Open Access Article
Open Access ArticleSodium alkoxide-mediated g-C3N4 immobilized on a composite nanofibrous membrane for preferable photocatalytic activity†
Xue Liab,
Qin Wuab,
Mushraf Hussain ac,
Liang Chenb,
Qiong Huangb,
Wei Huang
ac,
Liang Chenb,
Qiong Huangb,
Wei Huang d and
Tao Tao
d and
Tao Tao *ac
*ac
aSchool of Chemistry and Materials Science, Institute of Advanced Materials and Flexible Electronics, Nanjing University of Information Science and Technology (NUIST), Nanjing 210044, P. R. China. E-mail: taotao@nuist.edu.cn
bJiangsu Collaborative Innovation Centre of Atmospheric Environment and Equipment Technologies, Jiangsu Key Laboratory of Atmospheric Environmental Monitoring and Pollution Control, School of Environmental Science and Engineering, Nanjing University of Information Science and Technology, Nanjing 210044, P. R. China
cReading Academy, NUIST-UoR International Research Institute, Nanjing 210044, P. R. China
dState Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, P. R. China
First published on 20th May 2022
Abstract
g-C3N4 is a classic photocatalyst not only owing to the metal-free semiconducting electronic structure but also tunable multifunctional properties. However, strategies for chemical exfoliation of g-C3N4 based on organic bases have been rarely reported. A family of sodium alkoxide-mediated g-C3N4 has been prepared via a simple synthesis. The degradation rate of bulk g-C3N4 is 39.8% when irradiation lasts 140 minutes. However, the degradation rate of g-C3N4–MeONa, g-C3N4–EtONa, and g-C3N4–tBuONa is 55.1%, 68.6%, and 79.1%, respectively, under the same conditions. Furthermore, g-C3N4–tBuONa has been immobilized on flexible electrospun PAN nanofibers to prepare floating photocatalysts. SEM analysis shows that the paper-based photocatalyst PAN/g-C3N4–tBuONa becomes a nanofiber membrane (A4 size, 210 mm × 297 mm). The nanofiber is approximately 350 nm in diameter. Interestingly, once synthesized, the g-C3N4–tBuONa particles move into the spinning solution, where the nanofiber wraps around them to form a monodisperse structure that resembles beads, or knots of 1–2 μm, on a string. The degradation efficiency of 10 mg L−1 MB solution can reach 100% for 2 hours until the solution becomes colorless. In addition, the photocatalytic mechanism studies have been validated. Different from H2SO4 or HNO3, this work has proposed a facile strategy for designing preferable floating photocatalysts using sodium alkoxide.
Introduction
Semiconducting photocatalysis has been widely investigated in the field of multifunctional materials and environmental remediation in recent years.1–8 Particularly, pure organic photocatalysts have been extensively developed not only in dye degradation but also in hydrogen evolution.9–11 For example, graphitic carbon nitride (g-C3N4) is a classic material not only owing to the metal-free semiconducting electronic structure, and tuneable optical and photoelectrochemical properties but also because of the low cost, and high thermal and chemical stability.2,12–20 However, bulk g-C3N4 possesses the intrinsic disadvantages of low charge mobility rate and high recombination rate, which limit the photocatalytic potential.21 Therefore, exploring new methods and strategies is a key point for highly efficient photocatalysis in this case.On the one hand, the strategy for constructing heterojunction has been provided to decrease the recombination between photogenerated electron and hole, such as metallic oxide, metal sulfide or oxyhalide, and so on.22–30 For example, Shao and Li reported g-C3N4/BiOI heterojunction on polyacrylonitrile (PAN) nanofibers via the electrospun method, showing a better photocatalytic property for degradation of dyes.21 On the other hand, materials scientists are inspired while dealing with bulk g-C3N4 using the similar exfoliation of graphite into one and/or a few layers of graphene according to the Hummers' method (KMnO4). Considering the weak layer-by-layer interactions, it is a widely used method to prepare g-C3N4 nanosheets on a large scale.31 Liu's group reported that g-C3N4 nanosheets can be easily prepared with a thickness of approximately 2 nm by a thermal oxidation etching in the air.32 Zhu and co-workers synthesized g-C3N4 nanosheets by the sulfuric acid chemical stripping method. With the help of ultrasonic processing, concentrated sulfuric acid was inserted between massive g-C3N4 layers, and the photocatalytic experiment showed that the photocatalytic H2 production capacity of single-layer nanosheets increased by three times.33
Bulk g-C3N4 has improved the photocatalytic performance by the easy exfoliation using oxidant and/or concentrated acid, namely, concentrated H2SO4, HNO3, and HCl.31,34–38 Given the principle of acid-base catalysis, it makes sense for g-C3N4 to deal with a strong base rather than to posit themselves in opposition to the media. The question is whether the carbon nitride by soaking can be exfoliated in alkaline environments for photocatalysis.39–42 Interestingly, Sano and co-workers have treated g-C3N4 by hydrothermal method with sodium hydroxide, resulting in a porous structure with eight times surface area bigger than that of bulk g-C3N4.43 Herein, we present a series of sodium alkoxide-mediated (MeONa, EtONa, and tBuONa) g-C3N4 photocatalysts for fabricating flexible electrospun polyacrylonitrile nanofibers (Scheme 1). The motivation is not only to explore the structure–property study of functionalized g-C3N4 but also to adjust the photogenerated hole and electron carriers44 by the alternation of recombination rate and charge mobility rate. It is for the first time that sodium alkoxide-mediated g-C3N4 photocatalysts are synthesized.
