 Open Access Article
Open Access ArticleA new green approach for the reduction of consumed solvents and simultaneous quality control analysis of several pharmaceuticals using a fast and economic RP-HPLC method; a case study for a mixture of piracetam, ketoprofen and omeprazole drugs†
Mohamed A. Abdelgawad*a,
Eglal A. Abdelaleemb,
Mohammed Gamal *b,
Mohammed A. S. Abourehabc and
Nessreen S. Abdelhamidb
*b,
Mohammed A. S. Abourehabc and
Nessreen S. Abdelhamidb
aDepartment of Pharmaceutical Chemistry, College of Pharmacy, Jouf University, Aljouf 72341, Saudi Arabia. E-mail: mhmdgwd@ju.edu.sa
bPharmaceutical Analytical Chemistry Department, Faculty of Pharmacy, Beni-Suef University, Alshaheed Shehata Ahmad Hegazy St., 62514, Beni-Suef, Egypt. E-mail: mgamalm3000@yahoo.com
cDepartment of Pharmaceutics, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia
First published on 1st June 2022
Abstract
One of the main aims of green analytical chemistry (GAC) is the reduction of solvents and chemicals consumed. Recycling the mobile phase in chromatographic techniques provides an efficient way to implement GAC principles. However, this is not an easy job, particularly in the case of the gradient mode. Analysis of multi-pharmaceuticals for the same manufacturer using one mobile phase system dramatically reduces consumed solvents, time, and cost for pharmaceuticals analysis in quality control laboratories. This work is an attempt to reduce time, cost and effort needed for quality control analysis of several dosage forms produced by the same manufacturer. Our novel and green RP-HPLC method is able to separate and quantify a tertiary mixture of piracetam, ketoprofen and omeprazole produced by the same manufacturers. The analyst can easily quantify the three drugs in the three dosage forms in one run using the gradient elution mode of methanol and water (from 50% methanol to 85% methanol in ten minutes) with a flow rate 1.5 mL min−1 on a non-polar C18 column. Suitable dilutions were done for the working solution of the mixed pharmaceutical formulations prior to chromatographic analysis. This procedure will dramatically reduce the consumed solvents and save time and money during pharmaceutical analysis. The calibration ranges are (5–25), (5–25) and (3–20) μg mL−1 for the three studied drugs. The International Council for Harmonization (ICH) procedures were followed in the validation process and the results were evaluated in comparison with official HPLC methods, where no noteworthy differences were found. The green profile of the method and pictograms of AGREE and Green Analytical Procedure Index (GAPI) approaches proved the eco-friendly character for the studied drugs. The simultaneous quantitative analysis for Stimulan® and Hyposec® capsules, and Ketolgin® tablets from the Amoun Pharmaceutical Company, Egypt, can be accomplished via the novel method. Also, Memoral® ampoules, Topfam® tablets, and Gastroloc® capsules from Sigma Pharmaceutical Industries, Egypt, could be analyzed simultaneously. Omez® capsules and Ketogesic® tablets from the Pharaonia Pharmaceuticals, Egypt, could be determined simultaneously too. Applying this RP-HPLC method, a significant reduction of the total cost is assured as the required amount of solvent is noticeably decreased when performing multi-analyses in comparison to single component analysis.
1. Introduction
High performance liquid chromatography (HPLC) is one of the most frequently used techniques for the determination of drugs,1–8 natural products,9–13 foods,14–18 and important environmental markers.19–24 Quality control analysis is a vital step to ensure correct dosage during production, but the routine analysis for each pharmaceutical formulation alone results in a large amount of hazardous chemical waste. Green analytical chemistry aims to reduce chemical consumption and prevent pollution.25–27 Green chemistry metrics were developed and evaluated in many analytical studies for the estimation of a method’s ‘greenness’.28–35 Therefore, our main goal in this project is to offer a simple approach to reduce the chemicals consumed in routine drug analysis. The selected drugs for the analytical case study in this work are piracetam, ketoprofen and omeprazole.Piracetam (PIR) is 2-(2-oxopyrrolidin-1-yl) acetamide. It is a psycho-pharmacological and nootropic medicine.36 PIR is a cyclic compound derived from gamma aminobutyric acid (GABA). PIR is a neuroprotective drug and used for the treatment of myoclonus.36 The chemical structure for PIR is illustrated in Fig. 1A. Many HPLC methods have been reported for PIR analysis in pharmaceuticals, either on its own or in addition to impurities or other drugs.37–43
Ketoprofen (KET) is 2-(3-benzoylphenyl) propionic acid. KET belongs is a nonsteroidal anti-inflammatory drug (NSAID). The medical uses for KET include dysmenorrhea, headaches associated with dental pain, migraines, and postoperative pain. KET is also commonly used for rheumatoid arthritis and osteoarthritis. It is also used to reduce fever.44 The chemical structure of KET is shown in Fig. 1B. Many analytical HPLC methods have been recorded for the KET analysis of its formulations and enantiomers.45–52
Omeprazole (OME) is 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole. It inhibits abdominal H+, K+, ATPase enzymes. OME is a potent medication for decreasing gastric acid secretion in cases of refractory gastroesophageal reflux disease, peptic ulcers, and Zollinger–Ellison disease.53 The chemical structure of OME is displayed in Fig. 1C. There are many recorded publications for OME analysis in pharmaceuticals using HPLC methods.54–59
Several drug manufacturers have produced all three drugs or at least two of them: Table 1 lists some examples of pharmaceutical formulations in Egyptian pharmacies.
| Drug manufacturer | Pharmaceutical formulations | Active ingredients |
|---|---|---|
| Amoun Pharmaceutical Company S.A.E | Stimulan® capsules | Piracetam |
| Ketolgin® tablets | Ketoprofen | |
| Hyposec® capsules | Omeprazole | |
| Sigma Pharmaceutical Industries | Memoral® ampoules | Piracetam |
| Topfam® tablets | Ketoprofen | |
| Gastroloc® capsules | Omeprazole | |
| Pharaonia Pharmaceuticals (Pharo Pharma for Pharmaceuticals) | Omez® capsules | Omeprazole |
| Ketogesic® tablets | Ketoprofen |
The suggested fast and green RP-HPLC method could separate and quantify the three active ingredients from the three mixed dosage forms successfully. The aim of the presented research is to enable the analyst to analyze more than one dosage form or pure drug in a single run, saving time, cost and the effort needed for analysis as well as rendering the analysis more eco-friendly through reducing the volume of the solvents used and the waste resulting from the analysis. The new and green RP-HPLC method is able to separate and quantify tertiary mixtures of piracetam, ketoprofen and omeprazole produced by the same manufacturer.
2. Experimental
2.1. Pure samples and pharmaceutical formulations
Pure standards of piracetam, ketoprofen, and omeprazole were provided by Amoun Pharmaceutical Company S.A.E, Egypt, with purities of 99.85%, 99.93% and 99.79%, correspondingly.Stimulan® capsules, batch number 184![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 071, containing 40 mg of piracetam; Ketolgin® tablets, batch number 182
071, containing 40 mg of piracetam; Ketolgin® tablets, batch number 182![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 724, containing 50 mg of ketoprofen; and Hyposec® capsules, batch number 170
724, containing 50 mg of ketoprofen; and Hyposec® capsules, batch number 170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 575 containing 20 mg of omeprazole were manufactured by Amoun Pharmaceutical Company S.A.E, Egypt.
575 containing 20 mg of omeprazole were manufactured by Amoun Pharmaceutical Company S.A.E, Egypt.
2.2. Solvents and chemicals
Methyl alcohol and water of HPLC purity were obtained from Fisher Scientific, Loughborough, United Kingdom.2.3. Working and stock standard preparations
Stock alcohol preparations of (1 mg mL−1) for PIR, KET, and OME were prepared by dissolving 25 mg of each investigated drug in 25 mL methyl alcohol, each in a separate container. While working preparations (0.1 mg mL−1) were adjusted by the dilution of 5 mL of each stock preparation to 50 mL by methyl alcohol.2.4. Preparation of dosage from solution (0.1 mg mL−1)
From the powdered tablets or capsules, accurate amounts of 25 mg of each drug were mixed and sonicated with 25 mL of methanol, and filtered to obtain stock solutions containing 1 mg mL−1 of each of PIR, KET, and OME. Then, 5 mL of each stock solution was diluted to 50 mL using methyl alcohol, to obtain a working solution containing 0.1 mg mL−1 of each drug.2.5. HPLC instrument
The analytical HPLC chromatographic system, Agilent brand 1260 Infinity (Waldbronn, Germany), with a model G1361A preparative pump, a model G131SD diode array detector VL, a model G1316A thermostatically controlled column compartment, and a model G2260A preparative autosampler. The analyses were completed on an Agilent brand Zorbax Eclipse plus C-18 stationary phase (5 μm particle size; 250 × 4.6 mm id, United States).2.6. Chromatographic procedures, establishment of calibration curves and application to the pharmaceutical formulations
In 3 separate 10 mL volumetric flasks, suitable dilutions with methanol were done for each pure drug working solution to obtain different concentrations of each pure drug. 20 μL of each solution was injected three times for the C-18 stationary phase under a gradient elution mode of methyl alcohol and water (from 50% methyl alcohol to 85% methyl alcohol in 10 minutes), using 1.5 mL min−1 regular flow rate. The PIR peaks were found at 220 nm, while those of KET and OME were identified at 270 nm. The peak areas of all the drugs were plotted against their concentration in μg mL−1 and the regression equations were calculated for all the drugs.Suitable dilutions were obtained from the working solution of the mixed dosage forms prepared under Section 2.4, and then treated by the same aforementioned procedures. Standard addition techniques were applied by the estimation of spiked samples at different concentration levels.
3. Results and discussion
3.1. Development of the chromatographic method and its optimization
This research presents an uncomplicated, eco-friendly, reduced cost and time saving RP-HPLC method which enables quality control analysis with reduced sample treatment steps by the simultaneous analysis of different drug doses from the same manufacturer in a single run.The novel RP-HPLC method was able to separate and quantify the tertiary mixture of PIR, KET, and OME. It used a gradient elution of methanol and water (from 50% methanol to 85% methanol in ten minutes only) with 1.5 mL min−1 flow rate on a C-18 column. The PIR peaks were found at 220 nm, while those of KET and OME were identified at 270 nm.
Several trials were carried out to determine the optimal parameters to achieve maximum separation in the shortest run time using the lowest possible amount of green solvent.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a good separation was achieved among the three components. However OME was retained for too long in the column. The best chromatographic separation with suitable run time and peak shape was achieved using a gradient elution of water and methanol where the methanol ratio was gradually increased from 50% to 85% in a period of 10 min. The retention times for PIR, KET, and OME were 2.0 min, 3.6 min and 7.0 min, respectively.
1, a good separation was achieved among the three components. However OME was retained for too long in the column. The best chromatographic separation with suitable run time and peak shape was achieved using a gradient elution of water and methanol where the methanol ratio was gradually increased from 50% to 85% in a period of 10 min. The retention times for PIR, KET, and OME were 2.0 min, 3.6 min and 7.0 min, respectively.3.2. Validation procedures for the novel chromatographic method
Experimental data for the validation were handled in line with the ICH criteria. The HPLC method proved to have good linearity as revealed by the values of the correlation coefficients which were near 1 and the low intercept values, as demonstrated in Table 2. Precision of the method was verified through the performance of intra-day and intermediate precision studies. The good values in Table 2 verify the precision of the RP-HPLC method. As per ICH procedures, detection and quantitation limits were computed using: detection limits = 3.3 × σ (the standard deviation of the y-intercept of regression line)/S (the slope of the calibration curve) and quantitation limits = 10 × σ/S.| Items | PIR | KET | OMP |
|---|---|---|---|
| a R.S.D.%: the same-day relative standard deviation of the measured concentrations (5, 10, and 20 μg mL−1) for each drug.b R.S.D.%: the inter-days relative standard deviation of the measured concentrations (5, 10, and 20 μg mL−1) for each drug. | |||
| Calibration range (μg mL−1) | 5–25 | 5–25 | 3–20 |
| Slope | 0.042 | 0.014 | 0.009 |
| Intercept | 0.126 | 0.023 | 0.016 |
| Mean | 99 | 100 | 101 |
| S.D. | 1 | 1 | 2 |
| Correlation coefficient | 0.9995 | 0.9995 | 0.9990 |
| R.S.D.%a | 0.435 | 0.543 | 0.346 |
| R.S.D.%b | 0.545 | 0.592 | 0.469 |
| Detection limits (μg mL−1) | 1.59 | 1.56 | 0.84 |
| Quantitation limits (μg mL−1) | 4.83 | 4.74 | 2.53 |
The accuracy was tested for the analysis of different concentrations of the pure samples of the three drugs as displayed in Table 3. Furthermore, the accuracy was also verified using the standard adding technique for the mixed dosages, as illustrated in Table 3. The good results illustrated in Table 3 show that no interference was caused by excipients.
| Dosage form | Taken active ingredient | Found%a ± SD | Pure added (μg mL−1) | Pure found recovery% |
|---|---|---|---|---|
| a Average of three estimations. | ||||
| Stimulan® capsules, B.N. 184071 | PIR, 15 μg mL−1 | 102.8 ± 0.3 | 5 | 100.7 |
| 7 | 99.9 | |||
| 9 | 98.2 | |||
| Pure found (mean ± SD) | 99.6 ± 1.3 | |||
| Ketolgin® tablets, B.N. 182724 | KET, 15 μg mL−1 | 100.0 ± 0.5 | 5 | 100.0 |
| 7 | 100.8 | |||
| 9 | 99.2 | |||
| Pure found (mean ± SD) | 100.0 ± 0.8 | |||
| Hyposec® capsules, B.N. 170575 | OMP, 15 μg mL−1 | 106.7 ± 0.5 | 3 | 99.0 |
| 4 | 101.1 | |||
| 5 | 100.8 | |||
| Pure found (mean ± SD) | 100.3 ± 1.1 | |||
The HPLC method achieved good resolution among the studied drugs as displayed in Fig. 2. The results of the system suitability tests presented in Table 4 assure good chromatographic separation. Additionally, the quantification of the three tested drugs in the mixtures was effective.
| System suitability parameters | PIR | KET | OMP | Reference values |
|---|---|---|---|---|
| Tailing factor (T) | 1.10 | 1.00 | 1.09 | ∼1 |
| Capacity factor (K′) | 0.82 | 2.27 | 5.36 | 1–10 |
| Resolution factor (RS) | 6.40 | 10.88 | >1.5 | |
| Selectivity factor (α) | 2.78 | 2.36 | >1 | |
| Column efficiency (N) | 1024 | 3318 | 5575 | Higher values refer to more efficient of the separation |
| Height equivalent to theoretical plates HETP (cm per plate) | 0.0244 | 0.0075 | 0.0045 | The column efficiency is inversely proportional to HETP |
Furthermore, the results of the novel RP-HPLC method were compared to those obtained from reference methods for the three drugs.43,52,55 The estimated values for both t and F were below the theoretical values demonstrating no notable significant differences between the novel and the reference methods in terms of precision and accuracy as displayed in Table 5.
| Item | PIR in Stimulan® capsules, B.N. 184071 | KET in Ketolgin® tablets, B.N. 182724 | OMP in Hyposec® capsules, B.N. 170575 | |||
|---|---|---|---|---|---|---|
| Suggested RP-HPLC method | Reference HPLC methodc43 | Suggested RP-HPLC method | Reference HPLC methodd52 | Suggested RP-HPLC method | Reference HPLC methode55 | |
a n = 6 for all investigated HPLC methods.b The values for t and F are the equivalent tabulated values for p = 0.05.c Isocratic system of 85% aqueous solution having 0.2 g L−1 of tri-ethylamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15% acetonitrile (ACN) controlled at pH 6.5 by adding phosphoric acid. The recorded rate of flow was 1.00 mL min−1 at 205.00 nm at room temperature.d Isocratic system of methyl alcohol, ACN and 1.5% sodium acetate aqueous solution (15 15% acetonitrile (ACN) controlled at pH 6.5 by adding phosphoric acid. The recorded rate of flow was 1.00 mL min−1 at 205.00 nm at room temperature.d Isocratic system of methyl alcohol, ACN and 1.5% sodium acetate aqueous solution (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, by volume). The noted rate of flow was 1.00 mL min−1 at 240.00 nm.e Isocratic system of acidic phosphate buffer 50, by volume). The noted rate of flow was 1.00 mL min−1 at 240.00 nm.e Isocratic system of acidic phosphate buffer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN (35 ACN (35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65, by volume ratio), pH = 6.8 and the noted rate of the flow was 1.0 mL min−1 at 300.00 nm. 65, by volume ratio), pH = 6.8 and the noted rate of the flow was 1.0 mL min−1 at 300.00 nm. |
||||||
| Mean | 102.8 | 103.4 | 100.0 | 100.8 | 106.7 | 106.2 |
| SD | 0.3 | 0.4 | 0.5 | 0.6 | 0.5 | 0.6 |
| Variance | 0.080 | 0.162 | 0.222 | 0.315 | 0.203 | 0.335 |
| Student’s t-test (2.228)b | 0.013 | 0.038 | 0.125 | |||
| F-value (5.050)b | 0.458 | 0.708 | 0.594 | |||
3.3. Green characteristics for the new RP-HPLC method
The greenness features of the novel chromatographic method were studied. The greenness profile is used to assess the eco-friendly features for any investigated analytical method in comparison to other recorded methods. It evaluates the four criteria for the consumed solvents: they should not be persistent, bio-accumulative or toxic (PBT) i.e. they are not hazardous solvents, not corrosive (the accepted pH range is usually from 2 to 12), and the quantity of the resultant waste should be less than 50 g per sample.34,62 During the present analysis, only methyl alcohol was utilized with pure water. Moreover, methyl alcohol is recorded in the second category of recommended solvents as in the CHEM21 guide for selecting classical- and less classical-solvents.63 Besides, the pH of the mobile phase was approximately 7 and consequently the aqueous/methanol mixture is described as non-hazardous and non-corrosive. Moreover, the resulting waste for this RP-HPLC method was about 15 mL per sample. Therefore, the suggested method complies well with the four standards of the greenness profile. Besides, comparison between the novel RP-HPLC method and the reference ones that quantified the studied drugs singly, shows that the new method is more eco-friendly than the other ones regarding run time and consumption of solvents. A summary of the reference HPLC methods for the three drugs is displayed in Table 5.Additionally, the final AGREE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 score of 0.72 refers to the greenness features for the new HPLC method, as illustrated in Fig. 3; a method is considered green if it scores over 0.6. Also, the weaker green-color of the middle zone refers to the same concept. The weakest sub-sectors in the coloured pictogram were sector 3 and 12. Sector 3 refers to off-line procedures for sampling while sector 12 denotes the operator safety where methanol is highly inflammable organic solvent and explosive. The authors did their best to use ethanol but unacceptable chromatogram was obtained. The orange color of subsection 7 is attributed to generation of 15 mL of waste per run.
30 score of 0.72 refers to the greenness features for the new HPLC method, as illustrated in Fig. 3; a method is considered green if it scores over 0.6. Also, the weaker green-color of the middle zone refers to the same concept. The weakest sub-sectors in the coloured pictogram were sector 3 and 12. Sector 3 refers to off-line procedures for sampling while sector 12 denotes the operator safety where methanol is highly inflammable organic solvent and explosive. The authors did their best to use ethanol but unacceptable chromatogram was obtained. The orange color of subsection 7 is attributed to generation of 15 mL of waste per run.
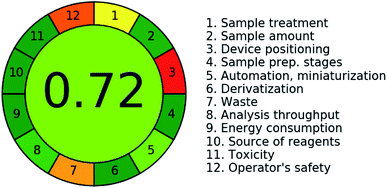 | ||
| Fig. 3 AGREE pictogram and score for assessment of the RP-HPLC method greenness for the concurrent analysis of a mixture of PIR, KET, and OME. | ||
Besides, the achieved GAPI pictogram28,64 for the novel RP-HPLC method as illustrated in Fig. 4, proves that only three subsections are red 1, 14, and 15 where sector 1 denotes off-line analysis, segment 14 symbolized to the quantity of mobile phase waste that exceeds ten mL per run (15 mL), and segment 15 refers to non-treatment of chemical waste as it is a gradient elution mode. The six green subsections refer to the overall greenness of the RP-HPLC method.
 | ||
| Fig. 4 GAPI pictogram for assessment of characteristics for the RP-HPLC method greenness for concurrent analysis of a mixture of PIR, KET, and OME. | ||
Certainly, AGREE and GAPI tools prove the achievement of the Green Chemistry Principles. However, the energy consumption parameter is a key point when using chromatographic techniques since the environmental impact generated can be analyzed and quantified by the so-called carbon footprint. It is a metric factor, expressed as kg CO2 equivalent, for the evaluation of the negative impact of a methodology that involves the power of the instrument, run time, and emission factor for electricity.65,66 The carbon footprint for this aforementioned method is computed by the expression provided in ESI S1,† within the HEXAGON tool described by Ballester-Caudet et al. in 2019.66 It equals 0.00659 kg CO2 equivalent. The overall qualification score is 0 on the 5-point scale as the total carbon footprint is less than 0.1. Regarding the carbon footprint, the shorter the analysis time (in our method it is only 8 minutes), the lower the carbon footprint score and the greener the method.66 Also, a significant reduction in the total cost is assured when using this HPLC method as the required amount of solvent is noticeably decreased when performing the multi-analysis in comparison to the single component analysis. Concerning the method’s sustainability with respect to the cost, many factors are considered e.g., the overall analysis time, the number of samples performed in seven days, and equipment cost. Performing 50 samples or more per week is considered the greenest approach. Generally, if the analysis time is less than 10 minutes, no penalty points should be assigned for the method. In our example the number of analyzed samples in seven days is about 300, which means a high green value for economic cost according to the HEXAGON tool descriptions.66
3.4. Potential future plans
This research is the first step in our project which depends on selecting many pharmaceuticals formulated by more than one common drug manufacturer, such that the research would be beneficial to several manufacturers for the conservation of time and cost.4. Conclusions
This research work presents a new, eco-friendly, simple and selective RP-HPLC chromatographic method for the simultaneous analysis of piracetam, ketoprofen, and omeprazole in bulk forms and in dosage form mixtures. The method provides a time effective and reduced cost simultaneous analysis of different dosage forms in a single run. Therefore it is recommended for repetitive quality control analyses. This novel research approach enables the analysis of more than one dosage form or pure drug in a single run saving time, cost and effort needed for the analysis, as well as rendering the analysis more eco-friendly through reducing the volume of solvent used and the resulting waste.Author contributions
Mohamed A. Abdelgawad: supervision, project administration, and writing—review and editing. Eglal A. Abdelaleem: data curation, conceptualization, methodology, investigation, project administration, writing—original draft. Mohammed Gamal: conceptualization, data curation, investigation, supervision, writing—review and editing. Mohammad A. S. Abourehab: supervision, funding acquisition, writing—review and editing. Nessreen S. Abdelhamid: project administration, investigation, data curation, writing—original draft and editing.Conflicts of interest
No conflict exists regarding this study.Acknowledgements
The authors would like to thank the Deanship of scientific research at Umm Al-Qura University for supporting this work under grant code (22UQU4290565DSR24).References
- M. R. Siddiqui, Z. A. AlOthman and N. Rahman, Arabian J. Chem., 2017, 10, S1409–S1421 CrossRef CAS.
- N. M. Hosny, N. N. Atia and S. M. El-Gizawy, Microchem. J., 2019, 149, 103955 CrossRef CAS.
- M. Gamal, Analyst, 2020, 145, 2025–2037 RSC.
- R. Nageswara Rao and V. Nagaraju, J. Pharm. Biomed. Anal., 2003, 33, 335–377 CrossRef CAS PubMed.
- M. Gamal, I. A. Naguib and R. M. Abdelfatah, Simultaneous analysis of oxytetracycline hydrochloride, lidocaine, and bromhexine hydrochloride in the presence of many interfering excipients, Arch. Pharm., 2021, 354, 2100131 CrossRef CAS PubMed.
- M. Gamal and L. M. A. Elhalim, Novel Eco-friendly HPLC Methods Using Refractive Index Detector for Analysis of Three Veterinary Antibiotics in Pharmaceutical Formulations and Rat Plasma, J. Chromatogr. Sci., 2020, 58, 940–950 CAS.
- M. M. Al-Sanea and M. Gamal, Critical analytical review: Rare and recent applications of refractive index detector in HPLC chromatographic drug analysis, Microchem. J., 2022, 178, 107339 CrossRef CAS.
- H. M. Ali, M. Gamal, M. M. Ghoneim and L. Mohammed Abd Elhalim, Quantitative Analysis of Abamectin, Albendazole, Levamisole HCl and Closantel in Q-DRENCH Oral Suspension Using a Stability-Indicating HPLC-DAD Method, Molecules, 2022, 27(3), 764 CrossRef CAS PubMed.
- J. L. Wolfender, Planta Med., 2009, 75, 719–734 CrossRef CAS PubMed.
- L. Nahar, A. Onder and S. D. Sarker, Phytochem. Anal., 2020, 31, 413–457 CrossRef CAS PubMed.
- M. Gamal, H. M. Ali, R. M. Abdelfatah and M. A. Magdy, A green approach for simultaneous analysis of two natural hepatoprotective drugs in pure forms, capsules and human plasma using HPLC-UV method, Microchem. J., 2019, 151, 104258 CrossRef CAS.
- M. Gamal, H.-A. H. Abd-ElSalam, I. A. Naguib, M. A. Al-Ghobashy, H. E. Zaazaa and M. Abdelkawy, Green and Cost-Effective Extraction Techniques of Quercetin from Mixture of Nutraceuticals with Yield Analysis via Spectrophotometry and High-Performance Liquid Chromatography Methods, J. AOAC Int., 2022, 105(1), 249–266 CrossRef PubMed.
- H. A. H. Abd-Elsalam, M. Gamal, I. A. Naguib, M. A. Al-Ghobashy, H. E. Zaazaa and M. Abdelkawy, Development of green and efficient extraction methods of quercetin from red onion scales wastes using factorial design for method optimization: A comparative study, Separations, 2021, 8, 137 CrossRef CAS.
- G. Garcia-Llatas, A. Alegría, R. Barberá and A. Cilla, Microchem. J., 2021, 168, 106377 CrossRef CAS.
- J. Peris-Vicente, E. Peris-García, J. Albiol-Chiva, A. Durgbanshi, E. Ochoa-Aranda, S. Carda-Broch, D. Bose and J. Esteve-Romero, Liquid chromatography, a valuable tool in the determination of antibiotics in biological, food and environmental samples, Microchem. J., 2022, 177, 107309 CrossRef CAS.
- S. A. Wadood, G. Boli, Z. Xiaowen, I. Hussain and W. Yimin, Microchem. J., 2020, 152, 104295 CrossRef CAS.
- A. M. Hegazy, R. M. Abdelfatah, H. M. Mahmoud and M. A. Elsayed, Development and validation of two robust simple chromatographic methods for estimation of tomatoes specific pesticides’ residues for safety monitoring prior to food processing line and evaluation of local samples, Food Chem., 2019, 125640 Search PubMed.
- I. Jalaludin and J. Kim, Comparison of ultraviolet and refractive index detections in the HPLC analysis of sugars, Food Chem., 2021, 365, 130514 CrossRef CAS PubMed.
- H. K. Chanduluru and A. Sugumaran, Assessment of greenness for the determination of voriconazole in reported analytical methods., RSC Adv., 2022, 12(11), 6683–6703 RSC.
- D. S. Francischini and M. A. Z. Arruda, When a picture is worth a thousand words: Molecular and elemental imaging applied to environmental analysis–A review., Microchem. J., 2021, 169, 106526 CrossRef CAS.
- S. A. Mansour, Environmental impact of pesticides in Egypt, Rev. Environ. Contam. Toxicol., 2008, 196, 1–51 CAS.
- M. A. Farajzadeh, N. Nouri and P. Khorram, TrAC, Trends Anal. Chem., 2014, 55, 14–23 CrossRef CAS.
- H. M. Ali, Efficiency Improvement of a Gas Chromatographic-Mass Spectrometric Method for Quantification of Nicotine in Hookah (Water Pipe) Tobacco Products, J. Eng. Appl. Sci., 2019, 15, 220–226 CrossRef.
- H. M. Ali, A. A. Essawy, T. A. S. Elnasr, A. M. Aldawsari, I. Alsohaimi, H. M. A. Hassan and I. B. Abdel-Farid, Selective and efficient sequestration of Cr(VI) in ground water using trimethyloctadecylammonium bromide impregnated on Artemisia monosperma plant powder, J. Taiwan Inst. Chem. Eng., 2021, 125, 122–131 CrossRef CAS.
- M. Yabré, L. Ferey, I. T. Somé and K. Gaudin, Greening reversed-phase liquid chromatography methods using alternative solvents for pharmaceutical analysis, Molecules, 2018, 23(5), 1065 CrossRef PubMed.
- J. Płotka-Wasylka, M. Fabjanowicz, K. Kalinowska and J. Namieśnik, History and milestones of green analytical chemistry, Green Analytical Chemistry, Springer, 2019, pp. 1–17 Search PubMed.
- H. M. Mohamed, TrAC, Trends Anal. Chem., 2015, 66, 176–192 CrossRef CAS.
- J. Płotka-Wasylka, A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index, Talanta, 2018, 181, 204–209 CrossRef PubMed.
- J. Płotka-Wasylka and W. Wojnowski, Complementary green analytical procedure index (ComplexGAPI) and software, Green Chem., 2021, 23, 8657–8665 RSC.
- F. Pena-Pereira, W. Wojnowski and M. Tobiszewski, AGREE - Analytical GREEnness Metric Approach and Software, Anal. Chem., 2020, 92, 10076–10082 CrossRef CAS PubMed.
- A. Gałuszka, Z. M. Migaszewski, P. Konieczka and J. Namieśnik, Analytical Eco-Scale for assessing the greenness of analytical procedures, TrAC, Trends Anal. Chem., 2012, 37, 61–72 CrossRef.
- M. Gamal, I. A. Naguib, D. S. Panda and F. F. Abdallah, Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine N -butyl bromide, Anal. Methods, 2021, 13, 369–380 RSC.
- A. B. Ahmed, M. Gamal, I. A. Naguib, H. M. Ali and F. F. Abdallah, Environmental Impact of the Reported Chromatographic Methods for the Determination of the First FDA-Approved Therapy for COVID-19 Patients, Remdesivir: A Comparative Study, Microchem. J., 2022, 176, 107242 CrossRef CAS PubMed.
- M. Sajid and J. Płotka-Wasylka, Talanta, 2022, 238, 123046 CrossRef CAS PubMed.
- M. Tobiszewski, Anal. Methods, 2016, 8, 2993–2999 RSC.
- B. Winblad, CNS Drug Rev., 2006, 11, 169–182 CrossRef PubMed.
- A. D. Lestari, A. T. Prasetyo, T. Palupi, E. Umayah, M. Yuwono and G. Indrayanto, HPLC determination of Piracetam in tablets; validation of the method, J. Liq. Chromatogr. Relat. Technol., 2005, 28, 1407–1416 CrossRef CAS.
- L. Gagliardi, D. Deorsi, G. Cavazzutti, D. Tonelli and S. Zappoli, HPLC determination of oxiracetam, its impurities, and piracetam in pharmaceutical formulations, Anal. Lett., 1994, 27, 879–885 CrossRef CAS.
- O. M. El-Hhoussini, N. H. Zawilla and M. A. Mohammad, Development and validation of RP-LC method for the determination of cinnarizine/piracetam and cinnarizine/heptaminol acefyllinate in presence of cinnarizine reported degradation products, Anal. Chem. Insights, 2013, 8, 99–106 Search PubMed.
- F. A. Siddiqui, N. Sher, N. Shafi, A. Wafa Sial, M. Ahmad, Mehjebeen and H. Naseem, Development of new method for simultaneous analysis of piracetam and levetiracetam in pharmaceuticals and biological fluids: Application in stability studies, Biomed Res. Int., 2014, 758283 Search PubMed.
- C. Lenzen, G. A. Winterfeld and O. J. Schmitz, Comparison of piracetam measured with HPLC-DAD, HPLC-ESI-MS, DIP-APCI-MS, and a newly developed and optimized DIP-ESI-MS, Anal. Bioanal. Chem., 2016, 408, 4103–4110 CrossRef CAS PubMed.
- P. Patil, M. Patil and D. D. Patil, Development and Validation of RP-HPLC Method for Simultaneous Estimation of Piracetam and Vinpocetine, Asian J. Pharm. Anal. Med. Chem., 2018, 8, 103 Search PubMed.
- M. S. Arayne, N. Sultana, F. A. Siddiqui, A. Z. Mirza, F. Qureshi and M. H. Zuberi, Simultaneous determination of piracetam and its four impurities by RP-HPLC with UV detection, J. Chromatogr. Sci., 2010, 48, 589–594 CAS.
- T. G. Kantor, Ketoprofen: A Review of Its Pharmacologic and Clinical Properties, Pharmacotherapy, 1986, 6, 93–102 CrossRef CAS PubMed.
- R. A. Shaalan, R. S. Haggag, S. F. Belal and M. Agami, Simultaneous Determination of Hyoscine, Ketoprofen and Ibuprofen in Pharmaceutical Formulations by HPLC-DAD, J. Appl. Pharm. Sci., 2013, 3, 38–047 CAS.
- Y. S. El-Saharty, F. H. Metwally, M. Refaat and S. Z. El-Khateeb, J. AOAC Int., 2007, 90, 102–112 CrossRef CAS PubMed.
- G. Andraws and S. Trefi, Ionisable substances chromatography: A new approach for the determination of Ketoprofen, Etoricoxib, and Diclofenac sodium in pharmaceuticals using ion–pair HPLC, Heliyon, 2020, 6(8), e04613 CrossRef PubMed.
- J. Dvořák, R. Hájková, L. Matysová, L. Nováková, M. A. Koupparis and P. Solich, Simultaneous HPLC determination of ketoprofen and its degradation products in the presence of preservatives in pharmaceuticals, J. Pharm. Biomed. Anal., 2004, 36, 625–629 CrossRef PubMed.
- D. Gherdaoui, H. Bekdouche, S. Zerkout, R. Fegas and M. Righezza, Chiral separation of ketoprofen on an achiral NH2 column by HPLC using vancomycin as chiral mobile phase additive, J. Iran. Chem. Soc., 2016, 13, 2319–2323 CrossRef CAS.
- C. Özlü, H. Basan, E. Şatana, N. Ertaş and N. G. Göǧer, Quantitative determination of ketoprofen in gels and ampules by using flow-injection UV spectrophotometry and HPLC, J. Pharm. Biomed. Anal., 2005, 39, 606–611 CrossRef PubMed.
- R. Mullangi, M. Yao and N. R. Srinivas, Biomed. Chromatogr., 2003, 17, 423–434 CrossRef CAS PubMed.
- B. Tsvetkova and L. Peikova, HPLC method for simultaneous determination of ketoprofen and preservatives in gel formulation, Int. J. Pharm. Pharm. Sci., 2014, 6, 199–202 CAS.
- F. Massoomi, J. Savage and C. J. Destache, Pharmacotherapy, 1993, 13, 46–59 CAS.
- J. Lun, S. Ma, M. Xue, P. Zhao, Y. Song and X. Guo, Simultaneous enantiomeric analysis of five proton-pump inhibitors in soil and sediment using a modified QuEChERS method and chiral high performance liquid chromatography coupled with tandem mass spectrometry, Microchem. J., 2021, 160, 105625 CrossRef CAS.
- A. H. Chaudhry, T. A. Malik, K. M. Ashfaq, M. Shafiq, M. Aslam and M. S. Akhtar, A simple hplc method for the determination of omeprazole in vitro, Int. J. Pharm. Chem., 2013, 2, 126–128 Search PubMed.
- A. H. Kamal, A. A. Marie and S. F. Hammad, Stability indicating RP-HPLC method for simultaneous determination of omeprazole and aspirin in the presence of salicylic acid as degradation product, Microchem. J., 2020, 152, 104350 CrossRef CAS.
- K. M. Darwish, I. Salama, S. Mostafa and M. El-Sadek, RP-HPLC/pre-column derivatization for analysis of omeprazole, tinidazole, doxycycline and clarithromycin, J. Chromatogr. Sci., 2013, 51, 566–576 CAS.
- F. S. Murakami, A. P. Cruz, R. N. Pereira, B. R. Valente and M. A. S. Silva, Development and validation of a RP-HPLC method to quantify omeprazole in delayed release tablets, J. Liq. Chromatogr. Relat. Technol., 2007, 30, 113–121 CrossRef CAS.
- B. Patel, M. Patel, J. Patel and B. Suhagia, Simultaneous determination of omeprazole and domperidone in capsules by RP-HPLC and densitometric HPTLC, J. Liq. Chromatogr. Relat. Technol., 2007, 30, 1749–1762 CrossRef CAS.
- M. Lennard, Clarke’s Analysis of Drugs and Poisons, Br. J. Clin. Pharmacol., 2004, 58, 99 CrossRef.
- Y. S. I. El-Saharty, Simultaneous determination of piracetam and vincamine by spectrophotometric and high-performance liquid chromatographic methods, J. AOAC Int., 2008, 91, 311–321 CrossRef CAS PubMed.
- M. Koel, in Green Chemistry, Royal Society of Chemistry, 2016, vol. 18, pp. 923–931 Search PubMed.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, CHEM21 selection guide of classical- and less classical-solvents, Green Chem., 2016, 18, 288–296 RSC.
- J. Płotka-Wasylka and W. Wojnowski, Complementary green analytical procedure index (ComplexGAPI) and software, Green Chem., 2021, 23, 8657–8665 RSC.
- N. Jornet-Martínez, S. Bocanegra-Rodríguez, R. A. González-Fuenzalida, C. Molins-Legua and P. Campíns-Falcó, in Processing and Sustainability of Beverages, Woodhead Publishing, 2019, pp. 275–317 Search PubMed.
- A. Ballester-Caudet, P. Campíns-Falcó, B. Pérez, R. Sancho, M. Lorente, G. Sastre and C. González, A new tool for evaluating and/or selecting analytical methods: Summarizing the information in a hexagon, TrAC, Trends Anal. Chem., 2019, 118, 538–547 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02395d |
| This journal is © The Royal Society of Chemistry 2022 |


