Open Access Article
Open Access ArticleAg@ZIF-8/g-C3N4 Z-scheme photocatalyst for the enhanced removal of multiple classes of antibiotics by integrated adsorption and photocatalytic degradation under visible light irradiation†
Xin Guo‡
,
Siyuan He‡,
Zhe Meng *,
Yinghui Wang and
Yuan Peng
*,
Yinghui Wang and
Yuan Peng
State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering, National Demonstration Center for Experimental Chemistry Education, College of Chemistry and Chemical Engineering, Ningxia University, Yinchuan 750021, China. E-mail: meng_z@nxu.edu.cn; Fax: +86 951 2061231; Tel: +86 951 2061224
First published on 16th June 2022
Abstract
By combining the plasmon resonance of Ag nanoparticles and orientation effects of ZIF-8, as well as the visible-light activity of g-C3N4, we constructed a direct Z-scheme heterojunction with a co-existing Ag+/Ag0 system by an in situ coprecipitation method. The presence of Ag+/Ag0 on the surface of ZIF-8 was confirmed by the exchange of Ag+ and Zn2+ ions. This promoted the reduction of the band gap of ZIF-8, according to X-ray diffraction (XRD) and X-ray photoelectron spectroscopy. The results reveal that the 12 wt% Ag@ZIF-8/g-C3N4 nanocomposite presented the best adsorptive–photocatalytic activity for the degradation of multi-residue antibiotics under visible light irradiation for 60 min. Its degradation efficiency reached 90%, and its average apparent reaction rate constant was 10.27 times that of pure g-C3N4. In the radical scavenger experiments, ˙O2− and ˙OH were shown to be important in the process of photocatalytic degradation. In addition, we proposed a possible direct Z-scheme photocatalytic mechanism, that is, an internal electric field was formed to compensate the mediators between the interfaces of Ag@ZIF-8 and g-C3N4. This improvement can be attributed to the direct Z-scheme heterojunction system fabricated between Ag@ZIF-8 and g-C3N4. This can accelerate photogenerated electron–hole separation and the redox capability of Ag@ZIF-8/g-C3N4. The integration of the adsorption and photocatalytic degradation of various antibiotics is a promising approach. ZIF-8 has been widely used in the integrated adsorptive–photocatalytic removal of various antibiotics due to its large surface area, high orientation adsorption capacity. Therefore, this study provides new insights into the design of enhanced redox capacity for the efficient degradation of multiple antibiotics under visible-light irradiation.
1. Introduction
The problem of antibiotic pollution in water is becoming more and more serious and has attracted widespread attention. Some antibiotics, such as sulfonamides, tetracyclines, and fluoroquinolones, are widely used in the treatment of infectious diseases of human and animals. In addition, they are added to feed in animal husbandry and aquaculture to promote animal growth and development.1,2 Moreover, antibiotic residues in environmental water have a long half-life. This not only affects the activity of organisms but also harms human health by entering the food chain. Therefore, research on the effective removal of various antibiotics in environmental water has gained public focus and needs to be urgently solved. Currently, semiconductor-based photocatalytic technology has been considered as one of the promising routes to solve the degradation of organics pollutants in environmental water. Photocatalysts have great potential to convert light energy into chemical energy to decompose various harmful antibiotic contaminants.3–5 However, there are few literature reports on the simultaneous removal of multiple classes of antibiotic residues by photocatalysis.The two-dimensional (2D) graphitic-C3N4 (g-C3N4) semiconductor has a wide range of applications in the environmental and energy fields because of its visible-light activity, unique physicochemical properties, excellent chemical stability and low-cost.6,7 Some important limitations of the photocatalytic activity of g-C3N4 are its low specific surface area, fast recombination of electrons and holes and poor visible light absorption.8,9 To improve the above problems, the construction of a heterojunction with a suitable band gap semiconductor (co-catalyst) has been shown to be a good strategy to improve the photocatalytic performance of g-C3N4, such as g-C3N4-based conventional type II heterostructures, g-C3N4-based Z-scheme heterostructures, and g-C3N4-based p–n heterostructures, etc. The unique “Z” shape as the transport pathway of photogenerated charge carriers in Z-scheme photocatalytic systems is the most similar system to mimic natural photosynthesis in the many g-C3N4-based heterojunction photocatalysts. The construction of Z-scheme photocatalytic systems can promote visible light utilization and carrier separation, and maintain the strong reducibility and oxidizability of semiconductors.10–14 There are many studies on g-C3N4-based Z-scheme heterojunction photocatalysts, such as ZnO/g-C3N4,15,16 g-C3N4/ZnS,17 g-C3N4/graphene/NiFe2O4,18 NiCo/ZnO/g-C3N4,19 Bi2Zr2O7/g-C3N4/Ag3PO4,20 g-C3N4/NiFe2O4,21 and WO3/g-C3N4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 and etc. These Z-scheme heterojunction photocatalysts have been made to improve the photocatalytic activity by combining with other semiconductor materials. However, there are some problems with the single photocatalytic method, such as low adsorption ability, limited active sites and low removal efficiency. The integration of the adsorption and photocatalytic degradation of various organic pollutants is considered as a suitable and promising technology. Therefore, it is still essential to fabricate photocatalysts with superior adsorption and degradation efficiencies.
22 and etc. These Z-scheme heterojunction photocatalysts have been made to improve the photocatalytic activity by combining with other semiconductor materials. However, there are some problems with the single photocatalytic method, such as low adsorption ability, limited active sites and low removal efficiency. The integration of the adsorption and photocatalytic degradation of various organic pollutants is considered as a suitable and promising technology. Therefore, it is still essential to fabricate photocatalysts with superior adsorption and degradation efficiencies.
Metal–organic zeolitic imidazolate frameworks (ZIFs) are a kind of porous organic/inorganic hybrid material that forms a 3D tetrahedral framework. It is well known that ZIF-8 has the advantages of a high surface area, high crystallinity and numerous active sites on the surface. It has been intensively studied and widely used in adsorption and photocatalytic systems.23–25 ZIF-8, composed of the metal ions Zn2+ and 2-methylimidazole, is a typical member of the ZIF family.26 Compared with pure ZIF-8, our previous research proved that ZIF-8-based composite (ZIF-8@graphene) had a superior selective adsorption capacity for trace antibiotics in wastewater.27 However, ZIF-8 always has some disadvantages. For example, ZIF-8 shows barely photocatalytic activity under visible light irradiation because of its large band gap (Eg = 5.0).28 One strategy that can further improve the photocatalytic activity of ZIF-8 is to incorporate light-harvesting noble-metal nanoparticles in ZIF-8. The noble-metal with plasmonic nanostructures (Au and Ag nanoparticles) can control resonant photons and induce coherent conduction band electron oscillation.29,30 More and more attention has been paid to the localized surface plasmon resonance (LSPR), especially in the field of catalysis. Chang et al. reported that Ag encapsulated in ZIF-8 can help to create surface plasmon resonance on ZIF-8 nanocrystals.31 This approach not only expands the visible light absorption, but also further reduces the band gap. The band gap energy (Eg) of Ag@ZIF-8 nanohybrids is narrower than that of ZIF-8. This can effectively inhibit the recombination rate of the charge carriers (photogenerated electron–hole pairs) of bare porous ZIF-8 nanomaterials. Fan et al. reported that the photocatalytic activity of a nanocomposite made of Ag/AgCl@ZIF-8 was better than that of the bare Ag/AgCl photocatalyst.32 Since Ag@ZIF-8 nanoparticles are easy to aggregate in water, this leads to a reduction in their adsorption–photocatalysis performance.
Integrating Ag@ZIF-8, with orientation adsorption effects, and g-C3N4, with visible-light activity, is a very good and viable strategy for the selective degradation of multiple antibiotics under visible light irradiation. Coupling Ag@ZIF-8 on g-C3N4 can introduce multiple benefits: (1) providing numerous selective adsorption sites, (2) distributing and stabilizing the photocatalyst nanoparticles and (3) improving the photo-generated charge transfer process via the unique electronic properties of its direct Z-scheme. However, no study has used a direct Z-scheme heterogeneous system to improve the separation efficiency of carriers by encapsulating Ag nanoparticles into ZIF-8, and then coupling with graphitic-C3N4 to form a Ag@ZIF-8/g-C3N4 heterostructure.
In this paper, nanoparticles of the noble metal Ag were encapsulated into zeolitic imidazolate framework-8 to form a new composite Ag@ZIF-8 with two types of coexisting Ag species (Ag0 and Ag+) through a simple in situ self-assembly process. The visible light-driven Ag@ZIF-8/g-C3N4 photocatalysts exhibit superior photocatalytic performances. 12 wt% Ag@ZIF-8/g-C3N4 shows the highest degradation efficiency of 90% of various antibiotics from water within 60 min under visible light irradiation. On the basis of the detailed experimental analysis, a direct Z-scheme mechanism was proposed to enhance the photocatalytic behaviors of Ag@ZIF-8/g-C3N4 composites.
2. Experimental
2.1 Synthesis of Ag@ZIF-8/g-C3N4 photocatalysts
The synthesis of the composite photocatalyst is shown in Scheme 1. g-C3N4 was synthesized through thermal polycondensation with programmed temperatures. Melamine was calcinated in a quartz crucible with a cover, and placed in a tube furnace with quartz tubes. The program settings of the calcination temperature were: the initial temperature was 200 °C for 20 min. After that, it was heated at a rate of 2 °C min−1 to 350 °C for 90 min. Finally, it was heated at a rate of 2 °C min−1 to 550 °C for 120 min.Nanoscale ZIF-8 was prepared by the co-precipitation method. In the synthesis of ZIF-8, 1.78 g of Zn(NO3)2·6H2O and 5.26 g of 2-methylimidazole were dispersed in two beakers containing 40 ml of methanol. Then, the suspension was stirred at 30 °C for 4 h. The product was recovered by centrifugation, and washed with fresh methanol three times. Then, it was dried at 60 °C for 12 h.
The Ag@ZIF-8 hybrid was obtained by mixing ZIF-8 with AgNO3. 0.20 g of ZIF-8 and 0.17 g of AgNO3 were dispersed into two beakers containing 20 ml of methanol. The two components were mixed by stirring for 25 min in the dark. We centrifuged the precipitate, and washed it with fresh methanol several times. Then, it was dried at 60 °C for 12 h. We defined the samples as Ag-doped-ZIF-8 (Ag@ZIF-8) by encapsulating Ag nanoparticles into zeolitic imidazolate framework-8. According to the amount of AgNO3 added, the samples of Ag@ZIF-8 were defined as 1.5 mmol, 1.0 mmol, and 0.5 mmol Ag@ZIF-8.
The Ag@ZIF-8/g-C3N4 composites were obtained by a simple in situ self-assembly process at room temperature. Ag@ZIF-8 and g-C3N4 in a certain mass ratio were dispersed in methanol and stirred for 30 min. Finally, the obtained product was dried at 60 °C for 12 h. The Ag@ZIF-8/g-C3N4 heterostructures were denoted as x wt% Ag@ZIF-8/g-C3N4 (10 wt%, 12 wt%, 17 wt%, 25 wt%), where x represents the Ag@ZIF-8 content in the heterostructures.
2.2 Photocatalytic performance measurements
3. Results and discussion
3.1 Characterization of the photocatalysts
The X-ray diffraction (XRD) patterns of the synthesized samples are shown in Fig. 1(a). The two diffraction peaks of g-C3N4 at approximately 13.3° and 27.3° corresponded to the (110) and (002) crystal planes, respectively. These were the same as those reported.33 In Fig. 1(a), the diffraction peaks of ZIF-8 indicated that it has the sodalite zeolite type structure. The predominant diffraction peaks (011), (002), (112), (022), (013), (222), (114), (233), (134) and (044) identified in the XRD patterns were the same as those reported.34 Meanwhile, two strong coupling peaks were observed for the (002) and (100) planes of g-C3N4, combining with the (134) and (112) planes of ZIF-8 in the Ag@ZIF-8/g-C3N4 samples, as shown in Fig. 1(a). In Fig. 1(b), a typical diffraction peak at 8.7° was found in the Ag@ZIF-8 composite. Furthermore, with the increase of the silver content, the diffraction peak intensity (8.7°) was significantly increased. This was attributed to silver imidazolate oligomers. This is the only peak for Ag-containing phases in the XRD patterns. This is consistent with the literature.34 The XRD diffraction peaks of ZIF-8 and Ag@ZIF-8 were amplified at the small angle range from 3° to 8° to further determine the presence of Ag-containing phases. In Fig. 1(c), the angle of the main diffraction peak of Ag@ZIF-8 was smaller than that of ZIF-8. According to the Bragg equation, 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = nλ, when XRD peaks shift to a smaller angle, the distance between the parallel atomic planes (d) has increased. The ionic radius of Ag is greater than that of Zn. The Ag ions partially replaced Zn ions in the host crystal and the diffraction peak was shifted to a smaller angle than that of ZIF-8. However, the XRD peak intensities of ZIF-8 decreased with the addition of Ag. This indicates that the localized crystalline structure was modified after Ag ions were introduced. XRD diffraction spectrum analysis showed that two Ag-containing phases were found in Ag@ZIF-8. As shown in Fig. 1(d), the FT-IR spectra of the samples were used to study the chemical functional groups of bare g-C3N4 and ZIF-8, and the Ag@ZIF-8/g-C3N4 composite. Compared with the FT-IR spectra of bare g-C3N4 and ZIF-8, the characteristic absorption of typical functional groups was clearly observed in the Ag@ZIF-8/g-C3N4 composite. For example, the absorption band at 810 cm−1 and the absorption peak at 421 cm−1 resulted from the plane repeating arrangement of the tri-s-triazine rings for g-C3N4 and the Zn–N stretching vibration for ZIF-8, respectively. No impurity peaks were observed in the Ag@ZIF-8/g-C3N4 composite.