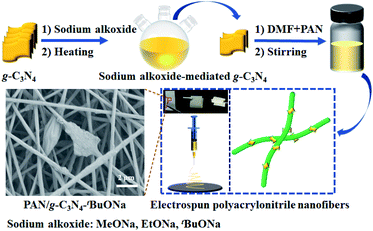 | ||
| Scheme 1 Schematic illustration for the synthesis of sodium alkoxide-mediated g-C3N4 immobilized on a composite nanofibrous membrane. | ||
Experimental
Syntheses of sodium alkoxide-mediated g-C3N4 photocatalysts
The bulk g-C3N4 powder was condensed from melamine.45–47 Sodium (1.00 g) was slowly added to MeOH, EtOH, and tBuOH solution (100 mL), respectively. The mixture was continuously stirred for 2 h until the sodium reacts to completed. Then, g-C3N4 powder (1.00 g) was added to the solution and heated to reflux overnight. After this reaction, the mixture was filtered to obtain the yellow precipitate, which was washed to neutral and dried at 105 °C for 24 h. The obtained samples were labelled g-C3N4–MeONa, g-C3N4–EtONa, and g-C3N4–tBuONa by the yield of 92–97%, respectively, according to different sodium alkoxide.Syntheses of PAN/g-C3N4–tBuONa floating photocatalysts
First of all, powder g-C3N4–tBuONa (0.80 g) was dissolved in N,N-dimethylformamide (DMF, 10 mL) and was exfoliated by ultrasonic treatment for 30 minutes followed by polyacrylonitrile (PAN, 1.20 g) powders. Secondly, the precursor PAN/g-C3N4–tBuONa solution was obtained for electrospun. PAN solution was also prepared by the same procedure without g-C3N4–tBuONa for comparison. Thirdly, a voltage of ∼10 kV and a distance of 6–8 cm were used between the syringe tip (10 mL). The counter electrode was covered by aluminium foil (supply speed: ∼0.6 mL h−1; roller speed: ∼300 rpm). After electrospun, finally, the products on the collector were placed in the oven and dried at 80 °C to prepare the g-C3N4–tBuONa floating photocatalyst (see ESI†).Photocatalytic measurements
The photocatalytic experiments were performed using the XPA series and the degradation of methylene blue48 (MB) was employed by T3 using a Xe lamp for floating photocatalysts. The wastewater was simulated in 10 mg L−1 of MB solution with 350 W of Xe lamp plus optical filters. The synthetic photocatalysts (50 mg) were added to the MB solution (50 mL). The mixture was performed for 40–60 minutes under the adsorption and desorption process in the dark, followed by illumination. After that, 4 mL of suspensions were extracted every 20 minutes and the solution was measured by UV-Vis.Results and discussion
Syntheses and characterizations
