 Open Access Article
Open Access ArticleLinear unit BN2: a novel birefringence-enhanced fundamental module with sp orbital hybridization†
Jianbang Chenab,
Mengfan Wubc,
Jie Zhangab and
Xuchu Huang *a
*a
aDepartment of Physics, Changji University, Changji 931100, China. E-mail: hxuchu@163.com
bCAS Key Laboratory of Functional Materials and Devices for Special Environments, Xinjiang Technical Institute of Physics & Chemistry, CAS, Xinjiang Key Laboratory of Electronic Information Materials and Devices, 40-1 South Beijing Road, Urumqi 830011, China
cCenter of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
First published on 16th May 2022
Abstract
Inorganic planar π-conjugated groups are advantageous to generate large birefringence in optical functional materials, and many excellent materials contain CO3, BO3 or B3O6, such as CaCO3, α/β-BaB2O4 (α/β-BBO), and KBe2BO3F2 (KBBF). In view of their microscopic structures, the common characteristics are the planar structures, which are regarded as birefringence-enhanced fundamental modules (FMs). Nowadays, exploring novel birefringence-enhanced FMs is becoming a burning issue. Herein, we investigated the birefringence-enhanced FMs in B–N systems and found that the BN2 linear unit could produce great birefringence. Through the investigation based on the Inorganic Crystal Structure Database, some compounds with the BN2 linear group were screened out with the formulas A3BN2 (A = Li, Na), A3BN3 (A = Mg, Ca), and Ba3(BN2)2. Particularly, Ca3(BN2)N exhibits a great birefringence of about 0.411 at 1064 nm, which is 3.5, 2.5 and 2.0 times those of the most commercially used birefringent crystals α-BaB2O4 (Δn = 0.116 at 1064 nm), CaCO3 (Δn = 0.164 at 1064 nm) and YVO4 (Δn = 0.208 at 1064 nm), respectively. To find the origins of the optical properties of compounds with the BN2 linear group, the first-principles, REDA and polarizability anisotropy analysis methods were used. Owing to the structural arrangement and the polarization anisotropy of the BN2 linear group, it can influence the birefringence significantly. This work will provide a general way for exploring birefringence-enhanced FMs in B–N compounds.
1. Introduction
In the field of optical functional crystals, birefringence plays an important role in material processing, laser phase-matching, optical measurements, photolithography, and polarizing microscopic observation.1–13 Standing out from the discovered birefringent crystal materials, CaCO3,14 YVO4,15 and α-BaB2O4,16,17 are widely applied in optical devices such as polarizers, optical isolators, and compensators.18–20Nowadays, many studies about birefringent crystals with large optical anisotropy have been carried out. For instance, Ca(BO2)2 (ref. 21) with the largest reported birefringence (0.247 at 193 nm) in the deep-ultraviolet (DUV) region is regarded as the most promising DUV birefringent material, while (NH4)2C2O4·H2O22 has also been identified as a promising UV birefringent material with a large birefringence (0.248 at 546 nm). Meanwhile, introducing F atoms into micro groups (e.g. from PO4 to PO3F and from SO4 to SO3F) can enhance the polarizability anisotropy significantly, and subsequently increase the birefringence to a certain extent.23 The original difference in the birefringence of Ba2BP3O11 and Ba2BP2O8F (from 0.013 to 0.034) mainly comes from the BO3F tetrahedra.24 Introducing different numbers of fluorine atoms into B–O groups can modulate the polarizability anisotropy.25–29 Consequently, the introduction of the F atom has become a feasible strategy to enlarge the polarizability anisotropy and enhance the birefringence.
Generally speaking, crystal structures and the corresponding microscopic groups are inextricably linked with the optical properties of materials, for instance, the arrangement of anionic frameworks or diverse combinations with metal cation polyhedrons will influence the birefringence in significant ways.30–32 Consequently, a feasible strategy to enhance the birefringence is introducing some functional modules with large optical anisotropy such as coplanar groups with π-conjugation systems (CO3, BO3, B3O6, C3N3O3, etc.).22,33–37 At the same time, the isolated parallel distribution, quasi-one dimensional (1D) chain, and quasi-2D plane of anionic groups are proper arrangement modes that contribute to the microscopic effects in FMs. The current research on π-conjugated microscopic groups mainly focuses on the B–O unit (BO2, BO3, B2O5 and B3O6)38 and C–O unit (CO3, C2O4, C2O6, C4O4, and C6O6),22 which make great contributions to the birefringence due to their large anisotropy of polarizabilities. Despite the enormous promise in their chemical and physical functionalities, the sheer amounts of boron nitrides are much smaller than those of the oxides, for reasons well known to chemists,39,40 and the units containing N have rarely been utilized in the optical functional crystals field. Nevertheless, it does not mean that the units containing N cannot make a contribution to the optical properties.
To the best of our knowledge, there are only a few systematic studies that unveil the contribution of B–N groups towards enhancing birefringence. Based on the analysis of B–O groups, we propose an idea that BN2 is a linear group containing sp orbital hybridization that contributes greatly to birefringence. Through the investigation of the BN2 unit based on the Inorganic Crystal Structure Database (Version 4.7.1 build 20211220-1548, ICSD), seven ternary nitride borates with the formulas A3BN2 (A = Li, Na), A3BN3 (A = Mg, Ca), and Ba3(BN2)2 were screened out.41–47 In order to explore the origin of the optical properties of the selected compounds, multiple analytical methods have been applied. The birefringence was calculated from the dielectric functions by the first-principles method, along with polarizability anisotropy and REDA analysis to further prove the contribution of the BN2 unit to birefringence. Essentially, a new birefringent-enhanced FM was screened.
2. Computational details
The electronic structures and optical properties of the target compounds were calculated by first-principles calculations based on the density functional theory (DFT) method48 implemented in the CASTEP package.49 The exchange-correlation functional50 was Perdew–Burke–Ernzerhof (PBE) within the generalized gradient approximation (GGA). The norm-conserving pseudopotentials (NCP)51 were selected for structural optimization and calculating the electronic and optical properties. The electronic configurations were treated as follows: Li 2s1, B 2s2 2p1, N 2s2 2p3, Na 2s2 2p6 3s1, Mg 2s2 2p6 3s2, Ca 3s2 3p6 4s2, and Ba 5s2 5p6 6s2, the cutoff energy was set at 880 eV for Ca3(BN2)N, 990 eV for Mg3BN3, and 770 eV for Li3BN2 (P21/c), Li3BN2 (P42/mnm), Li3BN2 (I41/amd), Na3BN2, and Ba3(BN2)2. The scissor operators of 1.18 eV for Li3BN2 (P21/c), 1.21 eV for Li3BN2 (P42/mnm), 1.22 eV for Li3BN2 (I41/amd), 1.24 eV for Na3BN2, 1.18 eV for Mg3BN3, 0.61 eV for Ca3(BN2)N and 1.00 eV for Ba3(BN2)2 were applied to move the simulated conduction bands to the right place. Furthermore, to reach the convergence of this calculation, the Brillouin zone comprised 3 × 3 × 3 Monkhorst–Pack k-point sampling with a separation of 0.04 Å−1. The other calculation parameters and convergent criteria were employed as the default values of the CASTEP code.To explore the optically anisotropic hierarchy and the functionality of the B–N units on birefringence, we investigated their polarizability anisotropy characteristics using the DFT method. The B–N units were obtained from primary crystal information without structure optimization. The polarization anisotropy was acquired from static polarization according to the following formula:
The so-called length-gauge formalism derived by Aversa and Sipe52,53 was adopted. At a zero frequency, the static second-order nonlinear susceptibilities can be ascribed to the virtual-hole (VH) and virtual-electron (VE) processes. The formulas for calculating the contributions from VE and VH are as follows:54
| χ(2)αβγ = χ(2)αβγ(VE) + χ(2)αβγ(VH) |
Here, α, β and γ are the Cartesian components, χαβγ(VH) and χαβγ(VE) denote the contribution from the VE process and the VH process, respectively, v and v′ represent the valence bands, c and c′ represent the conduction bands, and P(αβγ), ℏωij and pijα represent the full permutation, the difference of band energy and momentum matrix elements, respectively. Furthermore, the Born effective charges were also obtained using density functional perturbation theory (DFPT) with a linear response method performed using the CASTEP code.
3. Results and discussion
3.1. Crystal and electronic structure
To accelerate the process of advanced material discovery, computer-aided material design and prediction have recently emerged as particularly useful techniques and have exhibited various successful cases.55,56 In this work, in order to find the target compounds, the nitride borates with alkali and alkaline earth metal cations were screened in terms of the type of FMs with the BN2 units by investigating the ICSD. In total, 7 structures were screened out, as listed in Table 1.| Compounds | Space group | Group | Eg-GGA (eV) | Eg-HSE06 (eV) | Birefringence (@1064 nm) |
|---|---|---|---|---|---|
| Li3BN2 | P21/c | BN2 | 3.4 | 4.58 | 0.198 |
| Li3BN2 | P42/mnm | BN2 | 3.36 | 4.57 | 0.103 |
| Li3BN2 | I41/amd | BN2 | 3.16 | 4.38 | 0.116 |
| Na3BN2 | P21/c | BN2 | 1.52 | 2.76 | 0.157 |
| Mg3BN3 | P63/mcm | BN2 | 1.5 | 2.55 | 0.244 |
| Ca3(BN2)N | P4/mmm | BN2 | 0.55 | 1.16 | 0.411 |
| Ba3(BN2)2 | P212121 | BN2 | 2.46 | 3.46 | 0.185 |
As we can see, Fig. 1 shows the structure and arrangement of the seven compounds. Obviously, the BN2 units are all short straight linear groups. The two of Li3BN2 and Na3BN2, crystallize in the P21/c and P42/mnm space groups, and are composed of isolated and parallel BN2 units in opposite directions and A-site Li and Na cations (Fig. 1a, c and d). Mg3BN3 and Ca3(BN2)N belong to the space groups of P63/mcm and P4/mmm, respectively, and the BN2 units are parallel to each other along the c axis, while the Mg and Ca cations show symmetric distribution. Li3BN2 and Ba3(BN2)2 belong to the I41/amd and P212121 space groups, respectively, and the BN2 units are not only aligned in a parallel manner but also have angles of 172.8°and 170.1°, as shown in Fig. 1c and g.
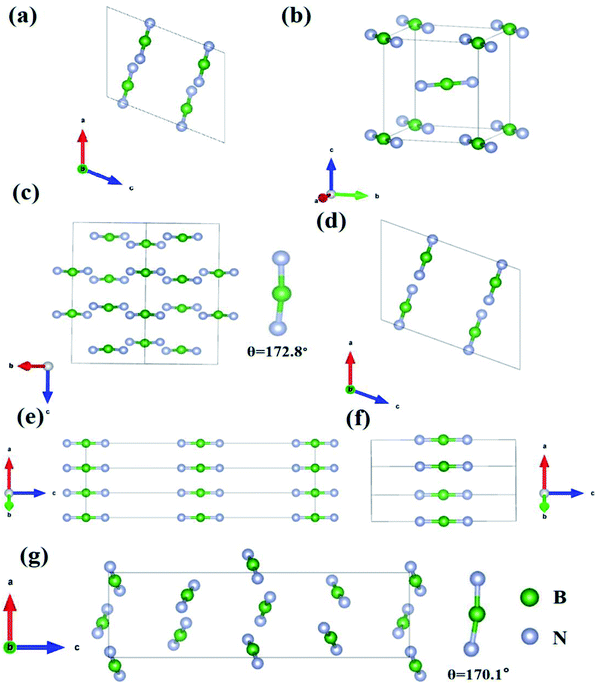 | ||
| Fig. 1 The arrangements of BN2 in the compounds (a) Li3BN2-14, (b) Li3BN2-136, (c) Li3BN2-141, (d) Na3BN2, (e) Mg3BN3, (f) Ca3(BN2)N and (g) Ba3(BN2)2. | ||
Since there is no data about the experimental bandgap, we employed the HSE06 hybrid functional to accurately predict the bandgaps of these compounds. Most of the title compounds possess a direct bandgap, while Li3BN2 (P21/c), Li3BN2 (P42/mnm) and Ba3(BN2)2 possess indirect ones. The calculated bandgaps using the GGA and HSE06 methods are shown in Table 1. The results from the HSE06 hybrid functional are 4.58 eV for Li3BN2 (P21/c), 4.57 eV for Li3BN2 (P42/mnm), 4.38 eV for Li3BN2 (I41/amd), 2.76 eV for Na3BN2, 2.55 eV for Mg3BN3, 1.16 eV for Ca3(BN2)N, and 3.46 eV for Ba3(BN2)2, which are larger than those from GGA because of the discontinuity of the derivative on the exchange-correlation energy within the GGA functional. The GGA bandgaps of those compounds are listed in Fig. S1.†
The partial density of states (PDOS) as well as the orbitals near the Fermi level show the electronic states that are related to the optical properties. The total and partial density of states with the respective atoms was were analyzed to clearly understand the electronic structures of the title compounds, and calculated to analyze the distribution of atomic orbitals in the energy range from −15 to 15 eV, as shown in Fig. S2.† Similar electronic structures demonstrated for the target compounds. It clear to see that the bands of the title compounds near the bandgap are mainly determined by the 2p orbital of the N at the valence bands (VBs) maximum, and the bottom of the conduction bands (CBs) primarily dominated by the B 2p orbital, which means that the B–N group will plays an important role in the optical properties of the title compounds.
To reveal the birefringence-related functions of the BN2 anionic group, we performed an intensive comparison with BN3, BO2 and BO3. In order to explore these anionic groups, we investigated the electronic structure and optical properties of the BN2, BN3, BO2 and BO3 anionic groups using DFT implemented by the Gaussian 09 package57 at the 6-31G basis set. As is well known to all, the optical property is closely related to the characteristic occupied and unoccupied states near the Fermi level. Fig. 2 shows the corresponding polarizability anisotropy and highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO–LUMO) gaps of the four anionic groups. The polarizability anisotropy Δα values are 23.7, 13.7, 14.4, 7.0 for BN2, BN3, BO2 and BO3, respectively. It is obvious that the BN2 unit possesses the biggest polarizability anisotropy among them. For BN2, BN3, BO2 and BO3, the HOMO–LUMO gaps are 5.1, 3.1, 9.2 and 8.5 eV, respectively. In particular, the BO3 unit has been recognized as a birefringence-preferential group, such as in Ca3(BO3)2 (ref. 58) with a large birefringence of 0.104 (at 1064 nm). It was also confirmed that Δα increases with the π-electron population of the conjugated system, which indicates the possibility of large birefringence for the corresponding compounds with B–N units. These calculated results suggest that the BN2 unit possesses a larger polarizability anisotropy and maintains a moderate HOMO–LUMO gap as well. Thus, we investigated the compounds with BN2 units and analyzed their optical properties.
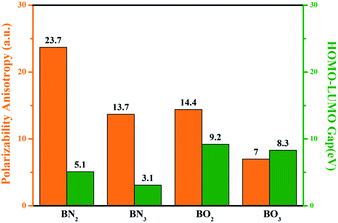 | ||
| Fig. 2 Comparison of the polarizability anisotropy and HOMO–LUMO gap for the BN2, BN3, BO2, and BO3 anionic groups. | ||
3.2. Optical properties
Using the GGA approach and scissors operator via the calculations with the CASTEP code, the birefringence Δn (Δn = nmax − nmin), which depends on the difference of the maximum and minimum values of the refractive index along the different optical principal axes, was obtained, and the title compounds possessed a large birefringence of about 0.1–0.4 (at 1064 nm). The birefringence values of Li3BN2 in different space groups are 0.198 (P21/c), 0.103 (P42/mnm), and 0.116 (I41/amd). For Na3BN2, Mg3BN3 and Ba3(BN2)2, their birefringence values are 0.157, 0.244, 0.185, respectively. The bandgap was calculated by GGA, and the birefringence of the compounds with BN2 units is shown in Fig. 3. Simultaneously, the results indicate that the birefringence of the title compounds is much larger than that of the birefringent materials. Ca3(BN2)N especially exhibits a gigantic birefringence of about 0.411 at 1064 nm, which is 3.5, 2.5, and 2.0 times those of the most commercially used birefringent crystals α-BaB2O4 (Δn = 0.116 at 1064 nm), CaCO3 (Δn = 0.164 at 1064 nm), and YVO4 (Δn = 0.208 at 1064 nm), respectively. Meanwhile, in comparison with the chain CNO,59 which possesses a birefringence of 0.3–0.4, and the compounds with planar C3N3O3, such as β-Sr3(C3N3O3)2 (Δn = 0.36 at 1064 nm) and Ca3(C3N3O3)2 (Δn = 0.35 at 1064 nm),60–62 the birefringence of Ca3(BN2)N is also larger than theirs, which indicates that the proposed compounds containing BN2 units such as Ca3(BN2)N are promising candidates for optical applications.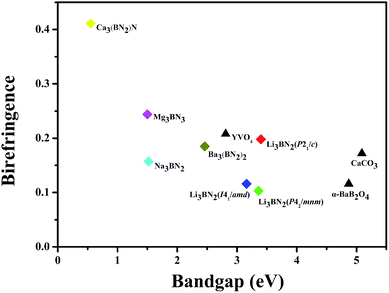 | ||
| Fig. 3 Bandgap and birefringence (@1064 nm) of the compounds with BN2 units and birefringent crystal materials. | ||
Since Ba3(BN2)2 belongs to a non-centrosymmetric group, the NLO coefficients were obtained with ab initio electronic structure calculations. Owing to the Kleinman symmetry, the P212121 space group for the investigated compounds yields three non-zero NLO coefficients. Similar to the calculation of linear optical properties, the same scissors were used to generate the NLO coefficients. At the zero-frequency limit, the calculated largest NLO coefficient d14 is 0.174 pm V−1 (∼0.44 × KDP) for Ba3(BN2)2, and B–N interactions contribute the most to the SHG coefficients (Fig. S4†).
As a post-processing tool, SHG-density (SHG63 stands for second harmonic generation) was performed to further explore the contribution of atoms or groups to the SHG response through the CASTEP package. SHG-density is divided into occupied and unoccupied states of virtual-electron (VE) and virtual-hole (VH), respectively. Then, the distribution of density can be visualized by highlighting the map of SHG-density in the occupied and unoccupied states. For the Ba3(BN2)2 VE and VH processes, VE occupies 57% in the SHG process for d14. Therefore, both of the SHG processes of VE and VH with the occupied and unoccupied states were examined, as shown in Fig. S4.† In Ba3(BN2)2, the density of SHG in the VE occupied and unoccupied states originate from B–N interaction and a small contribution from Ba–N interaction, respectively, while Ba–N interaction plays an important role in the VH process. The result indicates that the atoms in the asymmetric sublattice have an important contribution to the SHG response.64
3.3. Optical properties analysis
To the best of our knowledge, the methods of analysis and birefringence estimation can also be employed to evaluate the ability of the functional units, such as the response electron distribution anisotropy (REDA) model,65,66 real-space atom-cutting (RSAC) technique,67 Born effective charge analysis method,68 Bader charge integration69 and the sum of polarizability anisotropy (SPA).70The optical anisotropy of a crystal depends on the direction of the covalent bond in the anionic groups. Therefore, birefringence is sensitive to the anisotropy of the response electron distribution, corresponding to the REDA index = Σg[NcZaΔρb/(n1Eg)] g of the anionic groups contained in the same crystal. Here, Nc is the coordination number of the nearest neighbor cations to the central anion, Za is the formal chemical valence of the anion, Eg is the optical bandgap, Δρb = ρbmax − ρbmin, ρbmax and ρbmin are the maximum and minimum of the covalent electron density of the covalent bond on the optical principal axes of a crystal, and n1 is the minimum refractive index. Pan et al. proved the rationality of the REDA method, and that the birefringence is proportional to the REDA index. To check the above analysis and quantification, we employed the REDA method to calculate the bonding electron density difference (Δρb) of the covalent bonds along the optical principal axes in Li3BN2 (P21/c, P42/mnm, I41/amd), Na3BN2, Mg3BN3, Ca3(BN2)N and Ba3(BN2)2. The Δρb values of BN2 are 2.12–4.49 (×10−2), and we calculated the Δρb values of the remaining anionic groups (Li–N, Na–N, Mg–N, Ca–N and Ba–N), as shown in Table 2. As a result, the BN2 units contribute the most to Δρb, which indicates that the BN2 units are the main sources of birefringence in the title compounds. As is well known, the Δρb value obtained by the REDA method contains the arrangement of anionic groups, and the arrangement of BN2 units in the title compounds are aligned in a parallel manner; the parallel or antiparallel arrangement is beneficial to the superposition of micro polarizability anisotropy. These results are generally in agreement with the increase of the macro birefringence.
| Compounds | Group | Δρb (×10−2) of BN2 | Δρb (×10−2) of A (Li, Na, Mg, Ca, Ba) Nn | Birefringence (@1064 nm) |
|---|---|---|---|---|
| Li3BN2(P21/c) | BN2 | 3.07 | 0.09 | 0.198 |
| Li3BN2(P42/mnm) | BN2 | 2.51 | −0.18 | 0.103 |
| Li3BN2(I41/amd) | BN2 | 4.49 | 1.46 | 0.116 |
| Na3BN2 | BN2 | 2.15 | −0.13 | 0.157 |
| Mg3BN3 | BN2 | 3.28 | −0.77 | 0.244 |
| Ca3(BN2)N | BN2 | 2.65 | −0.16 | 0.411 |
| Ba3(BN2)2 | BN2 | 2.12 | 0.47 | 0.185 |
Particularly, comparing Mg3BN3 and Ca3(BN2)N, we find that the birefringence of Ca3(BN2)N is about 1.7 times that of Mg3BN3, while the values of Δρb of BN2 in Mg3BN3 and Ca3(BN2)N are 3.28 and 2.65, respectively. Therefore, the BN2 units contribute about 130% in Mg3BN3 and 110% in Ca3(BN2)N, which are roughly in agreement. According to the formula of REDA approximation, except for the values of the bandgap, the other indicators in the formula of the two compounds are very close to each other. Consequently, we performed a comparison of Δρb(BN2)/Eg with the birefringence, as shown in Fig. 4. As we can see, the proportion of Δρb(BN2)/Eg is 2.284 in Ca3(BN2)N, almost 1.7 times that in Mg3BN3 (1.286), which indicates that the tendency is consistent with the variation of birefringence. Simultaneously, there exists a significant difference between the bandgap of the two compounds, which is the main reason that Ca3(BN2)N exhibits an extraordinarily larger birefringence than Mg3BN3 while they have a similar contribution of BN2 units.
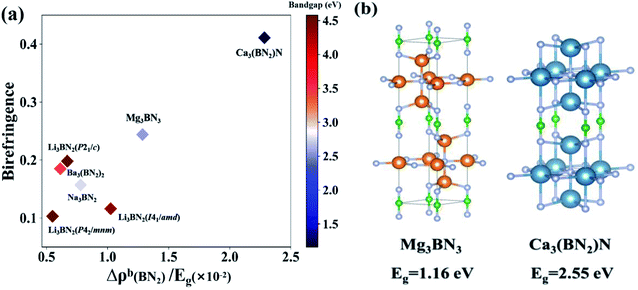 | ||
| Fig. 4 (a) A comparison of Δρb(BN2)/Eg (HSE06) with the birefringence of the selected BN2 compounds. (b) The structural comparison of Mg3BN3 and Ca3(BN2)N. | ||
4. Conclusion
In summary, we investigated the birefringence-enhanced FMs in a B–N system, and a linear group BN2 unit was proposed. Through the investigation based on the Inorganic Crystal Structure Database, seven ternary boron nitrides with the formulas A3BN2 (A = Li, Na), A3BN3 (A = Mg, Ca), and Ba3(BN2)2 were screened. Their birefringence values are in the range of 0.1–0.4 at 1064 nm, especially the birefringence of Ca3(BN2)N, which is 0.411 at 1064 nm, exceeding that of the commercial birefringent materials α-BaB2O4, CaCO3 and YVO4. Based on the analysis of the REDA and polarizability anisotropy, it was concluded that the large birefringence mainly originates from the BN2 unit. Hence, the functionality of the BN2 unit could be utilized in birefringence-enhanced FMs, which exhibits the possibility of applying B–N modules to the birefringent field. This study provides a feasible way to design optical materials with enlarged birefringence.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 11664001).References
- E. Collett, Field Guide to Polarization, SPIE, Bellingham, 2005 Search PubMed.
- D. H. Goldstein, Polarized Light, CRC, Boca Raton, 2011 Search PubMed.
- J. Hecht, A Short History of Laser Development, Opt. Eng., 2010, 49, 091002 CrossRef.
- Z. Y. Xie, L. G. Sun, G. Z. Han and Z. Z. Gu, Optical Switching of a Birefringent Photonic Crystal, Adv. Mater., 2008, 20, 3601–3604 CrossRef CAS.
- M. F. Weber, C. A. Stover, L. R. Gilbert, T. J. Nevitt and A. J. Ouderkirk, Giant Birefringent Optics in Multilayer Polymer Mirrors, Science, 2000, 287, 2451–2456 CrossRef CAS PubMed.
- F. Flossmann, U. T. Schwarz, M. Maier and M. R. Dennis, Polarization Singularities Generated from a Vortex Propagating through a Uniaxial Birefringent Crystal, Phys. Rev. Lett., 2005, 95, 253901 CrossRef PubMed.
- A. Tagaya, H. Ohkita, M. Mukoh, R. Sakaguchi and Y. Koike, Compensation of the Birefringence of a Polymer by a Birefringent Crystal, Science, 2003, 301, 812–814 CrossRef CAS PubMed.
- D. Brida, C. Manzoni and G. Cerullo, Phase-Locked Pulses for Two-Dimensional Spectroscopy by a Birefringent Delay Line, Opt. Lett., 2012, 37, 3027–3029 CrossRef PubMed.
- P. Hlubina, D. Ciprian and L. Knyblova, Interference of White Light in Tandem Configuration of Birefringent Crystal and Sensing Birefringent Fiber, Opt. Commun., 2006, 260, 535–541 CrossRef CAS.
- M. J. Katz, H. Kaluarachchi, R. J. Batchelor, A. A. Bokov, Z. G. Ye and D. B. Leznoff, Highly Birefringent Materials Designed Using Coordination Polymer Synthetic Methodology, Angew. Chem., Int. Ed., 2007, 46, 8804–8807 CrossRef CAS PubMed.
- L. H. Nicholls, F. J. Rodríguez-Fortuño, M. E. Nasir, R. M. Córdova-Castro, N. Olivier, G. A. Wurtz and A. V. Zayats, Ultrafast Synthesis and Switching of Light Polarization in Nonlinear Anisotropic Metamaterials, Nat. Photonics, 2017, 11, 628–633 CrossRef CAS.
- M. Li, H. F. Pan, Y. Q. Tong, C. Chen, Y. Shi, J. Wu and H. P. Zeng, All-Optical Ultrafast Polarization Switching of Terahertz Radiation by Impulsive Molecular Alignment, Opt. Lett., 2011, 36, 3633–3635 CrossRef CAS.
- A. Tudi, S. J. Han, Z. H. Yang and S. L. Pan, Potential Optical Functional Crystals with Large Birefringence: Recent Advances and Future Prospects, Coord. Chem. Rev., 2022, 459, 214380 CrossRef CAS.
- D. H. Goldstein, L. G. DeShazer, D. B. Chenault, W. G. Egan and M. J. Duggin, Improved Midinfrared Polarizers Using Yttrium Vanadate, Proc. SPIE, 2002, 4481, 10–16 CrossRef.
- G. Ghosh, Dispersion-equation Coefficients for the Refractive Index and Birefringence of Calcite Anquartz Crystals, Opt. Commun., 1999, 163, 95–102 CrossRef CAS.
- G. Q. Zhou, J. Xu, X. D. Chen, H. Y. Zhong, S. T. Wang, K. Xu, P. Z. Deng and F. X. Gan, Growth and Spectrum of a Novel Birefringent α-BaB2O4 Crystal, J. Cryst. Growth, 1998, 191, 517–519 CrossRef CAS.
- R. Appel, C. D. Dyer and J. N. Lockwood, Design of a Broadband UV-visible Alpha-Barium Borate Polarizer, Appl. Opt., 2002, 41, 2470–2480 CrossRef CAS.
- M. J. Dodge, Refractive Properties of Magnesium Fluoride, Appl. Opt., 1984, 23, 1980–1985 CrossRef CAS PubMed.
- Y. M. Cai, X. Z. Wang and H. J. Huang, Design of Wollaston Prism Used for Polarization Illumination System in ArF Lithography Tool, Chin. J. Lasers, 2014, 41, 16002 Search PubMed.
- D. L. Steinrnetz, W. G. Phillips, M. Wirick and F. F. Forbes, A Polarizer for the Vacuum Ultraviolet, Appl. Opt., 1967, 6, 1001–1004 CrossRef CAS PubMed.
- X. L. Chen, B. B. Zhang, F. F. Zhang, Y. Wang, M. Zhang, Z. H. Yang, K. R. Poeppelmeier and S. L. Pan, Designing an Excellent Deep-Ultraviolet Birefringent Material for Light Polarization, J. Am. Chem. Soc., 2018, 140, 16311–16319 CrossRef CAS.
- T. H. Tong, W. Y. Zhang, Z. H. Yang and S. L. Pan, Series of Crystals with Giant Optical Anisotropy: A Targeted Strategic Research, Angew. Chem., Int. Ed., 2020, 60, 1332–1338 CrossRef PubMed.
- W. Q. Jin, W. Y. Zhang, A. Tudi, L. Y. Wang, X. Zhou, Z. H. Yang and S. L. Pan, Fluorine-Driven Enhancement of Birefringence in the Fluorooxosulfate: A Deep Evaluation from a Joint Experimental and Computational Study, Adv. Sci., 2021, 8, 2003594 CrossRef CAS PubMed.
- W. Y. Zhang, Z. Z. Zhang, W. Q. Jin, R. N. Zhang, M. Cheng, Z. H. Yang and S. L. Pan, From Borophosphate to Fluoroborophosphate: A Rational Design of Fluorine-induced Birefringe-nce Enhancement, Sci. China: Chem., 2021, 64, 1498–1503 CrossRef CAS.
- Z. H. Yang, A. Tudi, B. H. Lei and S. L. Pan, Enhanced Nonlinear Optical Functionality in Birefringence and Refractive Index Dispersion of the Deep-Ultraviolet Fluorooxoborates, Sci. China Mater., 2020, 63, 1480–1488 CrossRef CAS.
- B. B. Zhang, G. Q. Shi, Z. H. Yang, F. F. Zhang and S. L. Pan, Fluorooxoborates: Beryllium-Free Deep-Ultraviolet Nonlinear Optical Materials without Layered Growth, Angew. Chem., Int. Ed., 2017, 56, 3916–3919 CrossRef CAS.
- X. F. Wang, Y. Wang, B. B. Zhang, F. F. Zhang, Z. H. Yang and S. L. Pan, CsB4O6F: A Congruent-melting Deep-ultraviolet Nonlinear Optical Material by Combining Superior Functional Units, Angew. Chem., Int. Ed., 2017, 56, 14119–14123 CrossRef CAS.
- Y. Wang, B. B. Zhang, Z. H. Yang and S. L. Pan, Cation-Tuned Synthesis of Fluorooxoborates: Towards Optimal Deep-Ultraviolet Nonlinear Optical Materials, Angew. Chem., Int. Ed., 2018, 57, 2150–2154 CrossRef CAS PubMed.
- M. Mutailipu, M. Zhang, B. B. Zhang, L. Y. Wang, Z. H. Yang, X. Zhou and S. L. Pan, SrB5O7F3 Functionalized with [B5O9F3]6− Chromophores: Accelerating the Rational Design of Deep-ultraviolet Nonlinear Optical Materials, Angew. Chem., Int. Ed., 2018, 57, 6095–6099 CrossRef CAS PubMed.
- J. Y. Guo, A. Tudi, S. J. Han, Z. H. Yang and S. L. Pan, Sn2B5O9Cl: A Material with Large Birefringence Enhancement Activated via Alkaline-earth Metal Substitution by Tin, Angew. Chem., Int. Ed., 2019, 58, 17675 CrossRef CAS PubMed.
- J. Y. Guo, S. C. Cheng, S. J. Han, Z. H. Yan and S. L. Pan, Sn2B5O9Br as an Outstanding Bifunctional Material with Strong Second-Harmonic Generation Effect and Large Birefringence, Adv. Opt. Mater., 2020, 9, 2001734 CrossRef.
- J. Y. Guo, A. Tudi, S. J. Han, Z. H. Yang and S. L. Pan, Sn2PO4I: An Excellent Birefringent Material with Giant Optical Anisotropy in Non π-Conjugated Phosphate, Angew. Chem., Int. Ed., 2021, 60, 24901–24904 CrossRef CAS PubMed.
- J. Lu, Y. K. Lian, L. Xiong, Q. R. Wu, M. Zhao, K. X. Shi, L. Chen and L. M. Wu, How to Maximize Birefringence and Nonlinearity of π-Conjugated Cyanurates, J. Am. Chem. Soc., 2019, 141, 16151–16159 CrossRef CAS.
- F. Kong, C. L. Hu, M. L. Liang and J. G. Mao, Pb4(OH)4(BrO3)3(NO3): An Example of SHG Crystal in Metal Bromates Containing π-Conjugated Planar Triangle, Inorg. Chem., 2016, 55, 948–955 CrossRef CAS PubMed.
- Y. C. Liu, Y. G. Shen and S. G. Zhao, Structure-Property Relationship in Nonlinear Optical Materials with π-conjugated CO3 Triangles, Coord. Chem. Rev., 2020, 407, 213152–213172 CrossRef CAS.
- Y. G. Shen, S. G. Zhao and J. H. Luo, The Role of Cations in Second-Order Nonlinear Optical Materials Based on π-conjugated [BO3]3− Groups, Coord. Chem. Rev., 2018, 366, 1–28 CrossRef CAS.
- S. Y. Zhang, X. Wu, Y. T. Song, D. Q. Ni, B. Q. Hu and T. Zhou, Growth of Birefringent Ca3(BO3)2 Crystals by the Czochralski Method, J. Cryst. Growth, 2003, 252, 246–250 CrossRef CAS.
- M. Mutailipu, K. R. Poeppelmeier and S. L. Pan, Borates: A Rich Source for Optical Materials, Chem. Rev., 2021, 121(3), 1130–1202 CrossRef CAS.
- D. B. Luo, X. J. Qiao and R. Dronskowski, Predicting Nitrogen-Based Families of Compounds: Transition-Metal Guanidinates TCN3 (T = V, Nb, Ta) and Ortho-Nitrido Carbonates T′2CN4 (T′ = Ti, Zr, Hf), Angew. Chem., Int. Ed., 2021, 60, 486–492 CrossRef CAS PubMed.
- L. Kang, F. Liang, Z. S. Lin, F. Liu and B. Huang, Cyano-Based Materials with Giant Optical Anisotropy and Second Harmonic-Generation Effect, Inorg. Chem., 2018, 57, 15001–15008 CrossRef CAS PubMed.
- H. Yamane, S. Kikkawa, H. Horiuchi and M. Koizumi, Structure of a New Polymorph of Lithium Boron Nitride, Li3BN2, J. Solid State Chem., 1986, 65, 6–12 CrossRef CAS.
- H. Yamane, S. Kikkawa, H. Horiuchi and M. Koizumi, High- and Low-Temperature Phases of Lithium Boron Nitride, Li3BN2: Preparation, Phase Relation, Crystal Structure, and Ionic Conductivity, J. Solid State Chem., 1987, 71, 1–11 CrossRef CAS.
- F. E. Pinkerton and J. F. Herbst, Tetragonal I41/amd Crystal Structure of Li3BN2 from Dehydrogenated Li–B–N–H, J. Appl. Phys., 2006, 99, 113523 CrossRef.
- J. Evers, M. Monsterkotter, G. Oehlinger, K. Polborn and B. Sendlinger, Natriumdinitridoborst Mit Dem Linear Gebauten, Symmetrischen BN23− – Anion, J. Less-Common Met., 1990, 162, L17–L22 CrossRef CAS.
- J. Li, J. Ding, B. Ma, Z. Zhao and Y. Wang, Design and Research of a Self-activated Orange Magnesium Boron Nitride Phosphor with Its Application in W-LEDs, Dalton Trans., 2018, 47, 15439–15447 RSC.
- M. Häberlen, J. Glaser and H. J. Meyer, Ca3(BN2)N – Eine Fehlende Verbindung im Quasi-Binären System Ca3N2-BN, Z. Anorg. Allg. Chem., 2002, 628, 1959–1962 CrossRef.
- O. Reckeweg, F. J. DiSalvo and M. Somer, Rthorhombic Ba3[BN2]2: a New Structure Type for an Alkaline Earth Metal Nitrido Borate, J. Alloys Compd., 2003, 361, 102–107 CrossRef CAS.
- L. J. Sham and M. Schluter, Density-Functional Theory of the Energy Gap, Phys. Rev. Lett., 1983, 51, 1888–1891 CrossRef.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. I. J. Probert, K. Refson and M. C. Payne, First Principles Methods Using CASTEP, Z. Kristallogr., 2005, 220, 567–570 CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- A. M. Rappe, K. M. Rabe, E. Kaxiras and J. D. Joannopoulos, Optimized Pseudopotentials, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 1227–1230 CrossRef PubMed.
- C. Aversa and J. E. Sipe, Nonlinear Optical Susceptibilities of Semiconductors: Results with a Length-Gauge Analysis, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 52, 14636–14645 CrossRef CAS PubMed.
- S. N. Rashkeev, W. R. Lambrecht and B. Segall, Efficient ab initio Method for the Calculation of Frequency-Dependent Second-Order Optical Response in Semiconductors, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 3905 CrossRef CAS.
- B. B. Zhang, M. H. Lee, Z. H. Yang, Q. Jing, S. L. Pan, M. Zhang, H. P. Wu, X. Su and Z. X. Li, Simulated Pressure Induced Blue-Shift of Phase-Matching Region and Nonlinear Optical Mechanism for K3B6O10X (X = Cl, Br), Appl. Phys. Lett., 2015, 106, 031906–031912 CrossRef.
- X. Dong, A. R. Oganov, A. F. Goncharov, E. Stavrou, S. Lobanov, G. Saleh, G. R. Qian, Q. Zhu, C. Gatti, V. L. Deringer, R. Dronskowski, X. F. Zhou, V. B. Prakapenka, Z. Konk pkov, I. A. Popov, A. I. Boldyrev and H. T. Wang, A Stable Compound of Helium and Sodium at High Pressure, Nat. Chem., 2017, 9, 440–445 CrossRef CAS PubMed.
- B. B. Zhang, X. D. Zhang, J. Yu, Y. Wang, K. Wu and M. H. Lee, First-Principles High-Throughput Screening Pipeline for Nonlinear Optical Materials: Application to Borates, Chem. Mater., 2020, 32, 6772–6779 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- S. Y. Zhang, X. Wu, Y. T. Song, D. Q. Ni, B. Q. Hu and T. Zhou, Growth of Birefringent Ca3(BO3)2 Crystals by The Czochralski Method, J. Cryst. Growth, 2003, 252, 246–250 CrossRef CAS.
- J. Tang, X. H. Meng, F. Liang, K. J. Kang, T. X. Zeng, M. J. Xia, W. L. Yin, Z. S. Lin and B. Kang, Structural Diversity and Giant Birefringence in Cyanates BaCNOX (X = Cl, Br, I, and CNO) Containing Linear π-Conjugated Units: A Combined Experimental and Theoretical Study, Cryst. Growth Des., 2020, 20, 1242–1247 CrossRef CAS.
- M. J. Kalmutzki, M. Strobele, F. Wackenhut, A. J. Meixner and H. J. Meyer, Synthesis, Structure, and Frequency-Doubling Effect of Calcium Cyanurate, Angew. Chem., Int. Ed., 2014, 53, 14260–14263 CrossRef CAS PubMed.
- M. J. Kalmutzki, K. Dolabdjian, N. Wichtner, M. Ströbele, C. Berthold and H. J. Meyer, Formation, Structure, and Frequency-Doubling Effect of a Modification of Strontium Cyanurate (α-SCY), Inorg. Chem., 2017, 56, 3357–3362 CrossRef CAS PubMed.
- F. Liang, L. Kang, X. Zhang, M. H. Lee, Z. S. Lin and Y. C. Wu, Molecular Construction Using (C3N3O3)3− Anions: Analysis and Prospect for Inorganic Metal Cyanurates Nonlinear Optical Materials, Cryst. Growth Des., 2017, 17, 4015–4020 CrossRef CAS.
- R. E. Cohen, Origin of Ferroelectricity in Perovskite Oxides, Nature, 1992, 358, 136–138 CrossRef CAS.
- B. H. Lei, S. L. Pan, Z. H. Yang, C. Cao and D. J. Singh, Second Harmonic Generation Susceptibilities from Symmetry Adapted Wannier Functions, Phys. Rev. Lett., 2020, 125, 187402 CrossRef CAS PubMed.
- B. H. Lei, Z. H. Yang and S. L. Pan, Enhancing Optical Anisotropy of Crystals by Optimizing Bonding Electron Distribution in Anionic Groups, Chem. Commun., 2017, 53, 2818–2821 RSC.
- B. H. Lei, Z. H. Yang, H. H. Yu, C. Cao, Z. Li, C. Hu, K. R. Poeppelmeier and S. L. Pan, Module-Guided Design Scheme for Deep-Ultraviolet Nonlinear Optical Materials, J. Am. Chem. Soc., 2018, 140, 10726–10733 CrossRef CAS PubMed.
- J. Lin, M. H. Lee, Z. P. Liu, C. T. Chen and C. J. Pickard, Mechanism for Linear and Nonlinear Optical Effects in β-BaB2O4 Crystals, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 13380 CrossRef CAS.
- Q. Jing, G. Yang, J. Hou, M. Z. Sun and H. B. Cao, Positive and Negative Contribution to Birefringence in a Family of Carbonates: A Born Effective Charges Analysis, J. Solid State Chem., 2016, 244, 69–74 CrossRef CAS.
- C. S. Lin, A. Y. Zhou, W. D. Cheng, N. Ye and G. L. Chai, Atom-Resolved Analysis of Birefringence of Nonlinear Optical Crystals by Bader Charge Integration, J. Phys. Chem. C, 2019, 123, 31183–31189 CrossRef CAS.
- X. X. Jiang, S. Y. Luo, L. Kang, P. F. Gong, H. W. Huang, S. C. Wang, Z. S. Lin and C. T. Chen, First-Principles Evaluation of the Alkali and/or Alkaline Earth Beryllium Borates in Deep Ultraviolet Nonlinear Optical Applications, ACS Photonics, 2015, 2, 1183–1191 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Calculated bandgap and PDOS, calculated birefringence, the NLO coefficients, the SHG response origins. See https://doi.org/10.1039/d2ra02135h |
| This journal is © The Royal Society of Chemistry 2022 |