θ = nλ, when XRD peaks shift to a smaller angle, the distance between the parallel atomic planes (d) has increased. The ionic radius of Ag is greater than that of Zn. The Ag ions partially replaced Zn ions in the host crystal and the diffraction peak was shifted to a smaller angle than that of ZIF-8. However, the XRD peak intensities of ZIF-8 decreased with the addition of Ag. This indicates that the localized crystalline structure was modified after Ag ions were introduced. XRD diffraction spectrum analysis showed that two Ag-containing phases were found in Ag@ZIF-8. As shown in Fig. 1(d), the FT-IR spectra of the samples were used to study the chemical functional groups of bare g-C3N4 and ZIF-8, and the Ag@ZIF-8/g-C3N4 composite. Compared with the FT-IR spectra of bare g-C3N4 and ZIF-8, the characteristic absorption of typical functional groups was clearly observed in the Ag@ZIF-8/g-C3N4 composite. For example, the absorption band at 810 cm−1 and the absorption peak at 421 cm−1 resulted from the plane repeating arrangement of the tri-s-triazine rings for g-C3N4 and the Zn–N stretching vibration for ZIF-8, respectively. No impurity peaks were observed in the Ag@ZIF-8/g-C3N4 composite.
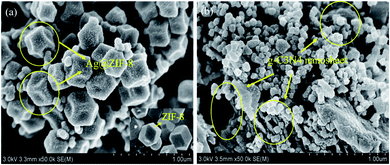
The morphological features and nanoparticle size distributions of the obtained Ag@ZIF-8 (1 mmol AgNO3) and 12 wt% Ag@ZIF-8/g-C3N4 composites were evaluated by SEM imaging. As shown in Fig. 2(a), Ag nanoparticles with a diameter of 10 nm were uniformly distributed on the surface of ZIF-8 with a dodecahedral structure (about 180 nm) by loading 1 mmol of silver (AgNO3). Compared with the pure ZIF-8 shown in Fig. 2(a), each crystal face of the Ag@ZIF-8 nanoparticles with the dodecahedral structure showed significant shrinkage. The conclusions of SEM and XRD are consistent. This further confirmed that the silver nanoparticles were embedded into the framework of ZIFs through the stable chemical bonds between Ag and ZIF-8. Therefore, with the addition of silver nitrate solution, Ag nanoparticles were embedded into the ZIF-8 framework, and ion exchange occurred between Ag+ in the solution and Zn2+ in the ZIF-8 framework. As shown in Fig. 2(b), an encouraging phenomenon was the emergence of g-C3N4 nanosheets in the Ag@ZIF-8/g-C3N4 composite, that is, contacting interfaces were formed between the g-C3N4 nanosheets and Ag@ZIF-8 nanoparticles.
We observed the morphology and composition of the 12 wt% Ag@ZIF-8/g-C3N4 composite by TEM and EDS. As shown in Fig. 3(a), Ag nanoparticles were uniformly dispersed and grown on the surface of ZIF-8 to form the Ag@ZIF-8 composite. Fig. 3(b) shows that Ag@ZIF-8 nanoparticles were anchored on g-C3N4 nanosheets to construct the Ag@ZIF-8/g-C3N4 composite. To further demonstrate the coexistence of Ag@ZIF-8/g-C3N4, HRTEM images of Ag@ZIF-8/g-C3N4 composites demonstrated the clear lattice fingerprints of the different species, as shown in Fig. 3(c and d). The spacings were 0.24 nm and 0.27 nm, corresponding to the (111) and (111) planes of Ag0 and Ag+ (Fig. 3(c)), respectively. In addition, the lattice fingerprints of g-C3N4 were the same as those reported in ref. 35. According to the EDX patterns in Fig. 3(e and f), C, N, Zn and Ag were uniformly distributed in its structure. The atomic percentages of the four elements were 49.67%, 46.62%, 3.30% and 0.41%, respectively. These findings confirmed the coexistence of Ag@ZIF-8 and g-C3N4 in the composite. In addition, SEM, TEM and EDS data supported the XRD data. The obtained composite showed Ag nanoparticles encapsulated into the zeolitic imidazolate framework and coupled on the surface of graphitic-C3N4.
 | ||
| Fig. 3 TEM images of (a) Ag@ZIF-8 and (b–d) Ag@ZIF-8/g-C3N4, (e) EDS spectrum of Ag@ZIF-8/g-C3N4 and (f) atomic percentages of Ag@ZIF-8/g-C3N4. | ||
The specific surface areas and pore structures of the samples were obtained by the study of the N2 adsorption–desorption isotherms. In Fig. 4(a and b), the specific surface areas of the prepared ZIF-8, Ag@ZIF-8, Ag@ZIF-8/g-C3N4, and pure g-C3N4 were evaluated through the Brunauer–Emmett–Teller (BET) method to be 492.43, 253.10, 104.51 and 5.60 m2 g−1, respectively. Compared with that of pure ZIF-8, the BET of the hybrid sample Ag@ZIF-8 was decreased greatly due to the introduction of Ag+. This verified the shrinkage of the ZIF-8 crystal plane observed in the SEM image (Fig. 4(a)). However, compared with that of bare g-C3N4 (5.60 m2 g−1), the Ag@ZIF-8/g-C3N4 hybrid composite had a high BET measurement of 104.51 m2 g−1, as shown in Fig. 4(b). The pore size distributions of the prepared ZIF-8, Ag@ZIF-8, Ag@ZIF-8/g-C3N4, and pure g-C3N4 were 4.05, 4.48, 6.17 and 23.23 nm, respectively. All samples showed characteristics of mesoporous structures because of the presence of the type IV isotherms. Compared with Ag@ZIF-8, the pore sizes of Ag@ZIF-8/g-C3N4 were mainly distributed around 6 nm. This indicated that the mesopores were possibly intergranular pores from Ag@ZIF-8 and g-C3N4. The significant increase in the pore sizes benefitted from the close contact interface between Ag@ZIF-8 nanoparticles and g-C3N4 nanosheets, since the demand for an electron mediator was eliminated, and the electron and ion migration lengths were shortened, which were very conducive to building a direct Z-scheme photocatalyst.