A series of g-C3N4 and sodium alkoxide-mediated g-C3N4–MeONa, g-C3N4–EtONa, g-C3N4–tBuONa have been prepared for comparison. These powders have been measured by XRD and FT-IR spectra. As shown in Fig. 1, all samples have similar diffraction profiles, which indicates that the crystal structures of the samples are similar. It is found that the (002) diffraction at around 27.3° relating to the stacking structure is shifted to 27.5°, which suggests that the interlayer distance is from 0.326 nm to 0.324 nm. The phase structures of bulk g-C3N4 (JCPDS No. 87-1526) remain in a stable state.49 FT-IR analysis in Fig. S1† also offers some support for this view.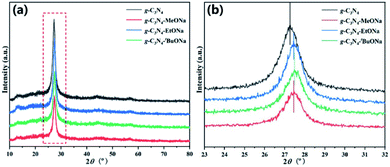 | ||
| Fig. 1 (a) XRD patterns of the bulk g-C3N4, g-C3N4–MeONa, g-C3N4–EtONa, and g-C3N4–tBuONa powders; (b) (002) magnified image of XRD patterns. | ||
However, scanning electron microscope (SEM) images present the change of morphology based on the stripping effect of sodium alkoxide in Fig. 2, 3, S2 and S3† (see ESI). Fig. 2 shows the block crystal of g-C3N4, while photocatalyst g-C3N4–tBuONa is surrounded by an irregular shape. Bulk g-C3N4 becomes smoother than sodium alkoxide-mediated g-C3N4. Fig. S2 and S3† show the same obvious etching phenomenon compared with that of bulk g-C3N4. Here, the etching results similar to wood laminations can be seen in Fig. S2.† Also, the image shows a fluffy morphology in Fig. S3.† The above changes may be attributed to the etching of sodium alkoxide-mediated study. More importantly, with increasing organic alkali, the surface roughness increases, where these spots are indicative of the crystal structure of g-C3N4–tBuONa and can be explained only by invoking the corrosion of tBuONa. For example, the mapping images of g-C3N4–tBuONa have made the strong case that C, N, Na, and O elements have amalgamated to form the composites in Fig. 3. Especially, the distribution of the C region (pink) coincides with the distribution of the N region (yellow). Also, the uneven Na (blue) and O (orange) areas have a similar distribution, which indicates that the Na and O components adhere to the surfaces and wrinkles of g-C3N4 and go on growth.
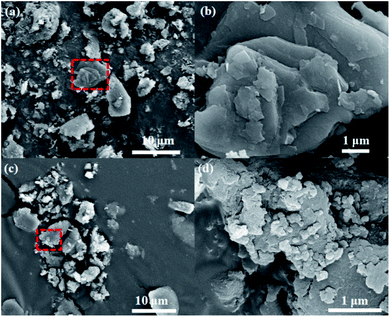 | ||
| Fig. 2 SEM images in different sizes of bulk g-C3N4: 10 μm (a) and 1 μm (b), g-C3N4–tBuONa: 10 μm (c) and 1 μm (d). | ||
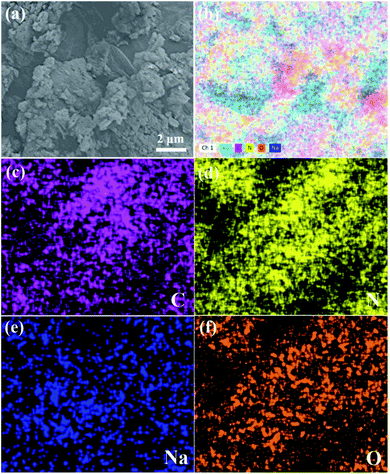 | ||
| Fig. 3 (a) SEM image of g-C3N4–tBuONa and (b–f) mapping images of the total, C, N, Na, and O elements, respectively. | ||
Photocatalytic degradation of sodium alkoxide-mediated g-C3N4
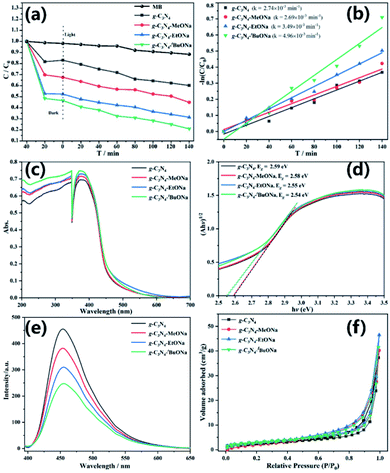
