Open Access Article
Open Access ArticleEffect of chloride ions on the corrosion behavior of carbon steel in an iron bacteria system
Ping Xu*,
Meihui Zhao†
 ,
Xue Fu† and
Chen Zhao
,
Xue Fu† and
Chen Zhao
Beijing University of Civil Engineering and Architecture, Beijing, 100044, China. E-mail: xuping@bucea.edu.cn
First published on 18th May 2022
Abstract
Reclaimed water used as circulating cooling water can effectively relieve water stress, but the corrosion problem in it is very prominent. In particular, Cl− and iron bacteria (IB) are important influencing factors of corrosion behavior in a circulating water environment, and both of them often coexist in circulating water systems, so it is crucial to study their synergistic effects. This paper investigated the effect of Cl− on the corrosion behavior of carbon steel in the IB system by use of weight loss measurements, electro-chemistry and X-ray photoelectron spectroscopy (XPS). In the first 1–9 days of the experiment, the increase of Cl− concentration led to an increase of corrosion rate and a decrease of anode potential and charge transfer resistance at the interface. The corrosion rate of the 4ClIB condition reached 0.45 mm a−1 in the 1st day, which was 1.47 and 1.15 times that of 3ClIB and 1ClIB, and its anode potential was 22.6% and 33.8% lower than that of 3ClIB and 1ClIB. This indicates that a higher concentration of Cl− made the anodic reaction easier and the corrosion more severe. However, after 9 days, a decline in the corrosion rate was recorded at similarly high Cl− concentrations. On the 15th day, the corrosion rates for 3ClIB and 4ClIB were 7.0% and 15.6% lower compared to the 1ClIB condition. At this stage, the anode potential and film resistance had increased significantly, to become the dominant factors controlling the corrosion reaction. On the 15th day, the βa values of 1ClIB, 3ClIB and 4ClIB were 1.2, 1.5 and 1.7 times higher than those of the 1st day, and the highest Rb value of 1592.1 Ω cm2 was obtained for the 4ClIB condition, which was 1.9 times higher than that of Rct. In the early stage of corrosion, the surface of the carbon steel was enriched in Cl− due to their high concentration, and the Cl− could easily destroy the developing corrosion product film and promote the generation of Fe2+. At the same early stage, the growth of IB was enhanced, and the metabolism of IB was promoting local corrosion. However, in the later stage of corrosion, biofilms had an increasing effect on corrosion. A high concentration of Cl− accelerated biofilm growth and densified the corrosion product layer which subsequently hindered the anodic reaction and thus inhibited corrosion.
1 Introduction
Industrial circulating cooling water in China accounts for about 70% of industrial water consumption.1 Recycling urban sewage water into industrial circulating cooling water can effectively alleviate the water shortage in China. However, the high content of organic matter in the reclaimed water provides conducive conditions for growth and adhesion of microorganisms, resulting in microbiological induced corrosion.2 At the same time, the reclaimed water contains corrosive ions such as Cl− and SO42−,3 which also promote the corrosion of metal pipes and equipment, which can result in equipment failure and associated risks and can cause large economic losses.4,5Many scholars have studied the corrosion mechanism of corrosive ions and microorganisms in reclaimed water. Cl− ions are one of the ions that have the greatest impact on corrosion.6–8 They have a small radius, strongly adsorb and easily penetrate the passivation layer aggravating metal corrosion. In the circulating water systems, the increase of Cl− concentration can accelerate the uniform corrosion of carbon steel pipes and pitting corrosion of stainless-steel pipes.9,10 Corrosion can also be aggravated by the presence of certain types of bacteria. Iron bacteria (IB) are one of the main corrosion inducing microorganisms, including iron-oxidizing bacteria (IOB) and iron-reducing bacteria (IRB). IOB can oxidize Fe2+ to form Fe(OH)3 precipitates. These precipitates cover the tube wall to form small anode points that act as galvanic cells with a large area where oxygen exists, causing local corrosion.11 On the other hand, IRB can reduce the insoluble Fe3+ precipitates to soluble Fe2+, leading to the destruction of the passivation film, thereby aggravating the corrosion.12 It has also been suggested that Fe2+ formed by the metabolism of IRB can form an ionic layer on the metal surface, which inhibits the attachment of dissolved oxygen and at the same time inhibits the dissolution and precipitation of anode iron, thus inhibiting corrosion.13,14 Changes in Cl− concentration can affect microbial growth, thereby altering the corrosion process.15 Some studies have found that there is an optimal concentration of Cl− that can promote the growth of sulfate reducing bacteria (SRB). Very low Cl− concentration was found to have little effect on the corrosion behavior of SRB, however, when the concentration of Cl− increased to 5–30 g L−1, the salinity increased, which promoted the growth and attachment of SRB enhancing corrosion.16,17 The concentration of Cl− over 30 g L−1 was found to inhibit the growth of SRB and reduces corrosion.17,18 Some studies suggest that the sulfide produced by the corrosion of SRB promoted the corrosion,19 while other studies suggest that the synergistic effect of Cl− and SRB caused pitting corrosion and accelerated the corrosion process.17 Excessive Cl− concentration will inhibit the diffusion of dissolved oxygen and SRB adhesion, resulting in a decrease in the corrosion rate.18
At present, the effect of Cl− on the corrosion behavior of the SRB system is relatively clear, but it's effect on the corrosion behavior of IB is uncertain. Most of the existing research on the behavior of IB is based on the analysis of experiments done for a limited amount of time. Therefore, this study investigated the effect of Cl− on the corrosion behavior of IB systems over the entire experimental duration. The corrosion process was analyzed by measuring corrosion weight loss and performing electrochemical tests. The components of corrosion products were analyzed by X-ray energy spectrometry. In addition, the amount of Cl− and IB in suspended and attached states was also detected and measured.
