Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
PVA/κ-carrageenan/Au/camptothecin/pegylated-polyurethane/paclitaxel nanofibers against lung cancer treatment
Mohammad Irani * and
Sina Mohammadrezaei Nodeh
* and
Sina Mohammadrezaei Nodeh
Faculty of Pharmacy, Alborz University of Medical Sciences, Karaj, Iran. E-mail: irani_mo@ut.ac.ir
First published on 1st June 2022
Abstract
Gold nanoparticles, paclitaxel (PTX), and camptothecin (CMPT) were loaded into the PVA/κ-carrageenan/pegylated-PU composite and core–shell nanofibers prepared by two-nozzle and coaxial electrospinning methods. The capability of composite and core–shell nanofibers was investigated for the targeted delivery of anticancer drugs in lung cancer treatment. In vitro and in vivo release of PTX and CMPT were investigated to find the release mechanism from nanofibers compared to direct administration of pristine PTX and CMPT. The mean fiber diameter for composite and core–shell nanofibers with shell feeding rates of 0.3, 0.5, and 0.7 mL h−1 was about 225, 330, 520, and 640 nm, respectively. In vivo release studies indicated that the blood concentration of CMPT and PTX for rats fed with core–shell nanofibers reached the highest values of 26.8 ± 0.04 μg mL−1, and 26.5 ± 0.05 μg mL−1 in 36 h, and 24 h and reduced slowly within 84 h, and 48 h, respectively. The maximum cytotoxicity was 75% in the presence PVA/κ-carrageenan/CMPT/Au/pegylated-PU/PTX core–shell nanofibers. In vivo antitumor activity results confirmed the synergic effect of Au, CMPT and PTX anticancer drugs on the reduction of tumor volume without change in mouse weight by the PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX core–shell nanofibers. The obtained results indicated that the simultaneous loading of CMPT and PTX anticancer drugs and Au nanoparticles is more beneficial for lung cancer treatment.
1. Introduction
The combination of paclitaxel (PTX) and camptothecin (CMPT) could provide several advantages over monotherapy. In previous studies, better treatment of various cancers, including lung, breast, and melanoma, is demonstrated by the co-delivery of PTX and CMPT compared to the monotherapy of PTX or CMPT1-4. For instance, the concentrations of PTX and CMPT could be controlled by their loading into the liposome.1 Borrelli et al.2 synthesized the squalene conjugates with PTX, CMPT, podophyllotoxin, and epothilone against A549 lung cancer cells. In other study, the combined effect of PTX and CMPT was investigated against melanoma,3 breast, pancreatic,4 and medullary thyroid carcinomas.5 Chen et al.6 attached PTX and CMPT anticancer drugs on the 2-nitroimidazole against hypoxic tumors. In another study, CMPT and PTX were conjugated with two cyclic cell-penetrating peptides to inhibit MCF-7 breast cancer cells.7 Hariri et al.8 investigated the targeted delivery of PTX and CMPT from a cross-linked sponge against lung cancer. The use of targeted drug delivery systems such as liposomes,9,10 microspheres,11,12 nanoparticles,13,14 and nanofibers15–18 could provide the sustained delivery of anticancer drugs.κ-Carrageenan, an anionic sulfonated polysaccharide, is extracted from red algae and used for biomedical applications such as food, tissue engineering and drug delivery systems.19–23 Carrageenan could also interact with inorganic materials such as graphene oxide (GO), metal–organic frameworks (MOFs) and proteins. κ-carrageenan, and κ-carrageenan-based composites have been used in various drug delivery systems. For instance, Sun et al.20 interacted κ-carrageenan with zein nanoparticles. Javanbakht et al.21 synthesized κ-carrageenan/MOFs composite hydrogel for oral delivery. Vinothini et al.22 used κ-carrageenan-grafted graphene oxide for drug delivery of DOX. Tort & Acartürk23 investigated the capability of electrospun κ-carrageenan nanofibers for oral mucositis treatment. The use of nanofibers prepared by electrospinning technique is an effective strategy for loading the high content of drugs due to their high porosity, high surface area, small size, good flexibility, and interconnecting channels.24 However, there are still limitations for electrospun nanofibers, due to their rapid degradation over time. The various nanofibers, including composite, multi-layered,25 and core–shell nanofibers,17,18 could provide the prolonged-release profiles. In recent studies, the hydrophobic polymers such as poly(ε-caprolactone),26 polyurethane (PU),27 poly(lactic acid) (PLA),28 and poly(lactic-co-glycolic acid) (PLGA)29 were used to increase the stability of polysaccharide nanofibers in aquatic systems.
Most PUs are non-degradable and have little use in biomedical applications. On the other hand, the natural/synthetic biodegradable polymers are stiff with low flexibility or soft with poor mechanical properties. To overcome these challenges, the biodegradable PUs, composed of a hard segment, soft segment, and chain extender, have been introduced as one of the most popular polymers due to their low cost, high biocompatibility, and excellent efficiency for drug delivery systems.30 The degradation rate of PUs is mainly due to the cleavage of hydrolytic bonds present in their soft segments. The composition of the soft segments could control the degradation rate of the PUs. The degradation rate of PUs with hydrophilic soft segments such as poly(ethylene glycol) is faster than PUs with hydrophobic soft segments such as polycaprolactone diol.31 Loading of anticancer drugs into the PUs, and its derivates could increase the availability of the anticancer agents at the tumor sites, suppress the cancer cells that did not kill by the initial dose of drug31,32 and decrease the adverse side effects. For instance, Gajbhiye et al.33 reviewed the performance of biodegradable PU nano-constructs for the targeted delivery of anticancer drugs. Yin et al.34 indicated that PTX molecules were released slowly from liposome-encapsulated PU scaffolds compared to PTX release from PU. Liu et al.35 investigated the potential PU functionalized with amine, and carboxyl group micelles for incorporating of doxorubicin hydrochloride (DOX) and PTX. In previous studies, the capability of drug-loaded PU nanofibers for various cancers treatment was studied by our group.17,18,36,37 Recently, inorganic materials such as metal oxides, zeolites, MOFs and noble metals were loaded into the nanofibrous matrix to increase the delivery of anticancer drugs on the surface of the cancerous tissues. Gold-based nanomaterials with various shapes, including nanoparticles,38–40 nanocages,41,42 and nanorods43–45 are incorporated into the different forms of drug delivery systems for targeted delivery and imaging in cancer therapy. Gold nanoparticles (Au NPs), due to their high stability, and easier synthesis were used to improve the attachment of cancerous cells and increase the therapeutic efficacy.46,47
In the present study, PTX and CMPT were loaded into the κ-carrageenan/Au/pegylated-PU composite nanofibers prepared by two-nozzle and coaxial electrospinning methods for targeted delivery of anticancer drugs against lung cancer treatment. It was hypothesized that the co-delivery of PTX and CMPT from nanofibers and the use of gold nanoparticles in the nanofibers structure could enhance the targeted delivery of anticancer drugs on cancerous tissues.