To further explore the photocatalytic activity, the experiment of dye degradation has been carried out for bulk g-C3N4, g-C3N4–MeONa, g-C3N4–EtONa, and g-C3N4–tBuONa powders in Fig. 4, where the photocatalytic activity has been evaluated by MB as a model pollutant and as a well-known dye under visible light irradiation. All the powders are adsorbed and saturated by MB solution to equilibrium in the dark for 40 min and the self-degradation of MB dye is negligible.As shown in Fig. 4a, the degradation rate of bulk g-C3N4 is 39.8% when irradiation lasts 140 minutes. However, the degradation rate of g-C3N4–MeONa, g-C3N4–EtONa, and g-C3N4–tBuONa is 55.1%, 68.6%, and 79.1%, respectively, at the same condition. Powder g-C3N4–tBuONa has better photocatalytic activity than g-C3N4, where the removal efficiency of MB for g-C3N4–tBuONa is double that for bulk g-C3N4. The kinetic linear curve shows that the first-order rate constants of four photocatalysts bulk g-C3N4, g-C3N4–MeONa, g-C3N4–EtONa, and g-C3N4–tBuONa are 2.74 × 10−3, 2.69 × 10−3, 3.49 × 10−3 and 4.96 × 10−3 min−1 in Fig. 4b, respectively. The first-order rate constants of g-C3N4–tBuONa powders are 1.81 times that of g-C3N4. Herein, the photocatalytic activity has increased with the increase of organic basicity. The capability of photocatalytic degradation is g-C3N4–tBuONa > g-C3N4–EtONa > g-C3N4–MeONa > g-C3N4 in the order. g-C3N4–tBuONa has an effective effect on improving its photocatalytic activity.
The optical properties of sodium alkoxide-mediated g-C3N4 also have been tested in Fig. 4c–e and BET analysis has been measured in Fig. 4f and Table S1.† According to UV-Vis DRS, the absorption regions of four photocatalysts are in the visible light range, with a slight bathochromic shift, which was attributed to the decrease of the bandgap. On the one hand, we calculated the corresponding bandgaps are 2.59, 2.58, 2.55, and 2.54 eV for bulk g-C3N4, g-C3N4–MeONa, g-C3N4–EtONa, and g-C3N4–tBuONa, respectively. Compared with bulk g-C3N4, the reduction of the bandgap can enhance the redox ability of photogenic radicals and then improve the photocatalytic activity, which provides a favourable basis for the photocatalytic degradation of MB.
The photoluminescent (PL) spectra enable us to further understand the photocarrier separation and transport process of sodium alkoxide-mediated carbon nitride samples with different alkalinity. Fig. 4e shows that PL intensity decreases obviously with the increase of organic basicity, indicating that g-C3N4–tBuONa has the lowest rate of electron–hole recombination, which is consistent with the results of the UV-Vis DRS test. BET analysis shows that the specific surface areas are 9.11, 10.97, 11.28, and 11.51 m2 g−1 for g-C3N4, g-C3N4–MeONa, g-C3N4–EtONa, and g-C3N4–tBuONa, respectively. Under the same conditions, the adsorption capacity of sodium alkoxide-mediated g-C3N4 agrees with the results of photocatalytic degradation in the dark reaction, indicating that the effect of strong alkali is conducive to the formation of pore structure.