2 Experimental
2.1 Materials
The national type I carbon steel coupon (50 mm × 25 mm × 2 mm) were prepared for dynamic testing. The elemental composition of the coupon was 99.421% Fe, 0.29% Mn, 0.17% Si, 0.095% C, 0.012% P and 0.012% S. Before the experiment, the coupons were soaked in acetone for 5 s to remove the surface grease, and then wiped with medical absorbent cotton and dried on a filter paper. The dried coupons were then rinsed with distilled water, soaked in absolute ethanol for 1 min and dried as before. The coupons were then wrapped with a filter paper, put in a desiccator for 1 day and weighed. They were sterilized under UV light for 30 minutes before use.A 1 cm2 carbon steel electrodes were prepared for electrochemical testing. This was done by mounting a piece of carbon steel in epoxy resin and leaving 1 cm2 surface exposed to work as the electrode. Before use, the samples were polished step by step using a 180#–2000# abrasive paper, polished with 0.05 μm Al2O3 polishing powder and chamois, washed and placed in anhydrous ethanol for 1 min and placed in a dry dish for 1 d.
The IB used in this experiment was obtained from a bacteria bank of Huazhong University of Science and Technology. The IB medium used for separation and purification had the following composition: 0.5 g MgSO4, 0.5 g (NH4)2SO4, 0.5 g K2HPO4, 0.2 g CaCl2, 0.5 g NaNO3 and 10.0 g C6H8FeNO7. After 14 days of incubation at 29 ± 1 °C, the conical flask with black or brown precipitation and red-brown color fading in the medium and becoming colorless and transparent or with this trend was judged to have iron bacteria. After taking it out and repeating the above steps to isolate and purify, the experimental bacteria were obtained after 3–4 generations of repeated culture.
The experimental water was prepared according to the quality specifications of the reclaimed water used as circulating cooling water.20 Chemicals used in synthesizing the experimental water together with their respective concentrations are shown in Table 1. Three groups of tests were conducted: (1) the samples were in the conventional reclaimed water inoculated with IB, 1ClIB; (2) the samples were in the reclaimed water with 3 times the Cl− concentration inoculated with IB, 3ClIB; (3) the samples were in the reclaimed water with 4 times the Cl− concentration inoculated with IB, 4ClIB. Table 2 shows the actual ion concentrations and the dosage of IB for each experimental condition.
| Num. of IB (CFU mL−1) | Cl− (mg L−1) | SO42− (mg L−1) | NH4+ (mg L−1) | HCO3− (mg L−1) | pH | |
|---|---|---|---|---|---|---|
| 1ClIB | 105 | 118.2 | 122.2 | 1.9 | 199 | 7.9 |
| 3ClIB | 105 | 369.3 | 122.2 | 1.9 | 199 | 7.9 |
| 4ClIB | 105 | 501.6 | 122.2 | 1.9 | 199 | 7.9 |
| NaCl (mg L−1) | Na2SO4 (mg L−1) | (NH4)2SO4 (mg L−1) | NaHCO3 (mg L−1) | |
|---|---|---|---|---|
| 1ClIB | 194.7 | 173.4 | 6.9 | 274 |
| 3ClIB | 608.6 | 173.4 | 6.9 | 274 |
| 4ClIB | 826.7 | 173.4 | 6.9 | 274 |
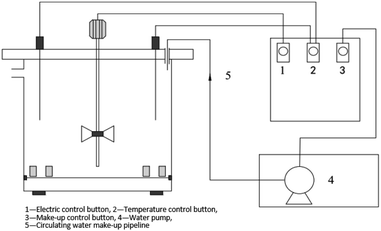
2.2 Experimental devices
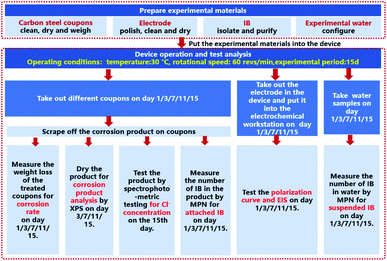
A combination of dynamic experiments and electrochemical tests were used. The dynamic test device, shown schematically in Fig. 1, is a self-built annular reactor with a volume of 2.5 L, the device simulates the hydraulic flow in the circulating cooling water pipe network. The temperature and rotational speed were set at 30 °C and 60 revs per min, respectively. The water pump supplied water to the device for each working condition every 2 hours. The experimental period was 15 days.The corrosion weight loss, the number of IB on the surface of the coupons and the number of suspended IB in the water were measured and analyzed on 1st, 3rd, 7th, 11th, and 15th day of the experiments. XPS was used to analyze the corrosion products on the surface of the coupons on 3rd, 7th, 11th, and 15th day. The Cl− concentration analysis on the surface of the coupons was carried out on the 15th day.