2. Experimental
2.1 Materials
Poly(ethylene glycol) (Mn = 2000 Da, PEG) supplied from Sigma-Aldrich (USA); hexamethylene diisocyanate (HDI) and 1,4-butanediol (BDO) purchased from Merck Company (Germany) were used to synthesize the pegylated polyurethane. Polyvinyl alcohol (99% hydrolyzed, Mn = 72 kDa) and κ-carrageenan were used to prepare PVA/κ-carrageenan nanofibers. Paclitaxel and camptothecin supplied from Sigma-Aldrich (USA) were utilized as anticancer drugs. HAuCl4, and sodium citrate provided from Sigma-Aldrich (USA) were applied to synthesize the gold nanoparticles.2.2 Preparation of core–shell solutions
The shell solution was the PTX-loaded pegylated polyurethane (PU). The pegylated polyurethane was synthesized by reacting PEG, HDI, and BDO as described previously by Yao et al.48 10 wt% pegylated PU electrospun solution was prepared by dissolving in N,N-dimethylacetamide (DMF, Merck). To load PTX molecules into the pegylated PU, 1 and 2 mg mL−1 PTX were added into the pegylated-PU solution under sonication for 30 min.The core solution was the CMPT/Au-loaded PVA/κ-carrageenan solution. The citrate reduction of HAuCl4 was used to synthesize Au nanoparticles.37 2 wt% κ-carrageenan (dissolved in distilled water) and 10 wt% PVA (dissolved in distilled water) were mixed under stirring for 6 h (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v). To load CMPT and Au nanoparticles into the PVA/κ-carrageenan solution, 2 wt% Au suspension (with respect to the PVA/κ-carrageenan solution v/v) and 1, 2 mg mL−1 CMPT were added into the PVA/κ-carrageenan solution under sonication for 30 min.
7 v/v). To load CMPT and Au nanoparticles into the PVA/κ-carrageenan solution, 2 wt% Au suspension (with respect to the PVA/κ-carrageenan solution v/v) and 1, 2 mg mL−1 CMPT were added into the PVA/κ-carrageenan solution under sonication for 30 min.
2.3 Preparation of composite nanofibers and core–shell nanofibers
To prepare PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX composite nanofibers, the prepared pegylated PU/PTX and PVA/κ-carrageenan/CMPT/Au solutions were separately transferred into the two syringes (needle tip: 19 gauge) and then placed at both counter sides of the cylindrical collector which was placed an aluminum foil on the collector. The applied high voltage was varied between 15 and 25 kV. Tip–collector distance was constant at 12 cm. The feeding rate was between 0.3–1 mL h−1 for both solutions to produce the PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX composite nanofibers under an electrospinning time of 4 h.To prepare the PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX core–shell nanofibers, the prepared core and shell solutions were transferred into the two syringes (tip needle: 27 and 14 gauges). The voltage, tip–collector distance, and core solution feeding rate were constant at 20 kV, 12 cm, and 0.3 mL h−1. The shell solution feeding rate varied between 0.3 and 0.7 mL h−1.
2.4 Instrumentation
Fourier transform infrared (FTIR) analysis was implemented ranging from 500–4000 cm−1 with a resolution of 2 cm−1 on the Fourier-transform infrared spectroscopy (Brucker, Tensor 27, Germany). X-ray diffraction (XRD) analysis ranged from 5° to 80° using an X-ray diffractometer (Intel, EQUINOX3000, France). Morphology of nanoparticles and nanofibers was carried out using a scanning electron microscope (SEM, AIS2100, SERON Technology, Republic of Korea) and a transmission electron microscope (TEM, H9500, Hitachi, Japan).2.5 Drug loading efficiency
To evaluate the drug loading measurements in the nanofibers, drug entrapment efficiency (DEE%) and drug loading efficiency (DLE%) were calculated as follows:
 | (1) |
 | (2) |
2.6 In vitro drug release and pharmacokinetic studies
In vitro release studies were implemented by immersion of 100 mg drug-loaded nanofibers in 500 mL of phosphate-buffered saline (PBS) solutions with a rotation rate of 50 rpm at the temperature of 37 °C, and pH values of 5.5 and 7.4. At preplanned times, 2 mL of aliquot was withdrawn, and 2 mL of fresh PBS was added. The final concentrations of PTX and CMPT were determined using High-performance liquid chromatography (HPLC, Agilent 1260, USA) and UV/Vis spectrophotometer (JAS.CO V-530, Japan) at 230 nm, and 360 nm, respectively. HPLC conditions are: a reversed-phase HPLC method, column: C-18 column, mobile phase: acetonitrile, methanol and water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v), temperature: 25 °C and flow rate of 1.0 mL min−1. Experiments were repeated three times, and the results were reported as mean ± S.D. The experimental data was statistically analyzed using an analysis of variance (ANOVA) as well as a t-test (P < 0.05).