Flexible electrospun polyacrylonitrile (PAN) nanofibers
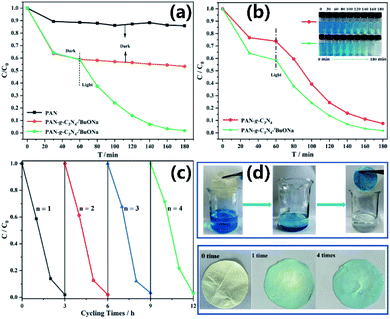
To promote the engineering applications, g-C3N4–tBuONa has been immobilized on a composite nanofibrous membrane to prepare the floating photocatalysts for preferable photocatalytic activity. Fig. 5 shows an SEM diagram of a paper-based floating photocatalyst PAN/g-C3N4–tBuONa (A4 size, 210 mm × 297 mm). Herein, the PAN/g-C3N4 nanofiber membrane has been prepared for comparison. As can be seen, the PAN polymer has a smooth and uniform linear structure to form a 2D nanofiber membrane by SEM. The nanofiber approximately is 350 nm in diameter. Interestingly, once synthesized, the g-C3N4–tBuONa particles move into the spinning solution, where the nanofiber wraps around them to form a monodisperse structure that resembles beads, or knots of 1–2 μm, on a string.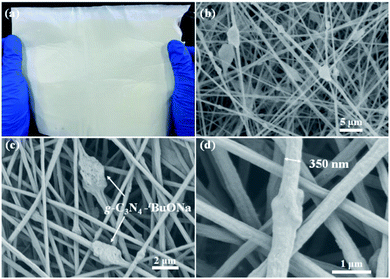 | ||
| Fig. 5 (a) Paper-based floating photocatalyst PAN/g-C3N4–tBuONa (A4 size, 210 mm × 297 mm) and SEM images for comparison: 5 μm (b), 2 μm (c) and 1 μm (d). | ||
To further understand the relationship between structure–property of nanomaterials, the adsorption of PAN and PAN/g-C3N4–tBuONa nanofibers, as well as photocatalytic degradation of paper-based floating photocatalyst PAN/g-C3N4–tBuONa, have been studied in Fig. 6. At first, the dark reaction lasts 60 minutes to reach the fully adsorbed equilibrium. Then, the films have been taken to test the concentration every 20 minutes by illumination for photocatalytic degradation. The degradation process monitoring of PAN/g-C3N4–tBuONa displays the efficiency of 10 mg L−1 MB solution can reach 85% for 1 hour, and can be completely degraded for 2 hours, until the solution becomes colourless. At the moment, the morphology of the paper-based floating photocatalyst was intact.
In addition, the cycling and stability experiment of floating photocatalyst has been studied in Fig. 6c. The paper-based photocatalysts float on solution surfaces to overcome sedimentation challenges and to maximize visible light absorption during photocatalysis in Fig. 6d. After the photocatalytic degradation, the paper-based floating photocatalyst can be taken out directly with tweezers and placed in the oven at 80 °C to dry for the next reaction.
After four cycling experiments, the adsorption performance of the catalyst is slightly weakened. The degradation rate of the first cycle is 98.08%, and that of the fourth cycle is 97.14%. However, the overall photocatalytic effect still keeps unchanged. It is noted that the degradation efficiency is approximately 100% and the morphology of the original thin film can be maintained. More importantly, loading the macroparticle catalyst onto the PAN film fails to hinder the better photocatalytic performance and to overcome the challenge brought by the difficult recycling of macroparticle catalyst.
Photocatalytic mechanism studies
As mentioned before, the corresponding bandgaps are 2.59, 2.58, 2.55, and 2.54 eV for bulk g-C3N4, g-C3N4–MeONa, g-C3N4–EtONa, and g-C3N4–tBuONa, respectively. At the same time, BET analysis shows that the specific surface areas are 9.11, 10.97, 11.28, and 11.51 m2 g−1 for bulk g-C3N4, g-C3N4–MeONa, g-C3N4–EtONa, and g-C3N4–tBuONa, respectively. Compared with bulk g-C3N4, lower bandgaps and bigger surface areas help promote photocatalytic activity.However, the medium of sodium alkoxide is the key element of modified g-C3N4, where sodium may promote the separation of photogenerated carriers and further participate in the process of photocatalytic oxidization. The presence of Na+ reduces the bandgap of g-C3N4 and also decreases the loading rate of photogenerated carriers. The above results have an agreement with the conclusion of alkali metal (Li+, Na+, K+) doping modification.50–52 Combined with the mechanism of alkali metal doping modified g-C3N4, the presence of sodium will increase the electron density of the carbon nitride plane, reduce the localization of electrons, and form an electron transport channel to facilitate the transport of carriers.
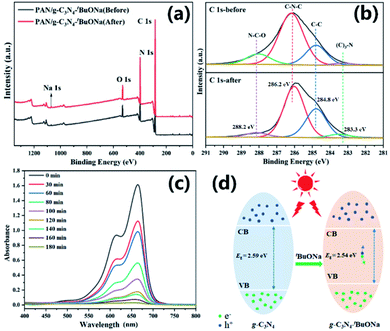
In the actual water treatment process, the potential safety risk of nanomaterials often is an inevitable bottleneck. Fortunately, sodium ions are less harmful to the environment than other toxic heavy metal ions. More importantly, XPS shows that the PAN/g-C3N4–tBuONa composite nanofibrous membrane keeps the chemical stability over the four cycles in Fig. 7a. As shown in Fig. 7b, for instance, the binding energy at about 286 eV might be attributed to the C 1s of g-C3N4, showing a good consistency before and after the photocatalytic degradation. Fig. 7c displays less absorption profile stepwise, which is in complete agreement with the results of photocatalytic degradation. The peak at 664 nm diminishes until it becomes a horizontal line and no new peaks as irradiation time goes on, indicating that no new pollutants are created. Therefore, we reckon the MB dye is most likely to be degraded directly to become carbon dioxide and water. It is undeniable that the mediated effect of sodium alkoxide plays an important role in decreasing the bandgap (Fig. 7d), improving the surface area, and enhancing the photogenerated electron–hole pairs.
