Open Access Article
Open Access ArticleTarget identification and occupancy measurement of necrosis avid agent rhein using bioorthogonal chemistry-enabling probes†
Cuihua Jiang‡
ab,
Jian Zhang‡ ab,
Shihe Huab,
Meng Gaoab,
Dongjian Zhangab,
Nan Yaoab and
Qiaomei Jin
ab,
Shihe Huab,
Meng Gaoab,
Dongjian Zhangab,
Nan Yaoab and
Qiaomei Jin *ab
*ab
aAffiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing 210028, Jiangsu, China. E-mail: jqmxy@163.com
bLaboratories of Translational Medicine, Jiangsu Province Academy of Traditional Chinese Medicine, Nanjing 210028, Jiangsu, China
First published on 6th June 2022
Abstract
Necrosis is an important biomarker, which only occurs in pathological situations. Tracking of necrosis avid agents is of crucial importance toward understanding their mechanisms. Herein, we developed a modular probe strategy based on bioorthogonal copper-free click chemistry. Structural modification of rhein with transcyclooctene (TCO) led to the identification of rhein-TCO2 as the most active probe with specific necrosis affinity. In a systematic evaluation, the colocalization of rhein-TCO2 in the nucleus (exposed DNA and rRNA) of necrotic cells was observed. This work provides a foundation for the development of target-identified of rhein compounds, and binding to exposed DNA and rRNA may be an important target of rhein compounds in necrotic cells.
Introduction
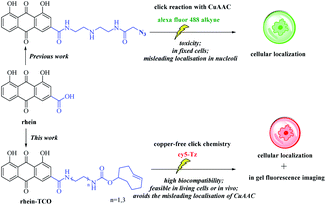
Natural products have always been the inspiration for the development of drugs and probes.1 In addition to their biological activity, structural determination, and total synthesis, it is above all the knowledge of their molecular targets and mechanism of action that is of central interest in current natural product research.2 Analysis of the clinical trials suggests that the late stage failures of candidate drug molecules in phase II or III clinical trials are frequently caused by off-target effects or inefficient target engagement in vivo. And major factors contributing to failures are insufficient proof of target engagement.3Necrosis, one mode of cell death, is a hallmark for severe diseases in many pathologies, such as cancers, cardiovascular disorders, neurodegeneration and also in the way malignant tumors respond to cancer therapy.4,5 As a result, noninvasive techniques for necrosis detection can be used to accomplish several goals including supporting disease diagnosis, assessing the extent of tissue damage and evaluating cancer therapeutic efficacy. But these noninvasive techniques are lacking in the clinic, only radiolabeled annexin V6 and [18F]ICMT-11 in clinical trials.7 Our group demonstrated that a certain class of natural necrosis avid agents like hypericin and rhein, which can specifically accumulate in the necrotic area of diseases (Fig. 1).8–14 Moreover, in order to understanding the necrosis avidity mechanism of rhein derivatives, we had explored the cellular localisation of rhein by synthesising a clickable derivative and labelling it in situ with an Alexa-fluor 488 click partner mediated by copper (CuAAC) in bioorthogonal click chemistry, that indicates the colocalization between rhein and nucleus including nucleoli.15 However, more recent studies show that the toxicity of Cu(I) has limited the use of this particular reaction for in vivo applications, and CuAAC also denatures and causes protein aggregation and precipitation, which may make it incompatible with some downstream applications.16 Moreover, the copper-based CuAAC methodologies in fixed cells implicated Cu(I)/alkyne intermediates in the non-specific localisation of ligands to the nucleoli.17 Therefore, our research on the necrosis targeting mechanism of rhein needs to be further proofed. Hence, we developed a strategy that allows identification of target occupancy measurement of rhein derivatives under physiological conditions, using bioorthogonal copper-free click chemistry in live cells (Fig. 2).
