar, Polym. Compos., 2022, 43, 1871–1886 CrossRef CAS.
- N. Domun, H. Hadavinia, T. Zhang, T. Sainsbury, G. H. Liaghat and S. Vahid, Nanoscale, 2015, 7, 10294–10329 RSC.
- Y. T. Park, Y. Qian, C. Chan, T. Suh, M. G. Nejhad, C. W. Macosko and A. Stein, Adv. Funct. Mater., 2015, 25, 575–585 CrossRef CAS.
- S. Jung, S. Kang, J. Kuwabara and H. J. Yoon, Polym. Chem., 2019, 10, 4506–4512 RSC.
- S. Kang, H. K. Moon and H. J. Yoon, Macromolecules, 2018, 51, 4068–4076 CrossRef CAS.
- Q. Shang, S. Hao, J. Zhang, J. Gong, F. Wang, Y. Wu and S. Gu, J. Adhes. Sci. Technol., 2015, 29, 910–923 CrossRef CAS.
- K. Tserpes and V. Tzatzadakis, Aerospace, 2022, 9, 64 CrossRef.
- G. Chen, H. Ma, Z. Zhou, F. Ren and W. Xu, Mater. Res. Express, 2022, 9, 016506 CrossRef CAS.
- M. Barcikowski and K. Rybkowska, Int. J. Fract., 2022, 223, 234–233 Search PubMed.
- G.-B. Lee, H. Kim, S. w. jae, J. Jeon, I.-S. Park and C. Nah, Elastomers Compos., 2021, 56, 179–186 CAS.
- Y. Liu, L. Yao, Y. Bu and Q. Sun, Materials, 2021, 14, 4601 CrossRef CAS PubMed.
- H. Xu, X. Zhang, G. Hu, L. Weng and L. Liu, Polym. Compos., 2020, 41, 4372–4378 CrossRef CAS.
- T. Farajpour and M. Abdollahi, Polym. Sci., Ser. A, 2018, 60, 655–662 CrossRef CAS.
- C.-H. Chen, J.-Y. Jian and F.-S. Yen, Polym. Bull., 2021, 78, 3821–3834 CrossRef CAS.
- X. Yin, Z. Xie, Q. Liu, X. Yuan, X. Hou and J. Zhao, J. Appl. Polym. Sci., 2021, 138, 51248 CrossRef CAS.
- R. Akbari, M. H. Beheshty and M. Shervin, Iran. Polym. J., 2013, 22, 313–324 CrossRef CAS.
- S. Xu, X. Song and Y. Cai, Materials, 2016, 9, 640 CrossRef.
- D. Ratna, A. B. Samui and B. C. Chakraborty, Polym. Int., 2004, 53, 1882–1887 CrossRef CAS.
- A. Kausar, J. Plast. Film Sheeting, 2020, 36, 391–408 CrossRef CAS.
- P. Xu, X. Du, P. Cong and Z. Zhou, Road Mater. Pavement Des., 2022, 23, 234–246 CrossRef CAS.
- P. Xu, X. Zhu, P. Cong, X. Du and R. Zhang, Constr. Build. Mater., 2018, 165, 295–302 CrossRef CAS.
- P. S. Achary, C. Gouri and R. Ramaswamy, J. Appl. Polym. Sci., 1991, 42, 743–752 CrossRef CAS.
- G. Hu, X. Zhang, H. Xu, L. Weng and L. Liu, Polymer Materials Science & Engineering, 2018, 34(11), 144–150 Search PubMed.
- G.-S. Chae, H.-W. Park, K. Kwon and S. Shin, Polymers, 2021, 13, 469 CrossRef CAS PubMed.
- S. Xiaoxue and X. Shiai, Adv. Mater. Res., 2015, 1120–1121, 568–571 Search PubMed.
- Y. Ebrahimabadi, M. Mehrshad, M. Mokhtary and M. Abdollahi, J. Appl. Polym. Sci., 2021, 138, 49932 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02915d |
| This journal is © The Royal Society of Chemistry 2022 |