 Open Access Article
Open Access ArticleHigh-quality all-inorganic CsPbBr3 single crystals prepared by a facile one-step solution growth method†
Mingming Chen ab,
Youwen Yuanb,
Yuan Liu
ab,
Youwen Yuanb,
Yuan Liu b,
Dawei Cao
b,
Dawei Cao *b and
Chunxiang Xu
*b and
Chunxiang Xu *a
*a
aState Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, Southeast University, Nanjing, Jiangsu 210096, China. E-mail: xcxseu@seu.edu.cn
bDepartment of Physics, School of Physics and Electronic Engineering, Jiangsu University, Zhenjiang, Jiangsu 212013, China. E-mail: dwcao@ujs.edu.cn
First published on 16th May 2022
Abstract
In this report, a facile one-step solution growth method has been proposed to synthesize high-quality all-inorganic CsPbBr3 single crystals. High-resolution X-ray diffraction, photoluminescence (PL), and current–voltage techniques have been performed to study the properties of CsPbBr3 single crystals. The results have shown that the as-grown CsPbBr3 single crystals exhibited a narrow X-ray rocking curve with a FWHM (full width at half maximum) of 0.043°, a narrow room-temperature PL spectrum with the FWHM of 18.9 nm, and extremely low density of traps (∼5.1 × 109 cm−3). The results shown in this work will provide a valuable strategy for the fabrication of high-quality all-inorganic CsPbBr3 single crystals.
Introduction
As emerging materials, lead halide perovskites (LHPs) (ABX3, A = Cs, CH3NH3 (MA), HC(NH2)2 (FA), etc.; B = Pb; X = Cl, Br, and I) have attracted tremendous attention in recent years because of their great success in the field of photovoltaic and photodetection technologies due to their remarkable properties such as long charge carrier diffusion length, high absorption coefficient, defects tolerance and low-temperature solution-processed ability.1–6 For example, the power conversion efficiency of solution-processed planar perovskite solar cells has reached up to ∼25.7% very recently,7 which is comparable to commercial silicon-based solar cells. Besides, LHPs have also been recognized as promising candidates for light emission applications owing to their competitive optical properties including efficient photoluminescence (PL), narrow-band emission, weak Auger recombination, high optical gain, and widely tunable energy bandgap.8–16Single crystals featured with improved crystalline quality and low density of defects are of vital importance in the development of high-performance optoelectronic devices. LHP single crystals can be grown via a low-temperature solution growth process.17–23 In general, the LHPs nucleate and grow inside the precursor solution containing raw materials (AX and BX2) and polar aprotic solvents (such as γ-butyrolactone (GBL), dimethyl sulfoxide (DMSO), and N,N-dimethylformamide (DMF)). In one typical growth process, the supersaturation of precursor solution is achieved through the slow vapor saturation of an antisolvent (VSA).18 In such a case, the nucleation and subsequent crystal growth can occur at room temperature (RT), but it produces several millimeter-sized single crystals and further limits the crystalline quality improvements due to the slow species transportation and diffusion at the solid/liquid interface under such a low temperature. In another growth process, the growth of organic–inorganic hybrid LHP single crystals has been reported through invariably heating the precursor solution (HPS). In this case, the supersaturation is realized by slow evaporation of solvent under a constant temperature heating process. Similar to the VSA method, the HPS method produces multiple small-sized single crystals. With the assistance of crystal seeding, millimeter-scale LHP single crystals have been obtained, but a high density of defects have been found at the seed boundaries.18 Recently, an improved method called inverse temperature crystallization has been proposed by Bakr et al.,19,22 in which the LHP single crystals can rapidly nucleate and grow. However, this method strongly depends on the retrograde solubility behaviors of LHPs, which may limit the variety and quality of as-grown LHPs owing to the limited aprotic solvents available.