 | ||
| Fig. 2 A new functionalized rhein probe containing a bioorthogonal moiety (copper-free click-probe) usable for assay in live cells. | ||
Results and discussion
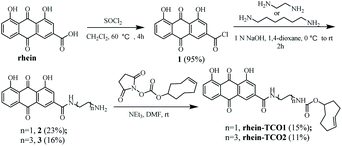
Base on previous structure–activity relationship, the rhein was equipped with a spacer bearing a terminal azide function, grafted onto position 3 of the anthraquinone core, so as to have a minimal effect on its necrotic affinity. We therefore designed and synthesized compound rhein-TCO1 and rhein-TCO2, which both contain the bioorthogonal moieties either trans-cyclooctene (TCO) (Fig. 2).The preparation of rhein-TCO1 and rhein-TCO2 is outlined in Scheme 1. The synthesis used the commercially available rhein as starting material. Rhein reacted with thionyl chloride to furnish an intermediate acyl chloride 1, which then coupled with ethylenediamine or 1,6-hexamethylenediamine to form amide 2 or 3 under basic conditions. Subsequently, amide 2 or 3 was reacted with (E)-cylooct-4-en-1-yl (2,5-dioxopyrrolidin-1-yl) carbonate to yield target compound rhein-TCO1 and rhein-TCO2. The structure of rhein-TCO1 and rhein-TCO2 was established by analysis of their NMR data (ESI†) and mass spectrometry.
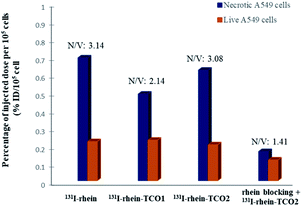
First, in order to investigate whether the modified rhein-TCO1 and rhein-TCO2 maintain the same necrotic targets as rhein, the necrosis avidities of rhein, rhein-TCO1 and rhein-TCO2 were evaluated in vitro and in vivo by labeling with radioisotope iodine-131, which has been proofed as feasible and convenient method with high detective sensitivity.18 The iodogen coating method was conducted successfully in this study.11 A radiochemical purity greater than 98% (labeling yield more than 90%) could be obtained as measured by analytical RP-HPLC for all tracers. The labeled compounds turned out to be stable after incubation in rat plasma at 37 °C for 24 h the data of 93.11% ± 0.47% (rhein), 96.07% ± 0.52% (rhein-TCO1) and 95.01% ± 0.38% (rhein-TCO2). Then, the necrosis affinities of rhein, rhein-TCO1 and rhein-TCO2 were assessed by determining the uptakes of 131I-rhein, 131I-rhein-TCO1 and 131I-rhein-TCO2 in necrotic A549 cells and normal A549 cells in vitro. Necrotic A549 cells were induced under intense hyperthermia at 57 °C for 1 h according to previous reports and the percentage of necrotic cells were in the range of 75–85% as determined by flow cytometry. As shown in Fig. 3 and Table S1,† the necrotic-to-viable uptake ratios of 131I-rhein, 131I-rhein-TCO1, 131I-rhein-TCO2 and 131I-rhein-TCO2 under rhein blocking were 3.14, 2.14, 3.08 and 1.41, respectively. 131I-Rhein-TCO2 features significant necrotic affinity in vitro as 131I-rhein. The ratios were considerable compared with other previously explored NAAs (2.50–2.80).19 Disappointingly, 131I-rhein-TCO1 with 2C alkyl chain spacer, which might hinder the binding of rhein to the target, showed lower necrotic affinity. Moreover, the significantly decreased uptake ratios in vitro rhein blocking experiment indicated that 131I-rhein-TCO2 and rhein should share the same specific target of necrotic cells.
