Open Access Article
Open Access ArticlePhotocatalysed direct amination of benzene and ammonia over Ti–V-MCM-41†
Chunhua Yang *,
Xiaomei Wang,
Chun-Hong Lin and
Wentao Zhu
*,
Xiaomei Wang,
Chun-Hong Lin and
Wentao Zhu
College of Chemical Engineering, Northeast Electric Power University, Jilin City, 132012, China. E-mail: y-chun-hua@126.com
First published on 28th June 2022
Abstract
Aniline is one of the important organic chemical raw materials and is widely used in the chemical production industry, including dyes, pharmaceuticals, pesticides, explosives, spices, etc. At present, the yield and selectivity of aniline synthesis by direct amination are relatively low, and how to improve the catalytic efficiency has become an urgent problem to be solved. A series of Ti–V-MCM-41 catalysts with different silicon–titanium ratios was prepared by the hydrothermal synthesis method. According to the analysis of XRD, FT-IR, UV-vis DRS, BET, and SEM characterization of Ti–V-MCM-41, it was found that V–O–Si and Ti–O–Si were distributed on Ti–V-MCM-41. The direct photocatalysis amination of benzene and ammonia to aniline can be realized under mild conditions with this catalyst. The yield and selectivity of aniline were improved to 6.11% and 90.7%, respectively, by optimizing the synthesis and reaction conditions.
1. Introduction
Aniline (C6H5NH2) is widely applied to the manufacturing of chemical products, such as dyes, drugs, resins, and rubber vulcanization accelerators.1,2 At present, there is a strong and robust market demand for aniline.3 By the end of 2018, the world's aniline production capacity was 8.647 million tons per year, and the output was 6.706 million tons, increasing by 4.1% and 2.2%, respectively, over the previous year.The synthesis methods of aniline mainly include multi-step amination and one-step amination. The multi-step amination mainly includes reduction of nitrobenzene, catalytic hydrogenation of nitrobenzene, and amination of phenol,4,5 all of which have the disadvantages of harsh reaction conditions, complicated operation, severe environmental pollution, many by-products, and low atom utilization. The one-step method is also called direct amination, which is the process of activating the C–H bond of the benzene ring, introducing the amino group into the benzene ring directly.6–8 But the yield of aniline synthesized by the one-step method is still low. Therefore, how to improve the yield of one-step amination of benzene has become the core problem in this field, and the key lies in the research of catalysts and amination conditions.9,10
Along with continuously going deep into the research, the yield of aniline has been improved11–13 by direct amination of benzene and hydroxylamine hydrochloride. Compared with the direct amination with hydroxylamine hydrochloride, the yield of aniline with ammonia is much lower. Although hydroxylamine is used as an aminating agent, the yield and selectivity of aniline have significantly been improved. However, the reaction is carried out in an acid medium,13,14 which leads to many problems, such as severe corrosion of instruments and equipment, dissolution of metal catalysts. Furthermore, the cost of hydroxylamine is much higher than that of ammonia. From the perspective of industrialization and green chemistry in the future, it is necessary to further study the one-step synthesis of aniline using ammonia as aminating agent under mild conditions.
According to the previous research, the photocatalysis reaction catalyzed by tetravalent titanium follows a radical reaction process,15–17 and the direct amination of benzene is also a radical substitution reaction process.18 Encouraged by previous work, titanium and vanadium loaded MCM-41 materials were designed and for the first time applied to the photocatalytic one-step synthesis of aniline from benzene using ammonia as the aminating agent under mild conditions, and the photocatalytic radical substitution mechanism has been proposed. The Ti–V-MCM-41 catalyst synthesized here improved the yield of aniline in this system, and provided a theoretical basis for the industrialization of one-step synthesis of aniline using ammonia as the aminating agent.