The elemental states, surface chemical composition and electronic environment of the constituent elements of the Ag@ZIF-8/g-C3N4 composites were elucidated by sensitive X-ray photoelectron spectroscopy (XPS) measurements. As shown in Fig. 5(a), the XPS spectrum of the Ag@ZIF-8/g-C3N4 composite proves that the surface chemical composition was composed of Ag 3d, Zn 2p, N 1s and C 1s. In Fig. 5(b), two distinct peaks at 284.8 and 287.9 eV are shown in the C 1s spectrum of g-C3N4. This corresponds to the graphitic carbon (sp2 C–C bonds) and the sp2 hybridized carbon in the N-containing aromatic rings (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N) of graphitic carbon nitride. In Fig. 5(c), the N 1s spectra shows a peak at 398.4 eV. This results from the bonding of sp2 hybridized aromatic N atoms to carbon atoms (C–N
C–N) of graphitic carbon nitride. In Fig. 5(c), the N 1s spectra shows a peak at 398.4 eV. This results from the bonding of sp2 hybridized aromatic N atoms to carbon atoms (C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). In Fig. 5(d), the typical high-resolution XPS signals of the Zn 2p spectra of the ZIF-8, Ag@ZIF-8, and Ag@ZIF-8/g-C3N4 samples are shown. The peak voltages of Zn 2p3/2 and Zn 2p1/2 were 1020.8 eV and 1043.8 eV for ZIF-8, respectively. Compared with ZIF-8 (2p1/2, 1043.8 eV; 2p3/2, 1020.8 eV), the Zn 2p states of Ag@ZIF-8 (2p1/2, 1044.8 eV; 2p3/2, 1021.8 eV) were shifted positively after the Ag nanoparticles were embedded into ZIF-8. This indicates that there was ion exchange between Ag+ in the solution and Zn2+ in the ZIF-8 framework. Therefore, the altered Zn 2p spectra of ZIF-8 and Ag@ZIF-8 further confirmed the formation of new chemical bonds between Ag and ZIF-8. This is accordance with the previous reports.31,36,37 In addition, there was no significant change in the Zn 2p spectra of Ag@ZIF-8 and Ag@ZIF-8/g-C3N4. This further confirmed the successful synthesis of the composite nanomaterials of Ag@ZIF-8 and Ag@ZIF-8/g-C3N4.
C). In Fig. 5(d), the typical high-resolution XPS signals of the Zn 2p spectra of the ZIF-8, Ag@ZIF-8, and Ag@ZIF-8/g-C3N4 samples are shown. The peak voltages of Zn 2p3/2 and Zn 2p1/2 were 1020.8 eV and 1043.8 eV for ZIF-8, respectively. Compared with ZIF-8 (2p1/2, 1043.8 eV; 2p3/2, 1020.8 eV), the Zn 2p states of Ag@ZIF-8 (2p1/2, 1044.8 eV; 2p3/2, 1021.8 eV) were shifted positively after the Ag nanoparticles were embedded into ZIF-8. This indicates that there was ion exchange between Ag+ in the solution and Zn2+ in the ZIF-8 framework. Therefore, the altered Zn 2p spectra of ZIF-8 and Ag@ZIF-8 further confirmed the formation of new chemical bonds between Ag and ZIF-8. This is accordance with the previous reports.31,36,37 In addition, there was no significant change in the Zn 2p spectra of Ag@ZIF-8 and Ag@ZIF-8/g-C3N4. This further confirmed the successful synthesis of the composite nanomaterials of Ag@ZIF-8 and Ag@ZIF-8/g-C3N4.
Additionally, we performed XPS analysis of the Ag@ZIF-8/g-C3N4 sample to determine the presence of Ag+ and Ag0 in the composite. The Ag 3d spectra are shown in Fig. 5(e). The observed Ag 3d spectrum of Ag@ZIF-8/g-C3N4 includes the two survey bands at 367.7 and 373.7 eV, which can be assigned to the Ag 3d5/2 and Ag 3d3/2 binding energies, respectively. However, each survey band can be further split into a pair of survey peaks (Ag 3d5/2, 367.5/368.4 eV and Ag 3d3/2, 373.5/374.1 eV).20,38–40 The first pair of survey peaks at 367.5 and 373.5 eV was attributed to the Ag+ of AgNO3. However, the second pair of survey peaks at 368.4 and 374.1 eV resulted from the metallic Ag0. All the XPS binding energies of Ag 3d indicated the coexistence of Ag+ and Ag0 phases. Such a result was consistent with the results in the literature and the results of XRD and TEM in this work.
The UV-vis diffuse reflectance spectra of bare g-C3N4 and ZIF-8, as well as those of Ag@ZIF-8 and Ag@ZIF-8/g-C3N4 with different mass ratios of Ag/g-C3N4, are presented in Fig. 6(a). The bare g-C3N4 nanosheets and ZIF-8 nanoparticles had absorption edges at 485 and 245 nm, which corresponded to band gaps of 2.61 and 4.90 eV, respectively. Compared with that of ZIF-8, the absorption band edge of Ag@ZIF-8 was significantly red-shifted to 380 nm. This indicates that Ag doping can enhance light absorption because of the LSPR effect of plasmonic metal nanoparticles. Compared with that of the bare g-C3N4, the band gaps of 10 wt%, 12 wt%, 17 wt% and 25 wt% Ag@ZIF-8/g-C3N4 were 2.58, 2.60, 2.69 and 2.59 eV, respectively. Obviously, we could adjust the ratio of g-C3N4 and Ag to enhance the ability to harvest visible light.
The recombination of the photogenerated electrons and holes was shown in the photoluminescence (PL) spectra. As shown in Fig. 6(b), compared to that of g-C3N4, the PL intensity of 12 wt% Ag@ZIF-8/g-C3N4 was the lowest. This further indicated that the recombination efficiency of photogenerated carriers was low. Therefore, an appropriate Ag/g-C3N4 ratio could effectively inhibit the rapid recombination of photogenerated electrons and holes (e−–h+) in the Ag@ZIF-8/g-C3N4 heterostructured composite. This would be beneficial to improving the photocatalytic performance.
In order to study the photoelectric separation and the charge migration efficiency of the prepared samples, we performed electrochemical impedance spectroscopy (EIS) analysis to measure the different mass ratios of bare g-C3N4, ZIF-8 and the Ag@ZIF-8/g-C3N4 composite. Generally, the smaller the EIS radius of a sample is, the higher the efficiency of photoelectric separation and migration is. In Fig. 6(c), the Nyquist diagram shows that the arc radius of 12 wt% Ag@ZIF-8/g-C3N4 was smaller than those of bare g-C3N4 and the other composites. This indicated that the photogenerated carrier transfer resistance of 12 wt% Ag@ZIF-8/g-C3N4 was the lowest. The results of EIS were consistent with those of PL. This indicated that 12 wt% Ag@ZIF-8/g-C3N4 had a better photoelectric separation efficiency.
3.2 Photocatalytic degradation properties
To evaluate the photocatalytic performance of the prepared products, we carried out photocatalytic degradation tests of 10 antibiotics of 3 categories in aqueous solution under light irradiation. The catalytic reaction system contained ten antibiotics (10 μg ml−1 for each), TC, OTC, CTC, CIP, ENR, OFL, SMZ, SDM, SCP and SMM. Before the photocatalysis experiment, we stirred the mixture in the dark for 30 min to reach the adsorption–desorption equilibrium. 12 wt% Ag@ZIF-8/g-C3N4 was used as the photocatalyst and the mixture was irradiated for 60 min. We took out 1.0 ml of the suspension and filtered it using a 0.22 mm filter membrane at given time intervals. UHPLC-MS/MS technology was used for the qualitative and quantitative analysis of each target antibiotic. C/C0 was calculated as the degradation removal rate, where C0 is the antibiotic concentration after dark adsorption and C is the actual concentration of the antibiotic after the photocatalytic degradation process. The photocatalytic performances of ZIF-8, g-C3N4, ZIF-8/g-C3N4 and Ag/g-C3N4 are shown in Fig. 7(a). The photocatalytic degradation performances at different times are shown in Fig. 7(b). In addition, the photocatalytic degradation abilities of Ag@ZIF-8 and 12 wt% Ag@ZIF-8/g-C3N4 were also measured after 60 min under the same conditions. By comparing bare ZIF-8 and g-C3N4 with the g-C3N4/ZIF-8 composite, ZIF-8, with good adsorption performance, was shown to be almost ineffective at increasing the photocatalytic activity of g-C3N4. However, compared with pristine ZIF-8 and g-C3N4, as Ag nanoparticles were introduced, Ag/ZIF-8 and Ag/g-C3N4 all showed significantly enhanced photoactivity performances under visible light irradiation. Under visible light irradiation (>490 nm), the optimized photocatalyst (12 wt% Ag@ZIF-8/g-C3N4) presented the highest photocatalytic activity. The removal rate for each antibiotic selected under visible light irradiation was over 90%. However, the removal efficiencies of all Ag@ZIF-8/g-C3N4 composites (10 wt%, 12 wt%, 17 wt%, 25 wt%) were significantly improved, with increased degradation rates of the various multi-residue antibiotics. When the amount of AgNO3 was 1.0 mmol, the 12 wt% Ag@ZIF-8/g-C3N4 photocatalyst showed excellent photocatalytic activity. This was because excessive AgNO3 will aggregate on the surface of Ag@ZIF-8/g-C3N4, resulting in a reduced photocatalytic performance.In addition, the degradation kinetics data were fitted using a pseudo first-order model, expressed as: ln(C0/Ct) = Kt. As shown in Fig. 7(b), the 12 wt% Ag@ZIF-8/g-C3N4 composite showed the highest apparent reaction rate constants (ENR k = 0.097 min−1, CIP k = 0.071 min−1, OFL k = 0.056 min−1, SMZ k = 0.037 min−1, SDM k = 0.056 min−1, SCP k = 0.047 min−1, SMM k = 0.062 min−1, TC k = 0.065 min−1, OTC k = 0.066 min−1 and CTC k = 0.072 min−1) compared with those of the other substances. They were 10.1, 13.8, 9.3, 7.4, 7.0, 7.8, 8.9, 13.0, 11.0 and 14.4 times those of pure g-C3N4.
Remarkably, compared with other photocatalysts (Table 1), the proposed Ag@ZIF-8/g-C3N4 could degrade the most antibiotics in the shortest time with the least catalyst. The results in this study showed an innovative Z-scheme heterojunction photocatalyst capable of efficiently degrading multiple classes of antibiotics under visible-light irradiation.
| Photocatalyst | Light | Dosage | Target | Degradation | Ref. |
|---|---|---|---|---|---|
| Ag/AgCl/ZIF-8 | 500 W, Xe, λ > 400 nm | 50 mg | RhB, 100 ml, 10 ppm | 99.12% (90 min) | 41 |
| ZIF-8@TiO2 | 300 W, Xe, λ > 420 nm | 60 mg | Tc, 100 ml, 100 ppm | 90% (120 min) | 42 |
| ZIF-8/g-C3N4 | 300 W, Xe, λ > 420 nm | 10 mg | U(VI), 100 ml, 10 ppm | 87.5% (120 min) | 43 |
| ZIF-8/TiO2 | UV | 10 mg | RhB, 30 ml, 10 ppm | 87.5% (120 min) | 44 |
| MB, 30 ml, 10 ppm | 66% (120 min) | ||||
| Ag/AgCl@ZIF-8/g-C3N4 | 150 W, Xe, λ > 420 nm | 50 mg | LVFX, 50 ml, 10 ppm | 87.3% (60 min) | 45 |
| MoS2/ZIF-8 | 300 W, Xe, λ > 420 nm | 20 mg | CIP, 50 ml, 20 ppm | 93.2% (180 min) | 46 |
| TC, 50 ml, 20 ppm | 75.6% (180 min) | ||||
| Ag@ZIF-8/g-C3N4 | 300 W, Xe, λ > 420 nm | 20 mg | Ten antibiotics, 30 ml, 10 ppm | 90% (60 min) | This work |
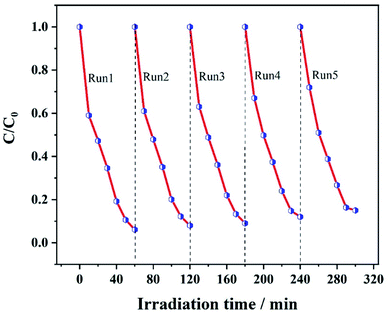
As requirements for practical applications, the stability and reusability of the catalyst are important. The 12 wt% Ag@ZIF-8/g-C3N4 composite for various multi-residue antibiotic degradation was reused five times under the same reaction conditions. As shown in Fig. 8, the Ag@ZIF-8/g-C3N4 composite has excellent stability and reusability. This is because the CIP photodegradation performance of the 12 wt% Ag@ZIF-8/g-C3N4 composite did not significantly decay after 5 cycles.