Conclusions
In summary, a family of sodium alkoxide-mediated g-C3N4 photocatalysts has been synthesized for the first time. Simultaneously, g-C3N4–tBuONa has been immobilized on PAN nanofibers to fabricate a composite membrane for preferable photocatalytic activity. The degradation rate of bulk g-C3N4 is 39.8% when irradiation lasts 140 minutes. However, the degradation rate of g-C3N4–MeONa, g-C3N4–EtONa, and g-C3N4–tBuONa is 55.1%, 68.6%, and 79.1%, respectively, at the same condition. BET analysis shows that the surface areas are 9.11, 10.97, 11.28, and 11.51 m2 g−1 for bulk g-C3N4, g-C3N4–MeONa, g-C3N4–EtONa, and g-C3N4–tBuONa, respectively. With increasing organic alkali from MeONa to tBuONa, the exfoliation effect increases. Also, the capability of photocatalytic degradation is g-C3N4–tBuONa > g-C3N4–EtONa > g-C3N4–MeONa > g-C3N4 in the order.SEM analysis shows that paper-based photocatalyst PAN/g-C3N4–tBuONa becomes a nanofiber membrane (A4 size, 210 mm × 297 mm). The nanofiber approximately is 350 nm in diameter. PAN/g-C3N4–tBuONa shows highly enhanced photocatalytic activity of a well-known dye MB. The degradation efficiency of 10 mg L−1 MB solution can reach 100% for 2 hours until the solution becomes colourless for paper-based floating photocatalysts. The structure of electrospun nanofibers was validated with SEM and XPS analysis. The improved performance could be partly owing to the larger surface area and sodium alkoxide participation that builds up the charge to effectively separate the photogenerated electron and hole carriers that synergistically lead to preferable visible light degradation. This work has opened up a new strategy to design preferable floating photocatalysts for metal-free semiconducting materials.
Author contributions
Xue Li: methodology, investigation, data curation. Qin Wu: formal analysis. Mushraf Hussain: writing – review & editing. Liang Chen: writing – review & editing. Qiong Huang: writing – review & editing. Wei Huang: supervision, writing – review & editing. Tao Tao: conceptualization, data curation, supervision, funding acquisition, project administration, writing – original draft & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21501097), the Natural Science Foundation of Jiangsu Province (No. BK20150890 and BK20201389), and the Qing Lan Project of the Jiangsu Higher Education Institutions of China (No. R2018Q03).Notes and references
- N. Serpone and A. V. Emeline, J. Phys. Chem. Lett., 2012, 3, 673–677 CrossRef CAS PubMed.
- W. J. Ong, L. L. Tan, Y. H. Ng, S. T. Yong and S. P. Chai, Chem. Rev., 2016, 116, 7159–7329 CrossRef CAS PubMed.
- Z. Zhang, C. Qiu, Y. Xu, Q. Han, J. Tang, K. P. Loh and C. Su, Nat. Commun., 2020, 11, 4722–4730 CrossRef CAS PubMed.
- Y. Qian, D. Li, Y. Han and H. L. Jiang, J. Am. Chem. Soc., 2020, 142, 20763–20771 CrossRef CAS PubMed.
- H. Ehtesabi, Mater. Today Chem., 2020, 17, 100342 CrossRef CAS.
- S. Y. Tameu Djoko, H. Bashiri, E. T. Njoyim, M. Arabameri, S. Djepang, A. K. Tamo, S. Laminsi, M. Tasbihi, M. Schwarze and R. Schomäcker, J. Photochem. Photobiol., A, 2020, 398, 112596 CrossRef CAS.