2. Experimental section
2.1 Preparation of catalyst material samples
Cetyltrimethylammonium bromide (CTAB), tetraethyl orthosilicate (TEOS), vanadyl sulfate (VOSO4), and tetra butyl titanate (TBOT) were required. The reagents and ratio of catalyst preparation were shown in Table S1.†All the catalysts sample were prepared according to the material ratio in the Table S1.† Firstly, CTAB was dissolved in 30 mL deionized water to prepare a template solution. The VOSO4 was dissolved in 5 mL deionized water. Next, the TEOS, VOSO4 solution and TBOT was added to the template solution according to the proportion in Table S1.† At the same time, the pH of the mixed solution was adjusted using ammonia. Then, the solution was continuously stirred at 60 °C for 1 hour and was left at room temperature for 24 hours. After these procedure, the solution was transferred to the reactor and crystallized at a specific temperature and specific time. After crystallization, the production was washed, filtered and dried. Finally, the catalyst was roasted in a muffle furnace at a specific temperature and specific time. After calcination, the product was ground continuously, and then catalyst powder was obtained. The optimal synthesis conditions will be discussed in ESI from Fig. S1–S5.† The optimal synthesis conditions are: pH = 10.0, crystallization temperature = 110 °C, crystallization time = 24 h, calcination temperature = 550 °C, calcination time = 6 h.
2.2 Characterization and analysis of catalyst
The catalysts prepared were characterized by XRD, FT-IR, UV-vis DRS, BET, and SEM. XRD is used to analyze the diffraction patterns of the materials, and the information of the composition of the materials, the structure or morphology of the atoms or molecules inside the materials can be obtained.19 The scanning angles 2θ are 1–8° and 10–80° respectively, the working voltage is 40 kV, the operating current is 50 mA, and the scanning rate is 1° min−1.Since the vibration or rotation of different functional groups and chemical bonds will absorb infrared light with different wave numbers, the functional groups or chemical bonds on the catalyst were characterized by FT-IR.19–21 The scanning wave number is 4000–400 cm−1.
2.3 Direct amination of benzene by photocatalysis
Benzene, ammonia, hydrogen peroxide was required for the direct amination. 365–370 nm ultraviolet lamps were installed at the mouth of the conical flask. To ensure that the reaction conditions are isolated from visible light, the conical flask is completely wrapped with aluminium foil. Then the catalyst and benzene were added to the conical flask, ammonia water was added, and hydrogen peroxide was added slowly. In this process, the amination reaction was carried out in a water bath by continuous stirring for three hours. After the reaction, the catalyst was filtered, and then the product was diluted for liquid chromatography and gas chromatography analysis.The effects of raw material ratio, titanium loading of catalyst, reaction temperature, and UV light intensity on the direct amination of benzene were studied.
2.4 Qualitative and quantitative analysis of products
The reaction products were qualitatively analyzed by gas chromatography. The test conditions were as follows: the vaporizer temperature was 280 °C, the detector temperature was 280 °C. The initial column temperature was 60 °C, and the temperature was kept for 3 min. Then, the temperature was programmed to 280 °C and kept for 10 min. The product was quantified by high performance liquid chromatography. The column temperature was 35 °C, the mobile phase was methanol and water with a ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, and the gradient elution method was adopted. The yield and selectivity of the product iscalculated as follows formula:
70, and the gradient elution method was adopted. The yield and selectivity of the product iscalculated as follows formula:3. Results and discussion
3.1 Characterization and analysis of catalyst
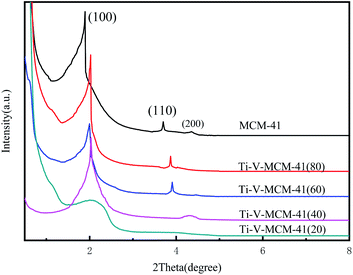
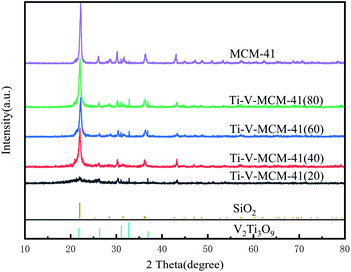
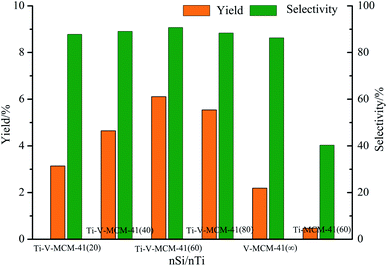
As shown in Fig. 2, the characteristic diffraction peaks of SiO2 appeared at 2θ = 22°, 28.4°, 31.4°, which is the framework of MCM-41 molecular sieve. At 2θ = 21.8°, 26°, 31°, the characteristic diffraction peaks of V2Ti3O9 appeared in the Ti–V-MCM-41, which proves that titanium and vanadium have been loaded on MCM-41.