40, v/v), temperature: 25 °C and flow rate of 1.0 mL min−1. Experiments were repeated three times, and the results were reported as mean ± S.D. The experimental data was statistically analyzed using an analysis of variance (ANOVA) as well as a t-test (P < 0.05).
The release data were fitted by pharmacokinetic models, including zero order (Q(t) = K0t), Higuchi (Q(t) = KHt0.5), and Korsmeyer–Peppas (Q(t) = Atn) equations.
2.7 In vivo release studies
For investigating the in vivo release of PTX and CMPT from nanofibers, first, the drug-loaded nanofibers were implemented into the 15 old mice with an average weight of 25 g (Tehran University) that were maintained under a maximum isolation environment for 6 weeks based on Institutional Animals Ethics Committee (94/PO/ReBi/S/1999/CPCSEA). All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Tehran University and approved by the Animal Ethics Committee of National Institute for Medical Research Development (NIMAD), Tehran, Iran. Then, nanofibers-implemented 15 old mice were divided into five groups of three (pure PVA/κ-carrageenan/pegylated PU nanofibers, PVA/κ-carrageenan/Au/pegylated PU nanofibers, PVA/κ-carrageenan/CMPT/Au/pegylated PU core–shell nanofibers, PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX core–shell nanofibers and PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX composite nanofibers). The mice dose of PTX and CMPT was 5 mg kg−1. The nanofibrous scaffolds were implanted into the lower right flank of each mouse. At predetermined intervals (1, 2, 3, 6, 12, 24, 36, 48, 60, 72, 84 and 96 h), 0.5 mL of blood samples were withdrawn and were stored in a centrifuge tube. The samples were centrifuged at 5000 rpm for 5 min to separate the plasma that was frozen at −20 °C until HPLC and UV assays to determine the concentrations of PTX and CMPT, respectively.2.8 Cell viability
To investigate the biocompatibility, and cytotoxicity of nanofibers, the pure drugs and drugs-loaded nanofibers were cut into flakes and placed in each well of L929 murine fibroblast and A549 lung cancer cell lines. Cells were purchased from the Institute Pasteur of Iran, and cultured in RPMI-1640 media under 5% CO2 and 37 °C for 24, 48 and 72 h. 5 × 104 cells per well were transferred in a 48-well plate and treated with 1 and 2 mg mL−1 concentrations of pure drugs and their equivalent doses of drugs-loaded nanofibers. The MTT assay was implemented by using a microplate reader (Multiskan MK3, Thermo Electron Corporation, USA) at the wavelength of 570 nm.27 Briefly, the MTT assay was carried out as follows: 20 μL of MTT solution with a concentration of 5 mg mL−1 in PBS (pH 7.4) was added to each well, and then, were incubated for a further 4 h. Then, the solution was aspirated cautiously from each well. After treating the cells with Sorenson buffer, the optical density of each well was readied at 570 nm.2.9 Antitumor efficiency in vivo
The tumor-bearing mice were divided into 5 groups of three. 2 × 106 A549 lung cancer cells were subcutaneously injected into the left hind flank of each anesthetized mice after isolation for 6 weeks. When the tumor volume reached 100 mm3, the mice were treated with nanofibrous samples, and experiments were continued for 20 days. The relative tumor volume with respect to the initial volume of tumor (V0 = 100 mm3) before treatment was calculated as V/V0. Briefly, animals were anesthetized by intra-peritoneal injection of pentobarbital at 20 mg kg−1, and a small incision was made on the skin to expose the tumor. A small incision was made into the tumor, and synthesized fibers were inserted into the tumor and then the wound was closed using subcutaneous suturing. The tumor volume is calculated as length × width × (length + width)/2.3. Results and discussion
3.1 Characterization of nanofibers
FTIR spectra of PVA, κ-carrageenan, Au, CMPT, PVA/κ-carrageenan/CMPT/Au, pegylated-PU, PTX, and pegylated-PU/PTX are illustrated in Fig. 1. For pure PVA, the detected peaks at 3250 cm−1, 2920 cm−1, 1690 cm−1, 1425 cm−1, 1080 cm−1 and 887 cm−1 were assigned to the –OH stretching, CH2 asymmetric stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, CH2 bending, C–O stretching, and C–C stretching, respectively. For pure κ-carrageenan, the observed peaks at around 3320 cm−1, 1420 cm−1, 1220 cm−1, 1150 cm−1, 930 cm−1, and 840 cm−1 were due to the –OH stretching, C–O–H bending, O
O stretching, CH2 bending, C–O stretching, and C–C stretching, respectively. For pure κ-carrageenan, the observed peaks at around 3320 cm−1, 1420 cm−1, 1220 cm−1, 1150 cm−1, 930 cm−1, and 840 cm−1 were due to the –OH stretching, C–O–H bending, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, C–O–C absorption, and O–SO3 stretching bands, respectively. The new peaks between 500–700 cm−1 after loading Au nanoparticles into the PVA/κ-carrageenan confirmed the presence of Au nanoparticles in the nanofibrous matrix. The shift of carbonyl stretching vibrations correspond to the ketone groups from 1610 cm−1 to 1660 cm−1, and the lactone groups from 1720 cm−1 to 1745 cm−1 demonstrated the interaction of CMPT molecules with nanofibrous matrix. For pegylated PU, the appeared peaks at 3350 cm−1, 2930 cm−1, 2840 cm−1, 2225 cm−1, 1730 cm−1, 1644 cm−1, 1550 cm−1, 1460 cm−1, and 1105 cm−1 were related to the N–H stretching, asymmetric CH2 stretching, symmetric CH2 stretching, –N
O stretching, C–O–C absorption, and O–SO3 stretching bands, respectively. The new peaks between 500–700 cm−1 after loading Au nanoparticles into the PVA/κ-carrageenan confirmed the presence of Au nanoparticles in the nanofibrous matrix. The shift of carbonyl stretching vibrations correspond to the ketone groups from 1610 cm−1 to 1660 cm−1, and the lactone groups from 1720 cm−1 to 1745 cm−1 demonstrated the interaction of CMPT molecules with nanofibrous matrix. For pegylated PU, the appeared peaks at 3350 cm−1, 2930 cm−1, 2840 cm−1, 2225 cm−1, 1730 cm−1, 1644 cm−1, 1550 cm−1, 1460 cm−1, and 1105 cm−1 were related to the N–H stretching, asymmetric CH2 stretching, symmetric CH2 stretching, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O absorption, C
C–O absorption, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, urea absorption, N–H bending, CH2 bending, and C–O–C absorption, respectively. The detected peaks, in the FTIR spectrum of pure paclitaxel, at 3630 cm−1, 3095 cm−1, 1721 cm−1, 1650 cm−1, 1245 cm−1 and 1078 cm−1 were due to the presence of O–H, CH2, C
O stretching, urea absorption, N–H bending, CH2 bending, and C–O–C absorption, respectively. The detected peaks, in the FTIR spectrum of pure paclitaxel, at 3630 cm−1, 3095 cm−1, 1721 cm−1, 1650 cm−1, 1245 cm−1 and 1078 cm−1 were due to the presence of O–H, CH2, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–C, C–N and C–O groups. The prominent peaks of pegylated PU and paclitaxel have overlapped.
O, C–C, C–N and C–O groups. The prominent peaks of pegylated PU and paclitaxel have overlapped.
 | ||
| Fig. 1 FTIR spectra of (A) PVA, κ-carrageenan, CMPT, Au, PVA/κ-carrageenan/CMPT/Au, and (B) pegylated PU, PTX, and pegylated PU/PTX. | ||
SEM images of PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX composite nanofibers under different applied voltages (15, 20 and 25 kV) and feeding rates (0.3, 0.5, and 1 mL h−1) are presented in Fig. 2. By increasing the applied voltage up to 20 kV, the more homogeneous fibers were prepared on the collector (mean = 225 nm, Fig. 2b). The higher applied voltage (25 kV) led to instability of jet solution and enhancement of fiber diameter (mean = 440 nm, Fig. 2c). However, an increase in the feeding rate from 0.3 to 1 mL h−1 led to increase the fiber diameter from 225 nm to 470 nm. Therefore, an applied voltage of 20 kV, tip–collector distance of 12 cm and feeding rate of 0.3 mL h−1 were selected to fabricate composite nanofibers for further experiments. SEM images from PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX core–shell nanofibers with different shell feeding rates (0.3, 0.5 and 0.7 mL h−1), core feeding rate of 0.3 mL h−1, the voltage of 20 kV, and the tip–collector distance of 12 cm are presented in Fig. 2f–h. As expected, an increase in shell feeding rate from 0.3 mL h−1 to 0.5 mL h−1, and 0.7 mL h−1 led to increased fiber diameter from 330 nm to 520 and 640 nm, respectively. The comparison of the PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX composite and core–shell nanofibers indicated that the composite nanofibers had a sharp distribution of fibers compared with broader distribution of core–shell nanofibers at studied condition. However, the non-alignment of fibers was detected for both composite and core–shell nanofibers, and thus have a randomly distributed nanofibrous structure for synthesized nanofibers. TEM image from core–shell nanofibers demonstrated the core-sheath structure of nanofibers prepared by coaxial electrospinning.
3.2 Release studies in vitro and in vivo
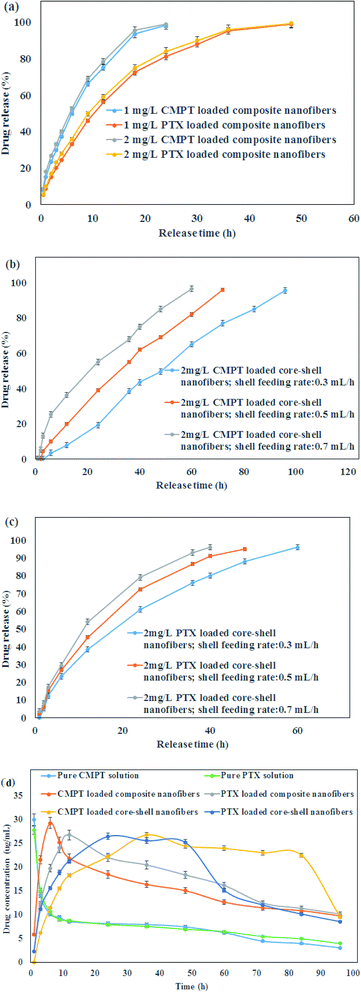
DEE (%) and DLE (%) are evaluated for PTX and CMPT-loaded nanofibers which results are presented in Table 1(a). The higher DEE than 95% for both PTX and CMPT loaded core–shell nanofibers confirmed an effective loading of anticancer drugs into the core–shell nanofibers. The lower DEE and DLE for drug-loaded composite nanofibers compared to drugs-loaded core–shell nanofibers could be attributed to the removing unattached molecule on the nanofiber surface. As expected, DLE was increased by increasing the initial drug content. There were no significant differences between the DEE of 1 mg L−1 and 2 mg L−1 anticancer drugs-incorporated nanofibers. The release behavior of PTX and CMPT from PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX composite and core–shell nanofibers produced by various shell feeding rates is implemented under physiological pH (pH: 7.4) (Fig. 3a–c). The obtained results suggested that prepared composite fibers exhibited the drug sustained-release profiles for PTX and CMPT in terms of initial burst release, followed by a sustained release period for composite nanofibers and a sustained release without initial burst release from core–shell nanofibers. There was no significant difference between the release behavior of 1 and 2 mg L−1 PTX and CMPT from composite nanofibers. Therefore, the loading of 2 mg L−1 PTX and CMPT did not change the release pattern but different rates compared with 1 mg L−1 PTX and CMPT-loaded nanofibers, and it is possible to control the release rate of PTX and CMPT from nanofibers by adjusting the concentration of drugs. On the other hand, the time durations for PTX & CMPT release from core–shell nanofibers with 0.3, 0.5 and 0.7 mL h−1 shell feeding rate were 60, 48, 40 h & 96, 72, 60 h, respectively. Because of higher drug loading efficiency and more sustained release F4 formulation with core–shell structure showed the best result and was selected for in vivo release studies. The release data of PTX from composite and core–shell nanofibers best described using Peppas' equation (Table 1(b)). The values of release exponent (n = 0.35, 0.32 and 0.29 for CMPT) were smaller than 0.45 from core–shell nanofibers which indicated that CMPT molecules were fled from the core–shell nanofibers following the Fickian mechanism. The linear release achieved for CMPT release from core–shell nanofibers demonstrated an excellent fitting of release data with the zero-order pharmacokinetic model. The “n” values for PTX release from both composite and core–shell nanofibers and “n” values higher than 0.45 for CMPT release from composite nanofibers confirmed the non-Fickian diffusion mechanism.| (a) | ||||
|---|---|---|---|---|
| Sample | Drug | Concentration (mg L−1) | DEE (%) | DC (%) |
| Composite (F1) | CMPT | 1 | 92.5 ± 0.5 | 2.628 ± 0.0142 |
| PTX | 1 | 93.4 ± 0.7 | 2.653 ± 0.0199 | |
| Composite (F2) | CMPT | 2 | 93.1 ± 0.8 | 5.290 ± 0.0455 |
| PTX | 2 | 94.8 ± 0.7 | 5.386 ± 0.0397 | |
| Core–shell (F3) | CMPT | 1 | 98.2 ± 0.3 | 2.790 ± 0.0085 |
| PTX | 1 | 97.9 ± 0.4 | 2.781 ± 0.0114 | |
| Core–shell (F4) | CMPT | 2 | 98.8 ± 0.2 | 5.614 ± 0.0114 |
| (b) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Nanofibrous carrier | Drug | Zero-order | Higuchi | Korsmeyer–Peppas | ||||
| K0 (h−1) | R2 | KH (h−0.5) | R2 | n | KKP | R2 | ||
| Composite nanofibers | CMPT | 0.5682 | 0.949 | 6.125 | 0.962 | 0.798 | 4.564 | 0.991 |
| PTX | 0.4651 | 0.932 | 4.589 | 0.958 | 0.685 | 4.251 | 0.994 | |
| Core–shell nanofibers | CMPT | 0.1842 | 0.989 | 2.897 | 0.941 | 0.293 | 2.251 | 0.990 |
| Shell feeding rate: 0.3 mL h−1 | PTX | 0.4180 | 0.948 | 4.052 | 0.960 | 0.625 | 4.155 | 0.991 |
| Core–shell nanofibers | CMPT | 0.1925 | 0.990 | 3.134 | 0.928 | 0.324 | 2.351 | 0.992 |
| Shell feeding rate: 0.5 mL h−1 | PTX | 0.4428 | 0.946 | 4.389 | 0.953 | 0.666 | 4.655 | 0.991 |
| Core–shell nanofibers | CMPT | 0.1989 | 0.992 | 3.379 | 0.944 | 0.352 | 2.846 | 0.993 |
| Shell feeding rate: 0.7 mL h−1 | PTX | 0.4859 | 0.938 | 4.827 | 0.955 | 0.702 | 4.989 | 0.991 |
The in vivo PTX and CMPT (10 mg of each drug) release profiles from nanofibers are presented in Fig. 3d. 5 mL of 2 mg mL−1 PTX and CMPT solutions were used as control. Using pure PTX and CMPT solutions, plasma concentrations of PTX and CMPT came to their highest within 1 h and lower to average level at about 6 h for both anticancer drugs. When rats were fed with composite nanofibers, the blood concentration of CMPT and PTX reached the highest values of 29.3 ± 0.05 μg mL−1 and 26.8 ± 0.03 μg mL−1 in 6 h and 12 h and reduced slowly within 24 h and 48 h, respectively. Additionally, owing to the drug concentration gradient distribution from the inner layer to shell layer and then granted by core–shell nanofibers, which stores most drug in the inner layer, the curve of CMPT and PTX release from core–shell nanofibers exhibited a sustained release for about 84 h and 48 h, respectively due to the continuous release of drugs from core–shell nanofibers.