- G.-Q. Zhao, J. Zou, J. Hu, X. Long and F.-P. Jiao, Sep. Purif. Technol., 2021, 279, 119769–119813 CrossRef CAS.
- J. Yang, H. Miao, J. Jing, Y. Zhu and W. Choi, Appl. Catal., B, 2021, 281, 119547 CrossRef CAS.
- X. Zhang, X. Yuan, L. Jiang, J. Zhang, H. Yu, H. Wang and G. Zeng, Chem. Eng. J., 2020, 390, 124475–124504 CrossRef CAS.
- J. Xu, Q. Gao, Z. Wang and Y. Zhu, Appl. Catal., B, 2021, 291, 120059 CrossRef CAS.
- X. Chen, J. Wang, Y. Chai, Z. Zhang and Y. Zhu, Adv. Mater., 2021, 33, 1–7 Search PubMed.
- F. Zhao, H. Cheng, Y. Hu, L. Song, Z. Zhang, L. Jiang and L. Qu, Sci. Rep., 2014, 4, 5882–5889 CrossRef CAS PubMed.
- D. Zhao, M. Wang, T. Kong, Y. Shang, X. Du, L. Guo and S. Shen, Mater. Today Chem., 2019, 11, 296–302 CrossRef CAS.
- C. Prasad, H. Tang, Q. Liu, I. Bahadur, S. Karlapudi and Y. Jiang, Int. J. Hydrogen Energy, 2020, 45, 337–379 CrossRef CAS.
- Q. Gao, J. Xu, Z. Wang and Y. Zhu, Appl. Catal., B, 2020, 271, 118933 CrossRef CAS.
- N. Rono, J. K. Kibet, B. S. Martincigh and V. O. Nyamori, Crit. Rev. Solid State Mater. Sci., 2020, 46, 189–217 CrossRef.
- J. Xu, J. Huang, Z. Wang and Y. Zhu, Chin. J. Catal., 2020, 41, 474–484 CrossRef CAS.
- J. Liang, X. Yang, Y. Wang, P. He, H. Fu, Y. Zhao, Q. Zou and X. An, J. Mater. Chem. A, 2021, 9, 12898–12922 RSC.
- X. Liu, R. Ma, L. Zhuang, B. Hu, J. Chen, X. Liu and X. Wang, Crit. Rev. Environ. Sci. Technol., 2020, 51, 751–790 CrossRef.
- K. A. Adegoke and N. W. Maxakato, Mater. Today Chem., 2022, 23, 100605 CrossRef CAS.
- X. Zhou, C. Shao, S. Yang, X. Li, X. Guo, X. Wang, X. Li and Y. Liu, ACS Sustainable Chem. Eng., 2018, 6, 2316–2323 CrossRef CAS.
- J. Xue, S. Ma, Y. Zhou, Z. Zhang and M. He, ACS Appl. Mater. Interfaces, 2015, 7, 9630–9637 CrossRef CAS PubMed.
- Y.-J. Yuan, Y. Yang, Z. Li, D. Chen, S. Wu, G. Fang, W. Bai, M. Ding, L.-X. Yang, D.-P. Cao, Z.-T. Yu and Z.-G. Zou, ACS Appl. Energy Mater., 2018, 1, 1400–1407 CrossRef CAS.
- S. Bai, N. Zhang, C. Gao and Y. Xiong, Nano Energy, 2018, 53, 296–336 CrossRef CAS.
- X. Jin, Q. Guan, T. Tian, H. Li, Y. Han, F. Hao, Y. Cui, W. Li, Y. Zhu and Y. Zhang, Appl. Surf. Sci., 2020, 504, 144241 CrossRef CAS.
- Z. Wang, Z. Lin, S. Shen, W. Zhong and S. Cao, Chin. J. Catal., 2021, 42, 710–730 CrossRef CAS.
- A. Gandamalla, S. Manchala, P. Anand, Y.-P. Fu and V. Shanker, Mater. Today Chem., 2021, 19, 100392 CrossRef CAS.
- Y. Li, C. Pan, G. Wang, Y. Leng, P. Jiang, Y. Dong and Y. Zhu, New J. Chem., 2021, 45, 6611–6617 RSC.
- Y. Bai, W. Mao, Y. Wu, Y. Gao, T. Wang and S. Liu, Colloids Surf., A, 2021, 610, 125481–125492 CrossRef CAS.
- D. Vaya, B. Kaushik and P. K. Surolia, Mater. Sci. Semicond. Process., 2022, 137, 106181–106208 CrossRef CAS.
- Q. Lin, L. Li, S. Liang, M. Liu, J. Bi and L. Wu, Appl. Catal., B, 2015, 163, 135–142 CrossRef CAS.
- P. Niu, L. Zhang, G. Liu and H.-M. Cheng, Adv. Funct. Mater., 2012, 22, 4763–4770 CrossRef CAS.
- J. Xu, L. Zhang, R. Shi and Y. Zhu, J. Mater. Chem. A, 2013, 1, 14766–14782 RSC.
- Y. Zhang, Q. Zhang, Q. Shi, Z. Cai and Z. Yang, Sep. Purif. Technol., 2015, 142, 251–257 CrossRef CAS.
- Y. Ma, E. Liu, X. Hu, C. Tang, J. Wan, J. Li and J. Fan, Appl. Surf. Sci., 2015, 358, 246–251 CrossRef CAS.
- X. Du, G. Zou, Z. Wang and X. Wang, Nanoscale, 2015, 7, 8701–8706 RSC.
- Y.-J. Yuan, Z. Shen, S. Wu, Y. Su, L. Pei, Z. Ji, M. Ding, W. Bai, Y. Chen, Z.-T. Yu and Z. Zou, Appl. Catal., B, 2019, 246, 120–128 CrossRef CAS.
- U. Ghosh and A. Pal, J. Photochem. Photobiol., A, 2020, 397, 112582 CrossRef CAS.
- J. Cui, D. Qi and X. Wang, Ultrason. Sonochem., 2018, 48, 181–187 CrossRef CAS PubMed.
- Y. Wang, B. Gao, Q. Yue and Z. Wang, J. Mater. Chem. A, 2020, 8, 19133–19155 RSC.
- X. Li, G. Huang, X. Chen, J. Huang, M. Li, J. Yin, Y. Liang, Y. Yao and Y. Li, Sci. Total Environ., 2021, 792, 148462–148482 CrossRef CAS PubMed.
- H. Liu, W. Huo, T. C. Zhang, L. Ouyang and S. Yuan, Mater. Today Chem., 2022, 23, 100729 CrossRef CAS.
- T. Sano, S. Tsutsui, K. Koike, T. Hirakawa, Y. Teramoto, N. Negishi and K. Takeuchi, J. Mater. Chem. A, 2013, 1, 6489–6496 RSC.
- Y. Li, M. Gu, X. Zhang, J. Fan, K. Lv, S. A. C. Carabineiro and F. Dong, Mater. Today, 2020, 41, 270–303 CrossRef CAS.
- Z. Chen, S. Zhang, Y. Liu, N. S. Alharbi, S. O. Rabah, S. Wang and X. Wang, Sci. Total Environ., 2020, 731, 139054–139075 CrossRef CAS PubMed.
- X. Xu, S. Wang, T. Hu, X. Yu, J. Wang and C. Jia, Dyes Pigm., 2020, 175, 108107–108118 CrossRef CAS.
- J. Singh, P. Kumari and S. Basu, J. Photochem. Photobiol., A, 2019, 371, 136–143 CrossRef CAS.
- E. Santoso, R. Ediati, Y. Kusumawati, H. Bahruji, D. O. Sulistiono and D. Prasetyoko, Mater. Today Chem., 2020, 16, 100233 CrossRef CAS.
- G. R. Surikanti, P. Bajaj and M. V. Sunkara, ACS Omega, 2019, 4, 17301–17316 CrossRef CAS PubMed.
- J. Jiang, S. Cao, C. Hu and C. Chen, Chin. J. Catal., 2017, 38, 1981–1989 CrossRef CAS.
- W. Yin, G. Zhou, L. Meng, X. Ning, J. Hou, S. Wang, Q. Xu and X. Wang, Res. Chem. Intermed., 2021, 47, 5189–5202 CrossRef CAS.
- M. Fronczak, E. Tálas, Z. Pászti, G. P. Szijjártó, J. Mihály, A. Tompos, P. Baranowski, S. K. Tiwari and M. Bystrzejewski, Diamond Relat. Mater., 2022, 125, 109006 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02441a |
| This journal is © The Royal Society of Chemistry 2022 |