3.2 Direct amination of benzene by photocatalysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nTi = 60 (yield and selectivity are 6.11% and 90.7%, respectively). It can be seen that the loading of Ti and V together improved the performance of the catalyst.
nTi = 60 (yield and selectivity are 6.11% and 90.7%, respectively). It can be seen that the loading of Ti and V together improved the performance of the catalyst.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3·H2O
NH3·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 = 1
H2O2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
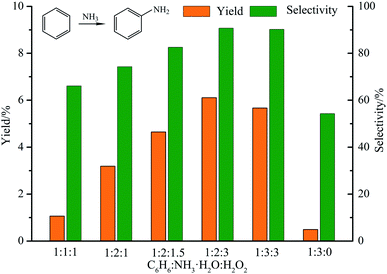
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which were 6.11% and 90.7%, respectively. The amounts of benzene, ammonia and hydrogen peroxide used were 0.0467, 0.1401 and 0.0934 moles, respectively.
2, which were 6.11% and 90.7%, respectively. The amounts of benzene, ammonia and hydrogen peroxide used were 0.0467, 0.1401 and 0.0934 moles, respectively.
We also discussed the effect of H2O2 on yield and selectivity, as shown in Fig. 6. Among all controls groups, the group without H2O2 (C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3·H2O
NH3·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 = 1
H2O2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) exhibited the lowest catalytic efficiency, the yield and selectivity are 0.49% and 54.3% respectively. Indeed, H2O2 promoted the ammoniation of benzene. In particular, when the ratio was C6H6
0) exhibited the lowest catalytic efficiency, the yield and selectivity are 0.49% and 54.3% respectively. Indeed, H2O2 promoted the ammoniation of benzene. In particular, when the ratio was C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3·H2O
NH3·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 = 1
H2O2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the catalytic efficiency began to decrease, which may be caused by unnecessary side reactions. That is, excess H2O2 lead to production of a large amount of phenol.17
3, the catalytic efficiency began to decrease, which may be caused by unnecessary side reactions. That is, excess H2O2 lead to production of a large amount of phenol.17
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
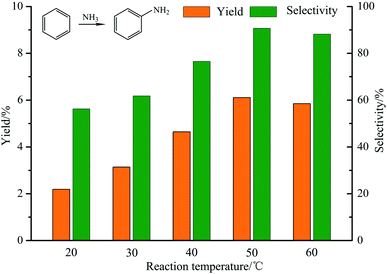
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the power of the ultraviolet lamp is 0.18 W. As shown in Fig. 7, when the temperature is lower than 50 °C, increasing the temperature promoted the direct amination of benzene. The yield and selectivity of aniline reached the highest at 50 °C (the yield and selectivity of aniline were 6.11% and 90.7%). On the contrary, when the temperature is higher than 50 °C, the increase of temperature inhibited the direct amination of benzene, which may be due to excessive volatilization of ammonia.
2, the power of the ultraviolet lamp is 0.18 W. As shown in Fig. 7, when the temperature is lower than 50 °C, increasing the temperature promoted the direct amination of benzene. The yield and selectivity of aniline reached the highest at 50 °C (the yield and selectivity of aniline were 6.11% and 90.7%). On the contrary, when the temperature is higher than 50 °C, the increase of temperature inhibited the direct amination of benzene, which may be due to excessive volatilization of ammonia.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
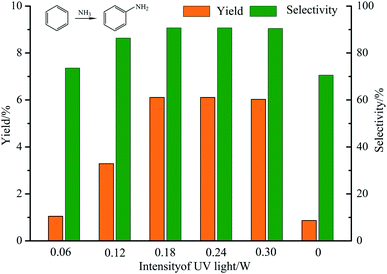
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the reaction temperature was 50 °C. The yield and selectivity of aniline are shown in Fig. 8. According to Fig. 8, the yield and selectivity of aniline increase with the increase of UV intensity before 0.18 W. When the ultraviolet emission power is 0.18 W, the yield and selectivity of aniline both reach the maximum value, which are 6.11% and 90.7%, respectively. The yield and selectivity of aniline will maintain a constant value, instead of continuing to increase while the UV intensity continues to grow. In order to prove that UV light played an important role in the reaction, the control group experiment was performed which was under heat and without UV light, the date was shown in Fig. 8. When the UV intensity was 0, the yield and selectivity are 0.87% and 70.6%, which was obviously lower than when UV intensity was 0.06 W. It shows that UV irradiation plays a great role in promoting the amination of benzene. Indeed, UV irradiation greatly accelerated the reaction.
2, the reaction temperature was 50 °C. The yield and selectivity of aniline are shown in Fig. 8. According to Fig. 8, the yield and selectivity of aniline increase with the increase of UV intensity before 0.18 W. When the ultraviolet emission power is 0.18 W, the yield and selectivity of aniline both reach the maximum value, which are 6.11% and 90.7%, respectively. The yield and selectivity of aniline will maintain a constant value, instead of continuing to increase while the UV intensity continues to grow. In order to prove that UV light played an important role in the reaction, the control group experiment was performed which was under heat and without UV light, the date was shown in Fig. 8. When the UV intensity was 0, the yield and selectivity are 0.87% and 70.6%, which was obviously lower than when UV intensity was 0.06 W. It shows that UV irradiation plays a great role in promoting the amination of benzene. Indeed, UV irradiation greatly accelerated the reaction.
3.3 Catalytic reaction mechanism
The reaction path of the photocatalytic amination reaction is proposed as follows: titanium(IV) in the Ti–V-MCM-41 catalyst generates electron–hole pair (e−/h+) under the excitation of the photon. Water molecules (H2O) and hydroxyl ions (OH−) generated a small number of hydroxyl radicals (˙OH) with substantial oxidation properties under the action of the hole. Electron and hydrogen peroxide (H2O2) produced a large number of hydroxyl radicals. These hydroxyl radicals oxidize ammonia molecules (NH3·H2O) into amino radicals (˙NH2), and then the amino radicals are converted into protonated amino radicals (˙NH3+) in the water. Benzene is activated into phenyl radical in the presence of the catalyst, and then protonated amino radicals can combine with phenyl radical to produce aminocyclohexanene intermediate. Under the action of the catalyst, the intermediate is transformed into aniline and water.4. Conclusions
In the present work, a series of Ti–V-MCM-41 catalysts different silicon–titanium ratios was prepared by hydrothermal synthesis. After different characterization analysis, Ti–V-MCM-41 has good crystallization condition, titanium is successfully loaded on MCM-41 support, and no non-framework titanium is generated. The pore structure is relatively complete, with a large specific surface area. Pore size distribution is uniform, mostly concentrated in 2–5 nm, which is beneficial to improve the yield of aniline. The N2 adsorption–desorption isotherm, pore size distribution, BET analysis and SEM can be seen in Fig. S6, S7, Table S2 and Fig. S8.†The synthesized Ti–V-MCM-41 catalysts were used in the experiment of direct amination of benzene by ultraviolet light, and the optimal synthesis conditions were as follows: the mixed solution pH = 10.0, the crystallization temperature = 110 °C, the crystallization time = 24 h, the calcination temperature = 550 °C, the calcination time = 6 h, and the nSi/nTi = 60. The optimal reaction conditions are as follows: C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3·H2O
NH3·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 = 1
H2O2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the reaction temperature is 50 °C, and the UV intensity is 0.18 W. Under these reaction conditions, the yield and selectivity can reach the maximum value, 6.11% and 90.7%, respectively.
2, the reaction temperature is 50 °C, and the UV intensity is 0.18 W. Under these reaction conditions, the yield and selectivity can reach the maximum value, 6.11% and 90.7%, respectively.
Conflicts of interest
The authors declare no competing financial interest.Author contributions
Conceptualization, C.-H. Y.; investigation, X.-M. W. and C.-H. Y.; methodology, C.-H. Y. and X.-M. W.; visualization, X.-M. W. and C.-H. L.; writing – original draft, X.-M. W.; writing – review & editing, C.-H. Y., C.-H. L., X.-M. W. and W.-T. Z. All authors reviewed the manuscript.Acknowledgements
This research was funded by Scientific Research Staring Foundation for PhDs of Northeast Electric Power University (BSJXM-201322) and Department of Education of Jilin Province for the “the Twelfth Five” Scientific and Technological Research Projects (2015-247).Notes and references
- L. Schmerling, US Pat.No. 2948755, 1960 Search PubMed.
- D. Edgren, H. Leeper, K. Nichols, et al. Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., 2000 Search PubMed.
- J. F. Hartwig, S. Shekhar, Q. Shen, et al. Synthesis of Anilines. Patai's Chemistry of Functional Groups, John Wiley & Sons, Ltd, 2009 Search PubMed.
- D. S. Surry and S. L. Buchwald, J. Am. Chem. Soc., 2007, 129, 10354–10355 CrossRef CAS PubMed.
- K. Weissermel and A. Hansmgürgen, Industrial Organic Chemistry, 4th edn, 2008 Search PubMed.
- S. Diao, W. Qian, G. Luo, W. Fei and W. Yao, Appl. Catal., A, 2005, 286, 30–35 CrossRef CAS.
- A. Hagemeyer, R. Borade, P. Desrosiers, S. Guan and U. Notheis, Catal. Today, 2003, 81, 319–328 CrossRef.
- Y. W. Zheng, B. Chen, P. Ye, K. Feng, W. Wang, Q. Y. Meng, L. Z. Wu and C. H. Tung, J. Am. Chem. Soc., 2016, 138, 10080–10083 CrossRef CAS PubMed.
- T. H. Yu, R. G. Yang, S. Xia, G. Y. Li and C. W. Hu, Catal. Sci. Technol., 2014, 4, 3159–3167 RSC.
- J. Becker and W. F. Hlderich, Catal. Lett., 1998, 54, 125–128 CrossRef CAS.
- C. Tong, C. Hu, Z. Fu and A. Tian, Chin. Sci. Bull., 2002, 47, 1937–1939 CrossRef.
- C. H. Yang, G. Chen and L. Zhang, Adv. Mat. Res., 2012, 550–553, 2607–2611 CAS.
- K. M. Parida, S. S. Dash and S. Singha, Appl. Catal., A, 2008, 351, 59–67 CrossRef CAS.
- H. Yuzawa and H. Yoshida, Chem. Commun., 2010, 46, 8854–8856 RSC.
- Y. Sohn, W. X. Huang and F. Taghipour, Appl. Surf. Sci., 2017, 396, 1696–1711 CrossRef CAS.
- S. J. Tan, H. Feng, Y. F. Ji, Y. Wang, J. Zhao, A. D. Zhao, B. Wang, Y. Luo, J. L. Yang and J. G. Hou, J. Am. Chem. Soc., 2012, 134, 9978–9985 CrossRef CAS PubMed.
- Q. W. Huang, S. Q. Tian, D. W. Zeng, X. X. Wang, W. L. Song, Y. Y. Li, W. Xiao and C. S. Xi, ACS Catal., 2013, 3, 1477–1485 CrossRef CAS.
- C. H. Yang and G. Chen, J. Chem. Soc. Pak., 2016, 38, 282–286 CAS.
- C. Y. Chen, H. X. Li and M. E. Davis, Microporous Mater., 1993, 2, 17–26 CrossRef CAS.
- K. Nakamoto, Theory & Applications in Inorganic Chemistry, 1977, vol. 5, pp. 88–97 Search PubMed.
- E. R. Lippincott, J. Am. Chem. Soc., 1963, 85, 3532 CrossRef.
- E. R. Lippincott, J. Am. Chem. Soc., 1963, 85, 3532 CrossRef.
- T. Blasco, A. Corma, M. T. Navarro and J. P. Pariente, J. Catal., 1995, 156, 65–74 CrossRef CAS.
- J. Zhang, D. S. Zhao, M. S. Liu and J. P. Li, Adv. Mat. Res., 2012, 557–559, 1411–1414 CAS.
- L. Wei, H. Zhang, Y. Dong, W. Song, X. Liu and Z. Zhao, RSC Adv., 2016, 6, 71375–71383 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02427f |
| This journal is © The Royal Society of Chemistry 2022 |