Among the typical growth processes mentioned above, the HPS method is simple, low-cost, and convenient, but it has difficulties in growing high-quality large-sized LHP single crystals. In this work, a modified and facile one-step solution growth method has been demonstrated to prepare high-quality centimeter-scale all-inorganic CsPbBr3 single crystals, where the supersaturation of precursor solution was realized via a combination of the HPS method and oil bath heating. Specifically, the as-grown CsPbBr3 single crystals exhibited a narrow X-ray rocking curve (XRC), narrow PL spectrum, and extremely low density of traps.
Experiments
Materials
Lead bromide (PbBr2, >99.5%), cesium bromide (CsBr, >99.5%), dimethyl sulfoxide (DMSO, anhydrous), dimethylformamide (DMF, anhydrous), and silicone oil were used as received.Growth of CsPbBr3 single crystals
2.4 mmol PbBr2 and 1.2 mmol CsBr were added into 3 mL anhydrous DMSO to form precursor solution. The precursor solution was firstly stirred at 80 °C, and then filtered and heated at 140 °C in a silicone oil bath (as schematically shown in Fig. 1a) in sequence. When invariably heating the precursor, the CsPbBr3 nucleated and grew due to the evaporation of DMSO solvent. The growth process lasted around 5 days. Then, the as-grown CsPbBr3 single crystals were washed by anhydrous DMF and dried in a vacuum for 2 hours in sequence, and finally stored in a vacuum before they were further characterized. For comparison, reference CsPbBr3 single crystals were prepared by HPS method with a same recipe. All experiments were carried out under ambient conditions.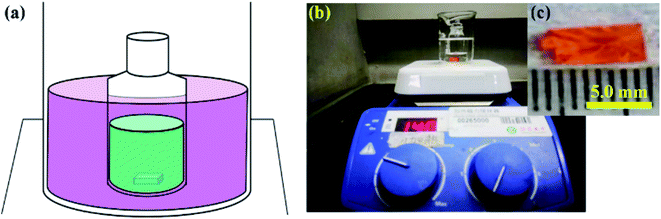 | ||
| Fig. 1 Growth of CsPbBr3 single crystal. (a) Schematic illustration of experimental setup. Optical images of CsPbBr3 single crystal captured (b) during the growth and (c) at the end of growth. | ||
Characterizations
The structural properties of as-grown CsPbBr3 single crystals were characterized by X-ray diffraction (XRD) with Cu Kα radiation of λ = 1.5406 Å. The optical properties of as-grown CsPbBr3 single crystals were investigated by homemade micro-PL spectroscopy with the excitation of a continuous-wave 405 nm laser. For the excitation power dependence of PL, the excitation power is tuned by an attenuator. The absorption spectra were collected on a UV-VIS spectrophotometer (Agilent Technologies, Cary-8454). The current–voltage (I–V) of Au/CsPbBr3/Au structured device is recorded by Keithley 2401 source-measure unit, where Au electrodes were deposited by a sputtering method at RT.Stimulation
The steady-state temperature field distribution of precursor solution (solution 1) in the silica glass beaker which is surrounded by silicone oil (solution 2), was numerically calculated using the COMSOL Multiphysics packages. For simplicity, both solution 1 and solution 2 are defined as water in the simulation process. The inner and outer radius of the beaker are 5.0 and 6.3 mm, respectively, and the solution 1 just fills half of the beaker. The beaker is placed in a silica glass container filled with solution 2. The radius of the container is 50 mm, and the height of solution 2 in the container is as twice as solution 1 in the beaker. The container is placed on a heat source. During the simulations, the module of heat transfer in solids and fluids is adopted, in which the heat flux is added at the interfaces between the system and the external environment. The ambient temperature is set as 293.15 K (20 °C), and the temperature of heat source keeps 413.15 K (140 °C). The material parameters for solution 1, solution 2, and silica glass are imported from the material library of COMSOL.Results and discussions
As reported, the all-inorganic cesium perovskite materials can exhibit various forms of structures such as CsPbBr3, CsBr-rich Cs4PbBr6, and PbBr2-rich CsPb2Br5 according to the connection manner of [PbBr6]4− octahedra in the unit cell.24–26 The molar ratio between raw materials (mCsBr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nPbBr2) is important for the final crystallization from DMSO solvent. In general, either Cs4PbBr6 or CsPb2Br5 is formed accompanied with the CsPbBr3 under a slight deviation from a perfect ratio of m
nPbBr2) is important for the final crystallization from DMSO solvent. In general, either Cs4PbBr6 or CsPb2Br5 is formed accompanied with the CsPbBr3 under a slight deviation from a perfect ratio of m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n = 1
n = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CsBr
1 CsBr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PbBr2.18,21 To obtain the pure phase CsPbBr3, it is necessary to add additional PbBr2 in the precursor solution since the solubilities of PbBr2 and CsBr in DMSO solvent are quite different (in fact, CsBr is less soluble than PbBr2 in the DMSO solvent). Herein, the precursor solution was prepared by dissolving CsBr and PbBr2 with a molar ratio of 1
PbBr2.18,21 To obtain the pure phase CsPbBr3, it is necessary to add additional PbBr2 in the precursor solution since the solubilities of PbBr2 and CsBr in DMSO solvent are quite different (in fact, CsBr is less soluble than PbBr2 in the DMSO solvent). Herein, the precursor solution was prepared by dissolving CsBr and PbBr2 with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in the DMSO solvent. Notably, only one CsPbBr3 single crystal can be found in the precursor solution during the whole growth process (Fig. 1b). The resulted CsPbBr3 single crystal is cuboid in shape with a size of ∼7 mm (Fig. 1c). We anticipate that the CsPbBr3 single crystal would grow even bigger when extending the growth time with sufficient raw materials.
2 in the DMSO solvent. Notably, only one CsPbBr3 single crystal can be found in the precursor solution during the whole growth process (Fig. 1b). The resulted CsPbBr3 single crystal is cuboid in shape with a size of ∼7 mm (Fig. 1c). We anticipate that the CsPbBr3 single crystal would grow even bigger when extending the growth time with sufficient raw materials.
XRD measurements were performed to study the structural properties of as-grown CsPbBr3 single crystal. As shown in Fig. 2a and S1 (ESI†), the XRD pattern of as-grown CsPbBr3 single crystal is dominated by the set of (0 0 l)/(h h 0) crystalline plane of orthorhombic CsPbBr3 without traces of Cs4PbBr6 and CsPb2Br5. Generally, CsPbBr3 crystallizes in the orthorhombic space group at RT and it transits to tetragonal and cubic phases at 361 K and 403 K, respectively.27,28 Interestingly, there is no trace of the tetragonal and cubic phase CsPbBr3 in the as-grown samples. According to the Bragg equation, the lattice constants are calculated to be a = 8.23 Å, b = 8.29 Å, and c = 11.83 Å, which are consistent with the values of CsPbBr3 bulk materials (a0 = 8.21 Å, b0 = 8.26 Å, and c0 = 11.76 Å, obtained from the PDF card: 01-072-7929, Fig. 2b). In contrast, orthorhombic phase CsPbBr3 with slight traces of CsPb2Br5 were obtained from the HPS method (Fig. S1, ESI†).
The crystalline quality of as-grown CsPbBr3 single crystal was further analyzed by high-resolution XRC measurement, and the result is shown in Fig. 2c. A narrower XRC spectrum suggests a higher crystalline quality. As can be seen, the full width at half maximum (FWHM) of (002) XRC is as small as 0.043° (155 arcsec). This is much smaller than CsPbBr3 single crystal obtained from the HPS method (623 arcsec, Fig. S2, ESI†) and other LHP bulk materials reported in the literature.18,21,29 Fig. 3a shows the RT PL spectrum of as-grown CsPbBr3 single crystal. As can be seen, the as-grown CsPbBr3 single crystal shows a strong and narrow excitonic emission centered at 532.5 nm (3.328 eV) with the FWHM as narrow as ∼18.9 nm (82.5 meV). The narrow PL spectrum can be an indication of the high crystalline quality of as-grown CsPbBr3 single crystal, which is well consistent with XRC results shown in Fig. 2c. In contrast, the PL spectrum of CsPbBr3 single crystals prepared by the HPS method slightly blueshifts (∼6 meV) and broadens (∼10 meV) (Fig. S3, ESI†), which may be associated with strain induced by the secondary phase (i.e., CsPb2Br5, shown in Fig. S1, ESI†). In addition, a redshift of the absorption edge is observed (Fig. S4, ESI†), which is caused by the optical absorption in the thick CsPbBr3 single crystal.
Besides, the type of exciton transition from as-grown CsPbBr3 single crystal was then investigated by the excitation power dependence of PL spectroscopy. In general, the relationship between the integrated PL emission intensities (I) and the excitation power (P) follows the eqn (1):30
| I ∼ Pk | (1) |
Then, the deep-level charged-trap densities in as-grown CsPbBr3 single crystal were further calculated with the space charge limited current (SCLC) theory.31 Shown in Fig. 4 is the I–V curve of an Au/CsPbBr3/Au structured device. As can be seen, an ohmic region (I ∼ Vn, n = 1) followed by a steep increase (I ∼ Vn, n > 3) can be observed, indicating the presence of a typical trap-filling region. According to SCLC theory, the trap density (ntrap) is calculated to be ∼5.1 × 109 cm−3, from the eqn (2):31
| ntrap = 2εε0VTFL/(ed2) | (2) |
 | ||
| Fig. 4 I–V curve of an Au/CsPbBr3/Au structured device. The I–V curve is fitted using a power law (I ∼ Vn) (shown as red line). | ||
In the end, the mechanisms of crystal growth of CsPbBr3 in the oil bath heating process are discussed. For the solution growth method (as shown in Fig. 1a), the growth kinetics of CsPbBr3 is generally governed by the species transportation and diffusion at the solid/liquid interface. As a result, an uniform distribution of temperature field of precursor solution is prerequisite for growing high crystalline quality and low defects single crystals.36 Here, the steady-state temperature field distribution of precursor solution shown in Fig. 1a was calculated using COMSOL Multiphysics packages, and the result is shown in Fig. 5a. It can be seen that the precursor solution is uniformly heated in the oil bath heating process. In this case, the crystal growth driving force is mainly related to constant and uniform supersaturation induced by the constant evaporation of solvent, which is responsible for the growth of high crystalline quality CsPbBr3 single crystals with extremely low density of defects. On this basis, the growth process is proposed as follows: initially, the CsPbBr3 nucleates when the precursor solution approaches saturation concentration (Fig. 5b). After that, a competitive CsPbBr3 nucleus survives (labelled by blue box) and acts as a seed simultaneously, maintaining the subsequent crystal growth. In contrast, a temperature gradient was found when directly heating the precursor solution on the hot table (Fig. S6, ESI†). In this case, additional crystal growth driving forces related to the precursor concentration gradient can be expected, which accelerates the growth of CsPbBr3 nucleus before they were re-dissolved. Meanwhile, the rapid growth rate will lead to a high defect density,37 and further deteriorates the crystalline quality of the as-grown CsPbBr3 single crystals.
Conclusions
In summary, we have shown a facile one-step solution growth method for growing high-quality all-inorganic CsPbBr3 single crystals. By employing the oil bath heating strategy, the as-grown CsPbBr3 single crystals have shown improved crystalline quality and extremely low density of defects, as verified by structural, optical, and electrical properties investigations. Simulation on the steady-state temperature field distribution indicated that the precursor solution was uniformly heated in the oil bath heating process, providing constant and uniform supersaturation as crystal growth driving force. This work provides a promising way to achieve high-quality all-inorganic CsPbBr3 single crystals via a facile solution growth method.Author contributions
Mingming Chen: conceptualization, methodology, writing – review & editing, funding acquisition. Chunxiang Xu: visualization, project administration. Youwen Yuan: investigation, writing – original draft. Yuan Liu: formal analysis, writing – review & editing. Dawei Cao: formal analysis, supervision, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the China Postdoctoral Science Foundation (2018M632196), and the Project of Faculty of Agricultural Equipment of Jiangsu University (NZXB20210202 and NZXB20200215).Notes and references
- G. Xing, N. Mathews, S. Sun, S. S. Lim, Y. M. Lam, M. Grätzel, S. Mhaisalkar and T. C. Sum, Science, 2013, 342, 344 CrossRef CAS.
- G. Sathiyan, A. A. Syed, C. Chen, C. Wu, L. Tao, X. Ding, Y. Miao, G. Li, M. Cheng and L. Ding, Nano Energy, 2020, 72, 104673 CrossRef CAS.
- C. Wu, C. Chen, L. Tao, X. Ding, M. Zheng, H. Li, G. Li, H. Lu and M. Cheng, J. Energy Chem., 2020, 43, 98–103 CrossRef.
- X. Ding, C. Chen, L. Sun, H. Li, H. Chen, J. Su, H. Li, H. Li, L. Xu and M. Cheng, J. Mater. Chem. A, 2019, 7, 9510–9516 RSC.
- C. Chen, C. Wu, X. Ding, Y. Tian, M. Zheng, M. Cheng, H. Xu, Z. Jin and L. Ding, Nano Energy, 2020, 71, 104604 CrossRef CAS.
- C. Chen, M. Cheng, H. Li, F. Qiao, P. Liu, H. Li, L. Kloo and L. Sun, Mater. Today Energy, 2018, 9, 264–270 CrossRef.
- https://www.nrel.gov/pv/assets/pdfs/cell-pv-eff-emergingpv-rev211214.pdf.
- G. Xing, N. Mathews, S. S. Lim, N. Yantara, X. Liu, D. Sabba, M. Grätzel, S. Mhaisalkar and T. C. Sum, Nat. Mater., 2014, 13, 476–480 CrossRef CAS.
- F. Zhang, H. Zhong, C. Chen, X.-g. Wu, X. Hu, H. Huang, J. Han, B. Zou and Y. Dong, ACS Nano, 2015, 9, 4533–4542 CrossRef CAS.
- Y. Wang, X. Li, J. Song, L. Xiao, H. Zeng and H. Sun, Adv. Mater., 2015, 27, 7101–7108 CrossRef CAS PubMed.
- J. Song, J. Li, X. Li, L. Xu, Y. Dong and H. Zeng, Adv. Mater., 2015, 27, 7162–7167 CrossRef CAS.
- M. Chen, Y. Yuan, Z. Wang, X. Shen, Y. Liu and D. Cao, Cryst. Growth Des., 2020, 20(8), 4855–4860 CrossRef CAS.
- X. Zheng, B. Chen, J. Dai, Y. Fang, Y. Bai, Y. Lin, H. Wei, X. C. Zeng and J. Huang, Nat. Energy, 2017, 2, 17102 CrossRef CAS.
- X. Shen, M. Chen, L. Shi, F. Chen, Y. Liu, D. Cao and C. Xu, Opt. Commun., 2019, 453, 124354 CrossRef CAS.
- V. G. V. Dutt, S. Akhil and N. Mishra, ChemNanoMat, 2020, 6, 1730–1742 CrossRef CAS.
- S. Akhil, V. G. V. Dutt and N. Mishra, ChemNanoMat, 2021, 7, 342–353 CrossRef CAS.
- O. Nazarenko, S. Yakunin, V. Morad, I. Cherniukh and M. V. Kovalenko, NPG Asia Mater., 2017, 9, e373 CrossRef CAS.
- Y. Rakita, N. Kedem, S. Gupta, A. Sadhanala, V. Kalchenko, M. L. Böhm, M. Kulbak, R. H. Friend, D. Cahen and G. Hodes, Cryst. Growth Des., 2016, 16, 5717–5725 CrossRef CAS.
- M. I. Saidaminov, A. L. Abdelhady, G. Maculan and O. M. Bakr, Chem. Commun., 2015, 51, 17658–17661 RSC.
- H. Zhang, X. Liu, J. Dong, H. Yu, C. Zhou, B. Zhang, Y. Xu and W. Jie, Cryst. Growth Des., 2017, 17, 6426–6431 CrossRef CAS.
- M. Zhang, Z. Zheng, Q. Fu, Z. Chen, J. He, S. Zhang, C. Chen and W. Luo, J. Cryst. Growth, 2018, 484, 37–42 CrossRef CAS.
- M. I. Saidaminov, A. L. Abdelhady, B. Murali, E. Alarousu, V. M. Burlakov, W. Peng, I. Dursun, L. Wang, Y. He, G. Maculan, A. Goriely, T. Wu, O. F. Mohammed and O. M. Bakr, Nat. Commun., 2015, 6, 7586 CrossRef PubMed.
- Y. Yuan, M. Chen, S. Yang, X. Shen, Y. Liu and D. Cao, J. Lumin., 2020, 226, 117471 CrossRef CAS.
- Z. Liu, Y. Bekenstein, X. Ye, S. C. Nguyen, J. Swabeck, D. Zhang, S.-T. Lee, P. Yang, W. Ma and A. P. Alivisatos, J. Am. Chem. Soc., 2017, 139, 5309–5312 CrossRef CAS.
- P. Acharyya, P. Pal, P. K. Samanta, A. Sarkar, S. K. Pati and K. Biswas, Nanoscale, 2019, 11, 4001–4007 RSC.
- X. Li, M. Chen, S. Mei, B. Wang, K. Wang, G. Xing and Z. Tang, Sci. China Mater., 2020, 63, 1510 CrossRef CAS.
- T. J. Whitcher, L. C. Gomes, D. Zhao, M. Bosman, X. Chi, Y. Wang, A. Carvalho, H. K. Hui, Q. Chang, M. B. H. Breese, A. H. Castro Neto, A. T. S. Wee, H. D. Sun, E. E. M. Chia and A. Rusydi, NPG Asia Mater., 2019, 11, 70 CrossRef.
- K. Wei, Z. Xu, R. Chen, X. Zheng, X. Cheng and T. Jiang, Opt. Lett., 2016, 41, 3821–3824 CrossRef CAS.
- M. Zhang, Z. Zheng, Q. Fu, Z. Chen, J. He, S. Zhang, L. Yan, Y. Hu and W. Luo, CrystEngComm, 2017, 19, 6797–6803 RSC.
- D. Damberga, R. Viter, V. Fedorenko, I. Iatsunskyi, E. Coy, O. Graniel, S. Balme, P. Miele and M. Bechelany, J. Phys. Chem. C, 2020, 124, 9434–9441 CrossRef CAS.
- J. Zeng, X. Li, Y. Wu, D. Yang, Z. Sun, Z. Song, H. Wang and H. Zeng, Adv. Funct. Mater., 2018, 28, 1804394 CrossRef.
- M. I. Saidaminov, M. A. Haque, J. Almutlaq, S. Sarmah, X.-H. Miao, R. Begum, A. A. Zhumekenov, I. Dursun, N. Cho, B. Murali, O. F. Mohammed, T. Wu and O. M. Bakr, Adv. Opt. Mater., 2017, 5, 1600704 CrossRef.
- A. R. Srimath Kandada, S. Neutzner, V. D'Innocenzo, F. Tassone, M. Gandini, Q. A. Akkerman, M. Prato, L. Manna, A. Petrozza and G. Lanzani, J. Am. Chem. Soc., 2016, 138, 13604–13611 CrossRef CAS.
- A. Solanki, S. S. Lim, S. Mhaisalkar and T. C. Sum, ACS Appl. Mater. Interfaces, 2019, 11, 25474–25482 CrossRef CAS PubMed.
- H. Jin, E. Debroye, M. Keshavarz, I. G. Scheblykin, M. B. J. Roeffaers, J. Hofkens and J. A. Steele, Mater. Horiz., 2020, 7, 397–410 RSC.
- K. Byrappa, in Springer Handbook of Crystal Growth, ed. G. Dhanaraj, K. Byrappa, V. Prasad and M. Dudley, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010, pp. 599–653, DOI:10.1007/978-3-540-74761-1_18.
- E. Huitema and J. P. van der Eerden, J. Cryst. Growth, 1996, 166, 141–145 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01900k |
| This journal is © The Royal Society of Chemistry 2022 |