The electrochemical test adopted the traditional three-electrode systems, and the experimental period was set to 15 days. On 1st, 3rd, 7th, 11th, and 15th day the polarization curve and electrochemical impedance spectroscopy (EIS) of different working conditions were measured.
2.3 Detection methods
The corrosion rate is calculated by the weight loss method, the calculation is as follows:
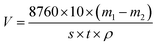 | (1) |
The variance is calculated from the triple sampling results and used as error bars for the test results.
The electrochemical workstation model was a CHI66C. The working electrode was a carbon steel electrode, the reference electrode was a saturated calomel electrode, and a platinum electrode was used as the auxiliary electrode. The polarization curve and EIS were measured for each working condition. For the determination of the polarization curve, the initial value of the open circuit potential should be set to −0.3 V, the termination value should be +0.3 V, and the scan rate should be adjusted to 1 mV s−1. The polarization resistance was obtained from the same samples throughout the 15 day period. When measuring EIS, the initial level was set to the open circuit potential value, the high and low frequencies were adjusted to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 0.01 Hz, and the amplitude was adjusted to 5 mV.
000 and 0.01 Hz, and the amplitude was adjusted to 5 mV.
The carbon steel coupon was finally dried by a freeze dryer and the corrosion products scrapped off and ground into a powder. The composition of the corrosion products was analyzed using a PHI Quantera II X-ray photoelectron spectrometer. The XPS spectrum needed to be corrected by C 1s external standard method, and then the peak position on the test curve was analyzed by CASA software.
On 15th day, the carbon steel coupon was taken out, then dried on a filter paper. The corrosion layer was then scraped off and placed in a dry centrifuge tube. The corrosion products were then shaken in water in the centrifuge and left to settle, the supernatant was then recovered for later use. A HACH DR6000 UV spectrophotometer was used to measure the Cl− concentration. The specific test method was carried out in accordance with the requirements of Hach Company's “Water Analysis Manual (Fifth Edition)”.
The MPN technique was used to detect the number of IB suspended in water and on the surface of carbon steel coupons respectively. Five points at different depths and different positions in the reactor were selected to collect water samples which were then mixed uniformly. The water samples were diluted and inoculated with IB medium. After culturing at 29 ± 1 °C for 14 days, the count was taken out and counted as the number of suspended IB. The coupons were taken out at the same time of the day each time measurements were done, and the biofilm and corrosion products were scraped from the surface of the coupons and placed in a sterile sampling tube. The attached IB were incubated and counted using the same method. The research flow chart as shown in Fig. 2.
3 Results and discussion
3.1 Corrosion rate test
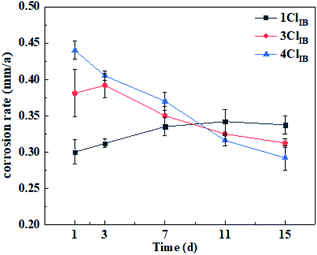
During the experiment, the average corrosion rate of carbon steel changed over time for all conditions as shown in Fig. 3.According to Fig. 3, the corrosion rate for 4ClIB and 3ClIB on the 1st day was 1.47 and 1.27 times that of 1ClIB respectively. The results show that, in the initial stages of the experiment, the higher the Cl− concentration, the faster the corrosion rate. However, as time progressed, a rapid decline in the corrosion rate for higher the Cl− concentrations was observed while the corrosion rate for 1ClIB remained steadily increasing. After about the 9th day, the corrosion rates of 3ClIB and 4ClIB were lower than that of 1ClIB. On the 15th day the corrosion rates of 3ClIB and 4ClIB were 7.0% and 15.6% lower than that of 1ClIB. At the beginning of the experiment, the IB biofilm was immature, and the carbon steel interface was mainly covered by corrosion products. Bo's result have shown that higher Cl− concentration dismantles the corrosion product layer and accelerates the corrosion of carbon steel.21 This means that the higher the concentration of Cl−, the more Fe2+ gets generated by corrosion.22 The IB are known to oxidize Fe2+ in order to obtain the energy required to sustain their lives. The Fe3+ generated precipitate as iron(III) oxide that forms anodic active sites on the metal surface, promoting local corrosion.23,24 However, in the later stages of corrosion, the results indicate that the higher the Cl− concentration in the IB system reduces the corrosion rate. Qi's study found that in the absence of bacteria, the high Cl− concentration promoted corrosion reaction over a period of 25 days,25 which was not consistent with the results in this study which was conducted in the presence IB. The reason why a decline in the corrosion rate was observed in the later stages of corrosion might be due to the increasing thickness of biofilm formed by IB which captures and immobilizes corrosion products and inhibit corrosion. Studies have found that IB biofilms were conducive to the precipitation of corrosion products and together form a denser structure, which has a greater inhibitory effect than pure iron oxides.26 In addition, the corrosion rate reached the peak in the earlier stages of corrosion for the higher the chloride ion concentration (4ClIB & 3ClIB) and reached a peak on the 11th day for the lower concentration (1ClIB), the peak values for 4ClIB, 3ClIB and 1ClIB were 0.450, 0.392 and 0.342 mm a−1 respectively, which appeared 1st, 3rd and 11th day. It is suggested that the more severe corrosion reaction observed at the initial stage of corrosion for higher concentrations causes the rapid growth of the protective film resulting in the early onset corrosion inhibition.