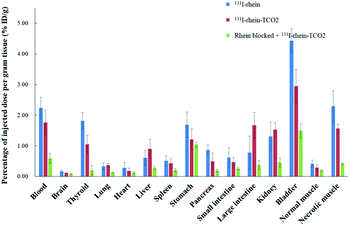
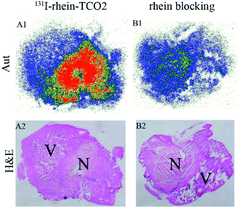
To investigate the necrosis targeting ability of rhein-TCO1 in vivo, biodistribution study was performed for accurate quantification of the tracer uptake in the selected organs in mice with muscular necrosis by gamma counting (p.i.) (Fig. 4 and Table S2†). As showed in Fig. 4, significantly higher and specific radioactivity uptakes of both compounds in necrotic muscle compared to normal muscle (P < 0.05). The concentrations of 131I-rhein in necrotic vs. normal muscle were 2.29 ± 0.51% ID/g vs. 0.42 ± 0.11% ID/g with the corresponding radioactivity ratios from necrotic to normal muscle were 5.49. And the necrotic-to-normal muscle ratios were 5.38 for 131I-rhein-TCO2. The organ that stand out was the excretion organ bladder. Other organs or tissues like kidneys, stomach, blood also showed high uptakes of 131I-rhein and 131I-rhein-TCO2. These prominent radioactivities revealed that both compounds showed high and specific necrosis affinity in necrotic organs or tissues and were mainly metabolized and/or excreted by a renal pathway. The average radioactivity uptakes of 131I-rhein-TCO2 were slightly lower than that of 131I-rhein in almost all organs and tissues, which would be consistent with the results obtained by uptakes in necrotic A549 cells. The reason might be that the prolongation of the linker increased the lipophilicity of the tracer, which had a slightly effect on its uptakes in cells and in vivo distribution properties. Furthermore, the blocking experiment, which was a strategy for target identification of rhein-TCO2 by competitive binding with rhein, was devised to demonstrate that rhein-TCO2 shares the same target in vivo as rhein. In good agreement with rhein-based competitive binding in vitro experiments, the excessive unlabeled rhein significantly reduced the uptake of 131I-rhein-TCO2 in necrotic muscle compared with the unblocked group. The results were also confirmed by autoradiography and H&E staining in Fig. 5. These data confirm that, in necrotic tissues, rhein-TCO2 and rhein should share the same specific targets.
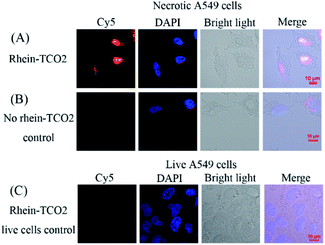
We next assessed the clickable compound rhein-TCO2 in necrotic A549 cells by confocal fluorescence microscopy imaging (Fig. 6). A negative control experiment was performed without rhein-TCO2. And confocal fluorescence microscopy studies were also conducted on live A549 cells to confirm the specific uptake of compound rhein-TCO2 on necrotic cells (Fig. 6B and C). Fig. 6A shows that the distribution of red fluorescence (Cy5) was basically consistent with that of blue fluorescence through the location of DAPI stained nucleus and the morphology of A549 cell observed under white light, and it could be inferred that rhein-TCO2 was mainly distributed in the nucleus (red fluorescence). We observed the same results in other fields of parallel experiments (Fig. S1†). As seen in Fig. 6 and S1A,† the nuclear marker DAPI could dye the entire shape of the nucleus and did not stain the nucleolar subcompartments, which appeared as dark areas at the same time. This is likely due to the fact that DAPI mainly performs nuclear staining by binding with DNA, and its binding ability with RNA is much weaker than that of DNA. Nucleoli are highly dense domains characterised by the strong spatial confinement of nucleic acids (mainly rRNA, very small amounts of DNA) and proteins, so the blue fluorescence of DAPI can hardly be observed in nucleoli.20 However, it is worth noting that red fluorescent signal of Cy5 located rhein-TCO2 was observed throughout the whole nucleus without nucleolar cavity, and the fluorescence intensity was even greater in the nucleolar region. These results indicated that rhein-TCO2 can be distributed throughout the nucleus. Similar results were obtained when the compound with 2C alkyl chain spacer (rhein-TCO1) was examined (see Fig. S1B†). But in this case, red fluorescence could also be observed in the cytoplasm, indicating that the targeting ability of rhein-TCO1 to the necrotic nucleus was weaker than rhein-TCO2. And this result was also consistent with the previous results of the necrotic targeting ability. Next, we investigated the interference of Cy5-Tz dye. Necrotic A549 cells were incubated with Cy5-Tz for 30 min without rhein-TCO2. There was almost no red fluorescence in the entire cell can be observed when no rhein-TCO2 was introduced (Fig. 6B). In contrast to the results in Fig. 6A, the cytoplasm of one of the cells showed weak red fluorescence, but by superposition comparison with DAPI, it could be seen that the nucleus presented a Cy5-Tz free (red fluorescence) void state, and thus most likely speculated that the red fluorescence in the nucleus was derived from the specific nuclear distribution of rhein-TCO2, excluding the interference of Cy5-Tz dye. Moreover, the same post labelling strategy was applied in the localization of rhein-TCO2 in live A549 cells. And not surprisingly, no significant staining and/or localization was observed, which proved the specific uptake of rhein-TCO2 on necrotic cells (Fig. 6C).
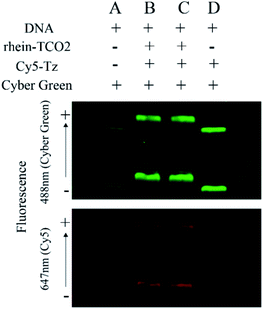
Up till now, the universal targets for developing molecular probes that can be used for visualization of necrotic cell include intracellular DNA, Hsp90 (90 kDa heat shock protein), fumarase, La antigen, histones and some unknown constituents.21,22 The conclusion to be drawn from the confocal experiments is that rhein-TCO2 is mainly distributed in the nucleus of necrotic cells. In addition, rhein is a primary anthraquinone, which has a planar structure and is likely to achieve necrosis targeting by binding to DNA. Then, we designed DNA electrophoresis experiments based on biological orthogonal reaction in a randomly linearized plasmid pUC19 DNA to further verify whether rhein-TCO2 can bind to DNA. The procedure involved incubation of the pUC19 DNA with rhein-TCO2 and reaction with Cy5-Tz, followed by purification of the mixtures and analysis of the labeled DNA on agarose gels (for details see the ESI†). A fluorescent image of the gel was shown in Fig. 7. DNA were stained with a green nucleic acid dye (cyber green, at 488 nm) for location on each lane and to distinguish the red fluorescence of Cy5 (at 647 nm). As illustrated in the gel at the top of Fig. 7, DNA presented two bands in each electrophoretic band (lane A–D), both of which were dyed green fluorescence. The lane A in the bottom gel shows that no red band was observed at the corresponding position of the green band when DNA was sampled separately in group A. Upon treatment with rhein-TCO2 and Cy5-Tz, red fluorescence could be obviously observed at the same position of green fluorescence in each lane of lane B and lane C. We also investigated only DNA and Cy5-Tz were added in group D and observed that no red fluorescence was observed. Taken together, these results clearly indicated that the red fluorescence came from the combination of DNA and rhein-TCO2, and undergo biological orthogonal reaction with dye Cy5-Tz, which was consistent with that observed by laser confocal experiments in cell experiments.
We also evaluated the DNA binding ability of rhein-TCO2. The DNA binding ability KSV value (Stern–Volmer quenching constant) for rhein-TCO2 was 1.20 × 104 M−1, which was similar to that of rhein (1.01 × 104 M−1) as reported in our previous studies.23 In addition, we observed that rhein-TCO2 could also be distributed into the nucleoli (mainly rRNA) of cells in laser confocal experiment. According to literature reports, anthraquinone compounds can not only bind to DNA, but also act as RNA embedders, such as antiviral compounds like ametantrone (HAQ) and mitoxantrone (DHAQ).24 Therefore, we speculated that ribaric acid necrosis probes might have certain binding ability to RNA. The well-established UV-vis protocol was used to test the binding ability of RNA with rhein, and the KSV value was 4 × 103 M−1, showing moderate binding ability (Fig. S2†).
Furthermore, to explain the observed results, the docking models were conducted to elucidate the possible binding mode of rhein-TCO2 or rhein with DNA and RNA (Fig. 8 and S3†). As shown in Fig. 8B and S3A,† compound rhein and rhein-TCO2 both had the same key interactions with DNA through interpolation. The compound rhein-TCO2 with 6C alkyl chain spacer retained higher target affinity likely because of enough space for TCO group protruding out of the DNA groove. This may also be the reason why the modification of substituents at the 3 site of rhein-like necrosis probes does not affect the necrosis targeting of these molecules. Fig. S3B† showed the binding mode of rhein in RNA, rhein can be well embedded into the base pair plane of RNA single strand.
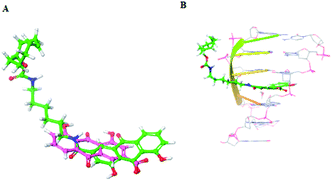 | ||
| Fig. 8 (A) Chemical structure of rhein and rhein-TCO2; (B) predicted binding modes of rhein-TCO2 with DNA (PDB code 282D). | ||
Conclusions
To conclude, we developed a biological orthogonal strategy to locate the distribution of rhein-like necrosis probes on necrotic cells and found that they were mainly distributed in the nucleus. And this copper-free method overcomes the limitation of the click-chemistry method of Cu(I) used in our previous study. Moreover, DNA electrophoresis and DNA binding ability experiments elucidated specific binding of the rhein-like necrosis probes to DNA in the nucleus and some rRNA in the nucleoli to achieve their necrosis targeting. In addition, molecular docking experiments also showed the binding events between rhein and DNA or rRNA through intercalation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (No. 21907053 and 82003989), and we also thank the China Pharmaceutical University for providing spectra and Schrodinger Suites for molecular docking.Notes and references
- M. H. Wright and S. A. Sieber, Nat. Prod. Rep., 2016, 33, 681–708 RSC.
- T. Böttcher, M. Pitscheider and S. A. Sieber, Angew. Chem., Int. Ed., 2010, 49, 2680–2698 CrossRef PubMed.
- A. Rutkowska, D. W. Thomson, J. Vappiani, T. Werner, K. M. Mueller, L. Dittus, J. Krause, M. Muelbaier, G. Bergamini and M. Bantscheff, ACS Chem. Biol., 2016, 11, 2541–2550 CrossRef CAS PubMed.
- R. K. Amaravadi and C. B. Thompson, Clin. Cancer Res., 2007, 13, 7271–7279 CrossRef CAS PubMed.
- M. C. M. Stroet, E. de Blois, J. Haeck, Y. Seimbille, L. Mezzanotte, M. de Jong, C. W. G. M. Löwik and K. M. Panth, Contrast Media Mol. Imaging, 2021, 2021, 2853522 Search PubMed.
- G. J. Kemerink, H. H. Boersma, P. W. Timister, L. Hofstra, I. H. Liem, M. T. Pakbiers, D. Janssen, C. P. Reutelingsperger and G. A. Heidendal, Eur. J. Nucl. Med., 2001, 28, 1373–1378 CrossRef CAS PubMed.
- Q. D. Nguyen, A. Challapalli, G. Smith, R. Fortt and E. O. Aboagye, Imaging apoptosis with positron emission tomography, Eur. J. Cancer, 2012, 48, 432–440 CrossRef CAS PubMed.
- Y. Ni, G. Bormans, F. Chen, A. Verbruggen and G. Marchal, Invest. Radiol., 2005, 40, 526–535 CrossRef CAS PubMed.
- C. Wang, Q. Jin, S. Yang, D. Zhang, Q. Wang, J. Li, S. Song, Z. Sun, Y. Ni, J. Zhang and Z. Yin, Mol. Pharmaceutics, 2016, 13, 180–189 CrossRef CAS PubMed.
- Q. Luo, Q. Jin, C. Su, D. Zhang, C. Jiang, A. F. Fish, Y. Feng, Y. Ni and J. Zhang, Anal. Chem., 2017, 89, 1260–1266 CrossRef CAS PubMed.
- Q. Wang, S. Yang, C. Jiang, J. Li, C. Wang, L. Chen, Q. Jin, S. Song, Y. Feng, Y. Ni, J. Zhang and Z. Yin, Sci. Rep., 2016, 6, 21341 CrossRef CAS PubMed.
- A. Zhang, T. Wu, L. Bian, P. Li, Q. Liu, D. Zhang, Q. Jin, J. Zhang, G. Huang and S. Song, Mol. Imaging Biol., 2020, 22, 515–525 CrossRef CAS PubMed.
- X. Zhang, B. Jin, W. Zheng, N. Zhang, X. Liu, T. Bing, Y. Wei, F. Wang and D. Shangguan, Dyes Pigm., 2016, 132, 405–411 CrossRef CAS.
- Q. Luo, Q. Jin, C. Su, D. Zhang, C. Jiang, A. F. Fish, Y. Feng, Y. Ni and J. Zhang, Anal. Chem., 2017, 89, 1260–1266 CrossRef CAS PubMed.
- Q. Jin, C. Jiang, M. Gao, D. Zhang, N. Yao, Y. Feng, T. Wuand and J. Zhang, New J. Chem., 2019, 43, 6121–6125 RSC.
- G. C. Rudolf and S. A. Sieber, ChemBioChem, 2013, 14, 2447–2455 CrossRef CAS PubMed.
- M. Carmo-Fonseca, L. Mendes-Soares and I. Campos, Nat. Cell Biol., 2000, 2, E107–E112 CrossRef CAS PubMed.
- N. Perek, O. Sabido, N. Le Jeune, N. Prevot, J. M. Vergnon, A. Clotagatide and F. Dubois, Eur. J. Nucl. Med. Mol. Imaging, 2008, 35, 1290–1298 CrossRef CAS PubMed.
- X. Duan, Z. Yin, C. Jiang, Q. Jin, D. Zhang, Z. Sun, W. Ye and J. Zhang, Eur. J. Pharm. Biopharm., 2017, 117, 151–159 CrossRef CAS PubMed.
- J. Kapuscinski, Biotech. Histochem., 1995, 70, 220–233 CrossRef CAS PubMed.
- A. A. Rybczynska, H. H. Boersma, S. de Jong, J. A. Gietema, W. Noordzij, R. A. J. O. Dierckx, P. H. Elsinga and A. van Waarde, Med. Res. Rev., 2018, 38, 1713–1768 CrossRef PubMed.
- D. Zhang, M. Gao, Q. Jin, Y. Ni and J. Zhang, Acta Pharm. Sin. B, 2019, 9, 455–468 CrossRef PubMed.
- L. Bian, M. Gao, D. Zhang, A. Ji, C. Su, X. Duan, Q. Luo, D. Huang, Y. Feng, Y. Ni, Q. Jin and J. Zhang, Anal. Chem., 2018, 90, 13249–13256 CrossRef CAS PubMed.
- J. M. Jamison, K. Krabill, K. A. Allen, S. H. Stuart and C. C. Tsai, Antiviral Chem. Chemother., 1990, 1, 333–347 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectroscopic data. See https://doi.org/10.1039/d2ra02844a. |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |