Open Access Article
Open Access ArticleHydrothermal synthesis of Bi2Se3 nanosheets by using gallic acid as a reductant†
Di Huo *ab,
Gongge Lina and
Mengfan Lv‡
*ab,
Gongge Lina and
Mengfan Lv‡
 a
a
aSchool of Materials Science and Engineering, Key Laboratory for Anisotropy and Texture of Materials (Ministry of Education), Northeastern University, Shenyang, 110819, Liaoning, PR China. E-mail: huod@atm.neu.edu.cn
bAdvanced Ceramics Research Center, Department of Materials Science, Northeastern University, Shenyang, 110819, Liaoning, PR China
First published on 18th May 2022
Abstract
In this work, a simple and reproducible hydrothermal synthesis was employed to synthesize two-dimensional Bi2Se3 nanosheets by using gallic acid as a reductant. Meanwhile, the effects of the amounts of gallic acid and sodium hydroxide and the surfactant Triton X-100 on phase composition and morphology of the obtained Bi2Se3 were also studied. The results reveal that gallic acid could effectively reduce Se4+ to Se2− and gave rise to the formation of Bi2Se3. Additionally, keeping the reaction conditions of molar ratio of gallic acid to the precursor elements (Bi + Se) at 1 to 1 (or higher) and using strong alkaline solutions were the key factors to synthesize high purity crystalline Bi2Se3 nanosheets. Furthermore, flower-like Bi2Se3 composed of nanosheets with a dozen nanometer thickness could be easily fabricated by adding appropriate amounts of Triton X-100. This work provides a novel approach for synthesis of ultra-thin Bi2Se3 nanosheets in a controllable manner.
1. Introduction
Bi2Se3, a VA-VIA direct semiconductor with a band gap Eg = 0.3 eV, has attracted much interest due to its great potential application in different fields such as optic-electronic sensitive devices,1 room temperature thermoelectrics,2 electrochemical hydrogen storage,3 lithium battery electrodes4 and photothermal therapy heating.5 Additionally, Bi2Se3 is recognized as one of the typical topological materials which is insulating in the bulk but conducting on a surface with great potential usefulness in quantum computing and spintronic devices.6,7 Bi2Se3 possesses the rhombohedral unit cell in which the equivalent atoms locate in a two-dimensional layer perpendicular to the c-axis. Five such layers arrange in the sequence of Se1–Bi–Se2–Bi–Se1. The Se atoms occupying two different sites form a tight bound unit that is termed quintuple layer (QL).8 The chemical bonds between the QLs generally belong to van der Waals forces and the insides of these QLs are considered to be predominantly covalent. Thus, the characteristic of Bi2Se3 structure usually leads to sheets-like morphology because of anisotropic crystal growth. Many researches have shown that the physical and chemical properties of Bi2Se3 are well related to its crystal size and morphology. For example, the enhanced thermoelectric performances were achieved on nanostructured Bi2Se3 ceramics that were constituted of ultra-thin plates.9,10 Therefore, it is important to synthesize Bi2Se3 with controlled crystal structure and morphology so as to realize the specific performances.A number of techniques have been used in controlling synthesis of nanostructured Bi2Se3.4,5,11–14 Therein the hydro/solvothermal synthesis was employed most popularly due to the advantages of simple, flexible, mild reactive condition and batch productivity. Meanwhile, crystal morphology of as synthesized samples can be controlled and modulated through changing reaction parameters including temperature, time, pH value, starting chemicals and surfactants. By now, Bi2Se3 with various morphologies such as nanoparticles, nanorods, nanobelts, and flowers-like have been fabricated by hydro/solvothermal route.14–16 Even though successfully used in preparation of various nanostructured Bi2Se3, further improvement of hydro/solvothermal method needs to make is to exclude the utilization of many toxic and hazardous reducing agents such as N2H4·H2O, NaBH4, ethylenediamine and some organic chemicals that are detrimental to natural environments. There has been a few researches on trying the green synthesis to make nanostructured Bi2Se3,17,18 but it is still necessary to develop novel synthetic route of nanostructured Bi2Se3 in a reliable and sustainable way.
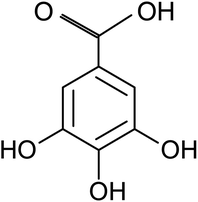
Gallic acid (GA), or 3, 4, 5-trihydroxybenzoic acid with molecular formula C7H6O5, is a naturally occurring polyphenolic compound which wildly distributes in various plants and natural herbs such as Vitisvinifera, Syzagiumcordatum, Caesalpiniasappan, and Chinese gallnuts, Terminalia Chebulu Retz.19 It is also abundant in various fruits and beverages including walnut, strawberry, apple, grapes, green tea, and red wine.20 GA in its free form or derivatives can be produced easily by extracting from natural plant sources or biosynthesis production at industrial level. As one of the natural active compounds, the biological and pharmacological properties of GA have been well recognized. Currently it is mainly used in fields of biomedicine and function food as antiviral, antibacterial and antioxidative agents.19 Besides its important biochemical value, GA has also found wild applications in the packaging, cosmetic, and textile industries.21 GA is a planar molecule that consists of an aromatic ring with three phenolic hydroxyl groups and a carboxylic acid group, as shown in Fig. 1. There are many chemical reaction pathways due to the existence of the active groups on it. It is generally believed that mild reducing effect of GA molecule comes from these phenolic hydroxyl groups in the process of chemical reaction in aqueous solutions.20 Over the last few years, noble metallic particles including Au, Pt and Ag have been prepared using GA as reducing and capping agent,22–24 and graphene oxide was also reduced to graphene by GA.25 However, to the best of our knowledge there has been no report about preparing inorganic metal selenides nanosheets by using GA as reductant.
In this work, we report a novel green approach for hydrothermal synthesis of Bi2Se3 nanosheets by using GA as reductant and crystal growth modifier. Meanwhile, the effect of the amounts of GA, NaOH and Triton X-100 surfactant on composition and morphology of Bi2Se3 was also studied. Gram-scale, high crystallinity, ultrathin Bi2Se3 nanosheets were obtained in a controllable manner.
2. Experimental
All of the chemicals including Selinium dioxide (SeO2, 99.99%), Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O, 99.9%), Sodium hydroxide (NaOH, 99%), Gallic acid monohydrate and Triton X-100 purchased from Shanghai Aladdin Bio-Chem Technology Co., LTD were used without further treatment. The aqueous solutions were made using deionized water.The hydrothermal synthesis procedure of Bi2Se3 nanosheets was as follows: firstly, NaOH (45 mmol, or 1.8 g) was dissolved in 70 ml deionized water under magnetic stirring at 70 °C for 3 min. Then, SeO2 (6 mmol, or 0.6658 g) and Bi(NO3)3·5H2O (4 mmol, or 1.9403 g) were added in sequence followed by further stirring for 20 min, and then, GA (10 mmol, or 1.8813 g) was added into the mixture solution accompanied by appropriate amount of Triton X-100 if necessary. After that, the mixed solution was transferred into a Teflon-lined autoclave of 100 ml capacity. The autoclave was sealed and maintained at 200 °C for 24 h and then allowed to cool to room temperature. The solid precipitate was separated by centrifugation, washed with deionized water for 3 times and absolute ethanol for 1 time, and finally about 1.2 g Bi2Se3 powder was obtained after drying at 60 °C for 8 h in vacuum. In order to control composition and to tailor morphology of the samples, GA was set in the range of 0.47–3.76 g; NaOH was of 0.6–2.4 g and Triton X-100 was of 0.005–5 g, respectively.
X-ray diffraction (XRD) pattern was carried out on a Japan Rigaku D/max X-ray diffractometer with graphite monochromatized Cu-Kα radiation (λ = 1.54056 Å) operating at tube voltage 40 KV and current of 20 mA. Field emission scanning electron microscopy (FE-SEM) image was taken on a Japan JEOL, JSM-7001F SEM to observe morphology of the samples. High resolution/Transmission electron microscopy (HRTEM/TEM) and selected area electronic diffraction (SAED) were performed with a Japan JEM-2100 F TEM at 100 kV accelerated voltage. X-ray energy dispersive spectroscopy (EDS) equipped on TEM was used to detect chemical elements of the samples. Fourier Transform Infrared (FTIR) spectra were performed with a RXI PerkinElmer spectrometer in the range of 500–4000 cm−1 at room temperature with the sample in a KBr disk. X-ray Photoelectronic Spectra (XPS) was collected on AXIS-SUPRA, Shimadzu-Kratos X-ray photoelectron spectrometer with Mono Al-Kα X-ray as the excitation source (1486.6 eV) to study chemicals and elemental valence of the products.
3. Results and discussion
3.1 Composition and morphology
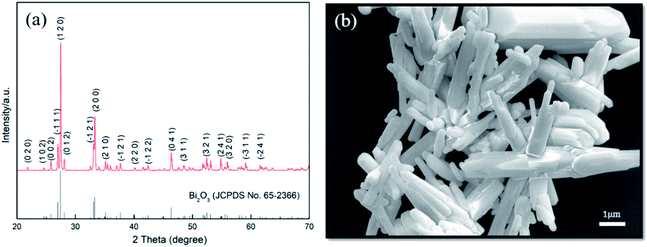
First of all, the reduction effect of GA is illustrated on successful acquisition of the target compound after hydrothermal reaction. Fig. 2a shows XRD pattern of the product synthesized at 200 °C for 24 h. All diffraction peaks can be indexed in accordance with standard Bi2Se3 (JCPDS No 89-2008) with hexagonal structure of a = b = 4.143 Å, c = 28.635 Å. There are no any other phases left in the sample, which suggests pure Bi2Se3 is obtained. Fig. 2b shows the SEM image of Bi2Se3, almost all of the Bi2Se3 crystallizes in two-dimensional nanosheets-like morphology and some of them display clearly hexagonal plate shape with lateral length of 1–3 μm. It can be seen that a number of smaller sheet-like crystals grow out perpendicularly on surface of the basal hexagonal plates, which exhibit similar shapes as reported by Liu et al.26 In most cases, the as synthesized Bi2Se3 grew into flower-like hierarchical nanostructures due to existence of dislocations generated in the process of Bi2Se3 growth.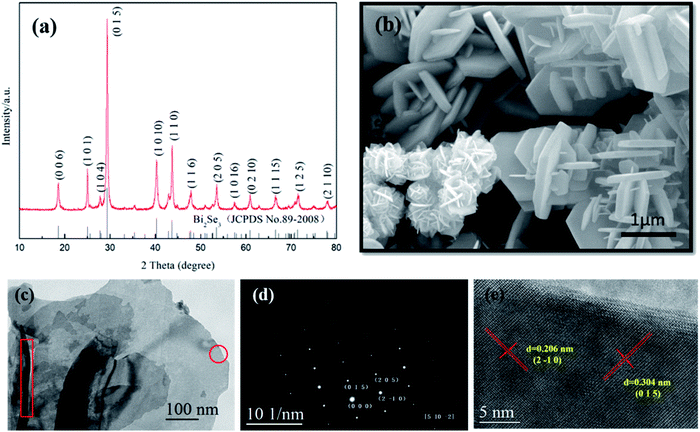 | ||
| Fig. 2 (a) XRD pattern, (b) SEM, (c) TEM, (d) SAED and (e) HRTEM images of as synthesized Bi2Se3 nanosheets. | ||
HRTEM/TEM were used to further characterize the as synthesized Bi2Se3 nanosheets. As shown in Fig. 2c–e, the nanosheets keep smooth and approximate hexagonal flat shape, SAED analysis demonstrates its single crystalline nature, and the crystal lamellar distances within the single crystal zone on the HRTEM image are 3.04 Å and 2.06 Å, which is corresponding to (015) plane and (2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) plane of Bi2Se3, respectively. Although the precise thickness of the Bi2Se3 plates was not directly determined, it still can be seen that the estimated average thickness was about 30 nm, as indicated in red rectangle on TEM image.
0) plane of Bi2Se3, respectively. Although the precise thickness of the Bi2Se3 plates was not directly determined, it still can be seen that the estimated average thickness was about 30 nm, as indicated in red rectangle on TEM image.
Fig. 3a shows the FTIR spectrum of as synthesized Bi2Se3 nanosheets, it appears that there are various vibration absorption peaks arising from different chemical groups. The absorption at around 3349 cm−1 can be assigned to the stretching vibration of O–H banding which is brought about by phenolic hydroxyl of GA.27 The other absorption peaks may be attributed to the residual organic groups or derivatives coming from the dissociated GA molecule under high pressure in hydrothermal reaction. For example, the peak appears at around 1355 cm−1 is attributed to C–O stretching vibration,28,29 and the strongest absorption band at around 1620 cm−1 can be well assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of COOH group.28–30 In addition, the absorption band at around 1851 cm−1 might be ascribed to the stretch vibration of C
O stretching vibration of COOH group.28–30 In addition, the absorption band at around 1851 cm−1 might be ascribed to the stretch vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O quinone carbonyl group conjugated to aromatic ring.31 All of these FTIR absorption bands confirm the presence of organic residues from GA.
O quinone carbonyl group conjugated to aromatic ring.31 All of these FTIR absorption bands confirm the presence of organic residues from GA.
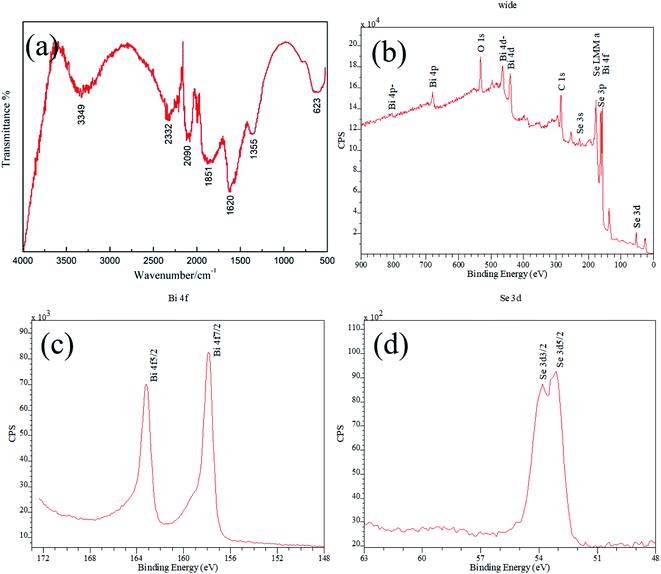 | ||
| Fig. 3 (a) Infrared spectroscopy and XPS (b) wide scan spectrum, (c) Bi 4f, (d) Se 3d of as synthesized Bi2Se3. | ||
XPS calibrated with reference to C1s (bonding energy 284.8 eV) was carried out to evaluate the composition and chemical valence of as synthesized Bi2Se3. The wide scan spectrum confirms that element Bi, Se, C and O signals presenting in the product, among which C and O elements should originate from the organic acid residues (Fig. 3b). Except for these two elements, the obtained XPS scan spectrum is identical with that reported in other works.13,32 The spectrum peaks of Bi 4f and Se 3p overlap at binding energy of about 160 eV, and the 4f have much higher intensities than the 3p transitions. In the fine spectrum of Bi element in Bi2Se3, the binding energy of Bi 4f7/2 at 157.9 eV and Bi 4f5/2 at 163.2 eV generate identical chemical shift of 1.3 eV compared to the binding energy at 156.6 eV and 161.9 eV of metallic Bi, with energy splitting of 5.3 eV (Fig. 3c). Similarly, the binding energy of Se 3d3/2 at 53.8 eV and Se 3d5/2 at 53.1 eV with energy splitting of 0.7 eV generate chemical shift of −1.5 eV and −1.4 eV, respectively, comparing to the binding energy at 55.3 eV and 54.5 eV of elemental Se (Fig. 3d). Additionally, Bi 4d levels with energy splitting of 23.5 eV can be observed in the spectrum. All of the features are accordance with the data that reported by other researchers,32,33 indicating that pure Bi2Se3 compound is synthesized.
3.2 Effects of the processing parameters
Based on the comparison experiment above, we can conclude that Bi2Se3 is generated by hydrothermal synthesis only when GA is present in solution. In order to understand the detailed effect of GA on phase composition and morphology, the samples with various GA contents were synthesized. The XRD patterns of these samples are shown in Fig. 5. Compared to the sample without adding GA, it can be seen the target compound Bi2Se3 appears promptly after adding GA. When 0.47 g GA was added (Fig. 5a), the phase composition of the product is consisted of dominated Bi2Se3, a small amount of Bi2O3 and trace of elemental Se (JCPDS No 73-0465). The diffraction peaks of Bi2O3 and Se become obviously less when GA content increases (Fig. 5b). When GA achieves at 1.41 g (Fig. 5c), the two intermediate phases disappear totally and only pure hexagonal Bi2Se3 exists. With further increasing of GA, as shown in Fig. 5d and e, the locations of diffraction peak keep unchanged except that their peaks become sharp as well as their HWFM become narrowed, which means highly crystallinity of Bi2Se3 is gotten. Therefore, adding sufficient amount of GA is necessary to provide enough reducing ability to finish the reaction. If setting the total precursor elements (Bi + Se) at a fixed value, the molar ratio of GA to the total elements had better to maintain at 1 to 1 (or higher) so that GA can reduce Se4+ to Se2− completely for getting pure Bi2Se3.
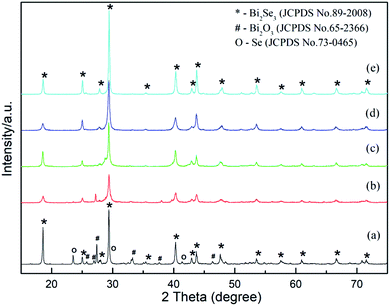 | ||
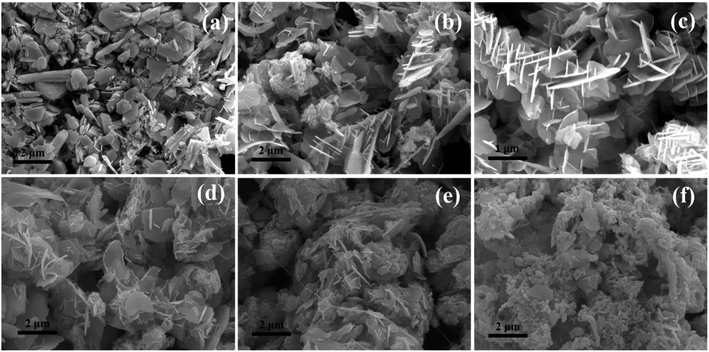
| Fig. 5 XRD patterns of Bi2Se3 added various amount of GA. (a) 0.47 g, (b) 0.94 g, (c) 1.41 g, (d) 1.88 g, (e) 3.76 g. | ||
The reductive effect of GA is generally believed coming from the phenolic hydroxyl groups of the molecular structure. GA is a strong natural antioxidant, and this property is related with the redox behavior.19 The hydroxyl radicals bonded to benzene ring can be readily oxidized along with deprotonation and releasing of electrons, simultaneously. It is known that GA is oxidized to quinone via semiquinone form. The oxidation process of GA can precede by transferring one electron via the formation of semiquinone radical cation (GA˙+), then the formed radical cation loses a proton to form the intermediate semiquinone radical (GA˙), irreversibly. This reaction will be followed by a second irreversible electron transferring by forming quinine cation (GA+), finally deprotonation of the quinine cation (GA+) completes the overall two-electron process to give a quinine (GAO).38 Therefore, here the released electrons from GA were transferred to Se4+ and the resulted Se2− was used to form Bi2Se3 in the process of hydrothermal synthesis. The appearance of the strongest absorption peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O radical in FTIR spectrograph may also support the presumption here.
O radical in FTIR spectrograph may also support the presumption here.
It has been proposed that in strong alkaline medium the reduction process of Se4+ to Se2− may proceed by either a two-step process of Se4+ → Se and Se → Se2− or a one-step process of Se4+ → Se2− by directly transferring six electrons.39 It seems that the two-step mechanism worked in this experiment, because elemental Se was observed as an intermediate product in the process of hydrothermal reaction. Some Se4+ ions just finished the first part of the two-step reduction process when GA and NaOH contents were less than a certain value.
Based on the evolvement of phase components in XRD patterns (Fig. 5) and analysis above, the hydrothermal reaction pathway of Bi2Se3 formation might be outlined as follows. When GA is absent in solution, the reactions (1)–(3) carry out and only Bi2O3 is formed.
| Bi(NO)3 + NaOH → Bi(OH)3 + Na+ + (NO)−3 | (1) |
| SeO2 + NaOH + H2O → Na2SeO3(soluble) + OH− | (2) |
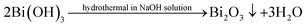 | (3) |
On the contrary, when GA is present in solution, the reactions (4)–(8) occur and Bi2Se3 is obtained.
| GA → GA˙+ + e′ → GA˙ + e′ + H+ | (4) |
| GA˙ → GAO + e′ + H+ | (5) |
| Se4+ + 4e′ → Se | (6) |
| Se + 2e′ → Se2− | (7) |
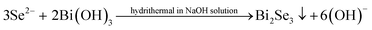 | (8) |
It is necessary to note that theoretically electrochemical reduction potential of Se (EoSe/se2−Se/se) is −0.924 V,39 and cyclic voltammogram (CV) electrochemical measurement for GA has confirmed reductive potentials of −1.20 V, −1.44 V, −1.65 V and −1.90 V for each hydroxyl group and carboxylic acid group in a strong alkaline solution.35 So increasing GA concentration are obviously favorable for completing the whole two-step reduction of Se4+ to Se2− and forming Bi2Se3 in terms of electrochemical reduction potentials, especially at high concentration of NaOH.
Besides the capability of reducing Se4+ to Se2− state, GA can also alter morphology of as synthesized Bi2Se3. With addition of 0.47 g GA, mixed morphologies composing predominated platelets are observed (Fig. 6a). When the GA contents continuously increase to 1.41 g and then to 1.88 g, nanosheets-like morphology of Bi2Se3 crystals become more prominence, as illustrated by their larger plate areas and thinner thickness. Additionally, their flower-like characteristics become obviously (Fig. 6b and c). However, when further increasing GA contents up to 2.35 g and 2.82 g (Fig. 6d and e), nanosheets-like morphologies gradually disappear. As adding 3.76 g GA, irregular agglomerated Bi2Se3 nanoparticles with few layered particles emerge in the sample (Fig. 6f). High concentration of GA will accelerate the reductive rate of Se4+ to Se2− and boost the reaction kinetics of Bi2Se3 formation. Thus, a large number of Bi2Se3 crystals nucleate in short time, these nucleus aggregate together quickly rather than grow into nanosheets in an anisotropic manner. In addition, when the amount of GA is excessive in solution, the freshly formed surface of inorganic Bi2Se3 particles may be covered by GA molecule or its derivatives, which may also lead to the inhibition of directional crystal growth. Overall, the suitable amount of GA for controllable synthesis of Bi2Se3 nanosheets are in range of 1.41 g ∼2.35 g.
Fig. S1† shows the XRD pattern of the samples added with different amounts of NaOH. When adding 0.6 g NaOH, the product is consisted of dominated Bi2Se3 with minor elemental Se residues, but none Bi2O3 exists (Fig. S1a†). Low concentration of NaOH does not only slow the degree of oxidation of GA, which may lead to poor reductive efficiency of Se → Se2−, but also make the precipitations of Bi2O3 unfavorable. When NaOH increases up to 1.2 g or above, hexagonal Bi2Se3 phase with high crystallinity is obtained, as shown in Fig. S1(b–d).†
Increasing the amount of NaOH or pH value of hydrothermal solution is beneficial for the formation of Bi2Se3 due to enhanced reducing ability of GA. Meanwhile, it shows that crystal morphologies of Bi2Se3 changes accordingly with different alkaline degree in aqueous solution (Fig. S2†). Apparently, the flat surface area of Bi2Se3 nanosheets increases with increasing amounts of NaOH. In Fig. S2a,† the nanoparticles dispersing on surfaces of Bi2Se3 nanosheets added with 0.6 g NaOH might be attributed to elemental Se, as indicated in the XRD pattern in Fig. 6. The rates of nucleation and crystal growth will be thermodynamic accelerated under the strong alkaline condition during synthesizing nanostructured chalcogenides.41 Similarly, the formation of Bi2Se3 would be facilitated as NaOH contents increase in solution, and then leading to the enlarged flat areas and decreased thickness of layered Bi2Se3 nanosheets. Their shapes become nearly hexagonal plate-like in the end, as shown in Fig. S2(b–d).†
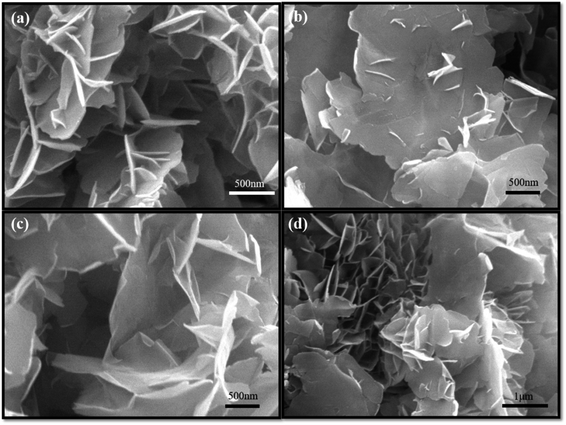
Fig. 7 shows SEM images of Bi2Se3 added with various amounts of Triton X-100. When 0.05 g Triton X-100 was added, the thickness of Bi2Se3 nanosheets started to decrease obviously (Fig. 7a). With continuously adding Triton x-100 to 0.2 g and further to 0.625 g, the plate area increases and the thickness decreases, accordingly. The size of a single nanosheet is about 3 μm in wide and a dozen of nanometer in thickness (Fig. 7b and c). As the surfactant increased up to 5 g, the lateral wide and thickness of the ultrathin nanosheets remain unchanged essentially (Fig. 7d). It is reasonable to postulate that hydrophobic side of the amphiprotic Triton X-100 absorbs directionally on nanosheets surfaces, thus forming ultrathin Bi2Se3 nanosheets with mainly lateral crystal growth. On the other hand, the hydrophilic side of Triton X-100 played a role on dispersion that led to less aggregation of Bi2Se3 nanosheets. From the TEM image, one can find much smoother and less curved plate-like characteristic of these ultrathin Bi2Se3 nanosheets. The SAED proves its single crystalline nature and EDS analysis features standard Bi/Se molar ratio of 2/3 (Fig. S4†).
4. Conclusions
In this work, we develop a simple and reproducible hydrothermal synthesis method for preparing Bi2Se3 nanosheets, in which GA is used as reductant to reduce Se4+ to Se2− and then the Se2− ion further reacts with Bi3+ to form Bi2Se3. Along with the reducing effect, GA molecule can change the morphology of as synthesized Bi2Se3. It was also found that keeping sufficient amounts of GA and NaOH in hydrothermal reaction system are the key factors to the synthesis of pure crystalline Bi2Se3 nanosheets, and thinner Bi2Se3 nanosheets with a dozen of nanometer thickness can be easily synthesized when adding appropriate amount of non-ionic surfactant Triton X-100.5. Author contributions
Di Huo: Conceptualization, Methodology, Investigation, Writing-review & editing, Funding acquisition, Supervision. Gongge Lin: Performing experiments, Validation. Mengfan Lv: Performing experiments, Data collection.Conflicts of interest
There are no conflict of interests to declare.Acknowledgements
The authors acknowledge Dr Yujie Liu for assistance on TEM operation and Dr Junqing Xia for collecting XPS data. This work was funded in part by National Nature Science Foundation of China (No 51872033).References
- A. K. Rath, M. Bernechea, L. Martinez and G. Konstantatos, Adv. Mater., 2011, 23, 3712–3717 CrossRef CAS PubMed.
- W. Liu, K. Lukas, K. McEnaney, S. Lee, Q. Zhang, C. P. Opeil, G. Chen and Z. F. Ren, Energy Environ. Sci., 2013, 6, 552–560 RSC.
- Z. Sun, F. Liu, X. Chen and L. Chen, Chem. Commun., 2010, 46, 3101–3103 RSC.
- H. M. Xu, G. Chen, R. C. Jin, J. Pei, Y. Wang and D. H. Chen, CrystEngComm, 2013, 15, 1618–1625 RSC.
- H. Zhou, L. Che, Z. M. Guo, M. H. Wu, W. Q. Li, W. P. Xu and L. F. Liu, ACS Sustainable Chem. Eng., 2018, 6, 4863–4870 CrossRef CAS.
- M. Salvato, M. Scagliotti, M. de Crescenzi, P. Castrucci, F. de Matteis, M. Crivellari, S. PelliCresi, D. Catone, T. Bauch and F. Lombardi, Nanoscale, 2020, 12, 12405 RSC.
- L. A. Wray, S. Y. Xu, Y. Q. Xia, Y. S. Hor, D. Qian, A. V. Fedorov, H. Lin, A. Bansil, R. J. Cava and M. Z. Hasan, Nat. Phys., 2010, 6, 855–859 Search PubMed.
- Z. G. Chen, G. Han, L. Yang, L. Cheng and J. Zou, Prog. Nat. Sci.: Mater. Int., 2012, 22, 535–549 Search PubMed.
- C. Bauer, I. Veremchuk, C. Kunze, A. Benad, V. M. Dzhagan, D. Haubold, D. Pohl, G. Schierning, K. Nielsch, V. Lesnyak and A. Eychmüller, Small Sci., 2020, 1, 2000021 Search PubMed.
- M. Hong, Z. G. Chen, L. Yang, G. Han and J. Zou, Adv. Electron. Mater., 2015, 1, 1500025 Search PubMed.
- R. Harpeness and A. Gedanken, New J. Chem., 2003, 27, 1191–1193 RSC.
- H. M. Cui, H. Liu, X. Li, J. Y. Wang, F. Han, X. D. Zhang and R. I. Boughton, J. Solid State Chem., 2004, 177, 4001–4006 CrossRef CAS.
- J. R. Ota, P. Roy, S. K. Srivastava, R. Popovitz-Biro and T. Reshef, Nanotechnology, 2006, 17, 1700–1705 CrossRef CAS PubMed.
- B. Belec, K. Ferfolja, T. Goršak, N. Kostevšek, S. Gardonio, M. Fanetti and M. Valant, Sci. Rep., 2019, 9, 19057 CrossRef CAS PubMed.
- H. Fan, S. X. Zhang, P. Ju, H. C. Su and S. Y. Ai, Electrochim. Acta, 2012, 64, 171–176 CrossRef CAS.
- Y. Xie, H. Su, B. Li and Y. T. Qian, Mater. Res. Bull., 2000, 35, 459–464 CrossRef CAS.
- M. Kuroda, S. Suda, M. Sato, H. Ayano, Y. Ohishi, H. Nishikawa, S. Soda and M. Ike, Appl. Microbiol. Biotechnol., 2019, 103, 8853–8861 CrossRef CAS PubMed.
- H. M. Xu, G. Chen, R. C. Jin, D. H. Chen, Y. Wang and J. Pei, RSC Adv., 2014, 4, 8922–8929 RSC.
- B. Badhani, N. Sharma and R. Kakkar, RSC Adv., 2015, 5, 27540 RSC.
- S. Dhiman and M. Mukherjee, Gallic acid (GA): A multifaceted biomolecule transmuting the biotechnologu era, in Recent Developments in microbial Technologies, Environmental and Microbial Biotechnology, ed. R. Prasad, Spinger Natural Singapore Pte Ltd, 2021, pp. 163–202 Search PubMed.
- B. R. Albuquerque, S. A. Heleno, M. B. Oliveira, L. Barros and I. C. R. Ferraira, Food Funct., 2021, 12, 14 RSC.
- K. Yoosaf, B. I. Ipe, C. H. Suresh and K. G. Thomas, J. Phys. Chem. C, 2007, 111, 12839–12847 CrossRef CAS.
- G. A. Martinez-Castanon, N. Nino-Martinez, F. Martinez-Gutierrez, J. R. Martinez-Mendoza and F. Ruiz, J. Nanopart. Res., 2008, 10, 1343–1348 CrossRef CAS.
- K. M. Kumar, B. K. Mandal and S. K. Tammina, RSC Adv., 2013, 3, 4033 RSC.
- J. Li, G. Y. Xiao, C. B. Chen, R. Li and D. Y. Yan, J. Mater. Chem. A, 2013, 1, 1481 RSC.
- X. L. Liu, J. W. Xu, Z. C. Fang, L. Lin, Y. Qian, Y. C. Wang, C. M. Ye, C. Ma and I. Zeng, Nano Res., 2015, 8, 3612–3620 CrossRef CAS.
- F. Billes, I. Mohammed-Ziegler and P. Bombicz, Vib. Spectrosc., 2007, 43, 193–202 CrossRef CAS.
- G. Cirillo, S. Hampel, R. Klingeler, F. Puoci, F. Iemma, M. Curcio, O. I. Parisi, U. G. Spizzirri, N. Picci, A. Leonhardt, M. Ritschel and B. Büchner, J. Pharm. Pharmacol., 2011, 63, 179–188 CrossRef CAS PubMed.
- K. M. Kumar, B. K. Mandal and S. K. Tammina, RSC Adv., 2013, 3, 4033–4039 RSC.
- A. H. Nadim, M. A. Al-Ghobashy, M. Nebsen and M. A. Shehata, RSC Adv., 2015, 5, 104981–104990 RSC.
- P. R. Griffiths and J. A. de Haseth, Fourier transform infrared spectroscopy, John wiley& Sons, Hoboken, NJ, 1986 Search PubMed.
- M. R. Thuler, R. L. Benbow and Z. Hurych, Chem. Phys., 1982, 71, 265–270 CrossRef CAS.
- V. B. Nascomento, V. E. de Carvslho, R. Paniago, E. A. Soares, L. O. Ladeira and H. D. Pfannes, J. Electron Spectrosc. Relat. Phenom., 1999, 104, 99–107 CrossRef.
- A. Aguiar and A. Ferraz, Chemosphere, 2007, 66, 947–954 CrossRef CAS PubMed.
- M. K. Carter, J. Mol. Struct., 2007, 831, 26–36 CrossRef CAS.
- C. Wu, L. Shen, Q. L. Huang and Y. C. Zhang, Mater. Lett., 2011, 65, 1134–1136 CrossRef CAS.
- M. Muruganandham, R. Amutha, G. J. Lee, S. H. Hsieh, J. Wu and M. Sillanpää, J. Phys. Chem. C, 2012, 116, 12906–12915 CrossRef CAS.
- R. Abdel-Hamid and E. F. Newair, J. Electroanal. Chem., 2011, 657, 107–112 CrossRef CAS.
- M. Bouroushian, Electrochemistry of Metal Chalcogenides, Springer-Verlag Berlin, Heidelberg, 2010 Search PubMed.
- P. Dwibedy, G. R. Dey, D. B. Naik, K. Kishore and P. N. Moorthy, Phys. Chem. Chem. Phys., 1999, 1, 1915–1918 RSC.
- Y. H. Wang, J. Zhang, Y. L. Yang, F. Huang, J. S. Zheng, D. G. Chen, F. B. Yang, Z. Lin and C. Wang, J. Phys. Chem. B, 2007, 111, 5290–5294 CrossRef CAS.
- A. M. Campos, E. Ponce and E. A. Lissi, J. Phys. Org. Chem., 2009, 22, 1208–1211 CrossRef CAS.
- G. Kedawat, P. Kumar, K. Nagpal, S. J. Paul, V. N. Singh, S. S. Kumar and B. K. Gupta, ChemistrySelect, 2019, 4, 6219–6226 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01129h |
| ‡ Present address: Advanced Institute of Information Technology PEKING University, Hangzhou 311215, Zhejiang, PR China. |
| This journal is © The Royal Society of Chemistry 2022 |