3.2 Electrochemical measurements
| Time/d | βa/mV | βc/mV | Icorr/(μA cm−2) | Ecorr/V |
|---|---|---|---|---|
| 1ClIB | ||||
| 1 | 166.82 | 238.35 | 8.559 | −0.733 |
| 3 | 133.50 | 192.35 | 8.922 | −0.793 |
| 7 | 130.27 | 147.71 | 9.522 | −0.828 |
| 11 | 187.52 | 104.88 | 9.710 | −0.831 |
| 15 | 202.83 | 136.47 | 9.634 | −0.803 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 3ClIB | ||||
| 1 | 129.13 | 240.46 | 9.964 | −0.772 |
| 3 | 125.08 | 198.08 | 10.285 | −0.787 |
| 7 | 124.55 | 82.63 | 11.165 | −0.834 |
| 11 | 196.66 | 97.60 | 9.307 | −0.816 |
| 15 | 198.28 | 107.28 | 8.969 | −0.807 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 4ClIB | ||||
| 1 | 110.39 | 244.87 | 12.061 | −0.783 |
| 3 | 85.21 | 217.34 | 12.584 | −0.842 |
| 7 | 148.05 | 93.48 | 10.357 | −0.813 |
| 11 | 175.94 | 115.34 | 9.007 | −0.795 |
| 15 | 191.03 | 128.32 | 8.268 | −0.789 |
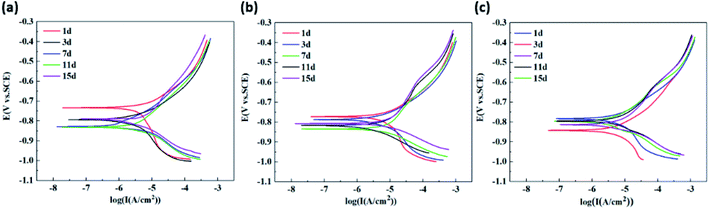
The anodic reaction on the surface of carbon steel is the dissolution of carbon steel, and the cathodic reaction is the reduction of oxygen. According to Table 3, the βa and βc of the three working conditions showed a similar trend of the values first decreasing and then increasing. It shows that the corrosion reaction in the early stage is gradually intensified, and the corrosion products in the later stage are gradually accumulated, which hinders the continuation of the cathodic and anode reactions. The results are consistent with the corrosion rate, Icorr and Ecorr.
At the same time, on the 1st day, the absolute value of the cathodic Tafel slope of the polarization curves (βc) for three working conditions were higher the value of the anodic Tafel slope of the polarization curves (βa), and they were in the range of 238.35–244.87 mV. Cathodic reactions are shown to be the controlling factor of corrosion at this time. However, with the extension of time, the increase of βa values was larger. By the 15th day, the βc values of the 1ClIB, 3ClIB and 4ClIB conditions were 0.6, 0.5 and 0.5 times higher than those of the 1st day, while the βa values were 1.2, 1.5 and 1.7 times higher than those of the 1st day. At this stage, the anode reaction becomes the controlling factor of corrosion process, and the higher the Cl− concentration, the greater the confinement effect of the anode. Therefore, it can be inferred that the reduction of the corrosion reaction rate in the later stages of the corrosion process is due to the inhibition of anodic iron dissolution reaction. This is most likely due to the hindering effect of the IB biofilm and corrosion product layer. In the later stages, the βc of the three working conditions all increased slightly, indicating that the dissolved oxygen mass transfer was also affected by the biofilm and corrosion product layers.
Comparison of βa values at the same time show that the values for 3ClIB and 4ClIB conditions were 22.6% and 33.8% lower than those for 1ClIB condition, which indicates that the higher the Cl− concentration at this stage, the easier it is for the anode to lose electrons.28 Jiang and Li also found that the erosive effect of Cl− and the metabolism of IB promoted the anodic reaction.29,30
However, over time, the corrosion rate slowed down suggesting that on the contrary, the high concentration of Cl− increased the impedance and inhibited the corrosion reaction. The results were consistent with the observations made in both the corrosion rate and polarization curves.
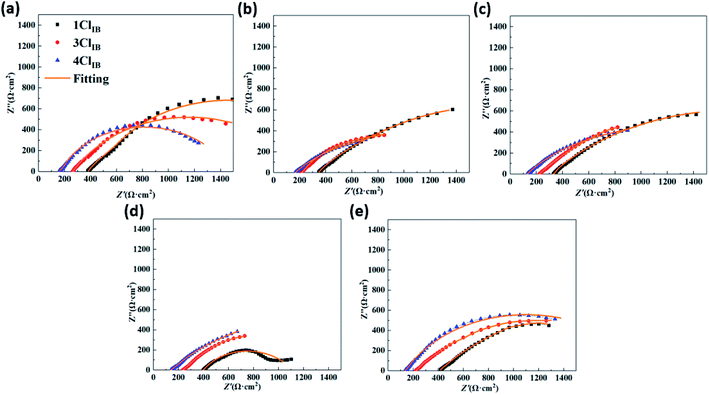
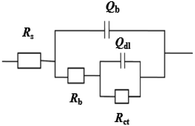
ZSimpWin software was used to fit the circuit. The equivalent circuit is shown in Fig. 6, and the fitted parameters are shown in Table 4. The meanings of the relevant parameters in Fig. 5 and Table 4 are as follows:33 Rs represents the solution resistance, Rct is the charge transfer resistance at the interface, Rb and Qb represent the film resistance and capacitance formed by the corrosion product layer and biofilm, and double layer capacitance are described by Qdl, nb and ndl are the dispersion index. The χ2 test indicates the fitting effect, and the χ2 values of ideal circuits are generally considered to be in the range of 10−3 to 10−5,34 and the χ2 values of all data in this study were within the required range.
| Time (d) | Qb (μF cm−2) | nb | Rb (Ω cm2) | Qdl (μF cm−2) | ndl | Rct (Ω cm2) |
|---|---|---|---|---|---|---|
| 1ClIB | ||||||
| 1 | 812 | 0.63 | 13.8 | 495 | 0.76 | 1848 |
| 3 | 1254 | 0.68 | 19.7 | 1100 | 0.67 | 1815 |
| 7 | 1907 | 0.71 | 63.8 | 2108 | 0.51 | 1627 |
| 11 | 1985 | 0.81 | 176.8 | 3189 | 0.60 | 1496 |
| 15 | 2757 | 0.94 | 442.0 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
0.61 | 1388 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| 3ClIB | ||||||
| 1 | 0.678 | 0.99 | 20.9 | 1067 | 0.67 | 1499 |
| 3 | 1.89 | 1.00 | 169.2 | 2372 | 0.62 | 1340 |
| 7 | 3599 | 0.63 | 383.1 | 2432 | 0.51 | 1258 |
| 11 | 5811 | 0.56 | 560.3 | 1136 | 0.80 | 1197 |
| 15 | 7415 | 0.57 | 869.0 | 580 | 0.89 | 954 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| 4ClIB | ||||||
| 1 | 2.98 | 1.00 | 42.1 | 171 | 0.61 | 1249 |
| 3 | 1119 | 0.69 | 280.5 | 194 | 0.56 | 1128 |
| 7 | 2601 | 0.62 | 530.4 | 2776 | 0.54 | 1033 |
| 11 | 3680 | 0.54 | 842.0 | 883 | 1.00 | 973 |
| 15 | 4484 | 0.51 | 1592.1 | 591 | 1.00 | 836 |
According to Fig. 5 and Table 4, although the fitted circuits conform to the R(Q(R(QR))) model for all three operating, there are large differences in the variation of their Rb and Rct values with increasing time. On the 1st day, the Rb of 1ClIB, 3ClIB and 4ClIB were only in the range 13.8–42.1 Ω cm2, all much smaller than the Rct values of 1249–1848 Ω cm2, demonstrating that the impedance in all three conditions at this time were chiefly from the interfacial charge transfer resistance, and the effect of film resistance was minimal. Moreover, the high Cl− concentration condition exhibited lower Rct values. However, thereafter, Rb kept reducing and Rct kept decreasing, and the higher the Cl− concentration, the greater the variation. On the 15th day, the Rct of the 4ClIB condition was 39.8% and 12.4% lower than the Rct of 1ClIB and 3ClIB, but the Rb was 2.60 and 0.83 times higher. The Rb was 1592.1 Ω cm2, which was 1.9 times higher than the Rct. It means that at this time, the impedance primarily comes concentration, the faster and denser the biofilm and corrosion product layer were formed, and therefore, the less likely corrosion would occur. It proves that the hypothesis of this study on the cause of corrosion rate decrease was sound. Both Ghafari et al.35 and Scheerder et al.36 found that the carboxyl groups of polysaccharides in extracellular polymers (EPS) secreted by IB contain C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds that can complex with Fe(II/III) and are responsible for the formation of a denser shielding layer.
O bonds that can complex with Fe(II/III) and are responsible for the formation of a denser shielding layer.
3.3 Corrosion product analysis
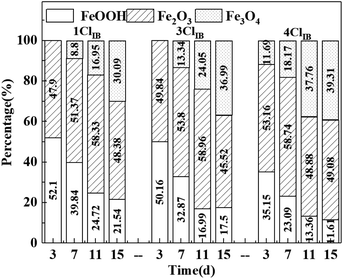
In order to evaluate the formation and transformation process of corrosion products under the different experimental conditions, the composition was analyzed semi-quantitatively using XPS technique. The Fe 2p spectra were fitted to the split peaks using CASA software. And Fig. 7 shows the results of the proportion of iron oxide content extracted based on the fitting results. The corrosion products initially consisted of FeOOH, Fe2O3, and later Fe3O4.As can be seen from Fig. 7, the corrosion products formed at different experimental conditions were of three distinct crystal types which are FeOOH, Fe2O3 and Fe3O4. The conversion of the unstable FeOOH to the more stable Fe2O3 and Fe3O4 occurred with increasing reaction time. Fe3O4 was detected on 3rd day for 4ClIB whilst it was detected on 7th day for 1ClIB and 3ClIB. The percentage of FeOOH was the lowest and the percentage of Fe3O4 was the highest in all 4ClIB conditions during the experiment. In comparison with 1ClIB and 3ClIB conditions, the percentage of Fe3O4 in 4ClIB was 9.22% and 2.32% higher on 15th day. The reason for this observation might be that the elevated Cl− concentration at the beginning of corrosion caused a high corrosion rate, that resulted in the anode surface accumulating too much Fe(OH)2 and Fe2+, promoting the conversion of corrosion products to Fe3O4.37 It was also likely that simultaneously the IOB metabolism rapidly consumed dissolved oxygen, creating an anaerobic environment for IRB bacteria whose metabolic activity also produced Fe3O4.38 The results of corrosion product analysis reveal that the high concentration of Cl− accelerates the conversion of the unstable corrosion product FeOOH to the stable product Fe3O4 in the IB system, making the corrosion product layer denser. This is consistent with the analysis of the cause of the rapid rise in film resistance in EIS, verifying the paper's conjecture of the cause of the corrosion rate decline.
3.4 Cl− concentration detection
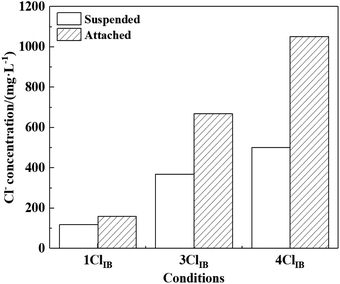
Corrosive ions impact the growth and attachment of IB, while the erosion of carbon steel by corrosive ions is also influenced by the growth of IB, especially their biofilm. The Cl− concentration on the carbon steel surface was measured on 15th day under different working conditions, and the results are shown in Fig. 8. The enrichment effect was evident after the increase of Cl− concentration, in which the Cl− concentration inside the biofilm was 1.81 times and 2.10 times higher than that in water under 3ClIB and 4ClIB conditions respectively. In the presence of high concentration of Cl− and IB, iron oxides precipitation increased, which lead to more pronounced adsorption.39,40In addition, both IRB and higher Cl− concentration stimulate the production of green rust, which allows a sustained flow of inorganic ions from the solution into the biofilm.41
The anodic reaction of metallic iron corrosion was originally reaction (2), but Darwish et al.42 and Burstein et al.43 found that Cl− can facilitate the dissolution of anodic iron by catalytic reaction (reactions (3)–(5)), which makes the corrosion reaction accelerate. In the late stage of corrosion, the protective film became the controlling factor of the corrosion reaction, preventing reactions (2)–(5) from occurring. The above demonstrates that high concentration of Cl− makes the corrosion product layer denser, and the effect of Cl− on biofilms will be further discussed in detail in 3.5.
| Fe → Fe2+ + 2e− | (2) |
| Fe + Cl− + H2O → [FeCl(OH)]ad− + H+ + e− | (3) |
| [FeCl(OH)]ad− → FeClOH + e− | (4) |
| FeClOH + H+ = Fe2+ + Cl− + H2O | (5) |
3.5 IB characteristics analysis
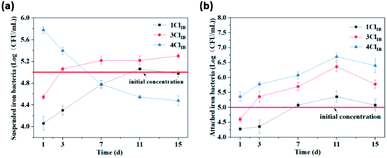
The trend of the number of IB in different working conditions is shown in Fig. 9. The number of suspended IB in 4ClIB on 1st day was 50 and 17 times higher than that of 1ClIB and 3ClIB, respectively. It indicates that high concentration of Cl− can promote the growth of suspended IB. Subsequently, the number of suspended IB in 1ClIB and 3ClIB both increased with time, but that for 4ClIB showed a decrease. The number of IB attached to the carbon steel interface within 15 days is shown in Fig. 9(b). Compared to the 1ClIB and 3ClIB conditions, the 4ClIB had an average of 1–1.5 orders of magnitude higher levels of attached IB. In comparison with the test results of Cl− concentration at the interface, the Cl− content at the interface of 4ClIB was 6.58 and 1.57 times higher than that of 1ClIB and 3ClIB on 15th day, which indicates that the high concentration of Cl− promoted the adhesion of IB on the surface of carbon steel. Initially, the high Cl− concentration, which released more Fe2+, led to faster growth of IB and faster biofilm formation. This resulted in less Fe2+ released into the water, leading to less IB in the water, but the number of IB in the biofilm continued to rise because of easier access to Fe2+.The optimum concentration of Cl− to promote the growth of IB was not established during the experiment. It may be due to the fact that this promotion is not mainly achieved by salt elevation, but because IB can oxidize the Fe2+ precipitated by corrosion to Fe3+ and derive energy from it,44,45 while this process also promotes the corrosion reaction to occur.
As time progressed, the increased Cl− concentration promoted the formation of IB biofilms, which, together with the dense corrosion products, formed a structurally dense protective film under the action of EPS, hindering corrosion occurrence. The synergistic slowing of corrosion by biofilm and corrosion products was also observed in the study by Wang et al.26 The experimental results of the IB characteristics analysis further prove the previous conjecture.
4 Conclusions
In this study, the effect of corrosive Cl− ions on the corrosion behavior of carbon steel in IB system was investigated in the context of reclaimed water reuse for circulating cooling water. It was found that the corrosion reaction was promoted by the increase of Cl− concentration in the early stage of corrosion, while with the extension of time, the high concentration of Cl− slowed down the corrosion rate instead.The increase in Cl− concentration, on one hand, leads to the enrichment of Cl− on the surface of carbon steel and in the corrosion product layer, which destroys the passivation film and increases the dissolved Fe2+. At the same time IB metabolic operations were also increased. Both of these make the anode potential and charge transfer resistance drop, accelerating the corrosion process. During this period, the anodic reaction may have been accelerated by the catalytic effect of Cl− and iron bacterial metabolism, and the iron solubilization reaction was accelerated. The rapid corrosion reaction results in a rapid accumulation of corrosion products and rapid consumption of dissolved oxygen. On the other hand, increased concentration of Fe2+ and oxygen starvation providing conditions for IRB to survive, this results in an increase of more dense corrosion products Fe3O4. Meanwhile, with the development of time, the higher the concentration of Cl−, which causes the higher number of attached IB in the system result in the faster development of the denser biofilm. The EPS secreted by IB can complex with corrosion products, forming a denser protective film and inhibiting the anodic Fe2+ leaching. The experimental results showed a significant increase in anode slope and membrane impedance. At the same time, the cathodic slope also increased, indicating that the reduction reaction of oxygen was also affected. In summary, high concentration of Cl− exhibits corrosion-inhibiting effect in the later stage of corrosion.
To further investigate the effect of chloride ions on growth factors affecting iron bacterial corrosion. Further studies are needed to clarify the mechanism of Cl− effects on metabolic behavior, biofilm structure, and adhesion behavior of iron bacteria.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 51578035).Notes and references
- S. J. Cai, X. Y. Zhao and Y. Y. Wang, Ind. Water Treat., 2009, 29, 4 CAS.
- J. Jin, G. Wu and Y. Guan, Water Res., 2015, 17, 207 CrossRef PubMed.
- X. Xu, S. Liu, K. Smith, Y. Cui and Z. Wang, J. Clean. Prod., 2020, 276(10), 124079 CrossRef CAS.
- Z. Panossian, N. Almeida, R. Sousa, G. Pimenta and L. Marques, Corros. Sci., 2012, 58, 1 CrossRef CAS.
- B. Hou, X. Li, X. Ma, C. Du, D. Zhang, M. Zheng, W. Xu, D. Lu and F. Ma, npj Mater. Degrad., 2017, 1, 4 CrossRef.
- H. Liu, C. Fu, T. Gu, G. Zhang, Y. Lv, H. Wang and H. Liu, Corros. Sci., 2015, 100, 484 CrossRef CAS.
- Y. Tang, Z. Yu, J. Wang, X. Zhao, B. Niu and L. Bing, Corros. Sci., 2014, 80, 111 CrossRef CAS.
- H. Li, C. Dong, K. Xiao, X. Li and P. Zhong, Int. J. Miner., Metall. Mater., 2016, 23, 1286 CrossRef CAS.
- Y. An, The study of localized corrosion mechanism of typical engineering metal material in seawater containing microorganism, Institute of Oceanology, Chinese Academy of Science, Qingdao, Doctoral Dissertation, 2010, p. 18 Search PubMed.
- H. X. Li, D. P. Li, L. Zhang, Y. W. Wang, X. Y. Wang and M. X. Lu, Int. J. Miner., Metall. Mater., 2019, 26, 329 CrossRef CAS.
- H. Wang, L. Ju, H. Castaneda, C. Gang and B. Newby, Corros. Sci., 2014, 89, 250 CrossRef CAS.
- S. Chen, The study of localized corrosion mechanism of typical engineering metal material in seawater containing microorganism, Doctoral Dissertation, Institute of Oceanology, Chinese Academy of Science, Qingdao, 2015, vol. 109 Search PubMed.
- J. Starosvetsky, R. Kamari, Y. Farber, D. Bilanovic and R. Armon, Corros. Sci., 2016, 102, 446 CrossRef CAS.
- H. Wang, C. Hu, L. Zhang, X. Li and Y. Min, Water Res., 2014, 65, 362 CrossRef CAS PubMed.
- Z. Li, J. Wang, Y. Dong, D. Xu and F. Wang, J. Mater. Sci. Technol., 2021, 71, 177 CrossRef CAS.
- T. I. Wu and J. K. Wu, Corrosion, 1995, 51, 185 CrossRef CAS.
- Y. H. Wang, Q. Wang, S. R. Yu and Y. L. Song, J. South China Univ. Technol., Nat. Sci., 2008, 36, 92 Search PubMed.
- Z. Zin, Y. Yu, Y. C. Wang, P. Q. Zhang, J. Z. Duan and B. R. Hou, Corros. Prot., 2014, 47, 57 Search PubMed.
- S. F. Zhou, X. F. Yin, W. G. Zhou, C. Sun and A. H. Han, Corros. Sci. Prot. Technol., 2004, 16, 4 Search PubMed.
- Y. Chu, P. Xu, Y. Ou, P. Bai and Z. Wei, Sci. Total Environ., 2020, 718, 136679 CrossRef CAS PubMed.
- S. C. Bo, China University of Petroleum, Qingdao, 2010, 41.
- J. L. Gao, Research of influencing factors on iron release in water distribution system, Zhejiang University, Hangzhou, 2013, p. 23 Search PubMed.
- H. Wang, C. Hu and X. Li, Eng. Failure Anal., 2015, 57, 423 CrossRef CAS.
- C. G. E. M. V. Beek, T. Hiemstra, B. Hofs, M. M. Nederlof, J. A. M. V. Paassen and G. K. Reijnen, Aqua, 2012, 61, 1 Search PubMed.
- Y. Qi, The study of influence caused by water quality factors on stainless steel material in reclaimed water and corrosion control, Beijing Jiaotong University, Beijing, 2017, 38 Search PubMed.
- H. B. Wang, C. Hu, X. X. Hu, M. Yang and J. H. Qu, Water Res., 2012, 46, 1070 CrossRef CAS PubMed.
- H. Qian, J. Zhang, T. Cui, L. Fan, X. Chen, W. Liu, W. Chang, C. Du and D. Zhang, Bioelectrochemistry, 2021, 140, 107746 CrossRef CAS PubMed.
- K. Pranav, S. S. Su, M. S. Mannan, H. Castaneda and S. A. Vaddiraju, Ind. Eng. Chem. Res., 2018, 57, 13895 CrossRef.
- Z. G. Jiang, H. H. Wang, L. H. Gong, G. P. Chen and X. J. Chen, Mater. Prot., 2017, 50, 74 Search PubMed.
- S. H. Li, Y. Y. Zhang, J. H. Liu and M. Yu, Acta Phys.-Chim. Sin., 2008, 24, 1553 CrossRef CAS.
- S. J. Yuan, A. M. F. Choong and S. O. Pehkonen, Corros. Sci., 2007, 49, 4352 CrossRef CAS.
- M. Zhu, C. W. Du, X. G. Li, Z. Y. Liu, S. R. Wang, T. L. Zhao and J. H. Jia, J. Mater. Eng. Perform., 2014, 23, 1358 CrossRef CAS.
- H. Liu and Y. F. Cheng, Electrochim. Acta, 2018, 10, 312 CrossRef.
- M. Behpour, S. Ghoreishi, N. Mohammadi, B. N. Soltani and N. M. A. Salavati, Corros. Sci., 2010, 52, 4046 CrossRef CAS.
- M. Ghafari, A. Bahrami, I. Rasooli, D. Arabian and F. Ghafari, Int. Biodeterior. Biodegrad., 2013, 80, 29 CrossRef CAS.
- J. Scheerder, R. Breur, T. Slaghek, W. Holtman, M. Vennik and G. Ferrari, Prog. Org. Coat., 2012, 75, 224 CrossRef CAS.
- M. Yamashita, H. Miyuki, Y. Matsuda, G. Nagano and T. Misawa, Corros. Sci., 1994, 25, 283 CrossRef.
- H. F. Sun, B. Y. Shi, D. A. Lytle, Y. H. Bai and D. S. Wang, Environ. Sci.: Processes Impacts, 2014, 16, 576 RSC.
- A. Jaiswal, S. Banerjee, M. Raj and M. C. Chattopadhyaya, J. Environ. Eng., 2013, 1, 281 CAS.
- K. Jafarzadeh, S. Shahrabi and M. M. Hadavi, J. Mater. Sci. Technol., 2007, 5, 623 Search PubMed.
- J. T. Jin, The biological-chemical coupling mechanism of microbiallly influenced corrosion in reclaimed wastewater pipeline, Tsinghua University, Beijing, 2015, p. 11 Search PubMed.
- N. A. Darwish, A. Hilbert, W. J. Lorenz and H. Rosswag, Electrochim. Acta, 1973, 18, 421 CrossRef CAS.
- G. T. Burstein and D. H. Davies, Corros. Sci., 1980, 20, 28 Search PubMed.
- S. C. Neubauer, D. Emerson and J. P. Megonigal, Appl. Environ. Microbiol., 2002, 68, 3988 CrossRef CAS PubMed.
- E. E. Roden, D. Sobolev, B. Glazer and G. W. Luther, Geomicrobiol. J., 2004, 21, 379 CrossRef CAS.
Footnote |
| † These authors contributed to the work equally and should be regarded as co-second authors. |
| This journal is © The Royal Society of Chemistry 2022 |