3.3 Cell viability
The cell viability of synthesized composite and core–shell nanofibers toward L929 normal cells is implemented to investigate the biocompatibility of synthesized nanofibers. The results are illustrated in Fig. 4A. The cell viability was higher than 90% for both composite and core–shell nanofibers, confirming the high biocompatibility of the synthesized nanofibrous samples. The cytotoxicity of PTX and CMPT loaded-nanofibers against A549 lung cancer cell lines is presented in Fig. 4B. The cancer cells were alive in the presence pure nanofibers without Au nanoparticles and anticancer drugs. A gradual decrease in the cell viability was observed after loading Au nanoparticles into the nanofibers, which indicated the anticancer activity of Au nanoparticles against A549 lung cancer cells. Whereas, the cell viability was reduced to 53%, 44% and 35% after treating A549 cancer cell lines with nanofibers containing CMPT, PTX and CMPT/PTX. The maximum cytotoxicity was about 75% in the presence PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX core–shell nanofibers (both core and shell feeding rates: 0.3 mL h−1). The sustained release of CMPT and PTX from nanofibers resulted in more cytotoxicity of core–shell nanofibers than composite nanofibers. In vitro cytotoxicity results showed that its inhibition effect on tumor cells by the PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX core–shell nanofibers was remarkable, but the toxicity for normal cells was slight. Similar trends are reported by other researchers.49,50 The simultaneous incorporation of Au nanoparticles and co-delivery of PTX and CMPT into the core–shell nanofibers led to effective treatment of A549 lung cancer cells. | ||
| Fig. 4 Cell viability of synthesized composite and core–shell nanofibers toward (A) L929 normal cells and (B) A549 lung cancer cells. | ||
3.4 In vivo studies
The in vivo antitumor efficacy of the PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX composite and core–shell nanofibers was investigated on the A549 tumor-bearing mice with an initial tumor volume of 100 mm3 which the results of tumor volume change and the weight of mice after treating with nanofibers are presented in Fig. 5. The pure nanofibers were selected as control. As shown in Fig. 5a, the maximum tumor inhibition was occurred in the presence PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX core–shell nanofibers compared to pure core–shell nanofibers, Au-loaded core–shell nanofibers, and composite nanofibers. The results indicated the synergistic effects of Au nanoparticles and anticancer drugs on tumor inhibition. Among the above four groups, the pure core–shell nanofibers did not show any tumor inhibition rate after 20 days. Furthermore, the nanofibers containing PTX and CMPT presented more effective tumor inhibition than Au nanoparticles loaded-nanofibers which revealed that the continuous release of CMPT and PTX from nanofibers could exert a better effect on the reduction of tumor volume and enhancement of tumor inhibition. Therefore, PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX core–shell nanofibers could be an effective localized delivery system for lung cancer treatment. The investigation of mice's body weight after therapy revealed that no significant change occurred for all mice after injecting tumors for 20 days (Fig. 5b). For comparison of the capability of the synthesized core–shell nanofibers for the treatment of lung cancer cells with other nanofibrous scaffolds, Qiu et al.51 prepared the mesoporous silica nanoparticles/doxorubicin hydrochloride (DOX)-loaded poly(L-lactic acid) nanofibers (PLLA/DOX@MSNs) against human spca-1 lung cancer cells. The maximum cell viability was 73.10%. The release trend of DOX from PLLA/DOX@MSNs composite nanofiber was similar to the release profiles of CMPT and PTX from PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX composite nanofibers (initial burst release followed by a long period of slow-release). The inhibition rate of titanocene dichloride-loaded PLLA nanofibers was 68.2% (240 mg L−1 drug concentration).52 The maximum cell viability of A549 lung cancer cells by the chitosan/PLLA/TiO2/DOX/GO nanofiber and a magnetic field was about 82% after 86 h.53 Therefore, the co-incorporation of Au nanoparticles and anticancer drugs into the nanofibers demonstrated an optimal therapy effect on the tumor inhibition without changing the body weight.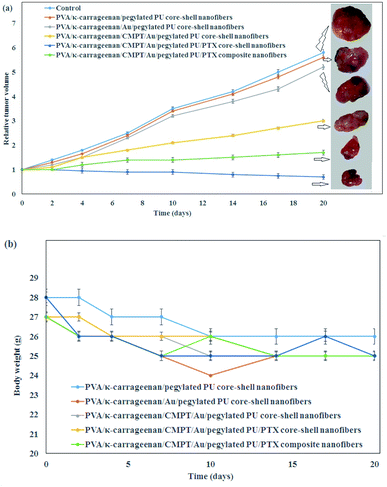 | ||
| Fig. 5 (a) In vivo antitumor efficacy of the PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX composite, and core–shell nanofibers and (b) mice body weight changes (number of trials = 3). | ||
4. Conclusion
In this work, Au nanoparticles, CMPT and PTX anticancer drugs were successfully incorporated into the PVA/κ-carrageenan/pegylated PU composite and core–shell nanofibers. The applied voltage of 20 kV, tip–collector distance of 12 cm and feeding rate of 0.3 mL h−1 were optimum values for the fabrication of PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX composite nanofibers with an average diameter of 225 nm. The mean fiber diameter for core–shell nanofibers produced under shell feeding rates of 0.3, 0.5, and 0.7 mL h−1 were 330, 520 and 640 nm, respectively. The higher DEE than 95% for PTX and CMPT confirmed an effective loading of anticancer drugs into the nanofibers. Time durations for PTX vb. CMPT release from core–shell nanofibers with 0.3, 0.5 and 0.7 mL h−1 shell feeding rates were 60, 48, 40 h vs. 96, 72, 60 h, respectively. The linear release is achieved for CMPT release from core–shell nanofibers. The PTX release data of composite, core–shell nanofibers and CMP release data of composite nanofibers were best described using the Peppas' equation. In vivo release studies indicated that rats fed with core–shell nanofibers, the blood concentration of CMPT and PTX reached the highest values of 26.8 ± 0.04 μg mL−1 and 26.5 ± 0.05 μg mL−1 in 36 h and 24 h and kept in the constant values between 36–84 h and 24–48 and finally reduced after 84 h and 48 h, respectively. The L929 cell viability higher than 90% for both composite and core–shell nanofibers confirmed the high biocompatibility of the synthesized composite and core–shell nanofibrous samples. The maximum cytotoxicity was 75% in the presence PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX core–shell nanofibers. In vivo antitumor efficacy results of A549 tumor-bearing mice treated with composite and core–shell nanofibers demonstrated the best effect on the reduction of tumor volume and enhancement of tumor inhibition without changing the mice's weight in the presence PVA/κ-carrageenan/CMPT/Au/pegylated PU/PTX core–shell nanofibers.Abbreviations
| ANOVA | Analysis of variance |
| BDO | 1,4-Butanediol |
| CMPT | Camptothecin |
| DOX | Doxorubicin hydrochloride |
| DEE | Drug entrapment efficiency |
| DLE | Drug loading efficiency |
| FTIR | Fourier transform infrared |
| Au NPs | Gold nanoparticles |
| GO | Graphene oxide |
| HDI | Hexamethylene diisocyanate |
| HPLC | High-performance liquid chromatography |
| MOFs | Metal–organic frameworks |
| DMF | N,N-Dimethylacetamide |
| PTX | Paclitaxel |
| PBS | Phosphate buffered saline |
| PEG | Poly(ethylene glycol) |
| PLA | Poly(lactic acid) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PU | Polyurethane |
| PVA | Polyvinyl alcohol |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
| XRD | X-ray diffraction |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Research reported in this publication was supported by Elite Researcher Grant Committee under award number [996215] from the National Institute for Medical Research Development (NIMAD), Tehran, Iran.References
- N. V. Koshkina, V. Knight, B. E. Gilbert, E. Golunski, L. Roberts and J. C. Waldrep, Cancer Chemother. Pharmacol., 2001, 47, 451 CrossRef CAS PubMed.
- S. Borrelli, M. S. Christodoulou, I. Ficarra, A. Silvani, G. Cappelletti, D. Cartelli, G. Damia, F. Ricci, M. Zucchetti, F. Dosio and D. Passarella, Eur. J. Med. Chem., 2014, 85, 179 CrossRef CAS PubMed.
- P. D. Senter, H. Marquardt, B. A. Thomas, B. D. Hammock, I. S. Frank and H. P. Svensson, Cancer Res., 1996, 56, 1471 CAS.
- D. P. Jeansonne, G. Y. Koh, F. Zhang, H. Kirk-Ballard, L. Wolff, D. Liu, K. Eilertsen and Z. Liu, Oncol. Rep., 2011, 25, 1473 CAS.
- K. Kaczirek, M. Schindl, A. Weinhausel, C. Scheuba, C. Passler, G. Prager and M. Raderer, J. Clin. Endocrinol. Metab., 2004, 89, 2397 CrossRef CAS PubMed.
- C. Jin, S. Wen, Q. Zhang, Q. Zhu, J. Yu and W. Lu, ACS Med. Chem. Lett., 2017, 8, 762 CrossRef CAS PubMed.
- N. S. El-Sayed, A. N. Shirazi, M. I. Sajid, S. E. Park, K. Parang and R. K. Tiwari, Molecules, 2019, 24, 1427 CrossRef CAS PubMed.
- G. Hariri, A. D. Edwards, T. B. Merrill, J. M. Greenbaum, A. E. van der Ende and E. Harth, Mol. Pharm., 2014, 11, 265 CrossRef CAS PubMed.
- M. Zhang, M. Li, L. Du, J. Zeng, T. Yao and Y. Jin, Int. J. Pharm., 2020, 578, 119177 CrossRef CAS PubMed.
- X. Liang, C. Gao, L. Cui, S. Wang, J. Wang and Z. Dai, Adv. Mater., 2017, 29, 1703135 CrossRef PubMed.
- D. Zahn, A. Weidner, Z. Nosrati, L. Wöckel, J. Dellith, R. Müller, K. Saatchi, U. O. Häfeli and S. Dutz, J. Magn. Magn. Mater., 2019, 469, 698 CrossRef CAS.
- F. Xie, K. D. Clercq, C. Vervaet, J. V. Bocxlaer, P. Colin and A. Vermeulen, Pharm. Res., 2019, 36, 1 CrossRef CAS PubMed.
- P. Chowdhury, P. K. B. Nagesh, E. Hatami, S. Wagh, N. Dan, M. K. Tripathi and S. Khan, J. Colloid Interface Sci., 2019, 535, 133 CrossRef CAS PubMed.
- M. Landgraf, C. A. Lahr, I. Kaur, A. Shafiee, A. Sanchez-Herrero, P. W. Janowicz and A. Ravichandran, Biomaterials, 2020, 240, 119791 CrossRef CAS PubMed.
- M. H. Azerbaijan, E. Bahmani, M. H. Jouybari, A. Hassaniazardaryani, P. Goleij, M. Akrami and M. Irani, Eur. J. Pharm. Sci., 2021, 164, 105914 CrossRef CAS PubMed.
- B. F. Dizaji, M. H. Azerbaijan, N. Sheisi, P. Goleij, T. Mirmajidi, F. Chogan, M. Irani and F. Sharafian, Int. J. Biol. Macromol., 2020, 164, 1461 CrossRef PubMed.
- A. Bazzazzadeh, B. F. Dizaji, N. Kianinejad, A. Nouri and M. Irani, Int. J. Pharm., 2020, 587, 119674 CrossRef CAS PubMed.
- L. F. Zhu, Y. Zheng, J. Fan, Y. Yao, Z. Ahmad and M. W. Chang, Eur. J. Pharm. Sci., 2019, 137, 105002 CrossRef CAS PubMed.
- A. Kalsoom Khan, A. U. Saba, S. Nawazish, F. Akhtar, R. Rashid, S. Mir, B. Nasir, F. Iqbal, S. Afzal, F. Pervaiz and G. Murtaza, Oxid. Med. Cell. Longevity, 2017, 2017, 1–13 CrossRef PubMed.
- X. Sun, C. Pan, Z. Ying, D. Yu, X. Duan, F. Huang, J. Ling and X. K. Ouyang, Int. J. Biol. Macromol., 2020, 146, 549 CrossRef CAS PubMed.
- S. Javanbakht, M. Shadi, R. Mohammadian, A. Shaabani, M. M. Amini, M. Pooresmaeil and R. Salehi, J. Drug Delivery Sci. Technol., 2019, 54, 101311 CrossRef CAS.
- K. Vinothini, N. K. Rajendran, M. A. Munusamy, A. A. Alarfaj and M. Rajan, Mater. Sci. Eng., C, 2019, 100, 676 CrossRef CAS PubMed.
- S. Tort and F. Acartürk, Carbohydr. Polym., 2016, 152, 802 CrossRef CAS PubMed.
- B. Ghafoor, A. Aleem, M. N. Ali and M. Mir, J. Drug Delivery Sci. Technol., 2018, 48, 82 CrossRef CAS.
- J. H. Seo, S. Y. Lee, C. R. Hwang, M. Yang, J. Lee, S. H. Lee and H. J. Cho, Int. J. Biol. Macromol., 2020, 162, 798 CrossRef CAS PubMed.
- Z. Amini, S. S. Rudsary, S. S. Shahraeini, B. F. Dizaji, P. Goleij, A. Bakhtiari, M. Irani and F. Sharifianjazi, Carbohydr. Polym., 2021, 258, 117680 CrossRef CAS PubMed.
- L. E. Aguilar, A. G. Nejad, C. H. Park and C. S. Kim, Nanomedicine, 2017, 13, 527 CrossRef CAS PubMed.
- J. Dai, J. Jin, S. Yang and G. Li, Mater. Res. Express, 2017, 4, 075403 CrossRef.
- S. Samadzadeh, H. Mousazadeh, S. Ghareghomi, M. Dadashpour, M. Babazadeh and N. Zarghami, J. Drug Delivery Sci. Technol., 2021, 61, 102318 CrossRef CAS.
- D. Feldman, Biointerface Res. Appl. Chem., 2020, 11, 8179 Search PubMed.
- J. Y. Cherng, T. Y. Hou, M. F. Shih, H. Talsma and W. E. Hennink, Int. J. Pharm., 2013, 450, 145 CrossRef CAS PubMed.
- Y. Niu, F. J. Stadler, J. Song, S. Chen and S. Chen, Colloids Surf. B., 2017, 153, 160 CrossRef CAS PubMed.
- K. R. Gajbhiye, B. P. Chaudhari, V. B. Pokharkar, A. Pawar and V. Gajbhiye, Int. J. Pharm., 2020, 588, 119781 CrossRef CAS PubMed.
- H. Yin, B. Du, Y. Chen, N. Song, Z. Li, J. Li, F. Luo and H. Tan, J. Biomater. Sci., Polym. Ed., 2020, 31, 2220 CrossRef CAS PubMed.
- C. Liu, Y. Guan, Y. Su, L. Zhao, F. Meng, Y. Yao and J. Luo, RSC Adv., 2017, 7, 11021 RSC.
- S. S. Qavamnia, L. R. Rad and M. Irani, Fiber. Polym., 2020, 21, 1634 CrossRef CAS.
- M. Irani, G. M. M. Sadeghi and I. Haririan, Int. J. Polym. Mater., 2018, 67, 361 CrossRef CAS.
- G. Chauhan, V. Chopra, A. Tyagi, G. Rath, R. K. Sharma and A. K. Goyal, Eur. J. Pharm. Sci., 2017, 96, 351 CrossRef CAS PubMed.
- J. Wang, Y. Zhang, N. Jin, C. Mao and M. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 11136 CrossRef CAS PubMed.
- M. Arumugam, B. Murugesan, N. Pandiyan, D. K. Chinnalagu, G. Rangasamy and S. Mahalingam, Mater. Sci. Eng., C, 2021, 123, 112019 CrossRef CAS PubMed.
- J. H. Park, H. Seo, D. I. Kim, J. H. Choi, J. H. Son, J. Kim, G. D. Moon and D. C. Hyun, Pharmaceutics, 2019, 11, 60 CrossRef CAS PubMed.
- T. H. L. Wu, M. Xie, S. Shen, W. Wang, M. Zhao, X. Mo and Y. Xia, Mater. Today Energy, 2019, 12, 129 CrossRef.
- Q. Yu, Y. M. Zhang, Y. H. Liu and Y. Liu, Adv. Thermoelectr., 2019, 2, 1800137 CAS.
- Y. Fu, H. Liu, Z. Ren, X. Li, J. Huang, S. Best and G. Han, J. Mater. Chem. B, 2017, 5, 5128 RSC.
- N. N. Mahmoud, H. Qabooq, S. Alsotari, O. A. Tarawneh, N. H. Aboalhaija, S. Shraim, A. M. Alkilany, E. A. Khalil and R. Abu-Dahab, RSC Adv., 2021, 11, 19956 RSC.
- N. Elahi, M. Kamali and M. H. Baghersad, Talanta, 2018, 184, 537 CrossRef CAS PubMed.
- R. A. Ovais, S. Naz, N. U. Islam, A. T. Khalil, S. Ali and Z. K. Shinwari, Appl. Microbiol. Biotechnol., 2017, 101, 3551 CrossRef PubMed.
- Y. Yao, D. Xu, C. Liu, Y. Guan, J. Zhang, Y. Su, L. Zhao, F. Meng and J. Luo, RSC Adv., 2016, 6, 97684 RSC.
- S. Shao, L. Li, G. Yang, J. Li, C. Luo, T. Gong and S. Zhou, Int. J. Pharm., 2011, 421, 310 CrossRef CAS PubMed.
- B. Ardeshirzadeh, N. A. Anaraki, M. Irani, L. R. Rad and S. Shamshiri, Mater. Sci. Eng. C, 2015, 48, 384 CrossRef CAS PubMed.
- K. Qiu, C. He, W. Feng, W. Wang, X. Zhou, Z. Yin, L. Chen, H. Wang and X. Mo, J. Mater. Chem. B, 2013, 1, 4601 RSC.
- P. Chen, Q. S. Wu, Y. P. Ding, M. Chu, Z. M. Huang and W. Hu, Eur. J. Pharm. Biopharm., 2010, 76, 413 CrossRef CAS PubMed.
- S. Samadi, M. Moradkhani, H. Beheshti, M. Irani and M. Aliabadi, Int. J. Biol. Macromol., 2018, 110, 416 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |