Open Access Article
Open Access ArticleIn vitro toxicity of carbon nanotubes: a systematic review†
Margarita R. Chetyrkina a,
Fedor S. Fedorov
a,
Fedor S. Fedorov *a and
Albert G. Nasibulin
*a and
Albert G. Nasibulin *ab
*ab
aSkolkovo Institute of Science and Technology, Nobel Str. 3, 143026, Moscow, Russia. E-mail: albert.nasibulin@aalto.fi
bAalto University, FI-00076, 15100, Espoo, Finland
First published on 31st May 2022
Abstract
Carbon nanotube (CNT) toxicity-related issues provoke many debates in the scientific community. The controversial and disputable data about toxicity doses, proposed hazard effects, and human health concerns significantly restrict CNT applications in biomedical studies, laboratory practices, and industry, creating a barrier for mankind in the way of understanding how exactly the material behaves in contact with living systems. Raising the toxicity question again, many research groups conclude low toxicity of the material and its potential safeness at some doses for contact with biological systems. To get new momentum for researchers working on the intersection of the biological field and nanomaterials, i.e., CNT materials, we systematically reviewed existing studies with in vitro toxicological data to propose exact doses that yield toxic effects, summarize studied cell types for a more thorough comparison, the impact of incubation time, and applied toxicity tests. Using several criteria and different scientific databases, we identified and analyzed nearly 200 original publications forming a “golden core” of the field to propose safe doses of the material based on a statistical analysis of retrieved data. We also differentiated the impact of various forms of CNTs: on a substrate and in the form of dispersion because in both cases, some studies demonstrated good biocompatibility of CNTs. We revealed that CNTs located on a substrate had negligible impact, i.e., 90% of studies report good viability and cell behavior similar to control, therefore CNTs could be considered as a prospective conductive substrate for cell cultivation. In the case of dispersions, our analysis revealed mean values of dose/incubation time to be 4–5 μg mL−1 h−1, which suggested the material to be a suitable candidate for further studies to get a more in-depth understanding of its properties in biointerfaces and offer CNTs as a promising platform for fundamental studies in targeted drug delivery, chemotherapy, tissue engineering, biosensing fields, etc. We hope that the present systematic review will shed light on the current knowledge about CNT toxicity, indicate “dark” spots and offer possible directions for the subsequent studies based on the demonstrated here tabulated and statistical data of doses, cell models, toxicity tests, viability, etc.
Introduction
Carbon nanotubes (CNTs) attracted the tremendous interest of the scientific community because of their diverse applications in electronics, photonics, composite materials, and as part of energy sources and storage systems.1–3 Landmark papers published by Iijima,4,5 where the structure of multiwalled carbon nanotubes (MWCNTs) and single-walled carbon nanotubes (SWCNTs) was visualized, ignited a great scope of R&D activities. A remarkable combination of physical and chemical properties, intensively studied in the next few years, pushed researchers' interest towards integrating CNTs into biosystems. Therefore, CNTs were also proposed for biomedical applications such as tissue engineering and regeneration, target drug delivery, hyperthermia treatment for selective cancer cell killing, gene therapy, bioimaging, biosensing, as electrodes for neural prosthetics, etc.6–11 Such great attention of researchers was driven by a unique alliance of nanoscale size and exceptional mechanical, optical and electrical characteristics that make CNTs attractive for a direct contact with living systems.12,13In 2000, scientists, for the first time, successfully combined the new material with the most sensitive living system, neurons, giving momentum to the relatively innovative cross-disciplinary field – nanotechnology for biomedical tasks.14 However, in the following years, a large number of publications also demonstrated a negative impact of CNTs on biosystems related to hydrophobicity of CNTs, low synthesis-to-synthesis reproducibility of the material characteristics and their unclear acute toxic and long-term biological impacts.15–26 On the contrary, some studies demonstrated non-toxic effects or apparent toxicity of CNTs in contact with biological systems.27–33 Metal catalyst impurities, CNT structure and geometry all stemming from the synthesis method, surfactants and functional groups can greatly affect the final CNT toxicity decision.24 Although the Materials Science and Engineering community continues to explore practical applications of the material, toxicologists are reasonably concerned with dangerous consequences and substantial negative impact of the CNTs on human health.
Nowadays, the scientific community has identified three possible mechanisms of cellular CNT toxicity. The first one is based on irreparable mechanical injury of membrane (cellular or nuclear).24 With high probability, the endocytosis, phagocytosis, or nano penetration, which are the main ways of the nanomaterial interaction with a lipid membrane, strictly depends on CNT geometry, especially length.21,34 The next proposed toxic mechanism is oxidative stress occurring because of the increased level of reactive oxygen species (ROS)24 and leading to numerous side effects in the cell such as apoptosis, necrosis, cytochrome c release, oxidative DNA damage, proliferation reduction, inhibition of cells growth, G2/M cycle arrest, etc. The final mechanism, genotoxicity mechanism, is somehow related to DNA damages characterized by a broad spectrum: the interaction of CNTs with proteins participating in chromosomes aberration, the impact of CNT on the mitotic spindle, micronuclei formation, indirect DNA oxidation, DNA breakage, etc. Despite the fact that the toxic mechanisms of CNTs are studied from several points of view, there is still a strong dependence of triggered or inhibited molecular pathways and cell types. A lot of the well-known signaling cascades are involved in different cell responses to materials, several of which are investigated in-depth in case of CNT impact: MAPK, AP-1, NF-κB, Akt, NLRP3 inflammasome, TGF-β1, and p53.24,35 Despite the described complexity of occurring processes inside the cells that were targeted by CNTs, some research papers propose ways to overcome the CNT toxicity impact by modification the material surface with functionalization groups,36 coating with metal oxides37 or with proteins attachment.38 For example, coating with recombinant C1q, which is a protein activating classic pathway of the complement system involved in innate immune system, is a perspective approach of the inflammation regulation.39 Besides, several theoretical studies, devoted to modelling of possible cellular response to CNTs, demonstrate a nanotubes mechanical interaction with a lipid layer40–42 or with proteins,43,44 proposing the safer CNT geometry, which deepen the understanding of CNT actions on cells.
In recent years a significant increase in CNT industrial production for electronics such as touch screens, composites fabrication, and other applications resulted in huge concerns about industrial workers' health hazards, environmental impact, and related needs of the development of the standardized protocols for safety guidance.45–51 In some publications, researchers have already highlighted that the main entry ways of nanomaterials into the human organism are mouth, nose, or skin, directing to the digestive tract, respiratory system or resulting in skin erosion, respectively (Fig. 1).52,53 Although the described exposure scenarios may have negative impact on human health, which is already traditionally comparable with asbestos because of the quite similar form, it is still essential to get more in-depth information about the toxicity mechanisms on a base cellular level (in vitro). This might help to reveal ways to overcome toxicity limits by the nanomaterial functionalization or surface modifications.7,54 Thus, role of CNT physical parameters (type of CNT, length, diameter, synthesis method, catalysts, etc.) towards substantial biological effects has been addressed in several reviews,7,15,21,55–59 along with cell type, toxic dose, mechanisms of toxicity and other essential parameters24,60 in attempt to make classification to reveal common trends.15,21,61 However, the numerous data related to substantial biological effects such as apoptosis, life cycle arrest, reactive oxygen species (ROS) production, and gene expression give only vague toxicity prognosis and uncertain identification of toxic doses. In addition, some original works hint at untouched parameters or characteristics of CNTs to be acknowledged. For example, Sweeney et al. demonstrated that carboxylated MWCNTs show reduced toxicity towards macrophages,62 but the work of Dong et al. demonstrated the fully vice versa effect for the same cell type.63 The difference between studies relies on MWCNTs produced by different companies (CheapTubes Inc. or Chengdu Organic Chemicals Co. Ltd), used cell viability assays (MTS assay or CellTiter-Glo test), types of macrophages (human or mice), and as a result culture medium (RPMI or DMEM). Likely, because of these reasons, according to the literature, the toxicity of CNT is located in the very broad range from 5 ng mL−1 up to 10 mg mL−1, which differs six orders of magnitude.21
At the same time, one more critical aspect that should be undoubtedly considered in the context of the material toxicity assessment and environmental impact is the so-called “bioaccumulation” phenomenon of material. The process is defined as the absorption of a chemical by a living organism through all possible routes happening in the natural environment.64 The recent review of Bjorkland et al., where 42 original references were collected to perform a potential examination of CNTs bioaccumulation in different species, revealed that trophic transfer of CNTs, or food chain, is negligible and absorption of CNTs through epithelial barriers is also low.65
According to the latest standards, every new drug or material proposed to somehow affect the human organism should be carefully tested for acute toxicity or long-term perspectives. Toxicology, historically established as a science of poisons,66 nowadays is a multidisciplinary field covering studies of all known synthetic and natural substances with a goal to test toxic effects and identify safety level.67 According to P. Paracelsus, well-known “Father of Toxicology,” all substances are poisons and the right dose differentiates a poison from a remedy.67 To verify chemical toxicity and identify safe doses, several guidelines were developed by European Centre for the Validation of Alternative Methods, Interagency Coordinating Committee on the Validation of Alternative Methods and Organization for Economic Cooperation and Development (OECD) for standardized methodology of laboratory practice for new compounds or drug testing. In particular, the ISO 10993-protocol formulated for “Biological evaluation of medical devices” in 2009 includes rigorously precise steps for toxicity evaluation. Depending on contact time, location in the organism, authors prescribe various tests for evaluation of cell morphology, cytotoxicity, genotoxicity, inflammation and other parameters (Fig. 1). As a result of the researchers' concern for applying nanomaterials in biosystems, nanotoxicology was also accepted as a distinct discipline in 2017.68 To study safeness or hazard effects of materials at the nanoscale, new protocols were recently formulated by the Food and Drug Administration (FDA) (ISO/TR 10993-22:2017).
Looking at the current examinations of the CNT toxicity for in vitro models in the frame of this mini-review, we have realized the absence of the prescribed protocols among the studies. This fact could be explained by the need to consider many characteristics, starting from the type of CNTs, type of contact with cells (on a substrate or in the form of dispersion) to long-term and high costs of these tests. Still, several publications demonstrated quite promising results of OECD prescribed tests employing CNTs synthesized by OCSiAl (Russia), by Bayer Technology Service GmbH (Germany), by Hanwha Nanotech Corp. (Korea), and by the European Commission Joint Research Centre (JRC, Italy).69–72 Consequently, every research group follows its own way of “toxicity evaluation” with various cell lines, cytotoxicity tests, incubation time, doses, etc. Therefore, the scientific community faces an extraordinary situation when many experiments to study CNT toxicity were conducted, but the straight doses causing toxic effects are still unclear, data about biological effects vary greatly and the toxicity question is scarcely addressed.
Hereby, we perceived the importance of competent assessment of the material's possible toxic effects to answer the question: “is the devil as black as he is painted?” We systematically evaluated studies performed in vitro and traced possible correlations between the type of CNTs (SWCNT or MWCNT), the way of contact with cells (substrate or dispersion), cell type, incubation time, dose and cytotoxic test that greatly influence the decision about the CNT toxicity. We also realized the need for a new approach of literature analysis in this field, so the present mini-review is a systematic collection of articles which establishes common trends regarding the CNT toxicity. The present mini-review focuses only on the results of in vitro toxicity studies following the PRISMA statements, a set of elements aimed to help authors how to perform and report systematic reviews,73 broadly used in the biomedical field (the methodology and search strategy are described in the next chapter in more details).74–76
The central part of the present work is divided into three chapters. Each chapter discusses in detail one of the critical parameters applied for original references classification. The first part examines CNT synthesis procedure to identify companies where materials were tested in vitro and industrial impact on the scientific field of CNT toxicology in time perspective. The second and the third parts summarize data about CNT applied in the form of substrate or dispersion, respectively. In addition, for these two chapters, we collected data about cell type, incubation time, the used design of CNT, synthesis procedure, applied cytotoxic tests, and dose/viability parameters. Thus, based on the collected data and references to original articles, one would be able to get the needed information quickly and orient in the broad field of CNT toxicity.
Methodology of search strategy
The databases MEDLINE, Cochrane Database of Systematic Reviews, PROSPERO were searched to guarantee this systematic review would not duplicate any works. Scopus and Web of Science databases were studied for the relevant publications; extraction date is 12 April 2021. Articles were independently searched by two authors (M. C. and F. F.).Criteria formulation
We used SPIDER formulation: sample (S), phenomenon of interest (PI), design (D), evaluation (E), research type (R), to identify our search strategy. Thus, we focus on in vitro CNT toxicity studies on mammalian cells with positive or negative outcomes and with quantitative or qualitative assessment.The results of studies with animals, in vivo, are less comparable than those ones in vitro using cells. This is because of numerous factors: different species (mice, rat, rabbit, guinea pig, etc.), way of drug administration (oral, intraperitoneal, intravenous, etc.), observation time (several hours, days or months), dose range and many others parameters that should be constantly controlled such as food supply, gender, light, etc. In contrast, conditions for cell studies are precisely guided: temperature, humidity, percent of CO2, nutrients supply, etc. Besides the more convenient use in laboratories, in vitro models could be applied for proposing effects and prediction for in vivo studies.77–79 Based on these facts, we have chosen the in vitro model for collecting existing literature data and our analysis.