3.3 Main reactive species
Hydroxyl radicals (˙OH), holes (h+) and superoxide radicals (˙O2−) are the main reactive species in the process of the photodegradative removal of multi-residue antibiotics. We designed trapping experiments of free radicals and holes to reveal the main active species during the photodegradation using the 12 wt% Ag@ZIF-8/g-C3N4 composite as a photocatalyst. We used BQ, EDTA–2Na and IPA as ˙O2−, h+ and ˙OH scavengers as probes to quantitatively trap radicals in the photocatalytic reaction system. As shown in Fig. 9(a), BQ, IPA and EDTA–2Na were separately added into the reaction system. The degradation efficiencies were reduced upon the addition of BQ and IPA into those systems, while they were almost unchanged for EDTA–2Na. These results indicate that ˙O2− and ˙OH were the predominant reactive species in the photodegradation systems of 12 wt% Ag@ZIF-8/g-C3N4.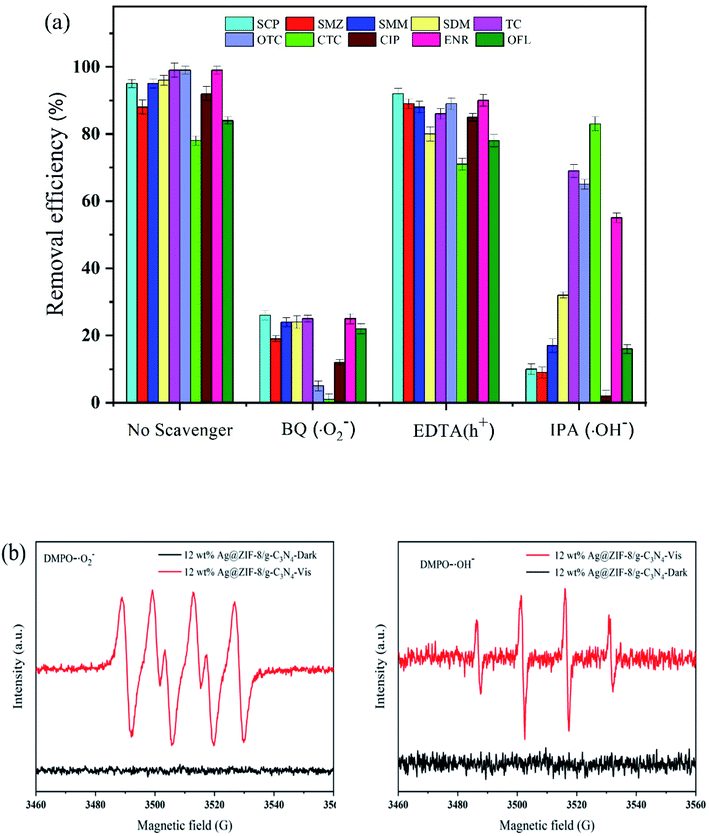 | ||
| Fig. 9 (a) The effects of scavengers on the photodegradation efficiency of antibiotics in the presence of the Ag@ZIF-8/g-C3N4 photocatalyst and (b) ESR spectra of DMPO–˙O2− and DMPO–˙OH. | ||
As shown in Fig. 9(b), we measured the electron spin resonance (ESR) spin spectra to further determine these active species (˙O2− and ˙OH) in the photodegradation systems of the Ag@ZIF-8/g-C3N4 composite. In particular, we could easily capture the superoxide radicals (˙O2−) and hydroxyl radicals (˙OH) produced in the photodegradation system using 5,5-dimethyl-L-pyrroline N-oxide (DMPO) in aqueous solution to produce the DMPO–˙OH and DMPO–˙O2− adducts, respectively. As shown in Fig. 9(b), the ESR signals of both the DMPO–˙O2− and DMPO–˙OH adducts can be monitored in the presence of the Ag@ZIF-8/g-C3N4 composite after 8 min of visible light irradiation. This indicates that Ag@ZIF-8/g-C3N4 can produce the stronger active species ˙O2− and ˙OH. Based on the results, the construction of the heterojunction between Ag@ZIF-8 and g-C3N4 can significantly promote the production of ˙O2− and ˙OH radicals. They are the main reactive species for the degradation of the antibiotic molecules in the photodegradation process.
3.4 Measurement of the photodegradation products
The transformation products of antibiotics were detected by UPLC-MS/MS (using SCP and CIP as examples) during the photocatalytic process. The possible degradation pathways and intermediate products of SCP and CIP are shown in Fig. S1 and S2,† respectively. Two transformation products with m/z of 251 (M1) and 221 (M2) for SCP (m/z = 285) were observed, and the corresponding molecular structures are shown in Fig. S1.† Six transformation products with m/z of 334 (M1), 306 (M2), 263 (M3), 164 (M4), 156 (M5) and 154 (M6) for CIP (m/z = 332) were observed, and the corresponding molecular structures are shown in Fig. S2.† The photodegradation products for all 10 selected antibiotics can be degraded into CO2, H2O and other small, mineralized molecules.3.5 Photocatalytic mechanism
Based on the M–S plots (Fig. 10(a and b)), the Efb of g-C3N4 and ZIF-8 are −1.10 and −0.70 V (vs. Ag/AgCl, pH = 7), corresponding to −0.49 and −0.09 V (vs. NHE, pH = 7), respectively. Typically, the CB potential of an n-type semiconductor is about 0.2 V greater than that of the Efb. Thus, the ECB of g-C3N4 and ZIF-8 are −0.69 and −0.29 V (vs. NHE, pH = 7). As shown in Fig. 10(c and d), after doping silver ions into ZIF-8, the band gap of ZIF-8 was reduced from 4.90 to 3.10 eV, because of the defect level above the VB of ZIF-8. Therefore, the VBs of g-C3N4 and ZIF-8 were determined to 1.92 and 2.81 V, respectively.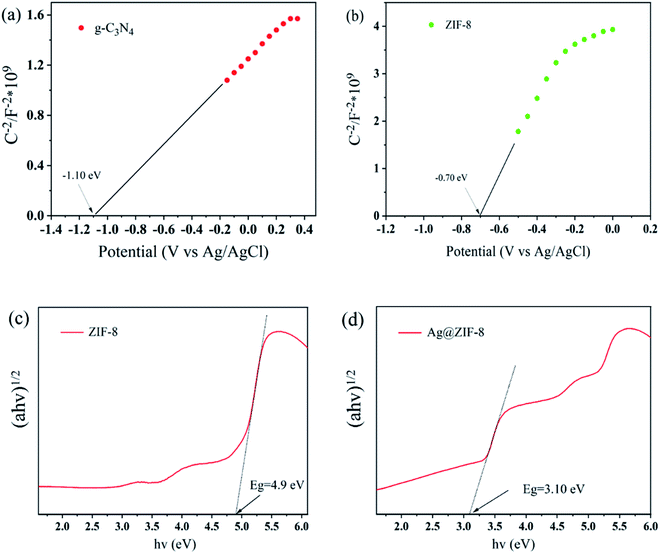 | ||
| Fig. 10 (a and b) Mott–Schottky (M–S) plots of g-C3N4 and ZIF-8, respectively. (c and d) Plot of (ahν)1/2 versus energy for ZIF-8 and Ag@ZIF-8, respectively. | ||
In light of the above analysis and test results, we proposed a potential Z-scheme charge-transfer path and photocatalytic mechanism for the elimination of multiple classes of antibiotics over the Ag@ZIF-8/g-C3N4 heterostructure under visible light irradiation (Fig. 11).
When g-C3N4 and Ag@ZIF-8 are in close contact, the charges are redistributed to reach the equilibrium of Ef levels. As a result, a built-in electric field directed from g-C3N4 to ZIF-8 is generated at the interface between the two. Meanwhile, the in situ generated Ag NPs have enough energy to surmount the Schottky barrier at the Ag@ZIF-8 interface and readily transfer to the CB of ZIF-8, and recombine with h+ on g-C3N4 under the built-in electric field. This provides evidence for Z-scheme charge transfer.
We used the shift of the binding energy in the XPS spectra to demonstrate the electron migration pathway for a direct Z-scheme photocatalyst. Compared with those of Ag@ZIF-8 (Fig. 5(d)), the Zn 2p states of Ag@ZIF-8/g-C3N4 were negatively shifted. This indicates that the electrons transfer from Ag@ZIF-8 to g-C3N4. Obviously, the charge-carrier migration mechanism of the Ag@ZIF-8/g-C3N4 direct Z-scheme photocatalyst was demonstrated by the noble metal loading and in situ XPS evaluation. In addition, the prepared 12 wt% Ag@ZIF-8/g-C3N4 showed the best photocatalytic degradation activity for multi-residue antibiotics because of its sufficient reduction potential (Fig. 7(a)). All the prepared composites showed better photocatalytic degradation efficiency (different silver nitrate addition amounts (Fig. S4†) and different mass ratios (Fig. S5†)). The efficient removal of multi-residue antibiotics is attributed to the structure of the direct Z-scheme heterojunction between Ag@ZIF-8 and g-C3N4.
In addition, ESR test results further confirmed the above results. According to the energy band position, electron-rich Ag@ZIF-8 cannot produce ˙O2−, because its CB position is more positive compared to the potential of O2/˙O2− (−0.33 V vs. NHE), and g-C3N4 cannot generate ˙OH, because its VB position is more negative than the potential of H2O/˙OH (2.40 V vs. NHE). However, ˙OH signals and ˙O2− signals were detected for the composite, indicating that the charge transfer mode follows a Z-scheme mechanism rather than the traditional type II.
4. Conclusions
In conclusion, by combining the catalytic properties of Ag nanoparticles and the molecular sieving and orientation effects of ZIF-8, the Ag@ZIF-8/g-C3N4 composite with a direct Z-scheme heterojunction was successfully prepared through a simple method. Using a range of characterization techniques, we investigated the morphology, chemical composition, and optical properties of the prepared samples by SEM, HRTEM, XRD, FTIR, UV-vis DRS, and photoluminescence techniques. The PL and EIS tests, as well as the UV-vis DRS test, proved that Ag, ZIF-8 and g-C3N4 had suitable band gaps that provided structures for the establishment of a Z-scheme heterojunction. Under visible light irradiation, the 12 wt% Ag@ZIF-8/g-C3N4 composite exhibited the highest photocatalytic performance for 10 antibiotics in 3 categories. The removal rates of the selected antibiotics were all over 90% under visible light illumination. Their average apparent reaction rates were 10.27 times that of pure g-C3N4. This further proved that the direct Z-scheme photocatalytic mechanism could significantly enhance the visible light absorption and e−–h+ separation efficiency. Through ESR tests, we found that Ag@ZIF-8/g-C3N4 could produce the stronger active species of ˙O2− and ˙OH radicals. This further confirmed the successful construction of the direct Z-scheme heterojunction. Therefore, this study prepared a Z-scheme photocatalyst by the integration of adsorption and photocatalytic degradation for antibiotic removal in ambient water, which is considered as a promising strategy.Conflicts of interest
The authors declare that they have no competing interests.References
- X. W. Liu, K. Lv, C. X. Deng, Z. M. Yu, J. H. Shi and A. C. Johnson, Environ. Pollut., 2019, 252, 1532–1538 CrossRef CAS PubMed.
- S. Zhong, J. J. Yang, H. Zhou, C. Y. Li and L. Bai, Sol. Energy Mater. Sol. Cells, 2022, 238, 111646 CrossRef CAS.
- Y. Li, K. Yin, L. L. Wang, X. L. Lu, Y. Q. Zhang, Y. T. Liu, D. F. Yan, Y. Z. Song and S. L. Luo, Appl. Catal., B, 2018, 239, 537–544 CrossRef CAS.
- T. Takata and K. Domen, ACS Energy Lett., 2019, 4, 542–549 CrossRef CAS.
- K. Yu, H. B. Huang, J. T. Wang, G. F. Liu, Z. Zhong, Y. F. Li, H. L. Cao, J. Lv and R. Cao, J. Mater. Chem. A, 2021, 9, 7759 RSC.
- Y. J. Ren, D. Q. Zeng and W.-J. Ong, Chin. J. Catal., 2019, 40, 289–319 CrossRef CAS.
- J. W. Fu, J. G. Yu, C. J. Jiang and B. Cheng, Adv. Energy Mater., 2018, 8, 1701503 CrossRef.
- Q. Hao, T. Chen, R. T. Wang, J. R. Feng and D. M. Chen, J. Clean. Prod., 2018, 197, 1222–1230 CrossRef CAS.
- A. S. Mestre and A. P. Carvalho, Molecules, 2019, 24, 3702 CrossRef CAS PubMed.
- Y. Chen, Z. X. Fan, Z. C. Zhang, W. X. Niu, C. L. Li, N. L. Yang and B. Chen, Chem. Rev., 2018, 118, 6409–6455 CrossRef CAS PubMed.
- J. X. Low, C. J. Jiang, B. Chen, S. Wageh, A. A. Al-Ghamdi and J. G. Yu, Small Methods, 2017, 1, 1700080 CrossRef.
- Q. L. Xu, L. Y. Zhang, J. G. Yu, S. Wageh, A. A. Al-Ghamdi and M. Jaroniec, Mater. Today, 2018, 21, 1042–1063 CrossRef CAS.
- W. H. Zhang, A. R. Mohamed and W.-J. Ong, Angew. Chem., Int. Ed., 2020, 59, 22894–22915 CrossRef CAS PubMed.
- D. L. Huang, S. Chen, G. M. Zeng, X. M. Gong, C. Y. Zhou, M. Cheng, W. J. Xue and J. Li, Coord. Chem. Rev., 2019, 385, 44–80 CrossRef CAS.
- W. L. Yu, D. F. Xu and T. Y. Peng, J. Mater. Chem. A, 2015, 3, 19936–19947 RSC.
- S. W. Liu, F. Chen, S. T. Li, X. X. Peng and Y. Xiong, Appl. Catal., B, 2017, 211, 1–10 CrossRef CAS.
- X. Q. Hao, J. Zhou, Z. W. Cui, Y. C. Wang, Y. Wang and Z. G. Zou, Appl. Catal., B, 2018, 229, 41–51 CrossRef CAS.
- G. Gebreslassie, P. Bharali, U. Chandra, A. Sergawie, P. K. Boruah, M. R. Das and E. Alemayehu, J. Photochem. Photobiol., A, 2019, 382, 111960 CrossRef CAS.
- J. Wu, J. Y. Hu, H. H. Qian, J. J. Li, R. Yang and L. B. Qu, Diamond Relat. Mater., 2022, 121, 108738 CrossRef CAS.
- Z. J. Qu, Z. Y. Jing, X. M. Chen, Z. X. Wang, H. F. Ren and L. H. Huang, J. Environ. Sci., 2023, 125, 349–361 CrossRef.
- G. Gebreslassie, P. Bharali, U. Chandra, A. Sergawie, P. K. Boruah, M. R. Das and E. Alemayehu, Appl. Organomet. Chem., 2019, 33, e5002 CrossRef.
- L. F. Cui, X. Ding, Y. G. Wang, H. C. Shi, L. H. Huang and Y. H. Zuo, Appl. Surf. Sci., 2017, 391, 202–210 CrossRef CAS.
- Y. Q. Zhang, Y. M. Sun, Y. Man, H. Yang, R. Y. Zhao, G. Q. Xiang, X. M. Jiang, L. J. He and S. S. Zhang, Chem. Eng. J., 2022, 440, 135723 CrossRef CAS.
- Y. F. Wang, W. Zhao, Z. Y. Qi, L. Zhang, Y. N. Zhang, H. O. Huang and Y. Z. Peng, Chem. Eng. J., 2020, 394, 124992 CrossRef CAS.
- G. Y. Liu, L. Y. Li, D. H. Xu, X. D. Huang, X. M. Xu, S. N. Zheng, Y. G. Zhang and H. Lin, Carbohydr. Polym., 2017, 175, 584–591 CrossRef CAS PubMed.
- H. Dai, X. Z. Yuan, L. B. Jiang, H. Wang, J. Zhang, J. J. Zhang and T. Xiong, Coord. Chem. Rev., 2021, 441, 213985 CrossRef CAS.
- Z. L. Wang, X. Y. Chen, Z. Meng, M. X. Zhao and H. J. Zhan, Water Sci. Technol., 2020, 82, 2322–2336 Search PubMed.
- C. C. Hu, Y. C. Huang, A. L. Chang and M. Nomura, J. Colloid Interface Sci., 2019, 553, 372–381 CrossRef CAS PubMed.
- P. Y. Jiang, K. F. Yu, H. B. Yuan, R. He, M. P. Sun, F. Tao, L. B. Wang and W. K. Zhu, J. Mater. Chem. A, 2021, 9, 9809 RSC.
- S. J. Li, C. C. Wang, Y. P. Liu, B. Xue, W. Jiang, Y. Liu, L. Y. Mo and X. B. Chen, Chem. Eng. J., 2021, 415, 128991 CrossRef CAS.
- N. Chang, Y. R. Chen, F. Xie, Y. P. Liu and H. T. Wang, Colloids Surf., A, 2021, 616, 126351 CrossRef CAS.
- G. D. Fan, X. M. Zheng, J. Luo, H. P. Peng, H. Lin, M. C. Bao, L. Hong and J. J. Zhou, Chem. Eng. J., 2018, 351, 782–790 CrossRef CAS.
- S. W. Liu, F. Chen, S. T. Li, X. X. Peng and Y. Xiong, Appl. Catal., B, 2017, 211, 1–10 CrossRef CAS.
- J. L. Shi, L. N. Zhang, N. N. Sun, D. Hu, Q. Shen, F. Mao, Q. Gao and W. Wei, ACS Appl. Mater. Interfaces, 2019, 11, 28858–28867 CrossRef CAS PubMed.
- C. H. Liu, H. L. Dai, C. Q. Tan, Q. Y. Pan, F. P. Hu and X. M. Peng, Appl. Catal., B, 2022, 310, 121326 CrossRef CAS.
- X. Meng, C. Duan, Y. L. Zhang, W. L. Lu, W. L. Wang and Y. H. Ni, Compos. Sci. Technol., 2020, 200, 108384 CrossRef CAS.
- D. S. Yuan, J. Ding, J. Zhou, L. Wang, H. Wan and W. L. Dai, J. Alloys Compd., 2018, 762, 98–108 CrossRef CAS.
- H. Zhang, G. Wang, D. Chen, X. J. Lv and J. H. Li, Chem. Mater., 2008, 20, 6543–6549 CrossRef CAS.
- W. Li, X. Wang, M. Li, S. A. He, Q. Ma and X. C. Wang, Appl. Catal., B, 2020, 268, 118384 CrossRef CAS.
- N. Q. Thang, A. Sabbah, L. C. Chen, K. H. Chen, C. M. Thi and P. V. Viet, Chemosphere, 2021, 282, 130971 CrossRef CAS PubMed.
- G. H. Zuo, A. Q. Wang, Y. Yang, H. L. Huang, F. B. Wang, H. W. Jiang, L. Zhang and Y. J. Zheng, J. Porous Mater., 2020, 27, 339–345 CrossRef CAS.
- R. Li, W. Li, C. Jin, Q. Y. He and Y. Z. Wang, J. Alloys Compd., 2020, 825, 154008 CrossRef CAS.
- M. Q. Qiu, Z. X. Liu, S. Q. Wang and B. W. Hu, Environ. Res., 2021, 196, 110349 CrossRef CAS PubMed.
- R. Chandra, S. Mukhopadhyay and M. Nath, Mater. Lett., 2016, 164, 571–574 CrossRef CAS.
- J. B. Zhou, W. Liu and W. Q. Cai, Sci. Total Environ., 2019, 696, 133962 CrossRef CAS PubMed.
- W. Q. Chen, L. Y. Li, W. H. Qiu, L. Tang, L. Xu, K. J. Xu and M. H. Wu, Engineering, 2019, 5, 755–767 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02194c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |