 Open Access Article
Open Access ArticleFunctions and performance of ionic liquids in enhancing electrocatalytic hydrogen evolution reactions: a comprehensive review
Kang Chen
ab,
Bin Xu
ab,
Linyu Shen
ab,
Danhong Shen
ab,
Minjie Li
*ab and
Liang-Hong Guo
 *ab
*ab
aCollege of Quality and Safety Engineering, China Jilliang University, Hangzhou, Zhejiang 310018, China. E-mail: mjli@cjlu.edu.cn; lhguo@cjlu.edu.cn
bInstitute of Environmental and Health Sciences, China Jiliang University, Hangzhou, Zhejiang 310018, China
First published on 6th July 2022
Abstract
As a green and renewable energy source, hydrogen can be produced by the electrolysis of water via the hydrogen evolution reaction (HER). Nevertheless, this method requires efficient and low-cost electro-catalysts to improve hydrogen production efficiency. Ionic liquids (ILs), with a unique combination of such superior properties as low vapor pressure, high electrical conductivity, high electrochemical stability, and a wide variety of functional groups, have found applications in electrochemical systems designed for efficient HER. Herein, we provide a comprehensive and updated review on the functions and performance of ILs used in electrochemical systems to enhance the HER. As the name suggests, ILs have been employed either as electrolytes by themselves, or as electrolyte additives. They also played many functional roles in the synthesis of HER electrocatalysts, including as the synthesis reaction solvent, reaction precursor as well as single/dual ion sources, binder and structure-directing agents of the catalysts. With the assistance of ILs, HER efficiency of electrocatalysts was improved significantly, resulting in decreased overpotentials in the range of 16–385 mV @ 10 mA cm−2 and increased Tafel slopes in the range of 30–210 mV dec−1. Lastly, the problems and challenges of ILs in electrocatalytic water electrolysis and HER are also discussed and their prospects considered.
1. Introduction
With the rapid economic development, the demand for and consumption of energy by mankind is increasing. Even today, fossil fuels remain the most in-demand energy source. Nevertheless, with increasing consumption and limited reserves, the lack and even depletion of fossil energy becomes an unavoidable problem. Moreover, combustion of fossil fuels has also caused rapid ecological deterioration.1 Under such circumstances, it has become an urgent task for all countries to explore an alternative and sustainable energy source.2 During the energy crisis (1970s), the hydrogen energy system began to attract extensive attention and has undergone tremendous development since then, because of its extremely high clean and renewable capacity, such as 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 J (kg−1 K−1) of high energy density and combustion products that are environmentally friendly and widely sourced.3–5 Even though hydrogen is abundant, it mainly exists in compounds, combined with oxygen and carbon, and thus cannot be used directly. Therefore, the production of pure hydrogen is regarded as an important undertaking. Of which, most of the current hydrogen production coming from fossil energy, which still falls short of what people expect. In contrast, electrolysis of water to produce high-purity hydrogen is a green and sustainable way. Whereas, because of the slow hydrogen evolution reaction (HER) kinetics, the hydrogen production efficiency is significantly reduced, resulting in a substantial increase in cost of hydrogen production.6,7 Therefore, many kinds of catalysts with catalytic activity for water electrolysis reactions have been studied to improve the production efficiency of hydroelectric analysis of hydrogen.
300 J (kg−1 K−1) of high energy density and combustion products that are environmentally friendly and widely sourced.3–5 Even though hydrogen is abundant, it mainly exists in compounds, combined with oxygen and carbon, and thus cannot be used directly. Therefore, the production of pure hydrogen is regarded as an important undertaking. Of which, most of the current hydrogen production coming from fossil energy, which still falls short of what people expect. In contrast, electrolysis of water to produce high-purity hydrogen is a green and sustainable way. Whereas, because of the slow hydrogen evolution reaction (HER) kinetics, the hydrogen production efficiency is significantly reduced, resulting in a substantial increase in cost of hydrogen production.6,7 Therefore, many kinds of catalysts with catalytic activity for water electrolysis reactions have been studied to improve the production efficiency of hydroelectric analysis of hydrogen.
The electrolysis water reaction is a multi-step reaction process involving multiple electrons. 1.23 V is the theoretical decomposition voltage of water.8 However, in actual operation, due to the polarization overpotential of the cathode and anode and the internal resistance of the electrolyte, the actual voltage of electrolyzed water is much higher than 1.23 V. Therefore, reducing the planned overpotential in the water electrolysis process is of great significance to reducing energy consumption. In the HER of water electrolysis, the Volmer–Heyrovsky and Volmer–Tafel mechanisms are two well-recognized hydrogen evolution mechanisms. In particular, the HER of electrolyzed water requires two reaction steps to generate a H2 molecule, including an electrochemical adsorption step and a desorption step.9,10 The first step of the reaction is to hydrate protons or water molecules (H3O+ or H2O) to obtain an electron and then adsorb on the surface of the catalyst to become an adsorbed hydrogen atom (H*) (Volmer step). The second step of the reaction is a composite desorption step (Tafel step) or an electrochemical desorption step (Heyrovsky step). The composite desorption step (Tafel step) is the direct combination of two adsorbed hydrogen atoms adsorbed on the surface of the catalyst to form a hydrogen molecule. The electrochemical desorption step (Heyrovsky step) is that a hydrogen atom adsorbed on the surface of the catalyst combines with nearby hydrated protons or water molecules to form a hydrogen molecule.
Ionic liquid is an organic solvent that is liquid at or near room temperature and are also salts composed of anions and cations. Accordingly, some ionic liquids have distinct physical properties compared to traditional electrolytes and organic solvents. Its melting point is lower than 100 °C, and it will hardly cause environmental pollution problems due to solvent volatilization at room temperature.11–13 Due to the asymmetry of certain substituents in the structure of ionic liquids, it cannot be stacked regularly, so its melting point is relatively low. Ionic liquids have almost no vapor pressure at room temperature, and have high electrical conductivity and electrochemical stability, they are widely used in the electrochemical field.14 It also includes high thermostability, wide liquidus range, tunable electrochemical window, and can dissolve and process a variety of materials. Ionic liquids have some special functional groups (such as amino or sulfonic acid groups), these groups can absorb protons in the aqueous solution.15 Based on these properties, ionic liquids have attracted much attention in the field of electrochemistry. Common ionic liquids are divided into two types: cations and anions.16 The cations include: quaternary ammonium ion, quaternary phosphonium ion, imidazolium ion and pyridinium ion, etc., and the anions are: halogen ion, tetrafluoroborate ion, hexafluorophosphate ion, etc. In theory, there may be more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 types of ionic liquids. People can choose the appropriate ionic liquid according to their own needs, and there is a large selection capacity. Studies have found that some ILs (imidazolium-based IL, ammonium-sulfonic-acid-based IL and so on) can be used as electrolytes or electrolyte additives in electrolyzed water hydrogen evolution systems. Some ionic liquids can be used as a reaction medium (such as solvents, precursors, ion sources, structure directing agents, binders, etc.) to prepare electrolytic water hydrogen evolution catalysts. In the process of preparing hydrogen evolution catalysts for electrolyzed water, the different properties of ionic liquids determine their different uses. Some ionic liquids have good solubility and thermal stability and are used as a solvent for reaction. Some ionic liquids can be directly involved in the preparation of catalyst materials and used as precursors. Some ionic liquids provide ions for reactions and are used as ion sources. Some ionic liquids help to change the structure of materials, are used as structure-directing agents, and so on. All in all, these ionic liquids play their respective roles in the preparation of hydrogen evolution catalyst materials. They make the prepared catalyst materials have more active hydrogen evolution sites, lower impedance, better conductivity and stability, so as to greatly improve the hydrogen production efficiency of electrolytic water hydrogen evolution system.
000 types of ionic liquids. People can choose the appropriate ionic liquid according to their own needs, and there is a large selection capacity. Studies have found that some ILs (imidazolium-based IL, ammonium-sulfonic-acid-based IL and so on) can be used as electrolytes or electrolyte additives in electrolyzed water hydrogen evolution systems. Some ionic liquids can be used as a reaction medium (such as solvents, precursors, ion sources, structure directing agents, binders, etc.) to prepare electrolytic water hydrogen evolution catalysts. In the process of preparing hydrogen evolution catalysts for electrolyzed water, the different properties of ionic liquids determine their different uses. Some ionic liquids have good solubility and thermal stability and are used as a solvent for reaction. Some ionic liquids can be directly involved in the preparation of catalyst materials and used as precursors. Some ionic liquids provide ions for reactions and are used as ion sources. Some ionic liquids help to change the structure of materials, are used as structure-directing agents, and so on. All in all, these ionic liquids play their respective roles in the preparation of hydrogen evolution catalyst materials. They make the prepared catalyst materials have more active hydrogen evolution sites, lower impedance, better conductivity and stability, so as to greatly improve the hydrogen production efficiency of electrolytic water hydrogen evolution system.
Herein, we review the application of ionic liquids in the hydrogen evolution catalysis of electrolyzed water in recent years. It is highlighted that ionic liquids play a pivotal role in enhancing the performance of electrolysis hydrogen as electrolyte or electrolyte additive and in the preparation of catalyst materials for electrolytic water hydrogen evolution. We summarize the roles of ILs as electrolytes and electrolyte additives in electrolytic systems and the diverse roles of ILs in the preparation of electrolytic water hydrogen evolution catalysts. It mainly includes ionic liquid as electrolyte/electrolyte additive, reaction solvent, precursor, single/dual ion sources, binder, morphological structure/phase structure directing agent. Finally, the problems and challenges of ionic liquids in water electrolysis and hydrogen evolution are analyzed and prospected.
2. Ionic liquids and their functions in electrocatalytic hydrogen evolution reactions
With the gradual exhaustion of traditional fossil fuels and the deteriorating ecological environment, hydrogen energy as a potential renewable energy source has attracted people's attention. The electrolytic water hydrogen evolution technology is considered to be the simplest and pollution-free method of hydrogen production among a variety of hydrogen production technologies. In order to improve hydrogen production efficiency and reduce production costs, it is necessary to develop efficient hydrogen evolution catalysts. Pt is an efficient hydrogen evolution catalyst, but its reserves are limited and it is a precious metal. Therefore, it is not widely used. It is hoped that a low-cost and high-efficiency catalyst can be prepared to replace Pt. In recent years, various metal compound materials have been extensively studied in the field of electrolytic water hydrogen evolution catalyst. However, there is still a gap between the hydrogen evolution performance of these synthetic materials and Pt. As an organic solvent, ionic liquid has good solubility, thermal stability and chemical stability, thus entering people's field of vision. It is found that different ionic liquids can promote the efficiency of hydrogen evolution in electrolytic water hydrogen evolution system. In this part, we mainly discuss ionic liquids as electrolyte and electrolyte additive in electrolytic system, and the different roles played by ionic liquids in the process of preparing electrolytic water hydrogen evolution catalysts, as shown in Table 1.| Ionic liquid | Function | Electrocatalyst | η @ 10 mA cm−2 (mV) | Tafel slope (mV dec−1) | Ref. |
|---|---|---|---|---|---|
| TEA-PS·BF4 | Electrolyte | — | — | — | 20 |
| DEAF | — | — | — | 9 | |
| [Dema-H]+[TfO]− | — | — | 22 | ||
| [Emim][MeSO3] | Electrolyte additive | — | — | — | 23 |
| [Bmim][Sal] | — | — | — | 25 | |
| [Beim][Br] | — | — | — | 26 | |
| [C4MIM][OTf] | Reaction solvent | MoS2 | — | 210.4 ± 3.1 | 28 |
| [BMIM]BF4 | MoS2/graphene | — | 52 | 35 | |
| [Bmim][BF4] | (MoS2)x(SnO2)1−x/RGO | 263 | 50.8 | 36 | |
| Ethaline | Ni–Cu alloy | 128 | 57.2 | 42 | |
| Ethaline | Ni–Mo alloy microsphere | — | 49 | 44 | |
| BMP-DCA | Ni–La alloy | 190 | 75.6 | 45 | |
| Ethaline | NiSx/CW | 54 | 54 | 46 | |
| [EMIM]HSO4 | Co–Ni alloy | 139 | — | 47 | |
| [Bmim]2[MoO4] | Reaction precursor | MoC | 110 | — | 48 |
| EMIM-DCA | Nitrogen-doped mesoporous carbons (NMCs) | 384.7 | 134 | 49 | |
| Biomass-based protic ionic liquids (BILs) | CoP@NPC-900 | 181 | 59 | 51 | |
| [BMIm]SCN | P–CoS1.097@MoS2/CC | 98/88 | 51/74.4 | 52 | |
| Divinyl-functionalized ionic liquids | Homo-PIL-Ru/C-600 | 16 | 42 | 53 | |
| poly(ionic liquid) (PIL) | Nitrogen/sulfur co-doped porous carbon (NSPC) | 146 | 64 | 54 | |
| PCMVIm-Tf2N | Nitrogen and sulfur-codoped mesoporous carbon (GCs) | 118.7 | 52 | 55 | |
| MBMG-FeCl3Br | Ion source | FePMBMG/CNT | 155 | 75.9 | 59 |
| [Omim]FeCl4 | Fe3O4/NCMTs-800(IL) | 170 | 72.65 | 60 | |
| [P(C6H13)3C14H29][FeCl4] | Fe2P/CNTs | 115 | 68 | 61 | |
| MBMG)2-CoCl2Br2 | Co(MGMB)/CNTs | 135 | 58 | 67 | |
| [P6,6,6,14]2[CoCl4] | Co2P/CNTs | 150 | 47 | 68 | |
| [P4,4,4,4]2[CoCl4] | Co2P/CNTs-1 | 135 | 58 | 69 | |
| MBMG-PF6 | N, P-double doped graphene (G(PF6)) | — | 88 | 70 | |
| 1-Buty-3-methylimidazolium hexafluorophosphate | NiCoP/NPC | 108/128 | — | 71 | |
| [P4444]Cl | Ni2P | 102 | 46 | 72 | |
| [BMIM][Tf2N] | Structure directing agent | MoO2_40 | 169 | 58 | 74 |
| [BMIM][Tf2N] | MoP/NPC | 188/150 | 60/65 | 75 | |
| BMIM-Tf2N | Ni2P | 107 | 70 | 76 | |
| [BPy]Br | 1T/2H-MoS2-3 | 259 | 59 | 77 | |
| PVEIB | NiS2-MoS2/PVEIB/PPy/GO | 205 | 49 | 78 | |
| AMIM-Br | Binder | AMIM-Br–MWCNTs | — | 125.6 | 81 |
| OPyPF6 | MWCNT/IL | — | 130 | 82 | |
| PIL | Other | Mo2C-RGO | 99 | 54.6 | 83 |
| [DBU][NTf2], [BMIm][NTf2] | [BMIm]@Pt/C and [DBU-H]@Pt/C | 33/36 | 30.2/30.8 | 84 | |
| 1-Butyl-3-methyl-imidazolium chloride | CNT-IM-Cl | 135 | 38 | 85 | |
| [EMIM]CF3COO | IL-AMNDs | 116 | 104 | 86 |
2.1 Ionic liquids as electrolytes/electrolyte additives
The electrolytic cell is a very important part of the hydrogen evolution system of electrolyzed water, and the use of different electrolytes will have different effects on the hydrogen evolution efficiency. Benefit from the unique properties of ionic liquids, studies had found that it could also be used as electrolyte/electrolyte additives to enhance the efficiency of hydrogen evolution. Moreover, compared with traditional electrolyte, ionic liquid has a great advantage in electrolysis of water for hydrogen evolution. At present, there are few studies on the electrolytic hydrogen production using ionic liquids as electrolytes. In previous years, Roberto F. de Souza et al. introduced imidazolium-based ILs as an electrolyte to produce hydrogen from water electrolysis.17–19 Imidazolium-based ILs was also the earliest ionic liquid as an electrolyte to enhance the efficiency of water electrolysis hydrogen evolution. Subsequently, their research group Fiegenbaum et al.20 based on the previous research, using ammonium-sulfonic-acid-based IL as an electrolyte to electrolyze water for hydrogen evolution. They compared ionic liquid (TEA-PS·BF4) as an electrolyte with conventional electrolytes. The results showed that compared with other electrolytes, TEA-PS·BF4 as an electrolyte significantly increased the current density, and at the same time increased the hydrogen production efficiency. Moreover, under such conditions, the ion mobility during electrolysis can be improved, and the energy barrier can also be reduced. They also investigated the performance of different electrodes in TEA-PS·BF4 electrolyte solution.21 The experimental results found that using TEA-PS·BF4 as the electrolyte, high efficiency (93–99%) was observed in all electrode systems, indicating a significant increase in hydrogen production. In addition, the activation energy (8.7 kJ mol−1) was the lowest and the cathode exchange current value (0.66 mA cm−2) was the highest when the electrochemical performance was tested using the glassy carbon cathode. These results showed that the use of IL not only leaded to increased catalytic activity, but also had a synergistic effect with glassy carbon. In recent years, Li et al.9 applied proton ionic liquid (PIL, diethylammonium formate (DEAF)) as a multifunctional electrolyte for water electrolysis for the first time. Other things being the same, a control experiment was designed using PIL (DEAF) and two other salts (Na2SO4 and KCl) as electrolytes for comparison. The experimental results showed that when using PIL (DEAF) as the electrolyte, the working electrode had the lowest onset potential (−0.002 V) and the smallest Tafel slope value (33.6 mV dec−1) (Fig. 1a). Next, the performance of aprotic IL (BMIM-Br) and PIL (DEAF) was also compared as electrolyte. It was found that PIL (DEAF) still showed excellent electrocatalytic hydrogen evolution performance with the highest current density (21 mA cm−2 at −0.5 V) and the most positive onset potential (−0.002 V). Moreover, it was also found that PIL (DEAD) interacts with the catalyst. Notably, there was a proton transfer equilibrium in the PIL aqueous solution. Of these, a small fraction of the PIL cations can act as a proton source to deprotonate and regenerate the Brønsted base (the amine B). Due to the increase of electron density, the affinity of protons in solution to catalyst will be enhanced (Fig. 1b). Therefore, it can be concluded that PIL can provide protons for water splitting and generate amines as co-catalysts to improve catalytic activity in hydrogen evolution reaction. In 2020, Thimmappa et al.22 used another proton ionic liquid ([Dema-H]+[TfO]−) as an electrolyte for intermediate temperature water electrolysis. In this work, two hydrogen evolution reactions were observed on Pt electrode in both “as prepared” [Dema-H]+[TfO]− and 10% [Dema-H]+[TfO]− in water. After testing, it was found that under the condition of 80 °C, the hydrogen evolution reaction has a lower onset potential (150 mV). This suggested that the reaction at higher temperature will further reduce the electrolysis potential of [Dema-H]+[TfO]−. In addition, it was found that adding water to electrolyte with ionic liquid can improve ionic conductivity and overall electrolytic performance. Two types of proton conduction could occur in water-PIL [Dema][TfO] solutions. The first is due to a small amount of free acid, via the hydronium ion, and the other via the [Dema-H]+ cation.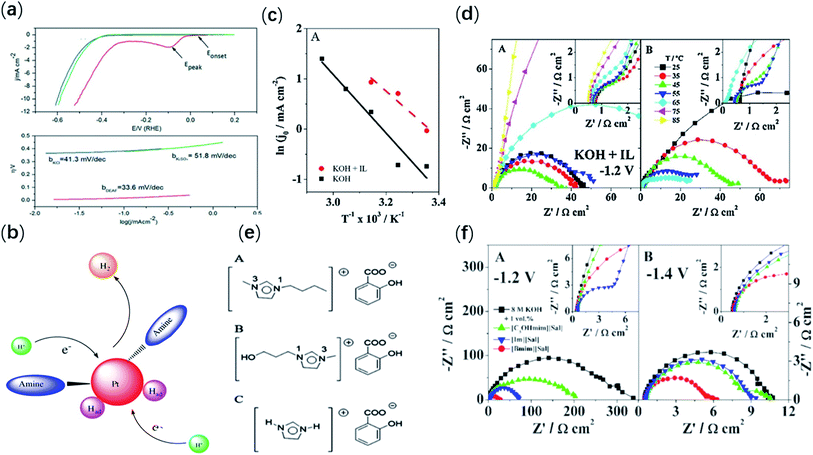 | ||
| Fig. 1 (a) Linear sweep voltammograms and Tafel plots of PIL and inorganic salts as electrolytes for HER. Red: 1 M DEAF, Blue: 1 M KCl, Green: 0.33 M K2SO4. (b) Interaction between amine and catalyst. Reproduced from ref. 9 with permission from WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright © 2016. (c) Arrhenius plots for the IL-free KOH and the IL-added electrolyte. (d) Nyquist (A, B) plots for the IL-added (A) and the IL-free (B) electrolytes at −1.2 V. Reproduced from ref. 24 with permission from Elsevier Ltd., Copyright © 2018. (e) Chemical structure of the studied ILs sharing the same anion: (A) [Bmim][Sal], (B) [C3OHmim][Sal], and (C) [Im][Sal]. (f) Nyquist (A, B) plots at −1.2 V (A) and −1.4 V (B) at 25 °C for all studied electrolytes, respectively. Reproduced from ref. 25 with permission from American Chemical Society, Copyright © 2018. | ||
Ionic liquids could not only be directly used as electrolytes, but also as electrolyte additives to enhance the performance of electrolytic water hydrogen evolution. Room temperature ionic liquid (RTIL) are semi-organic salts composed entirely of organic cations and organic or inorganic anions at room temperature. They have many excellent properties, including broad flow properties, good ionic conductivity, and excellent stability. In addition, there are high heat capacity and high cohesive energy density. In 2017, Amaral et al.23 investigated the effect of adding three different RTILs ([Emim][Ac](acetate), [Emim][EtSO4] (ethyl sulfate) and [Emim][MeSO3] (methane sulfonate)) to the KOH electrolyte on the catalytic performance of hydrogen evolution. Through controlled experiments, it was found that the addition of [Emim][MeSO3] as the additive had the best catalytic performance of hydrogen evolution. Compared with KOH electrolytes without IL, electrolyte with RTILs had lower Tafel slope value and higher current density. Among them, after adding ionic liquid ([Emim][MeSO3]), the catalyst showed small Tafel slope value (176 mV dec−1) and the largest current density (2.10 × 10−2 mA cm−2) in the electrolyte. Additionally, the addition of RTIL reduced the overall impedance of the system. This may be due to the fact that RTILs as an additive can modify the adsorption and charge transfer processes at the metal–electrolyte interface. The following year, they systematically investigated the effect of temperature on utilization of [Emim][MeSO3] as an electrolyte additive.24 The electrochemical measurement results showed that in the IL with 8 M KOH, the exchange current density of the hydrogen evolution catalyst increased with the increase of temperature, up to 45 °C. The exchange current density in the electrolyte was higher than that in the electrolyte without IL (Fig. 1c). Moreover, the total impedance associated with HER decreased significantly over the same temperature range (Fig. 1 d). Later, Amaral L. et al.25 prepared and investigated the salicylate-based ILs as electrolyte additives for HER. This had also not been reported before. The studied ILs, 1-butyl-3-methylimidazolium salicylate ([Bmim][Sal]), 1-(3-hydroxylpropyl)-3-methylimidazolium salicylate ([C3OHmim] [Sal]) and imidazolium salicylate ([Im][Sal]), composed of the same anion and different cations (Fig. 1e). There was a higher current in the electrolyte with IL addition compared to the KOH solution without IL. It was also proved that the charge transfers and polarization resistance generally decreased significantly in the solution with IL added, especially after the addition of [Bmim][Sal] (Fig. 1f). This indicated that ionic liquid additives actually improved the charge transport efficiency in the hydrogen evolution reaction. Then, in 2019, Amaral L. et al.26 prepared two bromine ionic liquids, 1-butyl-3-ethylimidazolium bromide [Beim][Br] and 1,3-diethylimidazolium bromide [Eeim][Br], and used them as electrolyte additives for HER in alkaline hydrolysis. Their effects on hydrogen evolution reaction (HER) as basic electrolyte additives were investigated. The electrolysis performance of KOH electrolyte with a small amount of ILs was compared with that without IL. The experimental results showed that lower overpotential values (−0.05 V) along with higher current densities (1.54 × 10−2 mA cm−2) were obtained after addition of 2%(vol) of [Beim][Br]. Electrical impedance test experiments showed that in the ionic liquid added with alkaline electrolyte, the total impedance of the system was significantly reduced. The reason was that the IL additives can stabilize the intermediate H atoms generated during the Volmer step through surface pre-adsorption. This work also proved that brominated ionic liquids used as electrolyte additives can improve electrolyte performance and improve electrolysis efficiency in alkaline solutions.
2.2 Ionic liquids as solvents of electrocatalyst synthetic reactions
Due to its chemical stability and thermal stability, ionic liquids can be used as a good solvent for preparing hydrogen evolution catalysts. Moreover, when using hydrothermal method, electrodeposition method and other methods to prepare materials, using ionic liquid as solvent has more advantages than traditional solvent. Although many studies on the synthesis of inorganic materials in ionic liquids have been reported, there is usually a lack of reasons for using such solvents.27 In response to this problem, in 2011, Lau et al.28 reported the formation of molybdenum disulfide (MoS2) by dissolving (NH4)2MoS4 in ionic liquids ([C4MIM][OTf],[C10MIM][OTf]) and then thermally decomposing it. This was also the first report of using ionic liquids to synthesize electrolyzed water hydrogen evolution catalysts. This focused on the research on the catalytic activity and morphology of hydrogen evolution of MoS2 crystals synthesized in and not in ionic liquids. Through control experiments, it was found that the active edge density of molybdenum disulfide samples synthesized with ionic liquid ([C4MIM] [OTf]) was higher than that of samples synthesized without ionic liquids. This was because IL increased the number of active sites by stabilizing active sites during the synthesis process, thereby increasing the activity of the catalyst. This work provided a theoretical basis for the synthesis of metal chalcogenides using ionic liquids, which was in contrast with traditional methods. Then, in the second year, Lau et al.29 studied how to comprehensively control the synthesis of different forms of MoS2 crystal layers by using different ionic liquids as reaction solvents. It showed that the MoS2 hydrogen evolution catalyst synthesized using non-coordinating aromatic ionic liquids was the most active, with higher crystallinity and a layered/unstacked morphology. This work also demonstrated that by choosing IL as solvent for thermal decomposition of (NH4)2MoS4 to MoS2, the crystallinity and morphology of the layered crystal MoS2 could be predicted and synthetically manipulated.After a long period of research, it was found that although some metal-based hydrogen evolution catalysts that have been studied have catalytic activity, their performance was not very good. It had been found that a composite material with good hydrogen evolution activity can be prepared by mixing some non-metallic materials with good conductivity and stability with metal-based catalysts. For example, graphene, carbon nanotubes, etc. In recent years, researchers had used solvothermal/hydrothermal methods to prepare some MoS2/graphene nanocomposites.30–34 However, due to some properties of MoS2, it is still a challenge to synthesize composite materials with a large number of active sites. To solve this problem, ionic liquids were applied in the synthesis reaction of MoS2. In 2016, Ye et al.35 reported the synthesis of MoS2/graphene composites by hydrothermal method using ionic liquid (1-butyl-3-methylimidazolium tetrafluoroborate, [BMIM]BF4). The prepared MoS2/graphene composite showed good electrocatalytic hydrogen evolution performance. There was a smaller Tafel slope value (52 mV dec−1) in acidic medium. The ionic liquid improved the charge compatibility between the graphene oxide sheet and the anions by modifying the surface of the graphene sheet during the reaction. Thus, the MoS2 layer can be better formed on the graphene surface. In addition, the ionic liquid could also reduce the surface energy of the molybdenum disulfide edge, thereby forming a delamination of molybdenum disulfide with more exposed active edge sites. Interestingly, in the second year, Ravula et al.36 studied the synthesis of stable few-layer (MoS2)x(SnO2)1−x nanoparticles by hydrothermal method using a series of ionic liquids as reaction solvents. The nanoparticles were anchored on the surface of the reduced and oxidized graphene (rGO) as a conductive carrier, as shown in Fig. 2a. This was also the first time that MoS2/SnO2 nano-hybrid materials had been applied to the hydrogen evolution catalyst for electrolysis of water. Compared with MoS2/rGO, the nanoparticles synthesized by this method had smaller overpotential (263 mV at 10 mA cm−2) and Tafel slope value (50.8 mV dec−1), as shown in Fig. 2b and c. The added IL acts can enhance the stability of the nanocrystals and inhibit aggregation/re-stacking, thereby achieving a layered morphology. Moreover, the catalytic activity can be significantly improved by combining Sn as discrete oxide nanocrystals into the nanocrystals. This indicated that the nano-scale heterojunction had great potential for application in hydrogen evolution catalysts. Recently, S. Díaz-Coello, et al.37 reported various composite materials produced by ionic liquid (octylpyridinium hexafluorophosphate (OPy)) as a reaction solvent and transition metal carbide (TMC). The previous work of their research group showed that the WC–OPy composite had the best hydrogen evolution catalytic performance.38 Therefore, the electrocatalytic performance study of metal carbide-ILs composites for HER was extended from WC–OPy to other TMCs. The experimental results showed that adding ionic liquid to the corresponding carbides will change the surface oxide layer, making the composites more stable. It was worth noting that in the composites synthesized using ionic liquids, OPy can serve as a protective layer and an effective corrosion inhibitor, greatly improving the electrocatalytic hydrogen evolution performance. Additionally, research had found that ionic liquids can be utilized as a solvent to prepare non-noble metal-based alloys by electrodeposition. Moreover, there are few reports on the application of alloys prepared with ionic liquids for electrocatalytic hydrogen evolution. The study found that the alloy membrane prepared by using ionic liquid as the solvent has better electrocatalytic hydrogen evolution performance than the alloy prepared by ordinary solvent. For example, because of the excellent properties of Ni–Cu alloy as electrode material, it was considered to be a potential Ni-based HER catalyst.39–41 In 2016, Gao et al.42 reported that a facile electrodeposition method, using ionic liquid (ethaline) as a solvent, successfully prepared Ni–Cu alloy nanosheet array with a porous structure on a copper substrate. The experimental results found that the deposits prepared in this ionic liquid as a solvent were significantly different from those obtained in other solvents. The prepared alloy films were composed of vertical nanosheets and exhibited a clear nanoporous structure (Fig. 2d). It showed a small Tafel slope of 57.2 mV dec−1 with a low onset potential of 48 mV in alkaline medium, as shown in Fig. 2e and f. The morphologies of different active substances in different chemical environments should be related to the changes of electroreduction and deposition kinetics.43 Therefore, the ionic liquid solvent in this work provided good thermodynamic conditions for the Ni–Cu alloy co-deposition. As a result, this vertical nanoporous structure with a high specific surface area was formed on the copper wire. Then, in 2017, Gao et al.44 reported the successful preparation of self-supported porous nickel–molybdenum alloy microspheres (Ni–Mo-MS) thin films on copper foil by means of electrodeposition using ionic liquid (ethaline) as solvent. The porous structure on the surface of the Ni–Mo alloy microsphere crystal film provided a large active area for HER. Electrochemical tests found that the porous Ni–Mo-MS/Cu showed a small Tafel slope (49 mV dec−1) in alkaline solution. Therefore, its hydrogen evolution performance was superior to deposited Ni/Cu and most Ni-based catalytic materials. Among them, ionic liquid as reaction solvent plays a certain role. Compared with ordinary solutions, a favorable chemical environment for favorably generating nanomaterials with high catalytic activity may be given by the interesting solvent properties of ethaline. In the same year, Gao et al.45 then prepared porous nanoparticle-packed Ni–La alloy films on copper foil substrate using ionic liquid (BMP-DCA) as solvent by electrodeposition method. Uniform alloy deposits were observed to grow on the surface of the Cu substrate. It consisted of a rough interconnected structure arrayed in which nanoparticles with a porous surface. By comparison, it was found that the catalytic activity of the prepared Ni–La/Cu electrode was better than that of recently reported Ni–RE (rare Earth) based HER catalyst in alkaline media. Intriguingly, Zeng et al.46 also reported that a highly active cauliflower-like S-doped nickel microsphere film directly grown on a copper wire (CW) substrate (labeled as NiSx/CW) by one-step electrodeposition in a choline chloride/ethylene glycol (ethaline)-based deep eutectic solvent (Fig. 2g). Control experiments showed that NiS0.25/CW with the best S doping amount exhibited a flower-like porous structure with high-density nanopores and a low onset potential of 18 mV (Fig. 2h). The good catalytic performance of this material was attributed to the cauliflower-like structure and the diffusion of the ECSA effect. And the active sites formed by the S-doping-induced 3D interconnected porous structure were effectively utilized. During the reaction, a new solvent environment was provided by this typical deep eutectic solvent (ethaline) for the formation of nanostructured electrodeposits with high hydrogen evolution catalytic activity. Subsequently, in 2019, Sun et al.47 reported the use of ionic liquid 1-ethyl-3-methylimidazolium bisulfate ([EMIM]HSO4) and ethylene glycol (EG) system to prepare nanocrystalline Co–Ni catalyst by the codeposition behavior. A series of experiments showed that the mechanism of co-deposition of cobalt-nickel was abnormal co-deposition. With the increase of the cobalt concentration in the system, the inhibitory effect on nickel reduction and the abnormal co-deposition phenomenon were significantly improved. This was because, during the deposition reaction, some species such as [EMIM] or/and EG molecules in the IL system could be adsorbed on the cathode surface and then inhibit the deposition of Co(II) to Co(0) and Ni(II) to Ni(0). In addition, the HER catalytic activity of the Co–Ni catalyst prepared by this method was tested, and the results showed that it had good hydrogen evolution catalytic activity and stability in alkaline solutions.
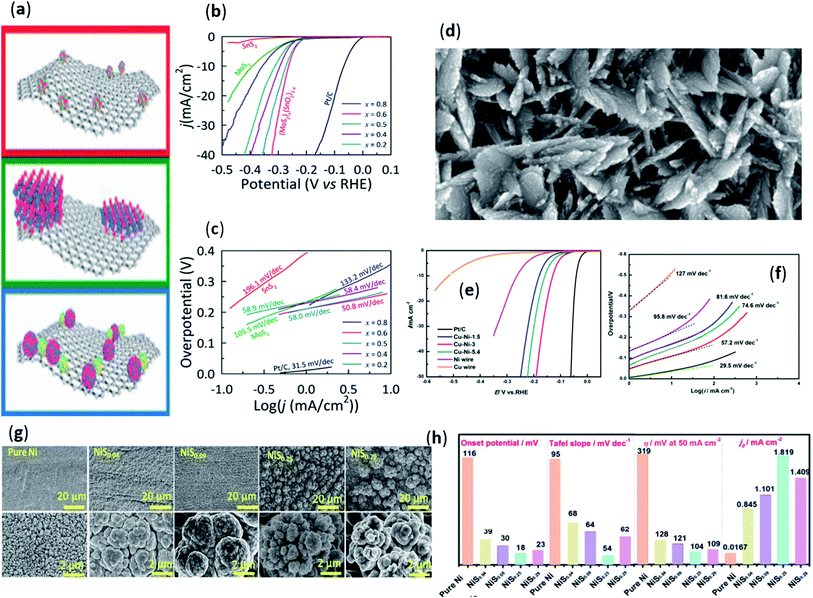 | ||
| Fig. 2 (a) Schematic diagram of (MoS2)x(SnO2)1−x nanoparticles anchored on the surface of rGO; (b) linear sweep voltammetry curves measured for (MoS2)x(SnO2)1−x/rGO nanohybrids prepared using [Bmim][BF4] for different values of x; (c) Tafel plots for (MoS2)x(SnO2)1−x/rGO nanohybrids prepared using [Bmim][BF4]. Reproduced from ref. 36 with permission from American Chemical Society, Copyright © 2017. (d) High magnification SEM images of Ni–Cu alloy film on the prepared Cu wire; (e) polarization curves of Pt wire, Ni wire, Cu wire and Ni–Cu alloy films with different charge density in 1.0 M KOH solution; (f) corresponding Tafel plots with linear fitting. Reproduced from ref. 42 with permission from Elsevier Ltd, Copyright © 2016. (g) The SEM images of NiSx by doping different S dopant concentrations; (h) comparison chart of the catalytic performance for hydrogen evolution of the NiSx/CW with different S doping levels. Reproduced from ref. 46 with permission from the Royal Society of Chemistry, Copyright © 2017. | ||
2.3 Ionic liquids as reaction precursors in electrocatalyst synthesis
As an organic molten salt with unique properties, ionic liquids (ILs) not only have excellent solubility, but also can react with other substances. In recent years, it has been found that ionic liquids can be used as a reaction precursor to prepare hydrogen evolution catalysts. In 2016, Zhang et al.48 thoroughly mixed and ground ionic liquids (1-butyl-3-methylimidazolium molybdate ([Bmim]2[MoO4])) and ordered mesoporous SBA-15 silica, and then Pyrolysis was carried out at a suitable temperature (1173 K), and finally MoC nanocrystals with a particle size of 5 nm were formed. In particular, they selected ionic liquid ([Bmim]2[MoO4]) with benign mobility and polarity as the only high-density precursor for the reaction of carbon and Mo, and the preparation process was shown in Fig. 3a. These ultra-fine nanoparticles could be connected with graphitic carbon to form an ordered mesoporous structure. Through characterization experiments, it was found that the porous material derived from [Bmim]2[MoO4] was composed of O, N-doped MoC materials and N-doped graphite carbons. The doping of oxygen and nitrogen heteroatoms on the surface of MoC@C nanostructure contributed to the increase of surface defects and the regulation of electronic structure. Therefore, more active sites of exposure could be provided. These characteristics could accelerate the reaction speed and improved the catalytic performance of hydrogen evolution. Similarly, Zhao et al.49 reported an approach to synthesize nitrogen-doped mesoporous carbon (NMC) electrocatalysts using ionic liquid (EMIM-DCA) as precursors. This method used a resole and 1-ethyl-3-methylimidazolium dicyanamide (EMIM-DCA) to achieve nitrogen atomic enrichment on the surface of the pore structure, as shown in the Fig. 3b. A control experiment showed that the HER catalytic activity of NMC-x samples was affected by the nitrogen content. The NMC-0.4 had a smaller Tafel slope (134 mV dec−1) in 0.5 M H2SO4 solution. This was because the mesoporous structure provided more surface activation sites and the accessibility of nitrogen atoms enriched on the carbon surface, thereby enhancing the catalytic activity.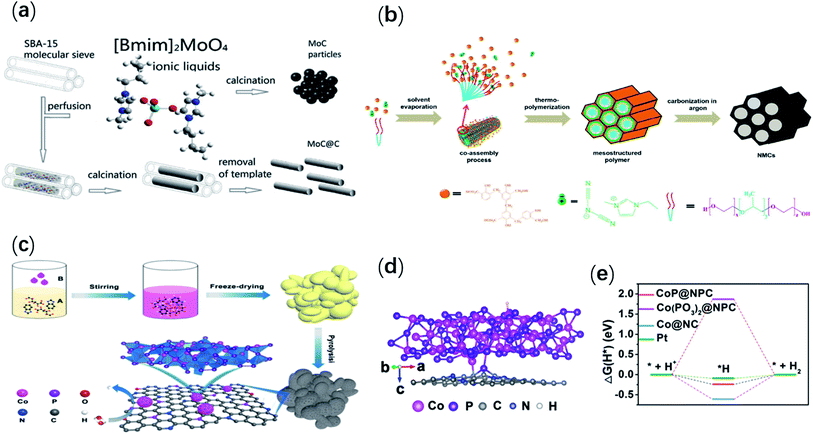 | ||
| Fig. 3 (a) The schematic representation of synthesis of MoC@C and MoC particles. Reproduced from ref. 48 with permission from Springer Science Business Media New York, Copyright © 2016. (b) Schematic illustration of the ionic liquid-assisted co-assembly process for the synthesis of mesoporous carbon with surface enriched nitrogen. Reproduced from ref. 49 with permission from American Chemical Society, Copyright © 2018. (c) Schematic of the fabrication process of CoP@NPC-900; (d) the theoretical models and adopted adsorption sites of H* on the surface of CoP@NPC-900. (e) Calculated free energy diagram of the HER on CoP@NPC, Co(PO3)2@NPC and Co@NC. Reproduced from ref. 51 with permission from The Author(s), under exclusive licence to Springer Science Business Media, LLC, part of Springer Nature, Copyright © 2021. | ||
It was also found that doping nitrogen, phosphorus and other heteroatoms can adjust the electron density of carbon atoms bound with heteroatoms, so as to improve the catalytic activity of carbon materials.50 Recently, Ma et al.51 synthesized porous carbon-coated CoP nanocrystals through a facile one-pot carbonization process, with BIL as an important precursor. Electrochemical tests showed that the prepared CoP@NPC-900 had the best catalytic performance for HER. In an acidic solution, it had a low overpotential (181 mV) at 10 mA cm−2 and a small Tafel slope (59 mV dec−1). Fig. 3c showed the preparation process of CoP@NPC-900. In the pyrolysis process, the precursor gradually formed NPC. This improved electron transfer capacity and also prevented cobalt phosphide from agglomeration. DFT calculation further proved that the catalytic material had excellent HER catalytic performance through the synergy between CoP nanocrystals and NPC (Fig. 3d and e). The excitation of electrocatalytic performance can not only be achieved by constructing heterogeneous nanostructures, but also by doping heteroatoms as a promising method. Therefore, Xu et al.52 reported a novel P-doped CoS1.097@MoS2 nanosheets heterostructure anchored on carbon cloth by a facile hydrothermal method. The realization of this process was first assisted by ionic liquids, followed by phosphating. Electrochemical tests revealed that the synthesized P–CoS1.097@MoS2/CC heterostructure exhibited good hydrogen evolution performance in both acidic and alkaline electrolytes with a low overpotential of 98 mV and 88 mV at the current density of 10 mA cm−2, respectively. During the reaction, the intrinsic properties of ionic liquids change the polarity of the synthesis system and the solubility of the reactants. This allowed the Co-compound to dissolve and participate in the reaction. Furthermore, CoS1.097@MoS2/CC can reduce aggregation profit from the steric hindrance effect of [BMIm]+. It was also beneficial to the adequate exposure of electro-catalytic active sites and rapid electron transport. Interestingly, Zhang et al.53 obtained uniformly distributed Ru nanoparticles (NPs) on a nitrogen-doped carbon framework by in situ confinement polymerization using divinyl-functionalized ionic liquid as a precursor. In the preparation process, the PIL-derived nitrogen-doped carbon networks can sever as a support for stable monodisperse Ru NPs. These results still demonstrated that IL was superior to neutral molecules in stabilizing Ru NPs. In addition, Dai et al.54 reported freestanding nitrogen/sulfur co-doped porous carbon (NSPC) films obtained by carbonizing an electrostatically cross-linked composite (consisted of hydrophobic poly(ionic liquid) (PIL) and alginic acid (AA)). The results showed that the proportion of PIL and AA had an important effect on the porous morphology of the prepared materials. This new NSPC membrane could be utilized as electrode for electrocatalytic HER directly, and had wonderful electrocatalytic performance, with a small Tafel slope value (64 mV dec−1). Recently, Liu et al.55 reported a mesoporous carbon electrocatalyst (GCs), which was synthesized by the direct carbonization of poly(3-cyanomethyl-1-vinylimidazole bis(trifluoromethane sulfonyl)-imide) (PCMVIm-Tf2N) and graphene oxide (GO) composite precursors. The experimental results showed that when the weight ratio of PCMVIm-Tf2N and GO in the precursor was 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the catalyst had the best hydrogen evolution activity, with a rather low overpotential of 118.7 mV at the current density of 10 mA cm−2 and a small Tafel slope of 52 mV dec−1 in 0.5 M H2SO4. This excellent HER performance was believed to have benefited from the synergistic effects of porous structure, good electronic conductivity, and N/S heteroatoms (provided by PIL) codoping.
1, the catalyst had the best hydrogen evolution activity, with a rather low overpotential of 118.7 mV at the current density of 10 mA cm−2 and a small Tafel slope of 52 mV dec−1 in 0.5 M H2SO4. This excellent HER performance was believed to have benefited from the synergistic effects of porous structure, good electronic conductivity, and N/S heteroatoms (provided by PIL) codoping.
2.4 Ionic liquids as single/dual ion sources in electrocatalyst synthesis
In recent years, ionic liquids containing transition metal elements such as iron and cobalt in anions have been extensively studied.56–58 It is found that these ionic liquids could provide a kind of ion source for synthesis of catalytic materials for hydrogen evolution, which is also different from ionic liquids generally used as precursors. In 2017, Cui et al.59 reported the reaction of ionic liquid (MBMG-FeCl3Br) with carbon nanotubes (CNTs) and sodium hypophosphate (NaH2PO2) to obtain CNT-supported iron phosphating (FePMBMG/CNT) catalyst. It was found by comparative experiments that FeP(MBMG)/CNTs exhibited high activity among all synthesized catalysts. It had the lowest onset overpotential (70 mV), Tafel slope (76 mV dec−1) and faradaic impedance (67 Ω), and the highest exchange current density (0.028 mA cm−2). The experimental results showed that MBMG-FeCl3Br provided ferrate(III) for the formation of the active component (FeP) in the FeP(MBMG)/CNTs catalyst. Furthermore, glucaminium cations were provided for the generation of amorphous carbon to enhance catalytic activity. In 2019, Liu et al.60 also reported a reactable ionic liquid ([Omim]FeCl4) insitu-induced synthesis of Fe3O4 nanoparticles decorated willow catkins derived N-doped 3D hollow porous carbon microtubes multifunctional electrocatalyst (Fe3O4/NCMTs-800(IL)), as shown in Fig. 4a. The prepared Fe3O4/NCMTS-800(IL) electrocatalyst retained the tubular structure of natural catkins. The iron-containing ionic liquid (Fe-IL) induces Fe3O4 nanoparticles to be uniformly distributed on the hollow porous carbon microtubes. Fe3O4 nanoparticles can provide hollow carbon nanotubes with many obvious pore structures and more active sites for electrocatalytic reaction (Fig. 4b). In addition, the 3D porous structure could accelerate electron transfer in electrocatalytic processes. Among the samples prepared, it also was found that Fe3O4/NCMTs-800 (IL) synthesized by nitrogen doping had the lowest onset potential of 170 mV (Fig. 4c). Later, it was discovered through research that ionic liquids can also be used as dual sources to prepare hydrogen evolution catalysts. In 2020, Chen et al.61 reported the preparation of Fe2P/CNTs catalysts using ionic liquid ([P(C6H13)3C14H29][FeCl4]) and carbon nanotubes. It was also the first time to use ionic liquid as iron source and phosphorus source to prepare iron phosphide. Through control experiments, it was found that Fe2P(IL6)/CNTs had the lowest Tafel slope of 68 mV dec−1, showing good hydrogen evolution catalytic activity. The reason may be that the carbonized IL cations improved the conductivity of the material during the pyrolysis process. Previous studies showed that the quaternary phosphonium cation of IL could be decomposed into trialkyl phosphonate (TAP) and the metal base anion could be reduced to metal nanoparticles (M-NPs).62,63 In the circumstances, Fe-TAP was produced during the pyrolysis step, and the P–C bond was broken to form phosphorus. At last, the metal reacts with phosphorus to get the TMP. In the preparation process, IL could also prevent aggregation of nanoparticles, as a solvent and stabilizer.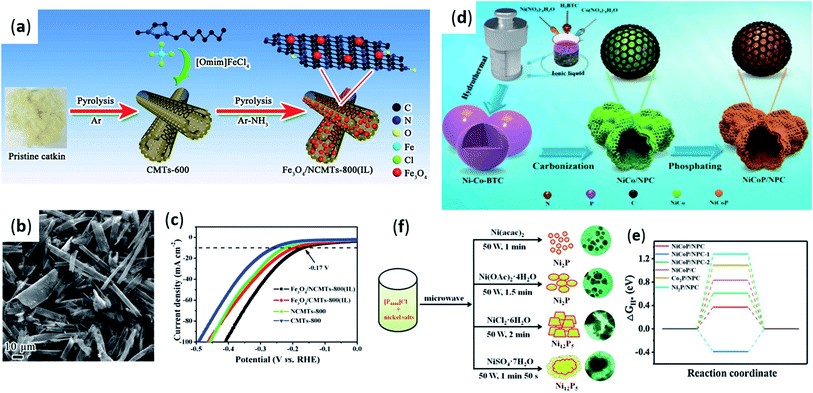 | ||
| Fig. 4 (a) Illustration of the preparation process of Fe3O4/NCMTs-800(IL); (b) TEM images of Fe3O4/NCMTs-800(IL); (c) electrolysis polarization curves for Fe3O4/NCMTs-800(IL), Fe3O4/CMTs-800(IL), NCMTs-800 and CMTs-800 in 1 M KOH solution. Reproduced from ref. 60 with permission from Elsevier B.V., Copyright © 2019. (d) Synthetic procedure for NiCoP/NPC HFSs. (e) Free energy diagram for hydrogen evolution on different catalysts. Reproduced from ref. 71 with permission form Elsevier B.V., Copyright © 2020. (f) The typical schematic illustration for various nickel phosphides tuned by counter anions of nickel salts. Reproduced from ref. 72 with permission form American Chemical Society, Copyright © 2018. | ||
It was found that the nanostructured transition metal phosphide (TMPs) material had good conductivity and stability, and could be used as a catalytic material for electrolysis of water and hydrogen evolution. Particularly, because of the high catalytic activity of hydrogen evolution, cobalt phosphide-based materials can be used as typical representative of TMPs. NaH2PO2, phosphorus, trioctylphosphine (TOP), and CoCl2, Co(NO3)2·6H2O, etc. were often used as P and Co sources to synthesize cobalt phosphides, respectively. Nevertheless, the synthesis of most of these substances had undergone high temperature/high pressure, and the cost required to obtain efficient hydrogen evolution catalysts was high.64–66 It was found that ionic liquid could replace the traditional cobalt and phosphorus compounds as the source to prepare catalyst materials for hydrogen evolution. In 2017, Li et al.67 mixed ionic liquid ((MBMG)2-CoCl2Br2) and carbon nanotubes to prepare CNTs-supported CoP (Co(MGMB)/CNTs). In the process of CoP synthesis, ionic liquid provided Co and P dual sources. It exhibited good HER activity with a low onset overpotential (55 mV), a small Tafel slope (58 mV dec−1) and high faradaic efficiency (FE) of 95%, and it maintained catalytic activity for more than 27 h. Electrochemical experiments and structure characterization indicated [CoCl2Br2]2− and (MBMG)+ in (MBMG)2-CoCl2Br2/CNTs formed CoP and amorphous carbon, respectively. The amorphous carbon also enhanced the conductivity of CoP(MBMG)/CNTs in the reaction. Then, in 2019, Tang et al.68 did similar work. They prepared a composite material (Co2P/CNTs) using another ionic liquid with carbon nanotubes for catalytic electrolysis of water for hydrogen evolution. They prepared Co2P/CNTs for the first time using a phosphorus-based ionic liquid containing cobalt ions ([P6,6,6,14]2[CoCl4]) as a P and Co dual-source. During the reaction, Co2P can be formed using only ionic liquid ([P6,6,6,14]2[CoCl4]) without the addition of other reagents. Co2P/CNTs showed good hydrogen evolution catalytic activity as electrocatalyst for HER, with an onset overpotential (55 mV) and a Tafel slope (47 mV dec−1). In the same year, Li et al.69 prepared Co2P/CNTs-1 by using ionic liquid ([P4,4,4,4]2[CoCl4]) and carbon nanotubes (CNTs) through a microwave-driven method. In acidic solution, Co2P/CNTs-1 prepared by microwave method exhibited excellent hydrogen evolution performance. During the preparation process, the ionic liquid ([P4,4,4,4]2[CoCl4) can be used as a source of P and Co, as well as a solvent and stabilizer in the reaction. It can effectively prevent the aggregation of cobalt phosphide nanoparticles. In addition, using this method of microwave heating, the preparation time of the material only takes 6 minutes. These results demonstrated that using this ionic liquid as a dual source combined with microwave heating to synthesize Co2P was a rapid, safe and effective method.
In addition, ionic liquids can also provide nonmetallic sources for preparing catalytic materials for hydrogen evolution. Therefore, there is great interest in the development of low cost, environmentally friendly and stable metal-free HER electrocatalysts. In 2018, Li et al.70 also reported a simple method of mixing ionic liquid (MBMG-PF6) and graphene in a vacuum to prepare a N,P-double doped graphene (G(PF6)). With N, P doping and high-density porous structure, the material exhibited high activity, good electron transfer ability, and strong stability when used as a metal-free electrocatalyst for HER. In this reaction, ionic liquid as nitrogen source and phosphorus source contributed greatly to the high HER activity of the catalyst. Similarly, in 2021, Yi et al.71 prepared hollow composites (NiCoP/NPC) by combining bimetallic NiCoP with N- and P-doped carbon using ionic liquid (1-buty-3-methylimidazolium hexafluorophosphate) as nitrogen and phosphorus sources. Three steps can be used to synthesize NiCoP/NPC HFSs by the solvothermal method, as shown in Fig. 4d. In the reaction, ionic liquid was involved as a source. Compared with the sample synthesized without ionic liquid, the folded structure of the synthesized material was richer and the active sites were increased. In addition, ionic liquids in the reaction can prevent NiCoP nanoparticles from aggregation during calcination. It can also avoid corrosion in corrosive environments and improve its electrical conductivity. Density functional theory (DFT) demonstrated that the doping of N and P atoms made the catalyst had more active area and accelerated electron transfer in the reaction. It also displayed a minimal |ΔGH*| value compared to other phosphides (Fig. 4e). Interestingly, ionic liquids could also be used as a single source in the preparation of hydrogen evolution catalysts. In 2018, Zhang et al.72 studied an ionic liquid tetrabutylphosphonium chloride ([P4444]Cl) as a new type of phosphorus source and reaction medium, and successfully synthesized a highly catalytically active Ni2P nanomaterial by microwave heating. In this method, ionic liquid ([P4444]Cl) was used as a phosphorus source to provide phosphorus atoms for the reaction (Fig. 4f). In addition, ionic liquids could serve as excellent stabilizers for nanoparticles in IL-mediated systems and could avoid aggregation of nickel phosphide nanocrystals, resulting in desirable dispersion. Therefore, compared with traditional preparation methods, Ni2P nanocrystals synthesized by this method using ionic liquid as phosphorus source had good HER electrocatalytic activity in acidic electrolyte.
2.5 Ionic liquids as structure directing agents in electrocatalyst synthesis
As we all know, the layered structure material not only have a larger contact area but also more active sites, which is beneficial to improve the electrocatalytic hydrogen evolution performance.73 Generally, hierarchical structures can be constructed by template methods or by surfactant-induced growth. It is found that ionic liquids can be used as structure-directing agent to participate in the preparation of electrolytic water hydrogen evolution catalytic materials. The addition of ionic liquid can change the morphology or phase structure of catalytic materials. Thereby, the active sites for hydrogen evolution of the material can be increased and the charge transport ability during the hydrogen evolution process can be enhanced. In 2017, Zhang et al.74 first reported the synthesis of hierarchical one-dimensional (1D) MoO2 in ionic liquids ([BMIM][Tf2N]). [BMIM][Tf2N] not only acted as a structure-directing agent to generate a layered structure in the synthesis reaction, but also served as a template for the formation of one-dimensional MoO2 nanoparticles. Through control experiments, it was found that the 1D MoO2_40 synthesized by the reaction of 40 mg MoO2(acac)2 with ionic liquid had the best nano-morphological structure. It could be seen through the transmission electron microscope (TEM) that the one-dimensional MoO2 nanostructure was composed of a large number of ultra-thin nanosheets with a porous structure (Fig. 5a), thereby increasing many exposed active sites. In addition, electrochemical tests found that it had a lower Tafel slope of 58 mV dec−1 and a lower onset potential of 115 mV in acidic media (Fig. 5b and c). Interestingly, in 2020, on the basis of the original preparation of one-dimensional (1D) structure MoO2. Jiang et al.75 used ionic liquid ([BMIM][Tf2N]) as a structure directing agent to synthesize hierarchically structured 1D MoP encapsulated in the N, P co-doped carbon layer. This work was to first react the ionic liquid with MoO2(acac)2 to generate a one-dimensional (1D) structure of MoO2, and then obtained a layered structure of one-dimensional (1D) MoP through the phosphating method. In addition, the hybrid multifunctional material (MoP/NPC) was formed by encapsulation with N, P co-doped carbon shells. To investigate the electrochemical performance of MoP/NPC nanomaterials, control experiments were designed according to the thickness of the carbon layer. Three samples (MoP/NPC-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, MoP/NPC-1
1, MoP/NPC-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and MoP/NPC-1
1, and MoP/NPC-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were obtained by the mass ratios of the MoO2 precursor to dopamine (DA) hydrochloride (MoO2/DA = 2
2) were obtained by the mass ratios of the MoO2 precursor to dopamine (DA) hydrochloride (MoO2/DA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). IL media played a role in stabilizing and inducing structures in synthesis reaction. Moreover, the one-dimensional layered morphology can be better formed through the “Ostwald ripening process” and the hydrogen bonding interaction within the ionic liquid.
2). IL media played a role in stabilizing and inducing structures in synthesis reaction. Moreover, the one-dimensional layered morphology can be better formed through the “Ostwald ripening process” and the hydrogen bonding interaction within the ionic liquid.
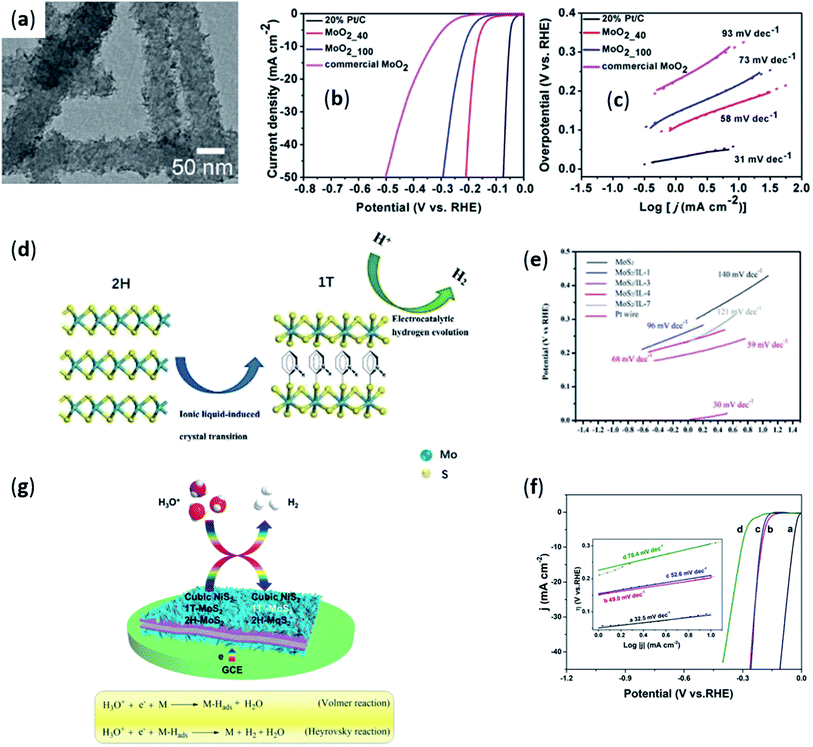 | ||
| Fig. 5 (a) High-magnification TEM images of MoO2_40. (b) Polarization curves; (c) Tafel slope of MoO2_40, MoO2_100, commercial MoO2, and 20% Pt/C. Reproduced from ref. 74 with permission from American Chemical Society, Copyright © 2017. (d) Schematic diagram of MoO2 changing from 2H phase to 1T phase under ionic liquid action; (e) Tafel plot of 1T/2H-MoO2. Reproduced from ref. 77 with permission from Wiley-VCH GmbH, Copyright © 2017. (f) Linear sweep voltammograms and Tafel plots (inset) of (a) Pt/C, (b) NiS2-MoO2/PVEIB/PPy/GO, (c) MoO2/PVEIB/PPy/GO and (d) NiS2/PVEIB/PPy/GO modified GCE in 0.5 M H2SO4. (g) The HER process on NiS2-MoS2/PVEIB/PPy/GO modified GCE in 0.5 M H2SO4. Reproduced from ref. 78 with permission from Elsevier B.V., Copyright © 2020. | ||
Ionic liquids could also be used as reaction media to change the phase of substances, thereby improving the catalytic activity of hydrogen evolution. In 2018, Roberts et al.76 used ionic liquid (BMIM-Tf2N) as the reaction medium successfully synthesized phase-pure nickel phosphide (Ni2P) nanocrystals. When the traditional organic solvent (ODE) was used to replace the ionic liquid as the reaction solvent, indeterminate, phase-impure nanocrystals were obtained. The catalytic activity of hydrogen evolution was compared between Ni2P nanocrystals prepared by ionic liquid and Ni2P nanocrystals synthesized by traditional methods. It was found that Ni2P nanocrystals prepared with ionic liquids had better catalytic hydrogen evolution performance with a low overpotential of 107 mV at the current density of 10 mA cm−2. In addition, the characterization results found that the size of the nanocrystals synthesized using BMIM-Tf2N were relatively small, which provided more active area for HER. This was also the first report of using IL instead of traditional organic solvents to obtain specific phase. In addition, Zhang et al.77 reported a simple and effective hydrothermal synthesis method, using ionic liquid (N-butyl pyridinium bromide, [BPy]Br) as a structure directing agent to prepare 1T/2H-MoS2 catalysts for the first time. It showed that the content of 1T crystal phase in MoS2 can be increased by adding [BPy]Br. This can be attributed to steric hindrance and the packing interaction based on aromatic rings (Fig. 5d). In general, the MoS2 of 1T phase had a higher number of active sites and a faster electron transfer rate. Therefore, more 1T phase of MoS2 enabled the synthesized material to have better catalytic performance for HER, with a lower Tafel slope value (59 mV dec−1) (Fig. 5e). More recently, Mao et al.78 also reported that using ionic liquids as structure-directing agents to change the phase of the material, thereby enhancing the catalytic hydrogen evolution performance of the material. They prepared a NiS2-MoS2/PVEIB/PPy/GO nanosheets using ionic liquid (PVEIB) as a structure directing agent through a hydrothermal method. The NiS2-MoS2 heterostructures was fixed on the poly(1-vinyl-3-ethylimidazolium bromide) functionalized polypyrrole/graphene oxide. The heterostructure was composed of cubic NiS2, metal 1T-MoS2 and semiconductor 2H-MoS2, and had a misfit lattice structure. According to observation, GCE modified by NiS2-MoS2/PVEIB/PPy/GO can achieve the best electrocatalytic activity for HER. ηonset and η10 were 151 and 205 mV, respectively, with a much lower Tafel slope of 49.0 mV dec−1 (Fig. 5f). In 0.5 M H2SO4 solution, the hydrogen evolution process of NiS2-MoS2/PVEIB/PPy/GO modified GCE can be described (Fig. 5g). During the synthesis, PVEIB can well connect the transition metal dichalcogenides (TMDCs) heterostructures and PPy/GO and induced the formation of 1T-MoS2. In addition, it stabilized more electrons, further promoting the production and release of hydrogen.
2.6 Ionic liquids as binders of composite electrocatalyst
Ionic liquids not only have good stability, but also have good conductivity and certain viscosity. Because of the good properties of ionic liquids, it had been found that it could also be used as a new binder. It could replace traditional non-conductive organic binders and be used to make carbon paste electrodes (CPE) that promote electrocatalytic efficiency. As early as 2006, Maleki et al.79 used pyridinium-based ionic liquid (OPFP) as a binder to prepare a new type of carbon composite electrode (CPE). After testing, it was found that this composite electrode could reduce the overpotential of the electroactive compound and also increased the rate of electron transfer. It was believed that the ionic liquid acted as a binder changed the microstructure of the electrode surface. Afterwards, they investigated the enhancement of the electrocatalytic activity of carbon paste electrodes (CPE) by using ionic liquids as a binder.80 It had been found that there were many factors that improved the electrochemical hydrogen evolution performance, such as increased ionic conductivity of the binder, reduced resistance of the electrode, the improvement of the ion exchange performance of the electrode, etc. In recent years, there had also been studies using ionic liquids as binders. In 2016, Li et al.81 respectively mixed and reacted five different ionic liquids with carbon nanotubes to prepare ionic liquid functionalized multi-walled carbon nanotubes (IL-MWCNTs), as shown in Fig. 6a. This was also the first time that metal-free electrocatalyst was applied to HER. The experimental results showed that AMIM-Br–MWCNTs prepared with ionic liquid (AMIM-Br) and carbon nanotubes had the best hydrogen evolution catalytic activity with the most positive onset potential of −0.3 V. It was also found that the conductivity of the material can be improved by adding ionic liquid in the preparation process. As shown in Fig. 6b, the significantly improved catalytic performance using AMIM-Br–MWCNTs was due to the synergistic effect of AMIM-Br and MWCNTs, which can enhance proton adsorption and electron transfer. Moreover, ionic liquids could also be utilized as a binder to replace expensive Nafion. This not only reduced the cost of water electrolysis hydrogen evolution, but also improved the stability of the catalyst. Then, in 2021, Gidi L. et al.82 studied the application of a catalyst prepared by using ionic liquid (OPyPF6) as a binder and a mixture of multi-carbon nanotubes (MWCNT) in the electrolysis of water for hydrogen evolution. It was not difficult to find that the image resolution of the MWCNT/IL system was higher, as shown in Fig. 6c and d. The difference would come from the role of adhesives because ionic liquids, unlike mineral oils, were highly conductive and thus had higher resolution. The voltammetric study showed the electrocatalytic activity of three systems (graphite/mineral oil (Gr), graphite/OPyPF6 (Gr/IL), MWCNT/mineral oil (MWCNT)) for HER (Fig. 6e). It was found that the prepared MWCNT/IL system had the highest electrocatalytic hydrogen evolution performances with an onset potential of −0.3 V vs. Ag/AgCl. This was because when the ionic liquid was used as a binder, it would facilitate the electrical interaction between different carbon materials in the electrode and made the electrode surface more conductive.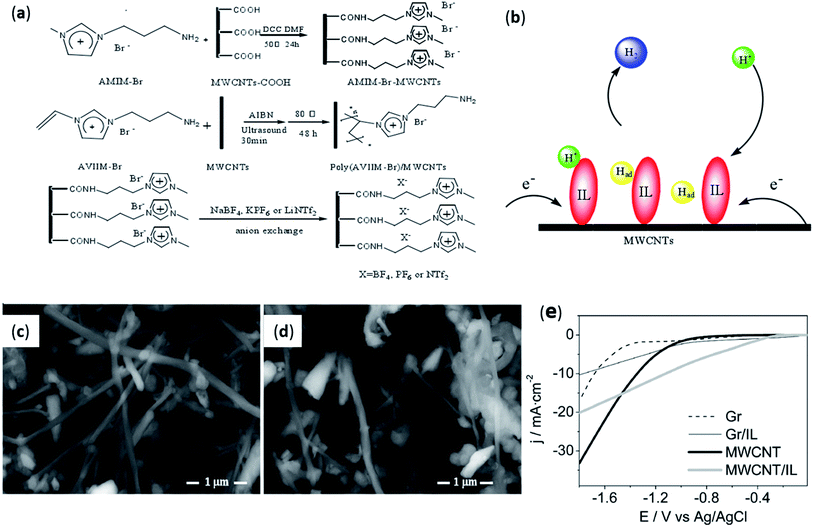 | ||
Fig. 6 (a) Preparation of five sorts of IL-MWCNTs; (b) mechanism of IL-MWCNTs catalyst. Reproduced from ref. 81 with permission from the Royal Society of Chemistry, Copyright © 2016. FESEM images for the systems (c) MWCNT and (d) MWCNT/IL at 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00×. (e) Voltammetric profiles for the graphite paste and MWCNT electrodes in absence and presence of ionic liquid, 0.1 M phosphate buffer pH = 7, scan rate = 0.1 V s−1. Reproduced from ref. 82 with permission from Elsevier Ltd., Copyright © 2021. 00×. (e) Voltammetric profiles for the graphite paste and MWCNT electrodes in absence and presence of ionic liquid, 0.1 M phosphate buffer pH = 7, scan rate = 0.1 V s−1. Reproduced from ref. 82 with permission from Elsevier Ltd., Copyright © 2021. | ||
2.7 Ionic liquid as other roles
In the process of preparing electrolytic water hydrogen evolution catalyst, ionic liquid has other functions besides the above mentioned. In 2019, Sun et al.83 reported a RGO-Supported Molybdenum Carbide (Mo2C-RGO) material synthesized using polyionic liquid. In the reported method, it was proposed for the first time to synthesize RGO-supported Mo2C by using PIL as a bridge between Mo2C and RGO. This process first was that PILs were coated on the surface of the RGO via π−π/noncovalent interaction or cation–π stacking to form PIL-modified RGO (PIL-RGO). Mo was then anchored on the RGO surface by ion-exchange reaction of PIL-RGO with polyoxometallates (POMs), which was then carbonized at high temperature (Fig. 7a). Through control experiments, it was found that Mo2C-RGO prepared by adding PIL with n-octyl side chain had the best catalytic activity and stability, and showed a lower onset potential of 40 mV, as shown in Fig. 7b. The analysis found that PIL can uniformly disperse Mo2C nanoparticles on the surface of RGO nanoparticles through ion exchange, thus improving the accessibility of the material. A clear image of the lattice fringe can be seen through the high-resolution transmission electron microscope (HRTEM), and the plane distance is 0.23 nanometers (Fig. 7c). Furthermore, the addition of PIL can maximize the prevention of RGO accumulation during the reaction process, thereby increasing the active sites of the structure. Recently, Wang et al.84 proposed a facile method, using hydrophobic protic ([DBU][NTf2]) or aprotic ([BMIm][NTf2]) ionic liquid to improve the hydrogen evolution performance of Pt-based electrocatalysts. In this work, ionic liquids were used as modifier to modify the-state-of-the-art and commercially available Pt/C. Controlled experiment found that the two catalysts ([BMIm]@Pt/C and [DBU-H]@Pt/C) synthesized by IL showed higher HER activity than the pristine Pt/C catalyst. The current density at 40 mV was 2.81 and 4.15 times higher than that of the pristine Pt/C catalyst, respectively. Additionally, IL@Pt/C also exhibited higher toughness and catalytic activity in acidic electrolyte. It may be due to the special structure of IL and the hydrophobic microenvironment in the electrolytic system.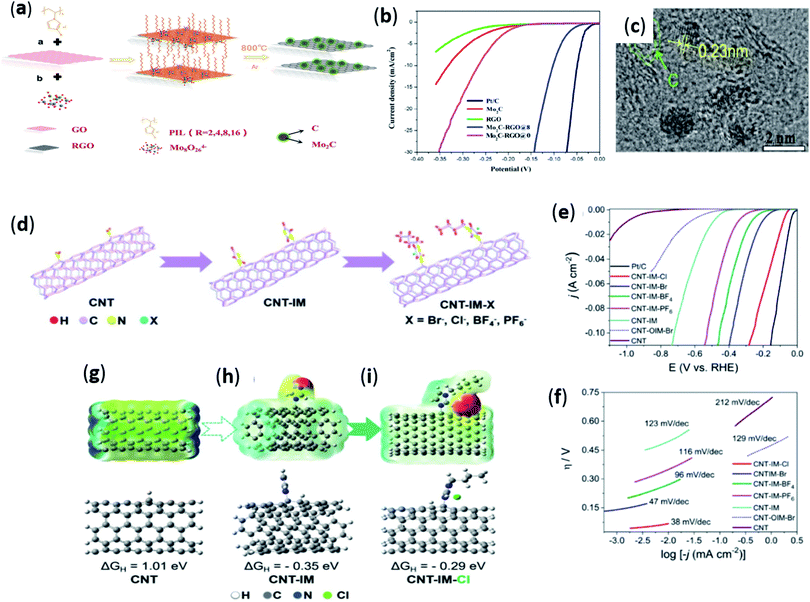 | ||
| Fig. 7 (a) Schematic illustration of the synthetic process of Mo2C-RGO; (b) polarization curves of Mo2C, RGO, Mo2C-RGO@0, Mo2C-RGO@8, and Pt–C; (c) HRTEM images of Mo2C-RGO@8. Reproduced from ref. 83 with permission from American Chemical Society., Copyright © 2019. (d) Graphic illustration of synthesis procedures for CNTs-IM-X (X = Br−, Cl−, BF4−, and PF6−); (e) linear sweep voltammograms, (f) corresponding Tafel plots of various catalysts in 0.5 M H2SO4 aqueous solution; (g–i) theoretical study of the surface electrostatic potential (blue: positive; red: negative; and green: neutral) and free energy of hydrogen adsorption on the surfaces of CNT, CNT-IM, and CNT-IM-Cl respectively. Reproduced from ref. 85 with permission from the Royal Society of Chemistry, Copyright © 2021. | ||
In recent years, carbon-based materials have been introduced as catalysts for HER. Among them, carbon nanotubes (CNTs) were regarded as a promising HER catalyst because of their numerous advantages (good electrical conductivity, high specific surface area, etc.). Nevertheless, the lack of active sites for efficient adsorption of H+ (electron acceptor) in the π-conjugated structure of CNTs results in chemical inertness and low activity. Therefore, Li et al.85 proposed a better method to improve the hydrogen evolution performance, namely, in situ functionalization of CNTs with ionic liquids (ILs). By a simple strategy, Imidazolium ILs were directly immobilised on the CNT skeleton, as shown in the Fig. 7d. Electrochemical tests showed that CNT-IM-Cl had a lower onset overpotential (80 mV) and a smaller Tafel slope (38 mV dec−1), with an overpotential of 135 mV at a current density of 10 cm−2 (Fig. 7e and f). In the process of hydrogen evolution, both anions and anions of ionic liquid played a role in the reaction. Cations caused electron and conjugation effects. In addition, the introduction of imidazole rings made CNTs more active. Theoretical calculations showed that the adsorption–desorption free energies of CNT-IM-Cl are close to 0 eV compared with other catalysts with various anions, as shown in the Fig. 7g–i. Very recently, Wang et al.86 employed active edge site engineering approach to expose abundant catalytically active edge sites by a novel catalyst of ultrasmall-sized antimonene nanodots (IL-AMNDs) modified with ionic liquid (1-ethyl-3-methylimidazole trifluoroacetate ([EMIM]CF3COO)). The catalyst showed superior activity for the HER, such as a low overpotential of 116 mV at 10 mA cm−2, a Tafel slope of 104 mV dec−1, and long term stability. By reducing the size of antimonene nanosheets, more edge active sites were exposed, which was beneficial to increasing the effective active surface area. In addition, the ionic liquids modified on the surface of AMNDs played a crucial role in facilitating the electron transfer process and acted as electron acceptors with excellent hydrogen adsorption capacity. Therefore, the electrocatalytic hydrogen evolution performance was enhanced through a synergistic effect.
3. Conclusions
In summary, ionic liquids have been extensively studied in the electrolysis of water hydrogen evolution due to their many excellent properties. In this review, we summarized the different roles played by ionic liquids in the process of preparing electrolytic water hydrogen evolution catalysts, and as electrolyte and electrolyte additive in electrolytic system. It mainly includes ionic liquid as reaction solvent, precursor, single/dual source, binder, structure directing agent, electrolyte/electrolyte additive. The role of ionic liquid in the efficient separation of hydrogen from water electrolysis was highlighted. The preparation of hydrogen evolution catalysts with good performance and stability has always been the goal of people's pursuit. Using its unique properties, ionic liquids can not only increase the active area of catalytic materials or change the phase structure of materials, but also affect the transport of electrons and the adsorption of protons on catalytic surfaces. Thus, the hydrogen evolution efficiency and catalytic performance of catalytic materials can be improved. Moreover, it can also make the whole electrolytic water hydrogen evolution system more stable by its stability.In theory, there are tens of thousands of types of ionic liquids. There is a large pool to choose for the right ionic liquid according to the needs. Here are some suggestions for designing suitable ionic liquids to promote the hydrogen evolution efficiency. Studies have found that some ionic liquids (TEA-PS·BF4 IL, protic ionic liquids (DEAF, [Dema-H]+[TfO]−) etc. can be used as electrolyte, and some ionic liquids ([Emim])-based room temperature ionic liquids (RTILs), salicylate ([Sal])-based ionic liquids, bromide-based ionic liquids, etc.) can be used as electrolyte additives, due to their good conductivity, wide range of fluidity, high chemical stability, etc. Some ionic liquids can be used as a reaction medium to prepare electrolytic water hydrogen evolution catalysts, and their different properties determine different uses. If it is necessary to provide stable chemical environment and thermodynamic conditions for synthetic materials, ionic liquids ([C4MIM][OTf], [BMIM]BF4, ethaline, etc.) can be selected as reaction solvents. And ionic liquids ([Bmim]2[MoO4], EMIM-DCA, poly(ionic liquids) (PILs), MBMG-FeCl3Br, [P6,6,6,14]2[CoCl4], etc.) can be directly involved in the preparation of catalyst materials and used as precursors or as ion sources. Additionally, some ionic liquids ([BMIM][Tf2N], [BPy]Br, [EMIM] CF3COO, AMIM-Br, etc.) can be used to change the material structure or modify the surface, thereby adding more catalytically active sites and accelerating electron transport.
Although great achievements have been made in the research field of ionic liquids in electrolysis of water for hydrogen evolution, there are still some problems and challenges: (1) the types of ionic liquids are diverse, and ionic liquids play different roles in the preparation of hydrogen evolution catalytic materials. Therefore, how to select and design the most suitable ionic liquid according to the structure and properties of the catalytic material, so as to maximize the hydrogen evolution catalytic performance of the material, is a challenge. (2) When preparing materials by electrodeposition, the potential window of some ionic liquids is not wide enough. Some active metals cannot be deposited directly, but can only be precipitated in the form of alloys. (3) There are high requirements for the purity of ionic liquids in the preparation of materials, but the purification steps of ionic liquids are complicated and difficult to operate. (4) The roles of various ionic liquids in promoting material progress are not fully understood. According to the governing mechanisms of ionic liquids in the synthetic reaction, the reaction conditions can be adjusted to achieve the best effect through theoretical modelling, which is also not yet achieved. (5) Stability is also a very important indicator for electrolyzed water hydrogen evolution materials. Although ionic liquids have good stability, it is still difficult to ensure that catalytic materials prepared by ionic liquids maintain good stability and high current density in relatively harsh environments. (6) In addition, some ionic liquids also have safety hazards during operation. For example, ionic liquids composed of dialkylimidazolium cation and BF4− and PF6− could be hydrolysed to produce highly corrosive HF when operated for a long time, which might cause harm to human health. Studies also indicated that some ionic liquids were difficult to degrade and showed mutagenic effects on biological systems. Therefore, the safety of various ionic liquids needs to be further studied. (7) Due to the complex synthesis process of ionic liquids, the cost is relatively high, which is also a key issue affecting its application. It is believed that, with continuous efforts by the international research community, these problems and challenges will be solved in the future, and ionic liquids will realize their potentials in facilitating hydrogen evolution in the electrolysis of water.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21878302).References
- W. Hua, H.-H. Sun, F. Xu and J.-G. Wang, Rare Met., 2020, 39, 335–351 CrossRef CAS.
- H. Yin and A. C. K. Yip, Catalysts, 2017, 7, 297 CrossRef.
- M. Momirlan and T. Veziroglu, Renew. Sustain. Energy Rev., 2002, 6, 141–179 CrossRef CAS.
- S. Dunn, Int. J. Hydrogen Energy, 2002, 27, 235–264 CrossRef CAS.
- K. Mazloomi and C. Gomes, Renew. Sustain. Energy Rev., 2012, 16, 3024–3033 CrossRef CAS.
- Y. Wang, B. Kong, D. Zhao, H. Wang and C. Selomulya, Nano Today, 2017, 15, 26–55 CrossRef CAS.
- Y. Shi and B. Zhang, Chem. Soc. Rev., 2016, 45, 1529–1541 RSC.
- M. W. Kanan and D. G. Nocera, Science, 2008, 321, 1072–1075 CrossRef CAS PubMed.
- T. Li, X. Wang, W. Yuan and C. M. Li, Chemelectrochem, 2016, 3, 204–208 CrossRef CAS.
- M. Grado-Caffaro and M. Grado-Caffaro, Optik, 2004, 115, 45–46 CrossRef CAS.
- J. S. Wilkes, Green Chem., 2002, 4, 73–80 RSC.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed.
- K. R. Seddon, Nat. Mater., 2003, 2, 363–365 CrossRef CAS PubMed.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- R. F. de Souza, J. C. Padilha, R. S. Gonçalves, M. O. de Souza and J. Rault-Berthelot, J. Power Sources, 2007, 164, 792–798 CrossRef CAS.
- J.-P. Belieres and C. A. Angell, J. Phys. Chem. B, 2007, 111, 4926–4937 CrossRef CAS PubMed.
- R. F. De Souza, J. C. Padilha, R. S. Gonšalves and J. Rault-Berthelot, Electrochem. Commun., 2006, 8, 211–216 CrossRef CAS.
- R. F. De Souza, G. Loget, J. C. Padilha, E. M. A. Martini and M. O. de Souza, Electrochem. Commun., 2008, 10, 1673–1675 CrossRef CAS.
- G. Loget, J. Padilha, E. Martini, M. Desouza and R. Desouza, Int. J. Hydrogen Energy, 2009, 34, 84–90 CrossRef CAS.
- F. Fiegenbaum, E. M. Martini, M. O. de Souza, M. R. Becker and R. F. de Souza, J. Power Sources, 2013, 243, 822–825 CrossRef CAS.
- F. Fiegenbaum, M. O. de Souza, M. R. Becker, E. M. A. Martini and R. F. de Souza, J. Power Sources, 2015, 280, 12–17 CrossRef CAS.
- R. Thimmappa, D. Walsh, K. Scott and M. Mamlouk, J. Power Sources, 2020, 449 Search PubMed.
- L. Amaral, D. S. P. Cardoso, B. Šljukić, D. M. F. Santos and C. A. C. Sequeira, J. Electrochem. Soc., 2017, 164, F427–F432 CrossRef CAS.
- L. Amaral, D. Cardoso, B. Šljukić, D. Santos and C. Sequeira, Mater. Res. Bull., 2019, 112, 407–412 CrossRef CAS.
- L. Amaral, J. Minkiewicz, B. Šljukić, D. M. F. Santos, C. A. C. Sequeira, M. Vraneš and S. Gadžurić, ACS Appl. Energy Mater., 2018, 1, 4731–4742 CrossRef CAS.
- L. Amaral, J. Minkiewicz, B. Šljukić, D. M. Santos, C. A. Sequeira, M. Vraneš and S. Gadžurić, J. Electrochem. Soc., 2019, 166, F1314–F1319 CAS.
- Z. Ma, J. Yu and S. Dai, Adv. Mater., 2010, 22, 261–285 CrossRef CAS PubMed.
- V. W.-h. Lau, A. F. Masters, A. M. Bond and T. Maschmeyer, ChemCatChem, 2011, 3, 1739–1742 CrossRef CAS.
- V. W. Lau, A. F. Masters, A. M. Bond and T. Maschmeyer, Chemistry, 2012, 18, 8230–8239 CrossRef CAS PubMed.
- Y. Li, H. Wang, L. Xie, Y. Liang, G. Hong and H. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- J. Guo, F. Li, Y. Sun, X. Zhang and L. Tang, J. Power Sources, 2015, 291, 195–200 CrossRef CAS.
- J. Zhang, L. Zhao, A. Liu, X. Li, H. Wu and C. Lu, Electrochim. Acta, 2015, 182, 652–658 CrossRef CAS.
- W.-H. Hu, R. Yu, G.-Q. Han, Y.-R. Liu, B. Dong, Y.-M. Chai, Y.-Q. Liu and C.-G. Liu, Mater. Lett., 2015, 161, 120–123 CrossRef CAS.
- J. Zhou, H. Xiao, B. Zhou, F. Huang, S. Zhou, W. Xiao and D. Wang, Appl. Surf. Sci., 2015, 358, 152–158 CrossRef CAS.
- J. Ye, Z. Yu, W. Chen, Q. Chen and L. Ma, Int. J. Hydrogen Energy, 2016, 41, 12049–12061 CrossRef CAS.
- S. Ravula, C. Zhang, J. B. Essner, J. D. Robertson, J. Lin and G. A. Baker, ACS Appl. Mater. Interfaces, 2017, 9, 8065–8074 CrossRef CAS PubMed.
- S. Díaz-Coello, M. M. Afonso, J. A. Palenzuela, E. Pastor and G. García, J. Electroanal. Chem., 2021, 898 Search PubMed.
- S. Díaz-Coello, J. Palenzuela, M. Afonso, E. Pastor and G. García, J. Electroanal. Chem., 2021, 880 Search PubMed.
- Ö. Öztop, G. H. Ağaoğlu and G. Orhan, Surf. Eng. Appl. Electrochem., 2019, 55, 410–417 CrossRef.
- R. Solmaz, A. Döner and G. Kardaş, Int. J. Hydrogen Energy, 2009, 34, 2089–2094 CrossRef CAS.
- K. Ngamlerdpokin and N. Tantavichet, Int. J. Hydrogen Energy, 2014, 39, 2505–2515 CrossRef CAS.
- M. Gao, C. Yang, Q. Zhang, Y. Yu, Y. Hua, Y. Li and P. Dong, Electrochim. Acta, 2016, 215, 609–616 CrossRef CAS.
- A. Pearson, A. P. O'Mullane, S. K. Bhargava and V. Bansal, Electrochem. Commun., 2012, 25, 87–90 CrossRef CAS.
- M. Gao, C. Yang, Q. Zhang, J. Zeng, X. Li, Y. Hua, C. Xu and P. Dong, J. Mater. Chem. A, 2017, 5, 5797–5805 RSC.
- M. Gao, C. Yang, Q. Zhang, J. Zeng, X. Li, Y. Hua, C. Xu and Y. Li, J. Electrochem. Soc., 2017, 164, D778 CrossRef CAS.
- J. Zeng, M. Gao, Q. Zhang, C. Yang, X. Li, W. Yang, Y. Hua, C. Xu and Y. Li, J. Mater. Chem. A, 2017, 5, 15056–15064 RSC.
- X. He, Z. Sun, Q. Zou, J. Yang and L. Wu, J. Electrochem. Soc., 2019, 166, D908–D915 CrossRef CAS.
- Y. Zhang, C. Li, Z. Chen, Y. Ni, F. Kong, A. Kong and Y. Shan, Catal. Lett., 2016, 147, 253–260 CrossRef.
- X. Zhao, S. Li, H. Cheng, J. Schmidt and A. Thomas, ACS Appl. Mater. Interfaces, 2018, 10, 3912–3920 CrossRef CAS PubMed.
- Y. Gao, Z. Xiao, D. Kong, R. Iqbal, Q.-H. Yang and L. Zhi, Nano Energy, 2019, 64 CrossRef.
- J. Ma, X. Chi, Y. Huang, R. Zou, D. Li, Z. Li, X. Li, C. Liu and X. Peng, J. Mater. Sci., 2021, 56, 18188–18199 CrossRef CAS.
- Q. Xu, Y. Liu, Z. Tian, Y. Shi, Z. Wang and W. Zheng, Electrochim. Acta, 2021, 385 Search PubMed.
- Y. Y. Zhang, N. Zhang, P. Peng, R. Wang, Y. Jin, Y. K. Lv, X. Wang, W. Wei and S. Q. Zang, Small Methods, 2021, 5 Search PubMed.
- Z. Dai, M. He, J. Yu, H. Song and Y. Xiong, New J. Chem., 2021, 45, 10349–10356 RSC.
- C. M. Liu, H. H. Song, Z. F. Dai and Y. B. Xiong, Ionics, 2022, 28, 1311–1321 CrossRef CAS.
- R. F. Frade, S. Simeonov, A. A. Rosatella, F. Siopa and C. A. Afonso, Chemosphere, 2013, 92, 100–105 CrossRef CAS PubMed.
- P. B. Hitchcock, K. R. Seddon and T. Welton, J. Chem. Soc., Dalton Trans., 1993, 2639–2643 RSC.
- S. A. Kozlova, S. P. Verevkin, A. Heintz, T. Peppel and M. Köckerling, J. Chem. Eng. Data, 2009, 54, 1524–1528 CrossRef CAS.
- Z. Cui, T. Li, D. Tang and C. M. Li, ChemistrySelect, 2017, 2, 1019–1024 CrossRef CAS.
- G. Liu, B. Wang, P. Ding, Y. Ye, W. Wei, W. Zhu, L. Xu, J. Xia and H. Li, J. Alloys Compd., 2019, 797, 849–858 CrossRef CAS.
- Y. Chen, T. Li, Q. Zhao, D. Liu and C. M. Li, RSC Adv., 2020, 10, 33026–33032 RSC.
- C. G. Read, J. F. Callejas, C. F. Holder and R. E. Schaak, ACS Appl. Mater. Interfaces, 2016, 8, 12798–12803 CrossRef CAS PubMed.
- F. Liang, L. Huang, L. Tian, J. Li, H. Zhang and S. Zhang, CrystEngComm, 2018, 20, 2413–2420 RSC.
- J. Wang, W. Yang and J. Liu, J. Mater. Chem. A, 2016, 4, 4686–4690 RSC.
- D. Zhou, L. He, W. Zhu, X. Hou, K. Wang, G. Du, C. Zheng, X. Sun and A. M. Asiri, J. Mater. Chem. A, 2016, 4, 10114–10117 RSC.
- J. Tian, Q. Liu, A. M. Asiri and X. Sun, J. Am. Chem. Soc., 2014, 136, 7587–7590 CrossRef CAS PubMed.
- T. Li, D. Tang and C. Li, Int. J. Hydrogen Energy, 2017, 42, 21786–21792 CrossRef CAS.
- D. Tang, T. Li and C. M. Li, Int. J. Hydrogen Energy, 2019, 44, 1720–1726 CrossRef CAS.
- T. Li, D. Tang and C. M. Li, Electrochim. Acta, 2019, 295, 1027–1033 CrossRef CAS.
- T. Li, D. Tang, M. Wang, Q. Song and C. M. Li, ChemistrySelect, 2018, 3, 6814–6820 CrossRef CAS.
- M. Yi, B. Lu, X. Zhang, Y. Tan, Z. Zhu, Z. Pan and J. Zhang, Appl. Catal., B, 2021, 283 Search PubMed.
- C. Zhang, B. Xin, Z. Xi, B. Zhang, Z. Li, H. Zhang, Z. Li and J. Hao, ACS Sustain. Chem. Eng., 2017, 6, 1468–1477 CrossRef.
- X.-Y. Yang, L.-H. Chen, Y. Li, J. C. Rooke, C. Sanchez and B.-L. Su, Chem. Soc. Rev., 2017, 46, 481–558 RSC.
- B. Zhang, Y. Xue, A. Jiang, Z. Xue, Z. Li and J. Hao, ACS Appl. Mater. Interfaces, 2017, 9, 7217–7223 CrossRef CAS PubMed.
- A. Jiang, Z. Wang, Q. Li and M. Dong, ACS Sustain. Chem. Eng., 2020, 8, 6343–6351 CrossRef CAS.
- E. J. Roberts, C. G. Read, N. S. Lewis and R. L. Brutchey, ACS Appl. Energy Mater., 2018, 1, 1823–1827 CrossRef CAS.
- X. Zhang, H. Li, H. Yang, F. Xie, Z. Yuan, L. Zajickova and W. Li, Chemelectrochem, 2020, 7, 3347–3352 CrossRef CAS.
- H. Mao, X. Guo, Q. Fan, Y. Fu, H. Yang, D. Liu, S. Wu, Q. Wu and X.-M. Song, Chem. Eng. J., 2021, 404 Search PubMed.
- N. Maleki, A. Safavi and F. Tajabadi, Anal. Chem., 2006, 78, 3820–3826 CrossRef CAS PubMed.
- N. Maleki, A. Safavi and F. Tajabadi, Electroanalysis, 2007, 19, 2247–2250 CrossRef CAS.
- T. Li, Z. Cui, W. Yuan and C. M. Li, RSC Adv., 2016, 6, 12792–12796 RSC.
- L. Gidi, R. Arce, J. Ibarra, M. Isaacs, M. J. Aguirre and G. Ramírez, Electrochim. Acta, 2021, 372 Search PubMed.
- Y. Sun, B. Wang, N. Yang, X. Tantai, X. Xiao, H. Dou, L. Zhang, B. Jiang and D. Wang, Ind. Eng. Chem. Res., 2019, 58, 8996–9005 CrossRef CAS.
- Q. Wang, Y. Gao, Z. Ma, Y. Zhang, W. Ni, H. A. Younus, C. Zhang, Z. Chen and S. Zhang, J. Energy Chem., 2021, 54, 342–351 CrossRef.
- T. Li, Y. Chen, W. Hu, W. Yuan, Q. Zhao, Y. Yao, B. Zhang, C. Qiu and C. M. Li, Nanoscale, 2021, 13, 4444–4450 RSC.
- J. K. Wang, C. Q. Wang, Y. H. Song, W. B. Sha, Z. Y. Wang, H. L. Cao, M. Zhao, P. Z. Liu and J. J. Guo, Chemcatchem, 2022, 14, e202101765 CAS.
| This journal is © The Royal Society of Chemistry 2022 |