Criteria for exclusion and inclusion
We excluded reviews, conference abstracts, opinions, commentaries, review book chapters from the search results. We included only original articles containing data with qualitative or quantitative assessments of cell viability.Requests for databases
For the Scopus database we used the next request: KEY ((“carbon nanotube*” OR swcnt* OR mwcnt* OR cnt*) AND (toxicity OR toxicology OR biocompatibility OR toxic OR cytotoxicity OR genotoxicity OR genotoxicology OR nanotoxicology OR nanotoxicity) AND (“cell interaction*” OR “in vitro” OR cell*) AND NOT (animal*)) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (LANGUAGE, “English”)). For the Web of Science: AK=((“carbon nanotube*” OR swcnt* OR mwcnt* OR cnt*) AND (toxicity OR toxicology OR biocompatibility OR toxic OR cytotoxicity OR genotoxicity OR genotoxicology OR nanotoxicology OR nanotoxicity) AND (“cell interaction*” OR “in vitro” OR cell*)).Data extraction
In the review, we collected the information about types of CNT, official distributors or manufacturers (companies), way of contact with cells (substrate or dispersion), type of cells, incubation time and type of tests for toxicity analysis.Results and discussion
In the Scopus database, we found 1124 articles that fulfilled our request; in the Web of Science database, the same request resulted in 196 articles. The reference search helped us to reveal 38 related publications. After filtering the publications, sorting, and lists combination, we obtained 194 articles with the required data forming the central core of the papers in the field of CNTs in vitro toxicity.CNT synthesis
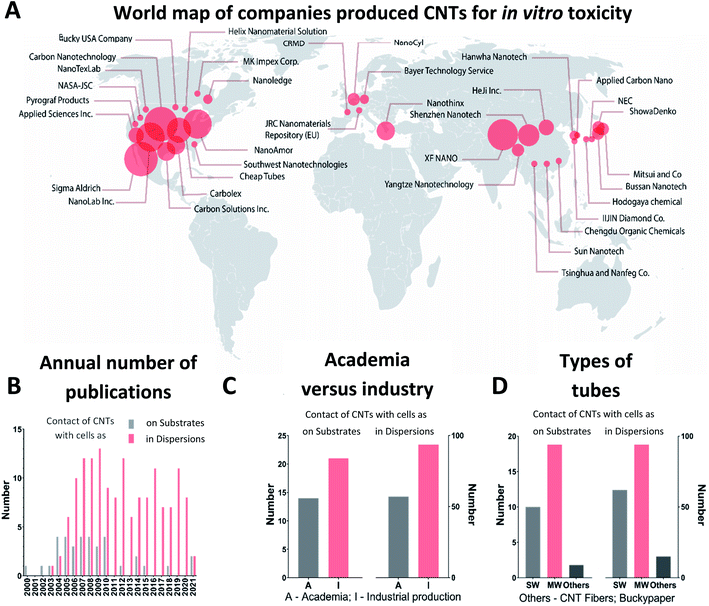
The CNT origin can be globally attributed to two groups: produced by a company or synthesized in a research laboratory. Commercially produced tubes were found to be more widespread among researchers for testing of the CNT toxicity, i.e., in about 60% of the found publications CNTs are purchased from companies. The most popular CNTs for cytotoxic studies were produced by Carbon Nanotechnologies Inc. (USA), Cheap Tubes Inc. (USA), Nanostructured and Amorphous Material (NanoAmor) Inc. (USA), Shenzhen Nanotech Port Co. Ltd (China), Sigma-Aldrich Inc. (USA) and Nanjing XFNANO Materials Tech Co. Ltd (China) as summarized in Table S1, ESI.† There are two largest centers serving as sources of commercial CNTs utilized in toxicology studies (Fig. 2A). The first one is located in the USA and the second one in Asia. While researchers from different disciplines address the toxicity of CNTs: engineering, biology, medicine, materials science, many laboratories are not related to the field of materials science, i.e., they do not synthesize the material. Therefore, the about the synthesis method, catalysts, and geometry of CNTs is often limited by the data provided by the manufacturer.80–82In Fig. 2B, we present the dynamics of appearance of the CNT toxicological studies for the time period, starting from the first publications in 2000 until 2021. The first pioneer works included data of cell cultivation directly on thin films made of CNTs.14,83–85 The results of these studies were quite promising because cell viability and morphology were similar to the control group kept without contact with CNTs. Intrigued by these first experiments, researchers brought greater attention to the topic, yielding the growth in publications in 2005–2007. Herein, researchers also studied the effects of CNTs on living cells in a dispersion related to possible applications in biology and medicine as drug carriers. The 2005–2007 period is characterized by an expected increase of CNTs industrial production driven by the developed CNTs synthesis technology at the macroscale and increased industry demands.36 For example, Nano Carbon Technologies Co. Ltd (Japan) and Shenzhen Nano Technologies Port Co. Ltd (China) grew quickly and were already producing 5 kg of CNTs per hour by 2007.86 Nowadays, worldwide CNT demand is reported to be more than 2000 tons per year for aerospace industry, composite production and battery manufacturing with market growth up to USD 9.84 billion by 2023.87 In many companies, such as Showa Denko, OCSiAl, Hanwha Nanotech, the annual volume of production is already greater than 100 tons.88
Starting from 2007, the scientists' interest in the CNT bio-effects was fully switched to CNTs in a form of dispersion facilitated by the increased industrial production and humanity fears about the toxic effect on the environment and on employees involved in the synthesis of the materials.89 Several years later, it was evident that being easily absorbed onto the skin surface, CNTs internalized through epithelial tissues forming barriers in a human organism (Fig. 1).15,46,90 The time was crucial for further applications of CNTs in the industry of composite materials, plastic, rubbers and biomedicine. From 2010, most publications were focused on the effect of CNTs in dispersions, and the interest in CNTs as a substrate for cell growth was reduced.
According to our analysis, MWCNTs were identified to be the most popular material in cytotoxicity studies (Fig. 2D). That fact can be explained by wider availability of the material and the proposed safer nature of MWCNTs because of their greater diameter, decreasing the chances of cell membrane damage and tube penetration into cells.18 Numerous studies proposed a toxic impact of SWCNTs in dispersions because of possible penetration into cells, while MWCNTs have an indirect impact by facilitating changes in cell microenvironment.18,23
Summing up, scientists' attention to the toxic effects of CNTs is driven by the massive industrial growth of application in energy storage systems, sensors, composites and transparent microsized electronics.91,92 Nearly 60% of all tubes employed in cytotoxicity tests are of industrial origin where the USA and Asia represent locations with the highest impact. Thus, MWCNTs were in more demand likely because of the availability.
Tested cells types
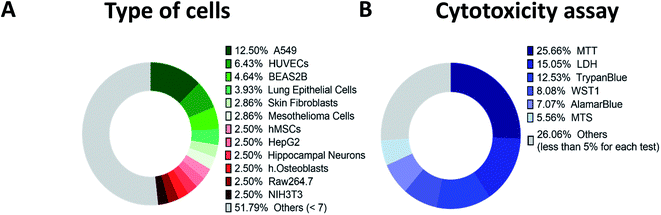
Fig. 3A and Table S2 (ESI†) summarizes all cell types tested in the collected articles published between 2000 and 2021. Nearly 80 different types of cells were tested by researchers for cytotoxic effects of CNTs. A large part of these studies was done using the regular A549 cell line, human alveolar epithelial cells.93 This cell line is widely used and recommended as a convenient model for toxicological studies.Human Umbilical Vein Endothelial Cells (HUVECs) is the second most common type of cells, usually used to study blood vessel regulation.94 The next one is BEAS2B epithelial line from a human lung that is also suitable for toxicity testing.95 Close to BEAS2B were other lung epithelial cell types and less than 3% of studies employed osteoblasts, skin fibroblasts, hippocampal neurons and several other cell types.
Thus, to verify the effects of pristine or functionalized CNTs, to check the impact of the synthesis approaches, catalysts, and CNT geometry, we identified the most employed models - A549, HUVECs or BEAS2B cells.
Impact of CNTs applied on substrates
As we mentioned earlier, one of the parameters influencing cytotoxic effects is the way the tubes contact with the cells – serving a substrate or in a dispersion. First studies included data of cell incubation on substrates covered with CNTs.14,83–85 We summarized these 36 publications in Table 1.| Type of cells or cell line | Incub. time (hours (h) or days (d)) | Type of CNTs, diameter (D), length (L) | Synthesis, producer, catalysts, functionalization (F) | Year | Ref. |
|---|---|---|---|---|---|
| Embryonic rat hippocampal neurons | 8 d | MWCNTs, D = 20 nm, L = 20–100 μm | Catalytic decomposition, ferrocene-xylene, 4-HNE (F) | 2000 | 14 |
| Osteoblasts | 3, 7 d | MWCNTs, D = 60–200 nm | CVD, Applied Sciences Inc., USA, pyrolytic aromatic hydrocarbon layer (F) | 2002 | 83 |
| Human osteoblasts, ovine bladder smooth muscle cell, mouse skin fibroblasts, human articular chondrocytes | 1 h | MWCNTs, D = 60–200 nm | CVD, Applied Sciences Inc., USA, pyrolytic aromatic hydrocarbon layer (F) (composite with polycarbonate urethane) | 2003 | 84 |
| Dissociated cortical cultures from one-day-old Charles River rats | Near 6 d (150 h) | No data about tube parameters | Clusters of CNT, iron nitrate as the catalyst | 2004 | 98 |
| Fibroblast mouse L929 | 1, 7 d | MWCNTs (vertically aligned), L = 50–35 μm | CVD, Ni catalyst, NanoLab Inc., USA | 2004 | 113 |
| Hippocampal neurons from 0 to 2 day Sprague-Dawley rats | 7 d | No data about tube parameters | AP-SWCNT, MWCNT-COOH (F), MWCNT with ethylene diamine(F), poly-m-aminobenzene, CVD | 2004 | 99 |
| Rat astrocyte cells, pheochromocytoma cells, human osteoblast cells (embryo 3T3) mouse fibroblasts | 1 h, 3 d | MWCNTs, D = 60 nm | CVD, Applied Sciences Inc., USA, pyrolytic aromatic hydrocarbon layer (F) | 2004 | 96 |
| Hippocampal neurons | 8, 10 d | MWCNTs, no data about tube parameters | Nanostructured and Amorphous Material (NanoAmor) Inc., USA | 2005 | 101 |
| Hippocampal neurons | 3, 7 d | SWCNTs, no data about CNT parameters | SWCNT-COOH (F), Carbon Solutions Inc., USA | 2005 | 102 |
| H19-7 (derived from hippocampi from embryonic day 17 Holtzman rats) | 6 h | MWCNTs (vertically aligned), L = 10 μm | CVD, iron catalyst | 2005 | 128 |
| NG108-15 neuroblastoma × glioma hybrid cells | 3, 5, 10 d | SWCNTs, no data about tube parameters | Arc-discharge, CarboLex Inc., USA | 2005 | 108 |
| NG108-15 neuroblastoma × glioma hybrid cells | 3, 5, 10 d | SWCNTs, no data about CNT parameters | Arc-discharge, CarboLex Inc., USA | 2006 | 109 |
| Human osteoblasts hFOB, human fibroblastic line HS-5 | 24, 48 h, 7 d | MWCNTs, no data about tube parameters | Catalytic decomposition on CoO/MgO catalyst | 2006 | 111 |
| Osteoblasts ROS 17/2.8 cells | 5 d | SWCNTs, D = 1.5 nm, length varied, MWCNTs, D = 10–30 nm | SWCNT-COOH(F); SWCNT-PABS(F); SWCNT-PEG(F); Carbon Solutions Inc., USA | 2006 | 81 |
| Mouse embryonic neural stem cells | 3, 5, 7 d | SWCNTs, no data about tube parameters | HiPCO, Carbon Nanotechnologies Inc., USA | 2007 | 104 |
| Hippocampal cultures from 0/3 d old Sprague Dawley rats | 8, 14 d | SWCNTs, no data about tube parameters | HiPCO, Carbon Nanotechnologies Inc., USA | 2007 | 103 |
| Human skin fibroblasts, Schwann cells from rats, cortical and cerebellar neurons, dorsal root ganglion neurons | 36–48 h, 14 d | MWCNTs, D = 10 nm, L = 300 μm | CVD | 2007 | 107 |
| Osteoblast | 21 d | MWCNTs, no data about tube parameters | CVD, cobaltous nitrate solution | 2007 | 112 |
| Immortal NIH3T3, primary, rat hippocampal neural cells | 14, 24 h, 7 d | SWCNTs, no data about tube parameters | Nanoledge Inc., Canada | 2008 | 106 |
| Fibroblast L929 mouse cells | 2, 24, 48, 72, 96 h | MWCNTs, no data about tube parameters | — | 2008 | 116 |
| Human osteoblast cells | 7 d | MWCNTs; D = 10–20 nm, L = 1–20 μm | CVD, NanoLab Inc., USA | 2008 | 110 |
| Human collateral cancer cell Caco-2, human breast adenocarcinoma MCF7 and HL-60, primary HA-SMCs | 24, 72, 120 h | — | CVD, NanoLab Inc., USA | 2008 | 97 |
| Hippocampal neurons of newborn Sprague-Dawley rats | 3 d | SWCNTs, no data about tube parameters | Carbon Solutions Inc., USA | 2009 | 105 |
| Neural stem cells | 12, 24 h, 7 d | SWCNTs, no data about tube parameters | HiPCO, Carbon Nanotechnologies Inc., USA | 2009 | 129 |
| Human embryonic stem cells | 1, 3, 5 d | — | — | 2009 | 121 |
| NIH-3T3 | 24, 48 h | MWNTs D = 5–15 nm | CVD, NanoLab Inc., USA | 2010 | 115 |
| Human mesenchymal stem cell | 1, 3, 7, 14 d | SWCNTs, no data about tube parameters | Carbon Solutions Inc., USA | 2010 | 122 |
| Human embryonic stem cells | 1, 7 d | MWCNTs, no data about tube parameters | Sigma-Aldrich Inc., USA | 2010 | 124 |
| Mouse fibroblast cells | 24, 48, 72, 96 h | MWCNTs, no data about tube parameters | Microwave plasma chamber, Ni or Fe layer | 2010 | 117 |
| Mouse fibroblast cells | 6, 48, 72 h, 7 d | — | Microwave plasma chamber, Ni or Fe layer | 2012 | 118 |
| Mouse embryonic fibroblasts, human bronchial epithelial cell line 24 h | 24 h | MWCNTs, D = 6–15 nm | — | 2014 | 119 |
| Human HCC lines (SNU182 and HUH7) | 3 d | Vertically aligned CNTs, no data about CNT parameters | CVD | 2014 | 125 |
| Human promyelocytic leukemia cell line HL-60, human 134 histiocytic lymphoma cell line U-937, the human chronic leukemia cell line K-562 | 24, 48, 72 h | — | CVD, NanoLab Inc., USA | 2015 | 123 |
| Pancreatic adenocarcinoma, PANC-1, AsPC-1, and BxPC-3 cell lines | 1, 4, 8 d | MWCNTs, forest, no data about tube parameters | CVD | 2018 | 126 |
| Murine L929 fibroblasts, human dermal fibroblast | 1, 3, 7 d | MWCNTs, no data about tube parameters | Nanostructured and Amorphous Material (NanoAmor) Inc., USA | 2021 | 114 |
| HEK-293 cells, neonatal rat ventricular myocytes (NRVMs), murine Bone marrow derived macrophages, Jurkat cells, SH-SY5Y | 2, 5 d | CNTF, D = 22.2 ± 0.7 μm | — | 2021 | 127 |
Among the listed publications, we found only several reports about the toxic effects of CNTs on substrates on cell physiology, meaning that CNTs play a role of a harmless surface for cell growth.84,96,97
The listed studies in Table 1 can be divided into four main groups according to the used cells types: cells related to nervous system,14,96,98–109 osteoblasts,81,83,84,96,110–112 fibroblasts,84,96,107,111,113–119 and other types.84,97,119–127
In the case of neurons, glia or cortical cultures growth, each study demonstrated a successful outcome. Thus, in 2000 the first attempts were made to study the interface between CNTs and living systems. Mattson et al. successfully applied CNTs as a substrate for growth of embryonic rat hippocampal neurons.14 The researchers used MWCNTs (diameter of 20 nm, length of 20–100 μm) prepared with the catalytic decomposition of a ferrocene-xylene precursor, dispersed in ethanol and applied to cultural plastic as substrates. Moreover, they covered some samples with 4-hydroxynonenal (4-HNE) to study the effects of modified tubes on neurite outgrowth.129 The molecule 4-HNE plays the role of a “crossroads” substance with numerous functions in cells, e.g. regulating gene expression, the proliferation of cells, cell death, and stress-mediated pathways.130 The scientists successfully demonstrated neuron adhesion, neurite outgrowth, and branching of cells grown on modified substrates. This first work opened the door connecting two fields, nanotube technology and neurobiology, which inspired many researchers to move further and study the interaction of the material with cells in more detail.
The other study was conducted with dissociated cortical cultures on a substrate patterned with CNTs “clusters”.98 After several days of incubation, researchers found that cells were located directly on the CNT clusters and formed interconnections, what demonstrated successful engineering of the neural network and its evolution.
In 2004, the research group tested the effect of chemically modified CNTs onto isolated hippocampal neurons.99 The researchers revealed that a pristine, or as-prepared (AP), SWCNT film was a permissive substrate; better branching of cells was observed onto the tubes modified with ethylenediamine (EN) (MWCNTs-EN). Based on these experiments, a vital conclusion followed - surface charge impacts the neurite outgrowth. In the same year, Webster et al.96 evaluated the possible application of CNTs as neural implants. The researchers revealed that the addition of CNTs in the composite resulted in inhibition of glial cells, which allowed to control glial to neuron cell ratio and glial scar formation. Increased postsynaptic activity of neurons grown onto CNTs101 and modulation of cell morphology102 was demonstrated for these cell cultures. Electrical stimulation through the modified substrate covered with CNTs enabled control over cell growth and cells differentiation.100,103–105,107–109,129 A good material biocompatibility with cells was also confirmed by Dubin et al.106 In the case of osteoblasts, researchers showed an enhanced proliferation83 and higher growth rates onto CNTs when compared with regular implant materials like Ti6Al4V or CoCrMo,84 and concluded the material to be promising for orthopedic applications.81,110–112
Fibroblast cultures were shown to have the same viability as in the control group,113,115–119 long-term growth without abnormalities in nuclei and regular morphology,106,107,114 so the material was also proposed to be promising for tissue restoration.
According to our analysis, the number of publications devoted to toxicity of CNTs placed onto substrates is four times lower when compared to works studying CNT effects in dispersion. The authors of the present review propose that such a shift could be due to the high rate of methods development to disperse CNTs and their possible impact in dispersion on the environment and human health by means of internalization through epithelial tissues.89
Furthermore, CNTs dispersed in liquids have wider application range than those placed onto the substrates or just in the form of thin films because of greater probabilities for contact with cell membranes and internalization into cells. For instance, CNTs are rather promising as a substrate for orthopedic or implant application, tissue engineering, and as electrodes or conductive substrates for electrically active tissues. At the same time, CNTs in the dispersion may be applied for target drug delivery, photonics, biosensing, bioimaging, gene therapy, etc.
Impact of CNTs in dispersion applied to cell cultures
To find some correlations and obtain a clear picture of the field, the authors of the present review made several assumptions. Firstly, we divided all cells into four groups according to tissue types – epithelial, connective, muscle, and nervous. Secondly, we considered a dose and incubation time accounting for the type of the CNTs only, i.e., disregarding the extra parameters such as functionalization or tube modifications, tube purification procedure and synthesis. Also, we roughly assessed the toxicity of materials as a factor influencing cell viability. However, in reality, the material may affect cell metabolism, gene expression and other aspects of cell physiology. Authors understand that such assumptions lead to significant simplification, but it seemed to be the only chance to identify some core trends of the CNT toxicity in dispersion in vitro.The most significant part of in vitro in dispersion studies was done with the commonly used MTT colorimetric assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) for cell viability identification; tetrazolium dye MTT is reduced to formazan with a purple color whose intensity correlates with a number of alive cells; near 26% of all utilized test types are MTT tests (Fig. 3B). However, in several works, researchers demonstrated that MTT is sometimes unreliable because of the reaction between reagent and CNTs, which results in color changes and false viability assessment131–133
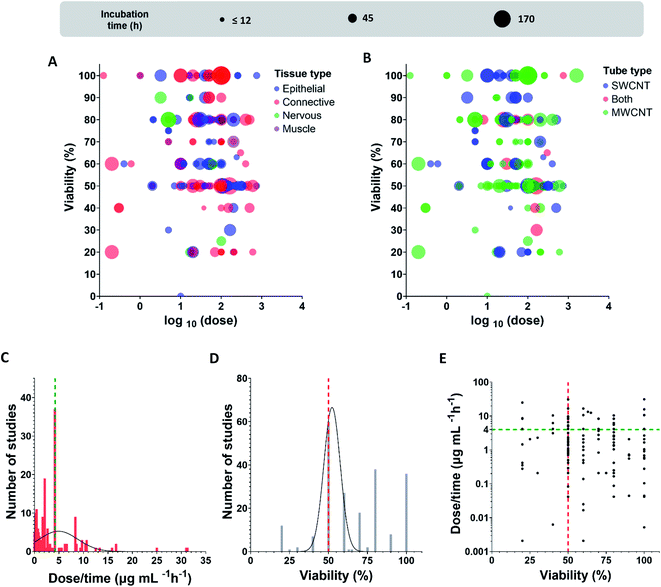
Fig. 4 displays the data of the toxicity of CNTs in dispersion as summarized in Table 2. Viability represents the percentage of alive cells after incubation time using a specified dose of CNTs. The dose is presented in μg mL−1 units using logarithmic scale (Fig. 4A and B), while incubation time indicates the duration of cells contact with the material. For both types of tubes, we have found only several studies where effects for SWCNTs and MWCNTs were similar in terms of cell viability.134–144
| Type | Cytotoxic test | Incub. time | Dose, viable (V)/dead (D) cell | Cell type | Year | Ref. |
|---|---|---|---|---|---|---|
| SWCNT | Alamar blue | 18 h | 0.24 mg mL−1 D 37.6% | Human epidermal keratinocytes | 2003 | 149 |
| SWCNT; MWCNT | Alamar blue | 24, 48, 72, 144 h | 144 h 0.16 mg mL−1 V 50% | hMSCs | 2008 | 135 |
| SWCNT (solution-indirect) | Alamar blue | 24, 48, 72 h, 96 h | Indirect 24 h 0.8 mg mL−1 V 90%; direct 24 h 0.4 mg mL−1 V 60% | A549 | 2008 | 150 |
| MWCNT | Alamar blue | 24 h | BEAS2B IC50 = 7 μg mL; mesothelioma IC50 = 17 μg mL−1 | BEAS2B; mesothelioma | 2012 | 151 |
| MWCNT | Alamar blue | 24 h | 50 μg mL−1; V 70% | BEAS2B | 2013 | 82 |
| MWCNT | Alamar blue | 24 h | Different data (1 μg mL−1 is safe; other conc varying data) | BEAS2B; MESO1 | 2014 | 152 |
| MWCNT | Alamar blue | 24 h | 10 mg L−1, V > 60% | Primary astrocytes | 2015 | 145 |
| SWCNT; MWCNT | Alamar blue | 24, 48 h | (SW)100 μg mL−1; V near 90% for both times; (MW) EC50 = 10 μg mL−1 | THP1 | 2017 | 153 |
| MWCNT | Alamar blue | 24 h | 24 μg mL−1; V > 50% | BEAS2B; SAEC | 2019 | 154 |
| MWCNT | Alamar blue | 24 h | 100 μg mL−1; V > 80% | Human lymph node endothelial cells | 2020 | 155 |
| SWCNT | Alamar blue (for substrates contact NIH3T3 and PC12) | 15 min RBC, 3 d | -;- (RBC lysis); V for NIH3T3; PC12 as in control | RBC; NIH3T3; PC12 | 2008 | 120 |
| MWCNT | Alamar blue, LDH | 24 h | BEAS2B 10 μg mL−1 V 0%; IMR32 100 μg mL−1 V 70%; THP1 10 μg mL−1 V > 60% | BEAS2B; mesothelioma; neuroblastoma (IMR32), THP1 | 2012 | 156 |
| SWCNT; MWCNT | Alamar blue, MTT | 24 h | A549 0.75 mg mL−1 V 100%; U937 varied data | A549; U937 | 2007 | 138 |
| SWCNT | Alamar blue, NR, MTT | 24 h | EC50 = 744 ± 91 μg mL−1; with serum > 800 μg mL−1 | A549 | 2007 | 157 |
| SWCNT | Annexin V + PI | 4 h–24 h | 10–50 μg mL−1, no cells activation | Primary immune cells | 2006 | 158 |
| SWCNT | Annexin V + PI | 24, 48 h, 72, 96 h | 50 μg mL−1 V 100% for all time points | A549 | 2007 | 159 |
| SWCNT; MWCNT | Annexin V + PI | 24, 48 h, 72 h | 48 h EC50 = 450 μg mL−1 (SW); 72 h EC50 = 600 μg mL−1 (MW) | Mononuclear cells | 2012 | 144 |
| MWCNT | Aqueous one | 24, 96 h | BEAS2B 4 d 200 μg mL−1 V near 20%; A549 4 d 200 μg mL−1 V 80% | BEAS2B; A549 | 2011 | 160 |
| MWCNT | CCK8 | 24 h | 50 mg mL−1 V > 60% (N); 50 mg mL−1 HeLa V 80% | Neuroblastoma (N); HeLa | 2012 | 161 |
| MWCNTs | CCK8 | 24 h | 40 μg mL−1 V 50% | HepG2 | 2016 | 162 |
| MWCNT | CCK8 | 24, 48 h | 100 μg mL−1 V > 80% for both times | NIH-3T3 | 2016 | 163 |
| MWCNT | CCK8 | 72 h (+26 weeks) | For 72 h 1.92 μg cm−2 V near 100% | HBEC-3KT | 2017 | 164 |
| MWCNT | CCK8 | 6 h | 64 μg mL−1 V near 60% | HUVECs | 2018 | 165 |
| SWCNT | CCK8 | 12 h | 50 μg mL−1 V near 50% | HUVECs | 2018 | 166 |
| SWCNT | CCK8 | 12 h | 50 μg mL−1 V near 60% | HUVECs | 2018 | 167 |
| MWCNT | CCK8 | 24 h | 60 μg mL−1 V near 80% | HUVECs | 2019 | 168 |
| MWCNT | CCK8 | 24 h | 64 μg mL−1 V > 80% | THP-1 | 2019 | 169 |
| MWCNT | CCK8 | 24 h | 64 μg mL−1 V > 60% | HUVECs | 2019 | 170 |
| MWCNT | CCK8 | 24 h | 64 μg mL−1 V > 60% | SMCs smooth muscle cells | 2019 | 171 |
| MWCNT | CCK8 | 24 h | 64 μg mL−1 V > 60% | HUVEC | 2019 | 172 |
| SWCNT | CellTiter blue assay | 6 h–48 h | -;- (factor increase units) | A549; NHBE | 2008 | 173 |
| MWCNT | CellTiter blue | 24, 72 h | 24 h 200 μg mL−1, V > 80% | Raw264.7 | 2012 | 174 |
| MWCNT | CellTiter-Glo | 24 h | 25 μg mL−1 V > 80% | Human brain microvasculature endothelial cells | 2017 | 175 |
| SWCNT | CellTiter-Glo | 72 h | 10 μg mL−1 V > 60% for the most cases (only one type decreased to 10%) | HEK293; MCF10A; MRC-5; HepG2 | 2017 | 176 |
| SWCNT | CyQUANT | 24 h | 25 μg mL−1 V 100% | Human lung fibroblasts; HUVEC | 2013 | 177 |
| SWCNT; MWCNT | Flow cytometry | 24, 48, 72 h | 24 h 100 μg mL−1 V 90%; 72 h 100 μg mL−1 V 80% | Jurkat | 2008 | 139 |
| MWCNT | Flow cytometry | 8, 24, 48 h | Jurkat S 100 μg mL−1 V 100% | Jurkat; primary splenocytes (S); primary neurons (N) | 2009 | 178 |
| MWCNT | Flow cytometry | 1 h | 100 μg mL−1 V near 50% | A549 | 2015 | 179 |
| MWCNT | Flow cytometry | 24 h | 200 μg mL−1 V near 50% | Normal liver cells | 2015 | 180 |
| MWCNT | Flow cytometry | 24 h | 120 μg mL−1 V near 50% | Raw264.7 | 2020 | 181 |
| SWCNT | Hemocytometer (cells recovery) | Up to 4 d | 50 μg mL−1 – (30% or 85% from control) | Murine lung epithelial cells | 2007 | 80 |
| MWCNT | Hoechst 33342 + microscope | 24, 48 h | 0.6 μg mL−1 V 60% for 48 h | Human skin fibroblasts | 2005 | 182 |
| MWCNT | Indirect assay (protein expression) | 24, 48 h | — | HEK keratinocytes | 2006 | 183 |
| MWCNT | Indirect assay (AP detection kit) | 2, 4 h | — | Mouse embryonic Stem cell | 2007 | 184 |
| SWCNT | Indirect assay (NO, free radicals, super oxide) | 1–2 h | 0.5 mg mL−1 no changes | Raw 264.7 | 2005 | 185 |
| SWCNT | Indirect assay (NO, free radicals, super oxide, apoptosis) | 24, 48, 72 h | 30 mg mL−1 D 10% for 48 h | J774; hMDM | 2006 | 186 |
| SWCNT | Indirect assay (oxidative stress) | 3 h | 10 μg mL−1 ROS increase in 12% | BJ Foreskin cells | 2007 | 187 |
| MWCNT | LDH | 24 h | 125 ng mL−1 no toxic effects | Human mononuclear cells | 2007 | 188 |
| MWCNT | LDH | 24 h | — | hMSCs | 2013 | 189 |
| MWCNT; SWCNT | LDH | 24 h | 40 μg mL−1 V 100% | MDMs | 2014 | 190 |
| MWCNT; SWCNT | LDH | 24 h | 100 μg mL−1 V 50% for both | A549; J774 | 2014 | 141 |
| MWCNT | LDH | 24 h | 80 μg mL−1 V 80% | Met-5A | 2016 | 191 |
| SWCNT | LDH | 24, 48 h | 2 μg mL−1 V 50% for both times | HeLa; HUVEC; Hep2G | 2018 | 192 |
| MWCNT | LDH | 24 h | — | Mesothelial LP9 | 2020 | 193 |
| MWCNT | LDH, trypan blue | 24 h | 12 μg mL−1 V > 60% | Bronchial epithelial primary cells | 2019 | 194 |
| MWCNT | LDH, CCK8 | 24 h | 100 μg mL−1 V 50% | HUVEC | 2014 | 195 |
| MWCNT | LDH, MTT | 48 h | 300 ng mL−1 V < 40% | HEK293 | 2010 | 196 |
| MWCNT | LDH, MTT, WST1, XTT | 1, 72 h | 48 h LDH 100 mg mL−1 V 60%; XTT 100 mg mL−1 V 80% | A549 | 2008 | 197 |
| MWCNT | LDH, trypan blue | 24 h | TC50 = 20–80 μg mL−1 (for both CNT) | A549; Hep3B; HEK | 2010 | 198 |
| MWCNT | LDH, WST1 | 24 h | EC50 near 200 μg mL−1 | A549 | 2017 | 199 |
| MWCNT; SWCNT | LDH, WST1 | 24 h | 256 μg mL−1 V 100% (MW); (SW) 64 μg mL−1 V 100% | 16HBE | 2018 | 143 |
| MWCNT | LDH; WST8 | 24 h | 32 μg mL−1 V near 80% | HUVECs | 2017 | 200 |
| MWCNT | Live/Dead | 12, 24 h | 5 μg mL−1 V 30% | Rat LE cells | 2010 | 201 |
| SWCNT | Microscopy | 6 d | — | Primary neurons (N); primary glia (G) | 2009 | 202 |
| MWCNT | Microscopy | 48 h | 17 μg mL−1 V 20% | DRG; PC12 | 2010 | 203 |
| MWCNT | Molecular probes cell viability | 24 h | 5.5–55.8 μg mL−1 V > 50% | Human osteoblasts | 2004 | 85 |
| SWCNT | MTS | 24, 48, 72 h | 0.04 mg mL−1 V 100% for all time points | MCF7; human epidermal keratino-s | 2004 | 204 |
| SWCNT | MTS | 24, 48, 72 h | 40 μg mL−1 V 90% | MCF7 | 2015 | 205 |
| MWCNT | MTS | 24 h | 100 μg mL−1 V for all > 50% | A549; lung V79 fibroblasts | 2015 | 206 |
| MWCNT | MTS | 24 h | 400 μg mL−1 V > 50% | Raw264.7 | 2016 | 207 |
| MWCNT | MTS | 24 h | IC50 10 μg mL−1 | A549 | 2021 | 208 |
| SWCNT | MTS, flow.cyt. | 24 h | 40 μg mL−1 V 100% | MCF7 | 2013 | 209 |
| MWCNT | MTS, LDH | 24 h | 100 μg mL−1 V 100% | BEAS2B; RLE6TN; THP1 | 2013 | 210 |
| MWCNT; SWCNT | MTS, live/dead | 24 h | (MW) 10 μg cm−2 V 90%; (SW) 10 μg cm−2 V 100% | HAEC | 2012 | 136 |
| SWCNT | MTS, NR, LDH | 24 h | 100 μg mL−1 resulted in first cytotoxic ef (varied EC50 233 μg mL−1 to 600 μg mL−1) | Caco2 | 2009 | 211 |
| SWCNT | MTT | 72 h | 20 μg mL−1 V 20% | Human epidermal keratino-s; HeLa; A549; H1299 | 2005 | 212 |
| SWCNT; MWCNT | MTT | 3 h, 6 h | 22.6 μg cm−2 V > 80% for 6 h (MWCNT); V 40% (SWCNT) | Alveolar macrophages | 2005 | 142 |
| SWCNT | MTT | 24 h–120 h | 100 μg mL−1 V 50% | HEK293 | 2005 | 213 |
| SWCNT | MTT | 24, 48 h | 20 μg mL−1 D 80% | Human dermal fibroblasts | 2006 | 36 |
| SWCNT; MWCNT | MTT | 24 h–120 h | 25 μg mL−1 V > 80% for both | Human dermis fibroblasts | 2006 | 134 |
| MWCNT | MTT | 24, 96 h | 0.2 μg mL−1 H596 24 h V > 60%; H466 24 h V > 20%; calu 24 h V > 20% | H596; H466; Calu-1 | 2006 | 214 |
| SWCNT | MTT | 3 d | 30 μg mL−1 V < 50% | Mesothelioma MSTO-211H | 2007 | 215 |
| SWCNT; MWCNT | MTT | 24 h | 100 μg mL−1 (N) V 80% for MW; V 40% for SW; (M) V 40% for MW; V 20% for SW | Neuroblastoma (N); Rat alveolar macrophages (M) | 2007 | 216 |
| SWCNT | MTT | 6 h, 48 h | 500 mg L−1; Hep3B and HepG2 V 80%; for Panc-1 40% | Hep3B; HepG2; Panc-1 | 2007 | 217 |
| SWCNT | MTT | 24, 48, 72 h | 24, 48 h 3.2 μg mL−1 V 90%; 72 h 3.2 μg mL−1 V 80% | Astrocytoma 1321N1 | 2008 | 218 |
| MWCNT | MTT | 24 h | 100 μg mL−1 V 50% | HeLa | 2009 | 219 |
| MWCNT | MTT | 24 h | 17.5 μg mL−1 V 90% | Primary cortical cultures | 2009 | 220 |
| MWCNT | MTT | 24, 48 h | 24 h D384 100 μg mL−1 V 25%; A549 100 μg mL−1 V 50% (same for 48 h) | A549; astrocytoma D384 | 2010 | 221 |
| MWCNT; SWCNT | MTT | 48, 72 h (up to 3 w for diff) | 24 h and 48 h 30 μg mL−1, V > 60% (for both CNT) | MSCs | 2010 | 137 |
| MWCNT | MTT | 72, 144 h | 30 μg mL−1 D near 15% | A549; Jurkat | 2011 | 222 |
| SWCNT; MWCNT | MTT | 24, 72, 168 h | (SW) 0.11 mg mL−1 V near 100% up to 168 h; (MW) 0.11 mg mL−1 V 50% | A549 | 2011 | 223 |
| MWCNT | MTT | 24 h | 100 μg mL−1 V 50% for both lines | A549; Raw264.7 | 2011 | 224 |
| MWCNT | MTT | 24, 48, 72 h | 40 μg mL−1 V 90%; 200 μg mL−1 V 90%; 400 μg mL−1 V 80% | NHDF | 2012 | 225 |
| MWCNT | MTT | 24 h | 100 μg mL−1 V 60% | A549 | 2012 | 226 |
| MWCNT | MTT | 24 h | 6 mg mL−1 for one type V 40%, for another near 20% | J774 | 2012 | 227 |
| MWCNT | MTT | 12, 24, 36 h | 24 h 100 μg mL−1 V 80%; 200 μg mL−1 V 70% | C6 rat glioma | 2012 | 228 |
| MWCNT | MTT | 24 h | A549 TC50 = 35.6 μg mL−1; HepG2 TC50 = 33.5 μg mL−1; HEK TC50 = 35 μg mL−1; P407 TC50 = 39 μg mL−1 | A549; HepG2; HEK; P407 | 2014 | 229 |
| SWCNT | MTT | 24 h | 50 μg mL−1 V 100% | A549; MCF7; SKBr3 | 2015 | 230 |
| MWCNT | MTT | 24 h | 2 μg mL−1, V (L) near 50%; V (S) near 70% | A549 | 2016 | 231 |
| MWCNT | MTT | 48 h | 200 μg mL−1 V 40% | HT29 | 2017 | 232 |
| MWCNT | MTT | 48 h | 256 μg mL−1 V 50% | A549 | 2019 | 233 |
| MWCNT; SWCNT | MTT | 48 h | 8 μg mL−1 V > 80% | A549 | 2020 | 234 |
| SWCNT | MTT | 24 h | 200 μg mL−1 V > 80% | A549 | 2020 | 235 |
| MWCNT | MTT, LDH | 24 h | 40 μg mL−1 V 60% | A549 | 2012 | 236 |
| SWCNT | MTT, LDH | 48 h | IC50 = 87.6 μg mL−1 | HEK293 | 2014 | 237 |
| MWCNT | MTT, LDH | 24 h | 100 μg mL−1 V 70% (TT1); V 70% (ATII), AM V 55% | Alveolar type-I like cells (TT1), ATII, alveolar macrophages | 2014 | 238 |
| MWCNT | MTT, LDH | 1, 3 h | — | A549 | 2020 | 239 |
| MWCNT | MTT, NR | 24 h | EC80 = 2.1 mg L−1 | H295R (adenocarcinoma); T47Dluc | 2014 | 240 |
| SWCNT | MTT, LDH, WST1 | 24 h | 50 μg mL−1 MTT V 60%; LDH V 100%; WST1 V 100% | A549; HUVEC; alveolar macrophage | 2006 | 133 |
| MWCNT | MTT, WST1 | 72, 96 h, 1–2 w | 3d 5 μg mL−1 V > 80%; 1 w 5 μg mL−1 V 80% | SH-SY5Y | 2009 | 241 |
| MWCNT; SWCNT | MTT, LDH | 24, 48 h | 24 h (SW) 300 μg mL−1 V 65%; 24 h (MW) 300 μg mL−1 V 65%; 48 h (SW) 150 μg mL−1 V 40%; 48 h (MW) 150 μg mL−1 V 50% | NIH3T3 | 2009 | 242 |
| SWCNT | MTT, LDH | 24, 48, 72 h | 50 μg mL−1 V 60% (for both tests) | HUVEC | 2010 | 243 |
| SWCNT | MTT, LDH | 24 h | 100 μg mL−1 V 20% | PC12 | 2010 | 244 |
| SWCNT | MTT, LDH; trypan blue | 24 h | 50 μg mL−1 V > 80% | Human umbilical cord MSCs | 2020 | 245 |
| SWCNT | MTT, WST | 24 h | MTT NR8383 5 μg mL−1 V > 70%; 10 μg mL−1 V > 40% for all samples; WST NR8383 100 μg mL−1 V > 70% for all | A549; rat macrophages (NR8383) | 2007 | 246 |
| SWCNT | NR, MTT | 24 h | 5 μg mL−1 V > 75% | HUVEC | 2006 | 247 |
| SWCNT | NR, MTT | 24 h | — | HUVEC | 2006 | 248 |
| SWCNT | NR, MTT | 96 h | 10 μg mL−1 V 100% | hMDM | 2009 | 249 |
| MWCNT | NR, live/dead | 48 h | 20 μg mL−1 V 80% | Macrophages | 2011 | 250 |
| SWCNT | NR; MTS | 24, 48 h | EC50 = 316 μg mL−1 for 24 h; EC50 = 81 μg mL−1 for 48 h | HUVEC | 2011 | 251 |
| MWCNT | NR, MTT; live/dead | 96 h | 20 μg mL−1 MTT V 50%; other tests V 70% | hMDM | 2009 | 252 |
| SWCNT | NR, MTT, alamar blue; aqueous one (AQO) | 24 h | 0.4 μg mL−1 V > 60% (AQO) | HEK keratinocytes | 2009 | 253 |
| SWCNT | Optical microscopy | 12, 24, 48, 60 h | 50 μg mL−1 V 100% | HeLa | 2007 | 254 |
| MWCNT | PI | 6 h | 50 μg mL−1 D 30% | A549 | 2017 | 255 |
| SWCNT; MWCNT | PI, acridine orange | 12, 48 h | 50 μg mL−1 V > 80% | H1299 | 2019 | 256 |
| MWCNT | Resazurin assay | 24, 48, 72 h | Raw IC50 > 80 μg cm−2 | Raw264.7; A549; Calu-3; alveolar macrophages | 2016 | 257 |
| SWCNT | Trypan blue | 24, 48, 72 h | 0.2 mg mL−1 V > 70% for 24 h and 72 h | Rat heart cell | 2005 | 258 |
| MWCNT | Trypan blue | 24, 48, 72, 96, 120 h | 10 ng per cell 24 h V > 80%, 48 h V 60% | T Lymphocytes | 2006 | 259 |
| MWCNT | Trypan blue | 18 h | 40 μg cm−2 V 70% | A549 | 2008 | 260 |
| MWCNT + SWCNT | Trypan blue | 24, 48, 72 h | 24 h 100 μg cm−2 V 30% | BEAS2B | 2009 | 261 |
| MWCNT | Trypan blue | 24 h | 100 μg mL−1 V 60% | HUVEC | 2011 | 262 |
| MWCNT SWCNT | Trypan blue | 24, 48, 72 h | 24 h 100 μg mL−1 V 50% | Raw264.7 | 2011 | 140 |
| SWCNT MWCNT | Trypan blue | 24, 48 h | 48 h BEAS2B 152 μg mL−1 V < 50% (SW); (SW) MeT5A 152 μg mL−1 V < 50%; (MW) MeT5A 152 μg mL−1 V 70% | BEAS2B; MeT5A | 2013 | 263 |
| SWCNT | Trypan blue | 36, 120 h | 30 μg mL−1 V near 80% for both times | A549 | 2013 | 264 |
| SWCNT | Trypan blue | 24, 48 h | 70 μg mL−1 V 20% | NIH3T3 | 2015 | 265 |
| MWCNT | Trypan blue | 13 w | 1.92 μg cm−2 V near 100% | HBEC-3-KT | 2018 | 266 |
| MWCNT | Trypan blue | 24, 48 h | 60 μg mL−1 V > 60% | Primary microglial cells | 2018 | 267 |
| MWCNT | Trypan blue | 24 h | 0.1 mg mL−1 V > 70% | HUVECs; human liver hepatocellular carcinoma | 2020 | 268 |
| MWCNT | Trypan blue, EZ-cytox assay | 24 h | EC20 for pristine 3 mg mL−1; EC20 for OH near 6 mg mL−1 | BEAS2B; HepG2 | 2016 | 269 |
| MWCNT (BP) | Trypan blue, NR | 48, 72 h | — | Blood lymphocytes | 2015 | 270 |
| MWCNT + fibers | Trypan blue, MTT | 24 h | 1 mg mL−1 V 80% | NIH3T3 | 2008 | 271 |
| MWCNT | Trypan blue, LDH | 24 h | 125 μg cm−2 V 20% | Mesothelial cells | 2008 | 272 |
| SWCNT | Trypan blue, LDH | 24, 48, 72 h | 50 μg mL−1 V 90% | T Lymphocytes | 2008 | 273 |
| MWCNT | Trypan blue, live/dead assay | 24, 48, 72 h | 72 h 50 μg mL−1 V 100% | THP-1 | 2016 | 274 |
| MWCNT | Trypan blue, WST1 | 24, 48, 72 h | 72 h 100 μg mL−1 V 50% | HPBCs (human peripheral blood cells) | 2016 | 275 |
| MWCNT | Trypan blue, WST1 | 24 h | A549 40 μg mL−1 V 80% (MTT 50%); BEAS2B 40 μg mL−1; V 80% (MTT 50%) | A549; BEAS2B | 2016 | 131 |
| MWCNT | Trypan blue, WST8, CCK8 | 168 h | 100 μg mL−1 V 100% | Dendritic cells | 2009 | 276 |
| MWCNT | WST1 | 24 h | GI50 = 0.0135% | NHBE | 2012 | 277 |
| SWCNT | WST1 | 72 h | 20 μg mL−1 V 100% | hMSCs | 2019 | 278 |
| MWCNT (+fibers) | WST1 | 24 h | 24 μg mL−1 V > 60% | BEAS2B | 2020 | 279 |
| MWCNT | WST1, CCK8 | 24 h | 100 μg mL−1 V 70% | Human bone osteosarcoma; human gingival fibroblasts (HGF-1) | 2016 | 280 |
| MWCNT SWCNT | WST1, LDH | 24 h | 150 μg per 106 cells V 60% | HAEC | 2009 | 281 |
| MWCNT | WST8 | 24 h | IC50 = 12 μg mL−1 | BEAS2B; CHO-K1 | 2010 | 282 |
| MWCNT | WST8 | 24 h | EC50 near 30 μg cm−2 | A549; HepG2 | 2019 | 283 |
| MWCNT | WST8, MTT | 16, 32 h | 24 h LC50 = 26 μg mL−1; 32 h LC50 = 22 μg mL−1 | J774.1; CHO-K1 | 2008 | 284 |
Earlier, it was shown that the composition of cell culture media and presence of bovine serum protein might strongly affect tube agglomeration, their bioavailability in dispersion and internalization in cells.82,138,145 We included different cell types in our analysis, so culture media might vary for cells cultures.
After dividing cells according to types of tissue, we realized that studies of connective tissue demonstrated high values of the CNT toxic impact (Fig. 4A). Researchers also registered small viability for epithelial tissues in some unique cases, but this is much rarer than in case of the connective ones.
To present the found data in a simplier way, we normalized the dose added to cells by incubation time. By using Spearman statistical criteria for analysis of relations between viability and doses, we have found a slight correlation between cells viability and dose per incubation time (r = −0.1648), i.e., a dose of tubes per hour (μg mL−1 h−1), (number of studies (N) = 214; statistical significance (p-value) = 0.0158). Cells viability and dose/incubation time parameters are weakly negatively correlated, meaning that dose increase results in the decrease of viability. We propose that the different dilution procedures could explain the absence of strong correlations. Undoubtedly, this is a rough estimation because cells meet the entire dose at the first interaction moment at incubation. However, cell adaptation to a prolonged regime may affect cells survival, so we also used dose/time characteristics for the analysis.
We further extracted only IC50 (inhibitory concentration) values, the mean value for studies with the known IC50 dose was 3.9 μg mL−1 h−1 (N = 60, 27.9% out of the entire sample); by calculating for 24 hours, it would be 93.8 μg mL−1.
By analyzing the extracted data in terms of frequency distribution for dose/time and viability parameters (Fig. 4C–E) we found that the majority of studies use IC50 values for cytotoxic analysis (27.9% out of the all revealed studies). Moreover, the most studied dose/time values are exactly in the region 4–5 μg mL−1 h−1 which is close to the found mean value for the viability 50% (3.9 μg mL−1 h−1). By applying a Gaussian distribution, we also revealed the maximum frequencies for the distribution given in Fig. 4C and D; for viability parameter, the maximum of distribution is 52% (amplitude 66.6, mean 52.3, SD 5.3), for dose/time, the maximum is precisely in the range 4–5 μg mL−1 h−1(amplitude 5.3, mean 4.9, SD 3.9). The intersection of green and red lines in Fig. 4E positions the maximum frequencies crossing for both characteristics, viability and dose/time; the maximum dots density is localized precisely in that region.
To sum up, we suggest CNT IC50 toxicity dose to be about 4–5 μg mL−1 h−1. Based on the found data, we also propose that the CNT toxic range is comparable with doxorubicin, a well-known agent for chemotherapy treatment (IC50 < 10 μg mL−1 for some cell lines after 24 h incubation).95,146–148
Conclusions
Thus, CNTs on a substrate are safe for many cell types. Nearly 90% of the publications included in the analysis reported the absence of a negative impact on cell cultivation on the CNT substrates.In the case of dispersions, we propose toxicity values comparable with the toxicity of a well-known chemotherapy agent called doxorubicin (IC50 < 10 μg mL−1). During our studies, we also identified the most suitable cell models (A549 and HUVECs) for testing the CNT toxicity and the comparison among research groups. Despite the examined phenomena of false-negative and unreliable results for cell viability obtained by the MTT test, near 26% of all works used the colorimetry assay to test cell viability.
There is no doubt that such CNT parameters as diameter, length, purification procedure, and synthesis may greatly affect toxicity, and should be carefully studied further employing a similar systematic approach.
The authors see an urgent need for standardization of materials and methods to investigate them. Guidelines such as OECD principles for proper laboratory practice are highly expensive and time consuming, therefore there is a strong need for a more convenient universal approach for testing the materials safety or toxic impact in a regular laboratory practice. Demonstrated results imply a need for practical toxicity assessment of CNTs with different geometry and functionalization, to deepen the understanding of what affects the CNT toxicity.
List of abbreviations
| 4-HNE | 4-Hydroxynonenal |
| Akt | Type of serine/threonine protein kinase |
| AP-1 | Activator protein 1 |
| CCK | Cell counting kit |
| CNT | Carbon nanotube |
| CVD | Chemical vapor deposition |
| EN | Ethylenediamine |
| FDA | Food and Drug Administration |
| HiPCO | High pressure carbon monoxide |
| ISO | International organization for standardization |
| LDH | Lactate dehydrogenase |
| MAPK | Mitogen-activated protein kinase |
| MTS | 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide |
| MWCNT | Multiwalled carbon nanotube |
| NF-kβ | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | Nod-like receptor family pyrin domain containing 3 |
| NR | Neutral red |
| OECD | Organization for economic co-operation and development |
| p53 | Tumor protein |
| PEG | Poly(ethylene oxide) |
| PEI | Poly(ethyleneimine) |
| PI | Propidium iodide |
| SWCNT | Single-walled carbon nanotube |
| TGF-β | Transforming growth factor beta |
| WST | 4-[3-(4-Iodophenyl)-2-(4-nitro-phenyl)-2H-5-tetrazolio]-1,3-benzene sulfonate |
| XTT | Sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-methoxy6-nitro) benzene sulfonic acid hydrate |
Author contributions
Margarita R. Chetyrkina carried out the literature search, wrote the initial manuscript and made subsequent modifications. Fedor S. Fedorov carried out the literature search, participated in writing and editing the manuscript. Albert G. Nasibulin supervised the work, participated in writing and editing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was partially supported by the Council on grants of the President of the Russian Federation grant number НШ-1330.2022.1.3. FSF thanks the Russian Science Foundation (grant no. 21-73-10288).Notes and references
- A. Venkataraman, E. V. Amadi, Y. Chen and C. Papadopoulos, Nanoscale Res. Lett., 2019, 14, 1–47 CrossRef CAS PubMed.
- S. Park, M. Vosguerichian and Z. Bao, Nanoscale, 2013, 5, 1727–1752 RSC.
- R. Rao, C. L. Pint, A. E. Islam, R. S. Weatherup, S. Hofmann, E. R. Meshot, F. Wu, C. Zhou, N. Dee, P. B. Amama, J. Carpena-Nuñez, W. Shi, D. L. Plata, E. S. Penev, B. I. Yakobson, P. B. Balbuena, C. Bichara, D. N. Futaba, S. Noda, H. Shin, K. Su Kim, B. Simard, F. Mirri, M. Pasquali, F. Fornasiero, E. I. Kauppinen, M. Arnold, B. A. Cola, P. Nikolaev, S. Arepalli, H.-M. Cheng, D. N. Zakharov, E. A. Stach, J. Zhang, F. Wei, M. Terrones, D. B. Geohegan, B. Maruyama, S. Maruyama, Y. Li, W. Wade Adams and A. John Hart, ACS Nano, 2018, 12, 11756–11784 CrossRef CAS PubMed.
- S. Iijima, Nature, 1991, 354, 737–740 CrossRef.
- S. Iijima and T. Ichihashi, Nature, 1993, 363, 603–605 CrossRef CAS.
- L. Lacerda, A. Bianco, M. Prato and K. Kostarelos, Adv. Drug Delivery Rev., 2006, 58, 1460–1470 CrossRef CAS PubMed.
- K. Kostarelos, A. Bianco and M. Prato, Nat. Nanotechnol., 2009, 4, 627–633 CrossRef CAS PubMed.
- K. Welsher, S. P. Sherlock and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 8943–8948 CrossRef CAS PubMed.
- M. Voge and J. P. Stegemann, J. Neural. Eng., 2011, 8, 011001 CrossRef PubMed.
- L. B. Keren and Y. Hanein, Front. Neural Circuits, 2012, 6, 1–16 Search PubMed.
- Y. Xue, Carbon Nanotubes for Biomedical Applications, Elsevier Inc., 2017 Search PubMed.
- Z. Liu, S. Tabakman, K. Welsher and H. Dai, Nano Res., 2009, 2, 85–120 CrossRef CAS PubMed.
- J. Ramón-Azcón, S. Ahadian, R. Obregón, H. Shiku, M. Ramalingam and T. Matsue, J. Biomed. Nanotechnol., 2014, 10, 2539–2561 CrossRef PubMed.
- P. Mattson, R. C. Haddon and A. M. Rao, J. Mol. Neurosci., 2000, 14, 175–182 CrossRef PubMed.
- C.-W. Lam, J. T. James, R. Mccluskey and R. L. Hunter, Crit. Rev. Toxicol., 2006, 36, 189–217 CrossRef CAS PubMed.
- P. Francis and T. Devasena, Toxicol. Ind. Health, 2018, 34, 200–210 CrossRef PubMed.
- A. Jafar, Y. Alshatti, A. Ahmad and U. Schumacher, Cogent Med., 2016, 3, 1217970 CrossRef.
- Y. Liu, Y. Zhao, B. Sun and C. Chen, Acc. Chem. Res., 2013, 46, 702–713 CrossRef CAS PubMed.
- H. J. Johnston, G. R. Hutchison, F. M. Christensen, S. Peters, S. Hankin, K. Aschberger and V. Stone, Nanotoxicology, 2010, 4, 207–246 CrossRef CAS PubMed.
- H. F. Cui, S. K. Vashist, K. Al-Rubeaan, J. H. T. Luong and F. S. Sheu, Chem. Res. Toxicol., 2010, 23, 1131–1147 Search PubMed.
- P. Firme and P. R. Bandaru, Nanomed. Nanotechnol. Biol. Med., 2010, 6, 245–256 CrossRef PubMed.
- D. Mohanta, S. Patnaik, S. Sood and N. Das, J. Pharm. Anal., 2019, 9, 293–300 CrossRef PubMed.
- K. Kostarelos, Nat. Biotechnol., 2008, 26, 774–776 CrossRef CAS PubMed.
- R. Alshehri, A. M. Ilyas, A. Hasan, A. Arnaout, F. Ahmed and A. Memic, J. Med. Chem., 2016, 59, 8149–8167 CrossRef CAS PubMed.
- M. A. Saleemi, M. Hosseini Fouladi, P. V. C. Yong, K. Chinna, N. K. Palanisamy and E. H. Wong, Chem. Res. Toxicol., 2021, 34, 24–46 Search PubMed.
- C. Kiratipaiboon, T. A. Stueckle, R. Ghosh, L. W. Rojanasakul and C. Chen, Environ. Sci.: Nano, 2019, 6, 2152–2170 RSC.
- P. Cherukuri, S. M. Bachilo, S. H. Litovsky and R. B. Weisman, J. Am. Chem. Soc., 2004, 126, 15638–15639 CrossRef CAS PubMed.
- Z. Liu, X. Sun, N. Nakayama-ratchford and H. Dai, ACS Nano, 2007, 1, 50–56 CrossRef CAS PubMed.
- N. Wong, S. Kam, M. O. Connell, J. A. Wisdom and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11600–11605 CrossRef PubMed.
- D. A. Heller, S. Baik, T. E. Eurell and M. S. Strano, Adv. Mater., 2005, 17, 2793–2799 CrossRef CAS.
- Z. Liu, M. Winters, M. Holodniy and H. Dai, Angew. Chem., Int. Ed. Engl., 2007, 46, 2023–2027 CrossRef CAS PubMed.
- M. Benincasa, C. Klumpp and J. Briand, Angew. Chem., Int. Ed. Engl., 2005, 44, 6358–6362 CrossRef PubMed.
- H. Dumortier, S. Lacotte, G. Pastorin, R. Marega, W. Wu, D. Bonifazi, J.-P. Briand, M. Prato, S. Muller and A. Bianco, Nano Lett., 2006, 6, 1522–1528 CrossRef CAS PubMed.
- K. Kostarelos, Nat. Biotechnol., 2008, 26, 774–776 CrossRef CAS PubMed.
- J. Dong and Q. Ma, Nanotoxicology, 2015, 9, 658–676 CrossRef CAS PubMed.
- M. Sayes, F. Liang, J. L. Hudson, J. Mendez, W. Guo, J. M. Beach, V. C. Moore, C. D. Doyle, J. L. West, W. E. Billups, K. D. Ausman and V. L. Colvin, Toxicol. Lett., 2006, 161, 135–142 CrossRef PubMed.
- J. Taylor, C. D. McClure, K. A. Shipkowski, E. A. Thompson, S. Hussain, S. Garantziotis, G. N. Parsons and J. C. Bonner, PLoS One, 2014, 9, e106870 CrossRef PubMed.
- C. Ge, J. Du, L. Zhao, L. Wang, Y. Liu, D. Li, Y. Yang, R. Zhou, Y. Zhao, Z. Chai and C. Chen, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 16968–16973 CrossRef CAS PubMed.
- K. M. Pondman, L. Pednekar, B. Paudyal, A. G. Tsolaki, L. Kouser, H. A. Khan, M. H. Shamji, B. ten Haken, G. Stenbeck, R. B. Sim and U. Kishore, Nanomed. Nanotechnol. Biol. Med., 2015, 11, 2109–2118 CrossRef CAS PubMed.
- W. Zhu, A. Von Dem Busschec, Y. Xin, Y. Qiu, Z. Wang, P. Weston, R. H. Hurt, A. B. Kane and H. Gao, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 12374–12379 CrossRef CAS PubMed.
- S. Kraszewski, A. Bianco, M. Tarek and C. Ramseyer, PLoS One, 2012, 7, 1–11 CrossRef PubMed.
- A. A. Tsukanov, B. Turk, O. Vasiljeva and S. G. Psakhie, Nanomaterials, 2022, 12, 650 CrossRef CAS PubMed.
- S. Ketabi and L. Rahmani, Mater. Sci. Eng., C, 2017, 73, 173–181 CrossRef CAS PubMed.
- J. Comer, R. Chen, H. Poblete, A. Vergara-Jaque and J. E. Riviere, ACS Nano, 2015, 9, 11761–11774 CrossRef CAS PubMed.
- M. Kumar and Y. Ando, J. Nanosci. Nanotechnol., 2010, 10, 3739–3758 CrossRef CAS PubMed.
- A. Helland, P. Wick, A. Koehler, K. Schmid and C. Som, Environ. Health Perspect., 2007, 115, 1125–1131 CrossRef CAS PubMed.
- E. J. Petersen, D. X. Flores-Cervantes, T. D. Bucheli, L. C. C. Elliott, J. A. Fagan, A. Gogos, S. Hanna, R. Kägi, E. Mansfield, A. R. M. Bustos, D. L. Plata, V. Reipa, P. Westerhoff and M. R. Winchester, Environ. Sci. Technol., 2016, 50, 4587–4605 CrossRef CAS PubMed.
- P. Laux, C. Riebeling, A. M. Booth, J. D. Brain, J. Brunner, C. Cerrillo, O. Creutzenberg, I. Estrela-Lopis, T. Gebel, G. Johanson, H. Jungnickel, H. Kock, J. Tentschert, A. Tlili, A. Schäffer, A. J. A. M. Sips, R. A. Yokel and A. Luch, Environ. Sci.: Nano, 2018, 5, 48–63 RSC.
- M. A. Zhilyaeva, E. V. Shulga, S. D. Shandakov, I. V. Sergeichev, E. P. Gilshteyn, A. S. Anisimov and A. G. Nasibulin, Carbon, 2019, 150, 69–75 CrossRef CAS.
- V. M. Gubarev, V. Y. Yakovlev, M. G. Sertsu, O. F. Yakushev, V. M. Krivtsun, Yu. G. Gladush, I. A. Ostanin, A. Sokolov, F. Schäfers, V. V. Medvedev and A. G. Nasibulin, Carbon, 2019, 155, 734–739 CrossRef CAS.
- S. Romanov, A. E. Aliev, B. Fine, A. S. Anisimov and A. G. Nasibulin, Nanoscale Horiz., 2019, 4, 1158–1163 RSC.
- A. Starón, O. Dlugosz, J. Pulit-Prociak and M. Banach, Materials, 2020, 13, 1–18 CrossRef PubMed.
- V. De Matteis, Toxics, 2017, 5, 29 CrossRef PubMed.
- M. Chen, G. Zeng, P. Xu, Y. Zhang, D. Jiang and S. Zhou, Environ. Sci.: Nano, 2017, 4, 720–727 RSC.
- C. Xiang, Y. Zhang, W. Guo and X. J. Liang, Acta Pharm. Sin. B, 2020, 10, 239–248 CrossRef CAS PubMed.
- D. Mohanta, S. Patnaik, S. Sood and N. Das, J. Pharm. Anal., 2019, 9, 293–300 CrossRef PubMed.
- Z. Liu, S. Tabakman, K. Welsher and H. Dai, Nano Res., 2008, 2, 85–120 CrossRef PubMed.
- S. F. Oliveira, G. Bisker, N. A. Bakh, S. L. Gibbs, M. P. Landry and M. S. Strano, Carbon, 2015, 95, 767–779 CrossRef CAS.
- R. Alshehri, A. M. Ilyas, A. Hasan, A. Arnaout, F. Ahmed and A. Memic, J. Med. Chem., 2016, 59, 8149–8167 CrossRef CAS PubMed.
- C. Darne, A. Desforges, N. Berrada, C. Fontana, Y. Guichard, L. Gaté, D. Bégin, F. L. Normand, F. Valsaque, J. Ghanbaja, J. Gleizee and B. Vigolo, Environ. Sci.: Nano, 2019, 6, 1852–1865 RSC.
- J. Kayat, V. Gajbhiye, R. K. Tekade and N. K. Jain, Nanomed. Nanotechnol. Biol. Med., 2011, 7, 40–49 CrossRef CAS PubMed.
- S. Sweeney, S. Hu, P. Ruenraroengsak, S. Chen, A. Gow, S. Schwander, J. (Jim) Zhang, K. F. Chung, M. P. Ryan, A. E. Porter, M. S. Shaffer and T. D. Tetley, Environ. Sci.: Nano, 2016, 3, 1340–1350 RSC.
- X. Dong, L. Liu, D. Zhu, H. Zhang, Y. Li and X. Leng, J. Nanomater., 2015, 2015, 1–8 Search PubMed.
- E. J. Petersen, M. Mortimer, R. M. Burgess, R. Handy, S. Hanna, K. T. Ho, M. Johnson, S. Loureiro, H. Selck, J. J. Scott-Fordsmand, D. Spurgeon, J. Unrine, N. W. Van Den Brink, Y. Wang, J. White and P. Holden, Environ. Sci.: Nano, 2019, 6, 1619–1656 RSC.
- R. Bjorkl, D. A. Tobias and E. J. Petersen, Environ. Sci.: Nano, 2017, 4, 747–766 RSC.
- K. D. Watson and P. Wexler, Information Resources in Toxicology, 2009, pp. 11–29 Search PubMed.
- L. J. Langman and B. M. Kapur, Clin. Biochem., 2006, 39, 498–510 CrossRef CAS PubMed.
- G. Oberdörster, E. Oberdörster and J. Oberdörster, Environ. Health Perspect., 2005, 113, 823–839 CrossRef PubMed.
- G. V. Kerckhove, J. Mater. Sci. Eng. B, 2019, 9, 41–49 Search PubMed.
- U. Wirnitzer, B. Herbold, M. Voetz and J. Ragot, Toxicol. Lett., 2009, 186, 160–165 CrossRef CAS PubMed.
- J. S. Kim, K. Lee, Y. H. Lee, H. S. Cho, K. H. Kim, K. H. Choi, S. H. Lee, K. S. Song, C. S. Kang and I. J. Yu, Arch. Toxicol., 2011, 85, 775–786 CrossRef CAS PubMed.
- Environment directorate joint meeting of the chemicals committee and the working party on chemicals, pesticides and biotechnology, https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2015)17/ann9%26doclanguage=en, (accessed October 2021) Search PubMed.
- D. Moher, A. Liberati, J. Tetzlaff, D. G. Altman and T. P. Group, PLoS Med., 2009, 6, e1000097 CrossRef PubMed.
- A. Phillips, P. Borry, I. Van Hoyweghen and D. F. Vears, Genet. Med., 2021, 23, 2038–2046 CrossRef PubMed.
- E. J. Dogterom, M. A. E. M. Wagenmakers, M. Wilke, S. Demirdas, N. M. Muschol, S. Pohl, J. C. van der Meijden, D. Rizopoulos, A. T. van der Ploeg and E. Oussoren, Genet. Med., 2021, 23, 2047–2056 CrossRef PubMed.
- N. Boy, K. Mengler, J. Heringer-Seifert, G. F. Hoffmann, S. F. Garbade and S. Kölker, Genet. Med., 2021, 23, 13–21 CrossRef CAS PubMed.
- M. H. Lahiani, S. Khare, C. E. Cerniglia, R. Boy, I. N. Ivanov and M. Khodakovskaya, Nanoscale, 2019, 11, 3639–3655 RSC.
- E. S. Kostewicz, B. Abrahamsson, M. Brewster, J. Brouwers, J. Butler, S. Carlert, P. A. Dickinson, J. Dressman, R. Holm, S. Klein, J. Mann, M. McAllister, M. Minekus, U. Muenster, A. Müllertz, M. Verwei, M. Vertzoni, W. Weitschies and P. Augustijns, Eur. J. Pharm. Sci., 2014, 57, 342–366 CrossRef CAS PubMed.
- W. Pfaller, P. Prieto, W. Dekant, P. Jennings and B. J. Blaauboer, Toxicol. in Vitro, 2015, 30, 1–3 CrossRef CAS PubMed.
- R. K. Saxena, W. Williams, J. K. McGee, M. J. Daniels, E. Boykin and M. I. Gilmour, Nanotoxicology, 2007, 1, 291–300 CrossRef CAS.
- L. P. Zanello, B. Zhao, H. Hu and R. C. Haddon, Nano Lett., 2006, 6, 562–567 CrossRef CAS PubMed.
- H. Haniu, N. Saito, Y. Matsuda, T. Tsukahara, K. Maruyama and Y. Usui, Toxicol. in Vitro, 2013, 27, 1679–1685 CrossRef CAS PubMed.
- K. L. Elias, R. L. Price and T. J. Webster, Biomaterials, 2002, 23, 3279–3287 CrossRef CAS PubMed.
- R. L. Price, M. C. Waid, K. M. Haberstroh and T. J. Webster, Biomaterials, 2003, 24, 1877–1887 CrossRef CAS PubMed.
- R. L. Price, K. M. Haberstroh and T. J. Webster, Nanotechnology, 2004, 15, 892 CrossRef CAS.
- P. Eklund, P. Ajayan, R. Blackmon, A. J. Hart, J. Kong, B. Pradham, A. Rao and A. Rinzler, International assessment of research and development of carbon nanotube manufacturing and applications, World Technology Evaluation Center, Baltimore, MD, USA, 2007, pp. 1–120 Search PubMed.
- International assessment of research and development of carbon nanotube manufacturing and applications, https://scienceus.org/wtec/docs/CNM_final_report.pdf, (accessed October 2021) Search PubMed.
- Global Carbon Nanotubes Market Report 2019: Production Capacities for MWCNTS and SWCNTs, Historical and Forecast to 2030, https://www.businesswire.com/news/home/20190611005526/en/Global-Carbon-Nanotubes-Market-Report-2019-Production-Capacities-for-MWCNTS-and-SWCNTs-Historical-and-Forecast-to-2030---ResearchAndMarkets.com, (accessed October 2021) Search PubMed.
- E. Bergamaschi, G. Garzaro, G. W. Jones, M. Buglisi, M. Caniglia, A. Godono, D. Bosio, I. Fenoglio and I. G. Canu, Nanomaterials, 2021, 11, 1–15 CrossRef PubMed.
- M. Bannunah, D. Vllasaliu, J. Lord and S. Stolnik, Mol. Pharm., 2014, 11, 4363–4373 CrossRef PubMed.
- M. Paradise and T. Goswami, Mater. Des., 2007, 28, 1477–1489 CrossRef CAS.
- V. N. Popov, Mater. Sci. Eng., R, 2004, 43, 61–102 CrossRef.
- ATCC, https://www.atcc.org/products/ccl-185 (accessed October 2021).
- H. Park, Y. Zhang, S. P. Georgescu, K. L. Johnson, D. Kong and J. B. Galper, Stem Cell Rev., 2006, 2, 93–102 CrossRef CAS PubMed.
- ATCC, https://www.atcc.org/products/crl-9609 (accessed October 2021).
- T. J. Webster, M. C. Waid, J. L. McKenzie, R. L. Price and J. U. Ejiofor, Nanotechnology, 2004, 15, 48–54 CrossRef CAS PubMed.
- S. Bellucci, M. Chiaretti, A. Cucina, G. A. Carru and A. Chiaretti, Nanomedicine, 2009, 4, 531–540 CrossRef CAS PubMed.
- T. Gabay, E. Jakobs, E. Ben-jacob and Y. Hanein, Physica A, 2005, 350, 611–621 CrossRef CAS.
- H. Hu, Y. Ni, V. Montana, R. C. Haddon and V. Parpura, Nano Lett., 2004, 4, 507–511 CrossRef CAS PubMed.
- X. Zhang, S. Prasad, S. Niyogi, A. Morgan, M. Ozkan and C. S. Ozkan, Sens. Actuators, B, 2005, 106, 843–850 CrossRef CAS.
- V. Lovat, D. Pantarotto, L. Lagostena, B. Cacciari, M. Grandolfo, M. Righi, G. Spalluto, M. Prato and L. Ballerini, Nano Lett., 2005, 5, 1107–1110 CrossRef CAS PubMed.
- H. Hu, Y. Ni, S. K. Mandal, V. Montana, B. Zhao, R. C. Haddon and V. Parpura, J. Phys. Chem. B, 2005, 109, 4285–4289 CrossRef CAS PubMed.
- A. Mazzatenta, M. Giugliano, S. Campidelli, L. Gambazzi, L. Businaro, H. Markram, M. Prato and L. Ballerini, J. Neurosci., 2007, 27, 6931–6936 CrossRef CAS PubMed.
- E. Jan and N. A. Kotov, Nano Lett., 2007, 7, 1123–1128 CrossRef CAS PubMed.
- E. B. Malarkey, K. A. Fisher, E. Bekyarova, W. Liu, R. C. Haddon and V. Parpura, Nano Lett., 2009, 9, 264–268 CrossRef CAS PubMed.
- R. A. Dubin, G. C. Callegari, J. Kohn and A. V. Neimark, IEEE Trans. Nanobiosci., 2008, 7, 11–14 CAS.
- P. Galvan-Garcia, E. W. Keefer, F. Yang, M. Zhang, S. Fang, A. A. Zakhidov, R. H. Baughman and M. I. Romero, J. Biomater. Sci., Polym. Ed., 2007, 18, 1245–1261 CrossRef CAS PubMed.
- M. K. Gheith, V. A. Sinani, J. P. Wicksted, R. L. Matts and N. A. Kotov, Adv. Mater., 2005, 17, 2663–2670 CrossRef CAS.
- M. K. Gheith, T. C. Pappas, A. V. Liopo, V. A. Sinani, B. S. Shim, M. Motamedi, J. P. Wicksted and N. A. Kotov, Adv. Mater., 2006, 18, 2975–2979 CrossRef CAS.
- I. Firkowska, E. Godehardt and M. Giersig, Adv. Funct. Mater., 2008, 18, 3765–3771 CrossRef CAS.
- J. Chłopek, B. Czajkowska, B. Szaraniec, E. Frackowiak, K. Szostak and F. Béguin, Carbon, 2006, 44, 1106–1111 CrossRef.
- S. Sirivisoot, C. Yao, X. Xiao, B. W. Sheldon and T. J. Webster, Nanotechnology, 2007, 18, 1–6 CrossRef.
- M. A. Correa-duarte, N. Wagner, C. Morsczeck, M. Thie and M. Giersig, Nano Lett., 2004, 4, 28–31 CrossRef.
- A. Benko, D. Medina-Cruz, J. Duch, T. Popiela, S. Wilk, M. Bińczak, M. Nocuń, E. Menaszek, L. D. Geoffrion, G. Guisbiers, A. Kotarba and T. J. Webster, Mater. Sci. Eng., C, 2021, 120, 111703 CrossRef CAS PubMed.
- S. R. Ryoo, Y. K. Kim, M. H. Kim and D. H. Min, ACS Nano, 2010, 4, 6587–6598 CrossRef CAS PubMed.
- A. O. Lobo, E. F. Antunes, M. B. S. Palma, C. Pacheco-Soares, V. J. Trava-Airoldi and E. J. Corat, Mater. Sci. Eng., C, 2008, 28, 532–538 CrossRef CAS.
- A. O. Lobo, M. A. F. Corat, E. F. Antunes, M. B. S. Palma, C. Pacheco-Soares, E. E. Garcia and E. J. Corat, Carbon, 2010, 48, 245–254 CrossRef CAS.
- A. O. Lobo, M. A. F. Corat, E. F. Antunes, S. C. Ramos, C. Pacheco-Soares and E. J. Corat, Mater. Sci. Eng., C, 2012, 32, 648–652 CrossRef CAS.
- Z. Tao, P. Wang, L. Wang, L. Xiao, F. Zhang and J. Na, J. Mater. Chem. B, 2014, 2, 471–476 RSC.
- T. Crouzier, A. Nimmagadda, M. U. Nollert and P. S. McFetridge, Langmuir, 2008, 24, 13173–13181 CrossRef CAS PubMed.
- T. I. Chao, S. Xiang, C. S. Chen, W. C. Chin, A. J. Nelson, C. Wang and J. Lu, Biochem. Biophys. Res. Commun., 2009, 384, 426–430 CrossRef CAS PubMed.
- C. Y. Tay, H. Gu, W. S. Leong, H. Yu, H. Q. Li, B. C. Heng, H. Tantang, S. C. J. Loo, L. J. Li and L. P. Tan, Carbon, 2010, 48, 1095–1104 CrossRef CAS.
- S. Dinicola, M. G. Masiello, S. Proietti, P. Coluccia, G. Fabrizi, A. Palombo, F. Micciulla, S. Bistarelli, G. Ricci, A. Catizone, G. De Toma, M. Bizzarri, S. Bellucci and A. Cucina, Toxicol. in Vitro, 2015, 29, 1298–1308 CrossRef CAS PubMed.
- T. I. Chao, S. Xiang, J. F. Lipstate, C. Wang and J. Lu, Adv. Mater., 2010, 22, 3542–3547 CrossRef CAS PubMed.
- G. Kucukayan-Dogu, D. Gozen, V. Bitirim, K. C. Akcali and E. Bengu, Appl. Surf. Sci., 2015, 351, 27–32 CrossRef CAS.
- B. Matta-Domjan, A. King, S. Totti, C. Matta, G. Dover, P. Martinez, A. Zakhidov, R. La Ragione, H. Macedo, I. Jurewicz, A. Dalton and E. G. Velliou, J. Biomed. Mater. Res., Part B, 2018, 106, 1637–1644 CrossRef CAS PubMed.
- J. S. Yan, M. Orecchioni, F. Vitale, J. A. Coco, G. Duret, S. Antonucci, S. S. Pamulapati, L. W. Taylor, O. S. Dewey, M. Di Sante, A. M. Segura, C. Gurcan, F. Di Lisa, A. Yilmazer, M. D. Mccauley, J. T. Robinson, M. Razavi, K. Ley, L. G. Delogu and M. Pasquali, Carbon, 2021, 173, 462–476 CrossRef CAS.
- X. Zhang, S. Prasad, S. Niyogi, A. Morgan, M. Ozkan and C. S. Ozkan, Sens. Actuators, B, 2005, 106, 843–850 CrossRef CAS.
- N. W. S. Kam, E. Jan and N. A. Kotov, Nano Lett., 2009, 9, 273–278 CrossRef CAS PubMed.
- L. H. S. Dalleau, M. Baradat, F. Gueraud and L. Huc, Cell Death Differ., 2013, 20, 1615–1630 CrossRef PubMed.
- C. L. Ursini, R. Maiello, A. Ciervo, A. M. Fresegna, G. Buresti, F. Superti, M. Marchetti, S. Iavicoli and D. Cavallo, J. Appl. Toxicol., 2016, 36, 394–403 CrossRef CAS PubMed.
- M. Davoren, E. Herzog, A. Casey, B. Cottineau, G. Chambers, H. J. Byrne and F. M. Lyng, Toxicol. in Vitro, 2007, 21, 438–448 CrossRef CAS PubMed.
- J. M. Wo, K. Pulskamp and H. F. Krug, Nano Lett., 2006, 6, 1261–1268 CrossRef PubMed.
- F. Tian, D. Cui, H. Schwarz, G. G. Estrada and H. Kobayashi, Toxicol. in Vitro, 2006, 20, 1202–1212 CrossRef CAS PubMed.
- E. Mooney, P. Dockery, U. Greiser, M. Murphy and V. Barron, Nano Lett., 2008, 8, 2137–2143 CrossRef CAS PubMed.
- A. K. Vidanapathirana, X. Lai, S. C. Hilderbrand, J. E. Pitzer, R. Podila, S. J. Sumner, T. R. Fennell, C. J. Wingard, F. A. Witzmann and J. M. Brown, Toxicology, 2012, 302, 114–122 CrossRef CAS PubMed.
- D. Liu, C. Yi, D. Zhang, J. Zhang and M. Yang, ACS Nano, 2010, 4, 2185–2195 CrossRef CAS PubMed.
- D. A. N. Elgrabli, S. Abella-gallart, O. Aguerre-chariol, F. Robidel, F. Rogerieux, J. Boczkowski and G. Lacroix, Nanotoxicology, 2007, 1, 266–278 CrossRef CAS.
- M. De Nicola, S. Bellucci, E. Traversa, G. De Bellis, F. Micciulla and L. Ghibelli, J. Phys.: Condens. Matter, 2008, 20, 474204 CrossRef.
- M. L. Di Giorgio, S. Di Bucchianico, A. M. Ragnelli, P. Aimola, S. Santucci and A. Poma, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2011, 722, 20–31 CrossRef CAS PubMed.
- P. Kumarathasan, D. Breznan, D. Das, M. A. Salam, Y. Siddiqui, C. MacKinnon-Roy, J. Guan, N. de Silva, B. Simard and R. Vincent, Nanotoxicology, 2014, 9, 148–161 CrossRef PubMed.
- G. Jia, H. Wang, L. Yan, X. Wang, R. Pei, T. Yan, Y. Zhao and X. Guo, Environ. Sci. Technol., 2005, 39, 1378–1383 CrossRef CAS PubMed.
- In vitro testing strategy for nanomaterials including database, https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2015)17/ann9%26doclanguage=en%<?pdb_no 0A32?>0A32<?pdb END?> (accessed October 2021) Search PubMed.
- J. Catalán, H. Järventaus, M. Vippola, K. Savolainen and H. Norppa, Nanotoxicology, 2012, 6, 825–836 CrossRef PubMed.
- X. Zhao, D. Lu, F. Hao and R. Liu, J. Hazard. Mater., 2015, 292, 98–107 CrossRef CAS PubMed.
- J. Lin, Y. Yu, S. Shigdar, D. Z. Fang, J. R. Du, M. Q. Wei, A. Danks, K. Liu and W. Duan, PLoS One, 2012, 7, 49277 CrossRef PubMed.
- B. Gajaraj and V. Nadumane, J. Pharm. Pharmacogn. Res., 2020, 8, 78–91 CAS.
- X. J. Fang, H. Jiang, Y. Q. Zhu, L. Y. Zhang, Q. H. Fan and Y. Tian, Oncol. Rep., 2014, 31, 2735–2742 CrossRef CAS PubMed.
- A. A. Shvedova, V. Castranova, E. R. Kisin, D. Schwegler-Berry, A. R. Murray, V. Z. Gandelsman, A. Maynard and P. Baron, J. Toxicol. Environ. Health, Part A, 2003, 66, 1909–1926 CrossRef CAS PubMed.
- A. Casey, E. Herzog, F. M. Lyng, H. J. Byrne, G. Chambers and M. Davoren, Toxicol. Lett., 2008, 179, 78–84 CrossRef CAS PubMed.
- H. Haniu, N. Saito, Y. Matsuda, Y. A. Kim, K. C. Park, T. Tsukahara, Y. Usui, K. Aoki, M. Shimizu, N. Ogihara, K. Hara, S. Takanashi, M. Okamoto, N. Ishigaki, K. Nakamura and H. Kato, Int. J. Nanomed., 2011, 6, 3487–3497 CrossRef CAS PubMed.
- H. Haniu, N. Saito, Y. Matsuda, T. Tsukahara, Y. Usui, K. Maruyama, S. Takanashi, K. Aoki, S. Kobayashi, H. Nomura, M. Tanaka, M. Okamoto and H. Kato, Int. J. Nanomed., 2014, 9, 1979–1990 CrossRef PubMed.
- K. Bhattacharya, G. Kiliç, P. M. Costa and B. Fadeel, Nanotoxicology, 2017, 11, 809–826 CAS.
- K. J. Siegrist, S. H. Reynolds, D. W. Porter, R. R. Mercer, A. K. Bauer, D. Lowry, L. Cena, T. A. Stueckle, M. L. Kashon, J. Wiley, J. L. Salisbury, J. Mastovich, K. Bunker, M. Sparrow, J. S. Lupoi, A. B. Stefaniak, M. J. Keane, S. Tsuruoka, M. Terrones, M. McCawley and L. M. Sargent, Part. Fibre Toxicol., 2019, 16, 36 CrossRef PubMed.
- M. Sano, M. Izumiya, H. Haniu and K. Ueda, Nanomaterials, 2020, 10, 1374 CrossRef CAS PubMed.
- H. Haniu, N. Saito, Y. Matsuda, Y. A. Kim, K. C. Park, T. Tsukahara, Y. Usui, K. Aoki, M. Shimizu, N. Ogihara, K. Hara, S. Takanashi, M. Okamoto, N. Ishigaki, K. Nakamura and H. Kato, Int. J. Nanomed., 2011, 6, 3487–3497 CrossRef CAS PubMed.
- M. Davoren, E. Herzog, A. Casey, B. Cottineau, G. Chambers, H. J. Byrne and F. M. Lyng, Toxicol. in Vitro, 2007, 21, 438–448 CrossRef CAS PubMed.
- H. Dumortier, S. Lacotte, G. Pastorin, R. Marega, W. Wu, D. Bonifazi, J. P. Briand, M. Prato, S. Muller and A. Bianco, Nano Lett., 2006, 6, 1522–1528 CrossRef CAS PubMed.
- K. Pulskamp, J. M. Wörle-Knirsch, F. Hennrich, K. Kern and H. F. Krug, Carbon, 2007, 45, 2241–2249 CrossRef CAS.
- X. He, S. Young, D. Schwegler-berry, W. P. Chisholm and J. E. Fernback, Chem. Res. Toxicol., 2011, 24, 2237–2248 Search PubMed.
- L. Q. Chen, P. P. Hu, L. Zhang, S. Z. Huang, L. F. Luo and C. Z. Huang, Sci. China: Chem., 2012, 55, 2209–2216 Search PubMed.
- J. Yu, S. Liu, B. Wu, Z. Shen, G. N. Cherr, X. X. Zhang and M. Li, Environ. Sci. Technol., 2016, 50, 3985–3994 CrossRef CAS PubMed.
- P. Zhao, L. Chen, H. Shao, Y. Zhang, Y. Sun, Y. Ke, S. Ramakrishna, L. He and W. Xue, Biomed. Mater., 2016, 11, 015021 CrossRef PubMed.
- S. Phuyal, M. Kasem, L. Rubio, H. L. Karlsson, R. Marcos, V. Skaug and S. Zienolddiny, Toxicol. in Vitro, 2017, 44, 230–240 CrossRef CAS PubMed.
- J. Long, X. Li, Y. Kang, Y. Ding, Z. Gu and Y. Caob, RSC Adv., 2018, 8, 9253–9260 RSC.
- N. Lu, Y. Sui, R. Tian and Y. Peng, Chem. Res. Toxicol., 2018, 31, 1061–1068 Search PubMed.
- N. Lu, Y. Sui, Y. Ding, R. Tian, L. Li and F. Liu, Chem.–Biol. Interact., 2018, 295, 64–72 CrossRef CAS PubMed.
- T. Wen, A. Yang, L. Piao, S. Hao, L. Du, J. Meng, J. Liu and H. Xu, Int. J. Nanomed., 2019, 14, 4475–4489 CrossRef CAS PubMed.
- J. Long, W. Ma, Z. Yu, H. Liu and Y. Cao, Nanotoxicology, 2019, 13, 938–951 CrossRef CAS PubMed.
- X. Zhao, S. Chang, J. Long, J. Li, X. Li and Y. Cao, Food Chem. Toxicol., 2019, 126, 169–177 CrossRef CAS PubMed.
- H. Yang, J. Li, C. Yang, H. Liu and Y. Cao, Toxicol. Appl. Pharmacol., 2019, 374, 11–19 CrossRef CAS PubMed.
- Y. Sun, J. Gong and Y. Cao, Int. J. Nanomed., 2019, 14, 9285–9294 CrossRef CAS PubMed.
- E. Herzog, H. J. Byrne, A. Casey, M. Davoren, A. G. Lenz, K. L. Maier, A. Duschl and G. J. Oostingh, Toxicol. Appl. Pharmacol., 2009, 234, 378–390 CrossRef CAS PubMed.
- D. Liu, L. Wang, Z. Wang and A. Cuschieri, Nanoscale Res. Lett., 2012, 7, 361 CrossRef PubMed.
- B. N. Eldridge, F. Xing, C. D. Fahrenholtz and R. N. Singh, Toxicol. In Vitro, 2017, 41, 223–231 CrossRef CAS PubMed.
- L. C. Ong, Y. F. Tan, B. S. Tan, F. F. L. Chung, S. K. Cheong and C. O. Leong, Toxicol. Appl. Pharmacol., 2017, 329, 347–357 CrossRef CAS PubMed.
- N. Azad, A. K. V. Iyer, L. Wang, Y. Liu, Y. Lu and Y. Rojanasakul, Nanotoxicology, 2013, 7, 157–168 CrossRef CAS PubMed.
- C. Gaiiiard, G. Celiot, S. Li, F. M. Toma, H. Dumortier, G. Spaliuto, B. Cacciari, M. Prato, L. Ballerini and A. Bianco, Adv. Mater., 2009, 21, 2903–2908 CrossRef.
- G. Visalli, M. P. Bertuccio, D. Iannazzo, A. Piperno, A. Pistone and A. di Pietro, Toxicol. in Vitro, 2015, 29, 352–362 CrossRef CAS PubMed.
- Z. Liu, Y. Liu and D. Peng, J. Biomed. Mater. Res., Part A, 2015, 103, 2770–2777 CrossRef CAS PubMed.
- O. Sabido, A. Figarol and J. Klein, Nanomaterials, 2020, 10, 319 CrossRef CAS PubMed.
- L. Ding, J. Stilwell, T. Zhang, O. Elboudwarej, H. Jiang, J. P. Selegue, P. A. Cooke, J. W. Gray and F. F. Chen, Nano Lett., 2005, 5, 2448–2464 CrossRef CAS PubMed.
- F. A. Witzmann and N. A. Monteiro-Riviere, Nanomed. Nanotechnol. Biol. Med., 2006, 2, 158–168 CrossRef CAS PubMed.
- L. Zhu, D. W. Chang, L. Dai and Y. Hong, Nano Lett., 2007, 7, 3592–3597 CrossRef CAS PubMed.
- V. E. Kagan, Y. Y. Tyurina, V. A. Tyurin, N. V. Konduru, A. I. Potapovich, A. N. Osipov, E. R. Kisin, D. Schwegler-Berry, R. Mercer, V. Castranova and A. A. Shvedova, Toxicol. Lett., 2006, 165, 88–100 CrossRef CAS PubMed.
- S. Fiorito, A. Serafino, F. Andreola and P. Bernier, Carbon, 2006, 44, 1100–1105 CrossRef CAS.
- S. Sarkar, C. Sharma, R. Yog, A. Periakaruppan, O. Jejelowo, R. Thomas, E. V. Barrera, A. C. Rice-Ficht, B. L. Wilson and G. T. Ramesh, J. Nanosci. Nanotechnol., 2007, 7, 584–592 CrossRef CAS PubMed.
- D. M. Brown, I. A. Kinloch, U. Bangert, A. H. Windle, D. M. Walter, G. S. Walker, C. A. Scotchford and K. Donaldson, Carbon, 2007, 45, 1743–1756 CrossRef CAS.
- A. A. Kroustalli, S. N. Kourkouli and D. D. Deligianni, Ann. Biomed. Eng., 2013, 41, 2655–2665 CrossRef PubMed.
- M. J. D. Clift, C. Endes, D. Vanhecke, P. Wick, P. Gehr, R. P. F. Schins, A. Petri-Fink and B. Rothen-Rutishauser, Toxicol. Sci., 2014, 137, 55–64 CrossRef CAS PubMed.
- M. Yu, R. Chen, Z. Jia, J. Chen, J. Lou, S. Tang and X. Zhang, Int. J. Toxicol., 2016, 35, 17–26 CrossRef CAS PubMed.
- F. Valentini, E. Mari, A. Zicari, A. Calcaterra, M. Talamo, M. G. Scioli, A. Orlandi and S. Mardente, Int. J. Mol. Sci., 2018, 19, 1316 CrossRef PubMed.
- S. M. Reamon-buettner, A. Hackbarth, A. Leonhardt, A. Braun and C. Ziemann, Mech. Ageing Dev., 2021, 193, 111412 CrossRef CAS PubMed.
- R. J. Snyder, K. C. Verhein, H. L. Vellers, A. B. Burkholder, S. Garantziotis and S. Management, Nanotoxicology, 2019, 13, 1344–1361 CrossRef CAS PubMed.
- M. Orecna, S. H. De Paoli, O. Janouskova, T. Z. Tegegn, M. Filipova, J. E. Bonevich, K. Holada and J. Simak, Nanomed. Nanotechnol. Biol. Med., 2014, 10, e939–e948 CrossRef PubMed.
- A. Rama, N. Reddy, Y. Narsimha, D. Rama and V. Himabindu, Toxicology, 2010, 272, 11–16 CrossRef PubMed.
- A. Simon-Deckers, B. Gouget, M. Mayne-L’Hermite, N. Herlin-Boime, C. Reynaud and M. Carrière, Toxicology, 2008, 253, 137–146 CrossRef CAS PubMed.
- A. R. N. Reddy, Y. N. Reddy, D. R. Krishna and V. Himabindu, Toxicol. Environ. Chem., 2010, 92, 1697–1703 CrossRef CAS.
- L. Zhou, H. J. Forman, Y. Ge, J. Lunec and L. Angeles, Toxicol. In Vitro, 2017, 42, 292–298 CrossRef CAS PubMed.
- J. Long, Y. Xiao, L. Liu and Y. Cao, J. Nanobiotechnol., 2017, 15, 80 CrossRef PubMed.
- P. Ravichandran, S. Baluchamy, B. Sadanandan, R. Gopikrishnan, S. Biradar, V. Ramesh, J. C. Hall and G. T. Ramesh, Apoptosis, 2010, 15, 1507–1516 CrossRef CAS PubMed.
- L. Belyanskaya, S. Weigel, C. Hirsch, U. Tobler, H. F. Krug and P. Wick, NeuroToxicology, 2009, 30, 702–711 CrossRef CAS PubMed.
- K. Matsumoto, C. Sato, Y. Naka, R. Whitby and N. Shimizu, Nanotechnology, 2010, 21, 115101 CrossRef CAS PubMed.
- Q. Lu, J. M. Moore, G. Huang, A. S. Mount, A. M. Rao, L. L. Larcom and P. C. Ke, Nano Lett., 2004, 4, 2473–2477 CrossRef CAS.
- M. Song, F. Wang, L. Zeng, J. Yin, H. Wang and G. Jiang, Environ. Sci. Technol., 2014, 48, 13978–13984 CrossRef CAS PubMed.
- M. Mrakovcic, C. Meindl, G. Leitinger and E. Roblegg, Toxicol. Sci., 2015, 144, 114–127 CrossRef CAS PubMed.
- M. Luo, P. Chen, J. Wang, X. Deng, L. Dong, M. Wu and X. Shen, Sci. China: Chem., 2016, 59, 918–926 CrossRef CAS.
- E. Rozhina, S. Batasheva, R. Miftakhova, X. Yan, A. Vikulina, D. Volodkin and R. Fakhrullin, Appl. Clay Sci., 2021, 205, 106041 CrossRef CAS.
- M. Song, L. Zeng, S. Yuan, J. Yin, H. Wang and G. Jiang, Chemosphere, 2013, 92, 576–582 CrossRef CAS PubMed.
- T. Xia, R. F. Hamilton Jr, J. C. Bonner, E. D. Crandall, A. Elder, F. Fazlollahi, T. A. Girtsman, K. Kim, S. Mitra, S. A. Ntim, G. Orr, M. Tagmount, A. J. Taylor, D. Telesca, A. Tolic, C. D. Vulpe, A. J. Walker, X. Wang, F. A. Witzmann, N. Wu, Y. Xie, J. I. Zink, A. Nel and A. Holian, Environ. Health Perspect., 2013, 121, 683–691 CrossRef PubMed.
- A. Jos, S. Pichardo, M. Puerto, E. Sánchez, A. Grilo and A. M. Cameán, Toxicol. in Vitro, 2009, 23, 1491–1496 CrossRef CAS PubMed.
- O. Jejelowo, A. C. Rice-ficht and G. T. Ramesh, 2005, 5, 1676–1684.
- D. Cui, F. Tian, C. S. Ozkan, M. Wang and H. Gao, Toxicol. Lett., 2005, 155, 73–85 CrossRef CAS PubMed.
- A. Magrez, S. Kasas, V. Salicio, N. Pasquier, J. W. Seo and M. Celio, Am. Chem. Soc., 2006, 6, 1121–1125 CAS.
- P. Wick, P. Manser, L. K. Limbach, U. Dettlaff-weglikowska, F. Krumeich, S. Roth, W. J. Stark and A. Bruinink, Toxicol. Lett., 2007, 168, 121–131 CrossRef CAS PubMed.
- A. M. Schrand, L. Dai, J. J. Schlager, S. M. Hussain and E. Osawa, Diamond Relat. Mater., 2007, 16, 2118–2123 CrossRef CAS.
- C. J. Gannon, P. Cherukuri, B. I. Yakobson, L. Cognet, J. S. Kanzius, C. Kittrell, R. B. Weisman, M. Pasquali, H. K. Schmidt, R. E. Smalley and S. A. Curley, Cancer, 2007, 110, 2654–2665 CrossRef CAS PubMed.
- L. Dong, K. L. Joseph, C. M. Witkowski and M. M. Craig, Nanotechnology, 2008, 19, 255702 CrossRef PubMed.
- Y. Zhu, W. Li, Q. Li, Y. Li and Y. Li, Carbon, 2009, 47, 1351–1358 CrossRef CAS.
- G. Bardi, P. Tognini, G. Ciofani, V. Raffa, M. Costa and T. Pizzorusso, Nanomed. Nanotechnol. Biol. Med., 2009, 5, 96–104 CrossRef CAS PubMed.
- T. Coccini, E. Roda, D. A. Sarigiannis, P. Mustarelli, E. Quartarone, A. Profumo and L. Manzo, Toxicology, 2010, 269, 41–53 CrossRef CAS PubMed.
- T. Thurnherr, C. Brandenberger, K. Fischer, L. Diener, P. Manser, X. Maeder-Althaus, J. P. Kaiser, H. F. Krug, B. Rothen-Rutishauser and P. Wick, Toxicol. Lett., 2011, 200, 176–186 CrossRef CAS PubMed.
- S. Wadhwa, C. Rea, P. O'Hare, A. Mathur, S. S. Roy, P. S. M. Dunlop, J. A. Byrne, G. Burke, B. Meenan and J. A. McLaughlin, J. Hazard. Mater., 2011, 191, 56–61 CrossRef CAS PubMed.
- B. Chen, Y. Liu, W. M. Song, Y. Hayashi, X. C. Ding and W. H. Li, Biomed. Environ. Sci., 2011, 24, 593–601 CAS.
- A. Patlolla, B. Knighten and P. Tchounwou, Ethn. Dis., 2010, 20, 1–17 Search PubMed.
- C. L. Ursini, D. Cavallo, A. M. Fresegna, A. Ciervo, R. Maiello, S. Casciardi, F. Tombolini, G. Buresti and S. Iavicoli, J. Nanomater., 2012, 12, 1–9 Search PubMed.
- S. L. Montes-Fonseca, E. Orrantia-Borunda, A. Duarte-Möller, A. Luna-Velasco, M. Román-Aguirre, C. González Horta and B. Sánchez-Ramírez, J. Nanomater., 2012, 12, 1–7 Search PubMed.
- Y. guang Han, J. Xu, Z. gui Li, G. gang Ren and Z. Yang, NeuroToxicology, 2012, 33, 1128–1134 CrossRef PubMed.
- B. Angoth, H. Lingabathula, D. Gandamalla and N. R. Yellu, Int. J. Pharm. Pharm. Sci., 2014, 6, 379–382 CAS.
- F. Charbgoo, M. Behmanesh and M. Nikkhah, Biotechnol. Appl. Biochem., 2015, 62, 598–605 CrossRef CAS PubMed.
- Y. Hiraku, F. Guo, N. Ma, T. Yamada, S. Wang and S. Kawanishi, Part. Fibre Toxicol., 2016, 13, 1–21 Search PubMed.
- O. M. Perepelytsina, A. P. Ugnivenko, A. V. Dobrydnev, O. N. Bakalinska, A. I. Marynin and M. V. Sydorenko, Nanoscale Res. Lett., 2018, 13, 286 CrossRef PubMed.
- M. R. Azari, Y. Mohammadian, J. Pourahmad, F. Khodagholi, H. Peirovi, Y. Mehrabi, M. Omidi and A. Rafieepour, Environ. Sci. Pollut. Res. Int., 2019, 26, 12709–12719 CrossRef PubMed.
- M. R. Azari and Y. Mohammadian, Environ. Sci. Pollut. Res. Int., 2020, 27, 15401–15406 CrossRef PubMed.
- M. Ahamed, M. J. Akhtar and M. A. M. Khan, Int. J. Environ. Res. Public Health, 2020, 17, 8221 CrossRef CAS PubMed.
- C. L. Ursini, D. Cavallo, A. M. Fresegna, A. Ciervo, R. Maiello, G. Buresti, S. Casciardi, F. Tombolini, S. Bellucci and S. Iavicoli, Toxicol. in Vitro, 2012, 26, 831–840 CrossRef CAS PubMed.
- A. R. N. Reddy, D. R. Krishna and V. Himabindu, Toxicol. Environ. Chem., 2015, 96, 931–940 CrossRef.
- S. Sweeney, D. Berhanu, S. K. Misra, A. J. Thorley, E. Valsami-Jones and T. D. Tetley, Carbon, 2014, 78, 26–37 CrossRef CAS PubMed.
- K. Kyriakidou, D. Brasinika, A. F. A. Trompeta, E. Bergamaschi and I. K. Karoussis, Food Chem. Toxicol., 2020, 141, 111374 CrossRef CAS PubMed.
- A. Simon, S. X. Maletz, H. Hollert, A. Schäffer and H. M. Maes, Nanoscale Res. Lett., 2014, 9, 396 CrossRef PubMed.
- O. Vittorio, V. Raffa and A. Cuschieri, Nanomed. Nanotechnol. Biol. Med., 2009, 5, 424–431 CrossRef CAS PubMed.
- P. Taylor, D. Ren, M. Li, Y. Dong, Y. Cheng, X. Yang, H. Zheng, H. Bai, X. Chu and J. Wang, 37–41.
- A. Albini, V. Mussi, A. Parodi, A. Ventura, E. Principi, S. Tegami, M. Rocchia, E. Francheschi, I. Sogno, R. Cammarota, G. Finzi, F. Sessa, D. M. C. Noonan and U. Valbusa, Nanomed. Nanotechnol. Biol. Med., 2010, 6, 277–288 CrossRef CAS PubMed.
- Y. Zhang, S. F. Ali, E. Dervishi, Y. Xu, Z. Li, D. Casciano and A. S. Biris, ACS Nano, 2010, 4, 3181–3186 CrossRef CAS PubMed.
- J. Czarnecka, M. Wiśniewski, N. Forbot, P. Bolibok, A. P. Terzyk and K. Roszek, Materials, 2010, 13, 2060 CrossRef PubMed.
- K. Pulskamp, S. Diabaté and H. F. Krug, Toxicol. Lett., 2007, 168, 58–74 CrossRef CAS PubMed.
- E. Flahaut, M. C. Durrieu, M. Remy-Zolghadri, R. Bareille and C. Baquey, J. Mater. Sci., 2006, 41, 2411–2416 CrossRef CAS.
- E. Flahaut, M. C. Durrieu, M. Remy-Zolghadri, R. Bareille and C. Baquey, Carbon, 2006, 44, 1093–1099 CrossRef CAS.
- A. E. Porter, M. Gass, J. S. Bendall, K. Muller, A. Goode, J. N. Skepper, P. A. Midgley and M. Welland, ACS Nano, 2009, 3, 1485–1492 CrossRef CAS PubMed.
- S. Boncel, K. H. Müller, J. N. Skepper, K. Z. Walczak and K. K. K. Koziol, Biomaterials, 2011, 32, 7677–7686 CrossRef CAS PubMed.
- D. Gutiérrez-Praena, S. Pichardo, E. Sánchez, A. Grilo, A. M. Cameán and A. Jos, Toxicol. in Vitro, 2011, 25, 1883–1888 CrossRef PubMed.
- K. K. K. Koziol, J. N. Skepper, P. A. Midgley, C. Cheng, K. H. Mu, M. E. Welland and A. E. Porter, 2009, 30, 4152–4160.
- A. O. Inman and L. W. Zhang, Toxicol. Appl. Pharmacol., 2009, 234, 222–235 CrossRef PubMed.
- H. N. Yehia, R. K. Draper, C. Mikoryak, E. K. Walker, P. Bajaj, I. H. Musselman, M. C. Daigrepont, G. R. Dieckmann and P. Pantano, J. Nanobiotechnol., 2007, 5, 1–17 CrossRef PubMed.
- G. Visalli, A. Facciolà, D. Iannazzo, A. Piperno, A. Pistone and A. Di Pietro, J. Trace Elem. Med. Biol., 2017, 43, 153–160 CrossRef CAS PubMed.
- K. Lee, P. Lo, G. Lee, J. Zheng and E. Cho, J. Biotechnol., 2019, 296, 14–21 CrossRef CAS PubMed.
- M. G. Bianchi, M. Chiu, A. Pagliaro, M. A. Koklioti, A. A. T. Enrico, B. Ovidio and B. Constantinos, Toxicol. Rep., 2016, 3, 230–243 CrossRef PubMed.
- S. Garibaldi, C. Brunelli, V. Bavastrello, G. Ghigliotti and C. Nicolini, Nanotechnology, 2006, 17, 391–397 CrossRef CAS.
- M. Bottini, S. Bruckner, K. Nika, N. Bottini, S. Bellucci, A. Magrini, A. Bergamaschi and T. Mustelin, Toxicol. Lett., 2006, 160, 121–126 CrossRef CAS PubMed.
- H. L. Karlsson, P. Cronholm, J. Gustafsson, L. Möller and L. Mo, Chem. Res. Toxicol., 2008, 21, 1726–1732 Search PubMed.
- H. K. Lindberg, G. C. M. Falck, S. Suhonen, M. Vippola, E. Vanhala, J. Catalán, K. Savolainen and H. Norppa, Toxicol. Lett., 2009, 186, 166–173 CrossRef CAS PubMed.
- Y. Y. Guo, J. Zhang, Y. F. Zheng, J. Yang and X. Q. Zhu, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2011, 721, 184–191 CrossRef CAS PubMed.
- H. K. Lindberg, G. C. M. Falck, R. Singh, S. Suhonen, H. Järventaus, E. Vanhala, J. Catalán, P. B. Farmer, K. M. Savolainen and H. Norppa, Toxicology, 2013, 313, 24–37 CrossRef CAS PubMed.
- J. P. Kaiser, T. Buerki-Thurnherr and P. Wick, J. King Saud Univ., Sci., 2013, 25, 15–27 CrossRef.
- J. G. Munguía-Lopez, E. Muñoz-Sandoval, J. Ortiz-Medina, F. J. Rodriguez-Macias and A. De Leon-Rodriguez, J. Nanomater., 2015, 2015, 1–7 CrossRef.
- S. Phuyal, M. Kasem, O. Knittelfelder, A. Sharma, D. de M. Fonseca, V. Vebraite, S. Shaposhnikov, G. Slupphaug, V. Skaug and S. Zienolddiny, Nanotoxicology, 2018, 12, 138–152 CrossRef CAS PubMed.
- S. Fiorito, J. Russier, A. Salemme, M. Soligo, L. Manni, E. Krasnowska, S. Bonnamy, E. Flahaut, A. Serafino, G. I. Togna, L. N. J. L. Marlier and A. R. Togna, Carbon, 2018, 129, 572–584 CrossRef CAS.
- C. Espinosa, L. M. Hoyos-palacio, L. López, J. A. Carlos-cornelio and I. C. Ortiz-trujillo, Integr. Med. Res., 2020, 9, 6059–6072 CAS.
- N. Chatterjee, J. Yang, D. Yoon, S. Kim, S. W. Joo and J. Choi, Biomaterials, 2017, 115, 167–180 CrossRef CAS PubMed.
- O. Zeni, A. Sannino, S. Romeo, F. Micciulla, S. Bellucci and M. R. Scarfi, Nanomedicine, 2015, 10, 351–360 CrossRef CAS PubMed.
- Y. Yun, Z. Dong, Z. Tan, M. J. Schulz and V. Shanov, Mater. Sci. Eng., C, 2009, 29, 719–725 CrossRef CAS.
- M. Pacurari, X. J. Yin, M. Ding, S. S. Leonard, D. Schwegler-berry, B. S. Ducatman, M. Chirila, M. Endo, V. Castranova and V. Vallyathan, Nanotoxicology, 2008, 2, 155–170 CrossRef CAS.
- O. Zeni, R. Palumbo, R. Bernini, L. Zeni, M. Sarti and M. R. Scarfì, Sensors, 2008, 8, 488–499 CrossRef CAS PubMed.
- S. Dekali, C. Bachelet, S. Maunoir-Regimbal, E. Flahaut, J. C. Debouzy and D. Crouzier, Toxicology, 2016, 365, 1–8 CrossRef CAS PubMed.
- J. S. Kim, K. S. Song and I. J. Yu, Int. J. Toxicol., 2016, 35, 27–37 CrossRef CAS PubMed.
- J. Wang, R. H. Sun, N. Zhang, H. Nie, J. Liu, J. N. Wang, H. Wang and Y. Liu, Carbon, 2009, 47, 1752–1760 CrossRef CAS.
- J. Sik, K. Kyung, S. Song and J. Kyu, 2012, 553–562.
- H. Kim, B. Jin, D. K. Patel, J. Kim, J. Kim, H. Seonwoo and K. Lim, IEEE Trans. NanoBioscience, 2019, 18, 463–468 Search PubMed.
- K. Fraser, V. Kodali, N. Yanamala, M. E. Birch, L. Cena, G. Casuccio, K. Bunker, T. L. Lersch, D. E. Evans, A. Stefaniak, M. A. Hammer, M. L. Kashon, T. Boots, T. Eye, J. Hubczak, S. A. Friend, M. Dahm, M. K. Schubauer-Berigan, K. Siegrist, D. Lowry, A. K. Bauer, L. M. Sargent and A. Erdely, Part. Fibre Toxicol., 2020, 17, 1–26 CrossRef PubMed.
- A. Warowicka, B. M. Maciejewska, J. Litowczenko, M. Kościński, A. Baranowka-Korczyc, M. Jasiurkowska-Delaporte, K. K. Koziol and S. Jurga, Compos. Sci. Technol., 2016, 136, 29–38 CrossRef CAS.
- V. G. Walker, Z. Li, T. Hulderman, D. Schwegler-berry, M. L. Kashon and P. P. Simeonova, Toxicol. Appl. Pharmacol., 2009, 236, 319–328 CrossRef CAS PubMed.
- S. Hirano, Y. Fujitani, A. Furuyama and S. Kanno, Toxicol. Appl. Pharmacol., 2010, 249, 8–15 CrossRef CAS PubMed.
- H. Requardt, A. Braun, P. Steinberg, S. Hampel and T. Hansen, Toxicol. in Vitro, 2019, 60, 12–18 CrossRef CAS PubMed.
- S. Hirano, S. Kanno and A. Furuyama, Toxicol. Appl. Pharmacol., 2008, 232, 244–251 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02519a |
| This journal is © The Royal Society of Chemistry 2022 |