Open Access Article
Open Access ArticleA comprehensive review on phytochemistry and pharmacology of genus Kopsia: monoterpene alkaloids – major secondary metabolites
Nguyen Quang Hopa and
Ninh The Son *bc
*bc
aFaculty of Chemistry, Hanoi Pedagogical University 2 (HPU2), Nguyen Van Linh, Xuanhoa, Phucyen, Vinhphuc, Vietnam
bInstitute of Chemistry, Vietnam Academy of Science and Technology (VAST), 18 Hoang Quoc Viet, Caugiay, Hanoi, Vietnam. E-mail: yamantson@gmail.com; ntson@ich.vast.vn
cGraduate University of Science and Technology, VAST, Vietnam
First published on 5th July 2022
Abstract
Kopsia belongs to the family Apocynaceae, which was originally classified as a genus in 1823. Kopsia consists of medicinal plants that can be traditionally used to treat rheumatoid arthritis, pharyngitis, tonsillitis, and dropsy. More than one hundred and twenty-five publications have been documented relating to the phytochemical and pharmacological results, but a systematic review is not available. The goal of this study is to compile almost all of the secondary metabolites from the plants of genus Kopsia, as well as the coverage of their pharmacological research. The document findings were conducted via reliable sources, including Web of Science, Sci-Finder, Science Direct, PubMed, Google Scholar, and publishers, while four words “Kopsia”, “monoterpene alkaloids”, “Phytochemistry” and “Pharmacology” are key factors to search for references. Most Kopsia secondary metabolites were collected. A total of four hundred and seventy-two, including four hundred and sixty-six monoterpene alkaloids, five triterpenoids, and one sterol, were summarized, along with their resource. Kopsia monoterpene alkaloids presented in various skeletons, but aspidofractinines, eburnamines, and chanofruticosinates are the three major backbones. Mersinines and pauciflorines are new chemical classes of monoterpene alkaloids. With the rich content of monoterpene alkaloids, Kopsia constituents were also the main objects in pharmacological studies since the plant extracts and isolated compounds were proposed for anti-microbial, anti-inflammatory, anti-allergic, anti-diabetic, anti-manic, anti-nociceptive, acetylcholinesterase (AChE) inhibitory, cardiovascular, and vasorelaxant activities, especially cytotoxicity.
1. Introduction
Natural products are chemical substances created by living organisms and found in nature. In the medicinal chemistry field, this concept is usually limited to secondary metabolites.1 The pharmacological studies on potential bioactive agents tend to find that lead molecules for drug development could arise from natural resources.Kopsia belongs to the subfamily Rauvolfioideae of the family Apocynaceae.2 This genus, containing about 30 species, is widely distributed in Southeast Asia, China, Australia, and some islands of the Western Pacific.3,4 Kopsia plants are recognized as a fertile reservoir of novel and bioactive secondary metabolite type alkaloids. Therefore, they have been traditionally used in each country. Chinese folk medicine deals with the use of parts of K. officinalis Tsiang & P. T. Li to treat rheumatoid arthritis, pharyngitis, tonsillitis, and dropsy.4 In Malaysia, the roots of four species, K. larutensis King & Gamble, K. macrophylla Hook.f., K. singapurensis Ridl., and K. paucifora Hook.f., were applied as a poultice to ulcerate noses in tertiary syphilis.2,5 Kopsia constituents are also well-known in pharmacological discoveries, in which they have a wide spectrum of pharmacological effects such as anticancer and anti-manic activities.6,7
Recently, the search for bioactive molecules from the genus Kopsia has drawn lots of interest to natural product chemists and pharmacists.8–13 Although there have been a variety of experimental studies, an overview of phytochemical and pharmacological assessments is not available now. The current review provides notes on basic knowledge about phytochemical research and sheds light on the pivotal role of Kopsia constituents in pharmacological examinations. More than one hundred twenty-five relevant publications have been used, as well as the data collection is from the 1950s to now.
2. Phytochemistry
Since the 1950s, a large number of phytochemical studies on Kopsia plants have been published. To some extent, this current paper provides basic knowledge about the isolation processes of Kopsia secondary metabolites. The results related to experimental reports are primarily based on chromatographic approaches, such as silica gel chromatography or HPLC procedure (high performance chromatography column), whereas the NMR structural elucidation of isolated compounds is due to the most utilization of spectral methods, such as 1D/2D-NMR, mass spectroscopy (MS), ultraviolet-visible (UV-Vis), optical rotation (OR), infrared (IR), circular dichroism (CD) and comparisons with previous literature. Among recorded thirty species, nineteen plants, including K. arborea, K. dasyrachis, K. deverrei, K. flavida, K. fruticosa, K. grandifolia, K. griffithii, K. hainanensis, K. jasminiflora, K. lancibracteolata, K. lapidilecta, K. larutensis, K. macrophylla, K. officinalis, K. pauciflora, K. profunda, K. singapurensis, K. teoi, and K. terengganensis, have been most widely utilized for phytochemical investigations. More than four hundred seventy metabolites were collected and tabulated in Table 1 and Fig. 1–9. Significantly, four hundred sixty-six isolated compounds have been categorized as monoterpene alkaloids, in which they have induced a diversity of chemical skeletons, including aspidofractinines 1–204, chanofruticosinates 205–241, aspidospermines 242–248, danuphyllines 249–252, eburnamines 253–301, akuammilines 302–322, sarpagines 323–326, aspidophyllines 327–331, strychnos 332–356, stemmadenine 357, mersinines 358–378, pauciflorines 379–390, skytanthines 391–400, rhazinilams 401–409, lundurines 410–426, aspidospermas 427–431, catharinensines 432–436, leuconoxines 437–442, pericines 443–446, alstonines 447–449, quebrachamines 450–452, arbophyllinines 453–454, arboflorines 455–456, andrasinines 457–458, corynantheines 459–460, carbolines 461–462, arbophyllidine 463, mersicarpine 464, azepane-fused tetrahydro-β-carboline 465, and andranginine 466. In each group, the name of the compound was alphabetically ordered in an arrangement. The similar chemical classes will be placed close to each other.| No. | Compounds | Species | References |
|---|---|---|---|
| Aspidofractinines | |||
| 1 | Arbolodinine A | K. arborea stem bark | 8 |
| 2 | Aspidofractinine | K. arborea stem bark, K. hainanensis twig and leaf, K. officinalis stem | 9–11 |
| 3 | (2β,5β)-Aspidofractinin-16-ol | K. hainanensis twig and leaf, K. officinalis leaf | 9, 12 and 13 |
| 4 | Aspidofractinine-1,3-dicarboxylic acid | K. officinalis stem | 11 |
| 5 | N-Carbomethoxy-11-hydroxy-12-methoxykopsinaline | K. griffithii leaf, K. officinalis twig, leaf and fruit | 14–16 |
| 6 | N-Carbomethoxy-11-methoxy-12-hydroxykopsinaline | K. officinalis fruit | 14 |
| 7 | N(1)-Carbomethoxy-11, 12-dimethoxykopsinaline | K. griffithii leaf, K. officinalis fruit | 14, 15 and 17 |
| 8 | N-Carbomethoxy-12-methoxykopsinaline | K. officinalis fruit | 14 |
| 9 | N-Carbomethoxy-5,22-dioxokopsane | K. dasyrachis stem, K. pauciflora stem | 18 and 19 |
| 10 | Dasyrachine | K. arborea stem bark, K. dasyrachis stem | 10 and 18 |
| 11 | Decarbomethoxykopsine (demethoxycarbonylkopsin) | K. fruticosa leaf, K. officinalis leaf and twig | 16 and 20 |
| 12 | Decarbomethoxyisokopsine | K. fruticosa leaf | 20 |
| 13 | Decarbomethoxykopsifine | K. arborea twig, K. dasyrachis stem, K. officinalis stem, K. pauciflora stem and stem bark | 11, 18, 19, 21 and 22 |
| 14 | N(1)-Decarbomethoxykopsamine | K. arborea stem bark, K. hainanensis stem and leaf, K. pauciflora leaf, K. singapurensis leaf | 7, 10, 22 and 23 |
| 15 | Na-Demethoxycarbonyl-12-methoxykopsine | K. jasminiflora stem bark, K. officinalis leaf and twig | 16, 24 and 25 |
| 16 | 10-Demethoxykopsidasinine | K. jasminiflora | 26 |
| 17 | 5,22-Dioxokopsane | K. hainanensis stem bark and twig, K. macrophylla bark, K. officinalis root, stem, twig and fruit, K. pauciflora stem bark | 11, 12, 14, 16, 19 and 27–29 |
| 18 | 11,12-Dimethoxykopsamine | K. dasyrachis leaf | 30 |
| 19 | 11,12-Dimethoxykopsinaline | K. pauciflora stem bark | 22 |
| 20 | 16-epi-Kopsinine | K. fruticosa stem bark, K. officinalis stem, K. singapurensis leaf | 11, 31 and 32 |
| 21 | 16-epi-Kopsinilam | K. jasminiflora stem bark | 24 |
| 22 | 16-epi-17α-Hydroxy-Δ14,15-kopsinine | K. teoi stem bark and leaf | 33 |
| 23 | 14,15-β-Epoxykopsingine | K. teoi leaf | 34 |
| 24 | N(1)-Formylkopsininic acid | K. singapurensis root | 35 and 36 |
| 25 | N(1)-Formylkopsininic acid-N(4)-oxide | K. singapurensis root | 35 and 36 |
| 26 | Fruticosamine | K. fruticosa leaf, K. jasminiflora leaf | 20 and 37–41 |
| 27 | Fruticosiamine A | K. fruticosa leaf | 41 |
| 28 | Fruticosine | K. jasminiflora leaf, K. fruticosa leaf, K. officinalis twig | 20 and 37–42 |
| 29 | 11-Hydroxykopsilongine | K. officinalis fruit and leaf | 13 and 25 |
| 30 | 11-Hydroxykopsingine | K. teoi leaf | 34 |
| 31 | 5β-Hydroxykopsinine | K. jasminiflora stem bark | 24 |
| 32 | 15-Hydroxykopsamine | K. singapurensis root | 35 and 36 |
| 33 | 15α-Hydroxykopsinine | K. arborea stem bark; K. fruticosa leaf and stem bark, K. singapurensis bark | 10, 31 and 36 |
| 34 | 17α-Hydroxykopsinine | K. teoi stem bark | 43 |
| 35 | 17α-Hydroxy-Δ14,15-kopsinine | K. singapurensis stem bark and leaf; K. teoi stem and stem bark | 23, 32, 34 and 44–48 |
| 36 | Jasminiflorine | K. jasminiflora leaf | 40 |
| 37 | Kopsamidine A | K. arborea stem bark | 10 |
| 38 | Kopsamidine B | K. arborea stem bark | 10 |
| 39 | Kopsamine | K. arborea twig and stem bark, K. dasyrachis stem and leaf, K. officinalis stem, root, leaf and fruit, K. griffithii leaf, K. pauciflora stem and stem bark, K. singapurensis leaf and root, K. teoi stem bark | 10, 13–15, 17–19, 21, 25, 30, 36, 43, 49 and 50 |
| 40 | Kopsamine N-oxide | K. arborea stem bark; K. dasyrachis stem and leaf, K. officinalis fruit, K. griffithii leaf, K. pauciflora stem, K. singapurensis root | 10, 14, 15, 17–19, 30, 36, 49 and 51 |
| 41 | Kopsanone | K. arborea stem bark; K. fruticosa stem bark, K. jasminiflora stem bark, K. hainanensis stem bark, K. pauciflora stem and stem bark, K. officinalis fruit | 10, 14, 19, 22, 24, 29 and 31 |
| 42 | Kopsaporine | K. singapurensis stem bark, K. teoi stem and stem bark | 32, 34, 44 and 45 |
| 43 | Kopsiafrutine A | K. fruticosa aerial part | 52 |
| 44 | Kopsiafrutine B | K. fruticosa aerial part | 52 |
| 45 | Kopsiafrutine C | K. fruticosa aerial part | 52 |
| 46 | Kopsiafrutine D | K. fruticosa aerial part | 52 |
| 47 | Kopsiafrutine E | K. fruticosa aerial part | 52 |
| 48 | Kopsiahainanin A | K. hainanensis twig and leaf | 53 |
| 49 | Kopsiahainanin B | K. hainanensis twig and leaf | 53 |
| 50 | Kopsiahainanin C | K. hainanensis twig and leaf | 53 |
| 51 | Kopsiahainanin D | K. hainanensis twig and leaf | 53 |
| 52 | Kopsiahainanin E | K. hainanensis twig and leaf | 53 |
| 53 | Kopsiahainanin F | K. hainanensis twig and leaf | 53 |
| 54 | Kopsiahainin A | K. hainanensis twig and leaf | 54 |
| 55 | Kopsiahainin B | K. hainanensis twig and leaf | 54 |
| 56 | Kopsiahainin C | K. hainanensis twig and leaf | 54 |
| 57 | Kopsiahainin D | K. hainanensis twig and leaf | 54 |
| 58 | Kopsiahainin E | K. hainanensis twig and leaf | 54 |
| 59 | Kopsiaofficine A | K. officinalis aerial part | 55 |
| 60 | Kopsiaofficine B | K. officinalis aerial part | 55 |
| 61 | Kopsiaofficine C | K. officinalis aerial part | 55 |
| 62 | Kopsiarborines A | K. arborea aerial part | 56 |
| 63 | Kopsidarine | K. singapurensis leaf | 48 |
| 64 | Kopsidasine | K. dasyrachis leaf | 57 |
| 65 | Kopsidasine-N-oxide | K. dasyrachis leaf | 57 |
| 66 | Kopsidasinine | K. dasyrachis leaf | 57 |
| 67 | Kopsidine A | K. singapurensis leaf, K. teoi leaf and stem bark | 34, 43, 45, 48 and 58 |
| 68 | Kopsidine B | K. teoi leaf | 34, 45 and 58 |
| 69 | Kopsidine C | K. singapurensis leaf, K. teoi leaf | 34, 48 and 58 |
| 70 | Kopsidine C N-oxide | K. singapurensis leaf | 48 |
| 71 | Kopsidine D | K. singapurensis leaf, K. teoi leaf | 32, 34 and 58 |
| 72 | Kopsidine E | K. arborea bark | 59 |
| 73 | Kopsifine | K. arborea stem bark, K. dasyrachis stem, K. hainanensis twig, K. officinalis stem, K. pauciflora stem and stem bark, K. singapurensis root | 10–12, 18, 22, 49 and 60 |
| 74 | Kopsiflorine | K. arborea stem bark; K. dasyrachis stem, K. hainanensis stem and leaf, K. officinalis leaf | 7, 10, 12, 13, 18 and 61 |
| 75 | Kopsiflorine N(4)-oxide | K. dasyrachis stem | 18 |
| 76 | Kopsifoline A | K. fruticosa leaf and aerial part, K. singapurensis leaf | 31, 36, 52, 62 and 63 |
| 77 | Kopsifoline B | K. fruticosa leaf | 31, 62 and 63 |
| 78 | Kopsifoline C | K. fruticosa leaf | 31, 62 and 63 |
| 79 | Kopsifoline D | K. fruticosa leaf | 31 and 63 |
| 80 | Kopsifoline E | K. fruticosa leaf | 31 and 63 |
| 81 | Kopsifoline F | K. fruticosa leaf | 31 and 63 |
| 82 | Kopsifoline G | K. hainanensis stem | 64 |
| 83 | Kopsihainin B | K. hainanensis stem | 65 |
| 84 | Kopsihainin C | K. hainanensis stem | 65 |
| 85 | Kopsihainin D | K. hainanensis twig | 12 |
| 86 | Kopsihainin E | K. hainanensis twig | 12 |
| 87 | Kopsihainin F | K. hainanensis twig | 12 |
| 88 | Kopsijasminine | K. teoi stem bark | 43 |
| 89 | Kopsijasmine | K. jasminiflora leaf | 40 |
| 90 | Kopsilarutensinine | K. larutensis stem bark and leaf | 66 |
| 91 | Kopsilongine | K. arborea twig and stem bark, K. dasyrachis stem, K. griffithii leaf and stem bark, K. officinalis leaf, K. pauciflora stem | 10, 13, 15, 17–19, 21, 22 and 32 |
| K. singapurensis leaf | |||
| 92 | Kopsilongine-N-oxide | K. singapurensis leaf | 32 |
| 93 | Kopsiloscine A | K. singapurensis leaf | 32 |
| 94 | Kopsiloscine B | K. singapurensis leaf | 32 |
| 95 | Kopsiloscine C | K. singapurensis leaf and stem bark | 32 and 48 |
| 96 | Kopsiloscine D | K. singapurensis leaf | 32 |
| 97 | Kopsiloscine E | K. singapurensis leaf | 32 |
| 98 | Kopsiloscine F | K. singapurensis leaf | 32 |
| 99 | Kopsiloscine G | K. singapurensis stem bark and leaf | 23 and 48 |
| 100 | Kopsiloscine H | K. singapurensis stem bark | 23 |
| 101 | Kopsiloscine I | K. hainanensis stem and leaf, K. singapurensis stem bark | 7 and 23 |
| 102 | Kopsiloscine J | K. singapurensis leaf | 23 |
| 103 | Kopsimaline A | K. singapurensis leaf | 23 |
| 104 | Kopsimaline B | K. singapurensis leaf | 23 |
| 105 | Kopsimaline C | K. singapurensis leaf | 23 |
| 106 | Kopsimaline D | K. singapurensis leaf | 23 |
| 107 | Kopsimaline E | K. singapurensis leaf | 23 |
| 108 | Kopsimaline F | K. singapurensis leaf | 48 |
| 109 | Kopsinarine | K. dasyrachis stem, K. hainanensis twig | 12 and 18 |
| 110 | Kopsine | K. dasyrachis stem, K. fruticosa leaf | 18, 20, 38, 39, 41 and 67 |
| 111 | Kopsinganol | K. singapurensis stem bark, K. teoi stem, stem bark and leaf | 32, 34, 43, 45, 47 and 48 |
| 112 | Kopsingine | K. singapurensis leaf and stem bark, K. teoi stem, stem bark and leaf | 32–34, 44, 45 and 48 |
| 113 | Kopsinginine | K. teoi stem and stem bark | 34, 43–45 and 47 |
| 114 | Kopsinginol | K. teoi stem and stem bark | 34, 45 and 47 |
| 115 | Kopsinidine A | K. arborea stem bark | 10 |
| 116 | Kopsinidine B | K. arborea stem bark | 10 |
| 117 | Kopsininic acid (kopsinic acid) | K. hainanensis stem bark, K. jasminiflora stem bark, K. officinalis stem, twig and leaf, K. singapurensis bark and leaf | 11, 13, 16, 24, 29 and 36 |
| 118 | Kopsinicine | K. singapurensis leaf | 23 |
| 119 | Kopsinidine A | K. arborea stem bark, K. officinalis leaf | 10 and 25 |
| 120 | Kopsinidine B | K. arborea stem bark, K. officinalis leaf | 10 and 25 |
| 121 | Kopsinidine C | K. officinalis leaf and twig | 16 |
| 122 | Kopsinidine D | K. officinalis leaf and twig | 16 |
| 123 | Kopsinidine E | K. officinalis leaf and twig | 16 |
| 124 | Kopsinilam | K. hainanensis stem bark and twig, K. jasminiflora stem bark, K. officinalis stem, twig, leaf and fruit | 11, 12, 14, 16, 24 and 29 |
| 125 | Kopisininate | K. hainanensis stem and leaf | 7 |
| 126 | Kopsinine | K. arborea twig and stem bark, K. dasyrachis stem, K. fruticosa stem bark, K. jasminiflora stem bark, K. grandifolia stem bark, K. griffithii leaf and stem bark, K. hainanensis leaf, stem, stem bark and twig, K. larutensis stem, stem bark and leaf, K. officinalis root, stem, twig, leaf and fruit, K. singapurensis stem bark and leaf, K. pauciflora stem, stem bark and leaf, K. teoi stem bark | 7, 9–11, 13–19, 21–25, 28, 29, 32, 36, 42, 43, 48, 50, 51, 64–66 and 68–72 |
| 127 | Kopsinine-N(4)-oxide | K. dasyrachis stem, K. griffithii stem bark, K. hainanensis stem and leaf, K. officinalis fruit and leaf, K. pauciflora stem, K. singapurensis bark | 7, 13, 15, 18, 25 and 36 |
| 128 | Kopsinine methochloride | K. officinalis leaf and twig | 16 |
| 129 | Kopsinine B | K. officinalis leaf and twig | 16 |
| 130 | Kopsinine F | K. hainanensis stem and leaf | 7 |
| 131 | Kopsinitarine A | K. singapurensis leaf, K. teoi leaf | 34, 48, 73 and 74 |
| 132 | Kopsinitarine B | K. singapurensis leaf, K. teoi leaf | 34, 48, 73 and 74 |
| 133 | Kopsinitarine C | K. teoi leaf | 34, 73 and 74 |
| 134 | Kopsinitarine D | K. teoi leaf | 34 and 74 |
| 135 | Kopsinitarine E | K. teoi stem bark | 43 |
| 136 | Kopsinol | K. teoi stem and stem bark | 34, 45 and 47 |
| 137 | (−)-Kopsinoline | K. hainanensis stem bark, K. officinalis stem, twig and leaf | 11, 16 and 29 |
| 138 | Kopsiofficine A | K. officinalis stem | 11 |
| 139 | Kopsiofficine B | K. officinalis stem | 11 |
| 140 | Kopsiofficine C | K. officinalis stem | 11 |
| 141 | Kopsiofficine D | K. officinalis stem | 11 |
| 142 | Kopsiofficine E | K. officinalis stem | 11 |
| 143 | Kopsiofficine F | K. officinalis stem | 11 |
| 144 | Kopsiofficine L | K. officinalis stem | 75 |
| 145 | Kopsofinone | K. singapurensis leaf | 23 |
| 146 | Kopsonoline | K. teoi stem bark | 43 |
| 147 | Kopsorinine | K. fruticosa leaf and stem bark | 31 |
| 148 | Lahadinine A | K. pauciflora leaf | 76 |
| 149 | Lahadinine B | K. pauciflora leaf | 76 |
| 150 | Mersingine A | K. singapurensis leaf, K. teoi leaf | 34, 49 and 74 |
| 151 | Mersingine B | K. teoi leaf | 34 and 74 |
| 152 | N(1)-Methoxycarbonyl-11,12-dimethoxykopsinaline | K. arborea stem bark, K. pauciflora stem | 10, 19 and 51 |
| 153 | N(1)-Methoxycarbonyl-11,12-methoxylenedioxykopsinaline | K. officinalis leaf, twig, stem and root, K. pauciflora stem and leaf | 11, 16, 42, 51, 69 and 76 |
| 154 | N(1)-Methoxycarbonyl-11,12-methylenedioxy-Δ16,17-kopsinine | K. profunda stem | 4 |
| 155 | N(1)-Methoxycarbonyl-12-methoxy-Δ16,17-kopsinine | K. griffithii leaf, K. pauciflora stem, K. profunda stem and leaf, K. teoi stem bark | 4, 17, 19, 43, 51 and 77 |
| 156 | N(1)-Methoxycarbonyl-12-methoxykopsinaline | K. officinalis root, stem, twig, leaf and fruit, K. pauciflora stem | 11, 16, 25, 51 and 69 |
| 157 | N(1)-Methoxycarbonyl-11,12-methylenedioxy-Δ16,17-kopsinine N(4) oxide | K. profunda stem and leaf | 77 |
| 158 | N(1)-Methoxycarbonyl-12-hydroxy-Δ16,17-kopsinine | K. pauciflora stem, K. profunda stem and leaf | 19 and 77 |
| 159 | N(1)-Methoxycarbonyl-12-methoxy-Δ16,17-kopsinine N(4) oxide | K. profunda stem and leaf | 77 |
| 160 | 11-Methoxykopsingine | K. teoi leaf | 34 |
| 161 | 11-Methoxykopsilongine | K. dasyrachis stem, K. officinalis stem and leaf | 11, 13 and 18 |
| 162 | 11-Methoxykopsilongine N(4)-oxide | K. dasyrachis stem | 18 |
| 163 | 11-Methoxy-12-hydroxy-kopsinol | K. teoi leaf | 34 |
| 164 | 12-Methoxykopsidasinine | K. griffithii leaf | 17 |
| 165 | (−)-12-Methoxykopsinaline | K. officinalis leaf and twig | 13, 16, 42 and 69 |
| 166 | 12-Methoxykopsine | K. arborea leaf, K. jasminiflora stem bark, K. officinalis root and stem, K. pauciflora leaf | 11, 22, 24 and 78 |
| 167 | 12-Methoxy-10-demethoxykopsidasinine | K. griffithii leaf, K. pauciflora stem | 15, 51 |
| 168 | 12-Methoxypleiocarpine | K. dasyrachis stem and leaf, K. hainanensis stem and leaf, K. griffithii leaf, K. pauciflora stem | 7, 15, 17–19 and 30 |
| 169 | (−)-Methylenedioxy-11,12-kopsinaline | K. arborea twig | 7 |
| 170 | N(4)-Methylkopsininate | K. officinalis leaf and twig | 16 |
| 171 | 11,12-Methylenedioxykopsaporine | K. singapurensis bark, K. teoi stem, stem bark and leaf | 33, 34 and 79 |
| 172 | (−)-11,12-Methylenedioxykopsinaline | K. dasyrachis stem, K. officinalis root, stem, leaf, twig and fruit | 11, 16, 18, 25 and 69 |
| 173 | 11,12-Methylenedioxykopsinaline N(4)-oxide | K. griffithii stem bark, K. officinalis stem, twig and leaf | 11, 15 and 16 |
| 174 | 11,12-Methylenedioxykopsine | K. arborea stem bark, K. dasyrachis stem, K. officinalis stem, K. pauciflora stem bark | 10, 11, 18 and 22 |
| 175 | Nitaphylline | K. teoi leaf | 34, 46 and 80 |
| 176 | 5-Oxokopsinic acid | K. jasminiflora stem bark, K. officinalis twig and leaf | 16 and 24 |
| 177 | Paucidactine A | K. pauciflora stem bark | 19 |
| 178 | Paucidactine B | K. arborea stem bark, K. pauciflora stem bark | 10 and 19 |
| 179 | Paucidactine C | K. arborea stem bark, K. pauciflora stem bark | 10 and 19 |
| 180 | Paucidactine D | K. pauciflora stem bark | 19 |
| 181 | Paucidactine E | K. pauciflora stem bark | 19 |
| 182 | Paucidactinine | K. pauciflora stem bark | 19 |
| 183 | Paucidisine | K. pauciflora stem bark | 19 |
| 184 | Paucidirinine | K. pauciflora stem bark | 19 |
| 185 | Paucidirisine | K. pauciflora stem bark | 19 |
| 186 | Pauciduridine | K. officinalis stem, K. pauciflora stem bark | 11 and 19 |
| 187 | Paucifinine | K. pauciflora leaf and stem bark | 22 and 76 |
| 188 | Paucifinine-N-oxide | K. pauciflora leaf | 76 |
| 189 | Pleiocarpine | K. arborea stem bark, K. dasyrachis stem and leaf, K. griffithii leaf, K. officinalis fruit, K. pauciflora stem, | 10, 14, 15, 17–19, 25 and 30 |
| 190 | Pleiocarpine N-oxide | K. pauciflora stem | 19 |
| 191 | Pseudokopsinine | K. pauciflora leaf and stem bark | 22 |
| 192 | 5,6-Secokopsinine | K. jasminiflora stem bark | 24 |
| 193 | Singaporentine A | K. singapurensis leaf | 36 |
| 194 | Singapurensine A | K. singapurensis bark | 79 |
| 195 | Singapurensine B | K. singapurensis bark | 79 |
| 196 | Singapurensine C | K. singapurensis bark | 79 |
| 197 | Singapurensine D | K. singapurensis bark | 79 |
| 198 | Venacarpine A | K. fruticosa leaf, K. singapurensis bark | 31 and 36 |
| 199 | Venacarpine B | K. fruticosa leaf | 31 |
| 200 | Venalstonidine | K. arborea stem bark | 10 |
| 201 | (−)-Venalstonine | K. arborea stem bark, K. fruticosa stem bark, K. lapidilecta stem and bark, K. singapurensis bark | 10, 31, 36 and 81 |
| 202 | Yunnanoffine A | K. officinalis leaf | 25 |
| 203 | Yunnanoffine B | K. officinalis leaf | 25 |
| 204 | Yunnanoffine D | K. officinalis leaf | 25 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Chanofruticosinates | |||
| 205 | Chanofruticosinic acid | K. officinalis leaf and twig | 16 |
| 206 | N1-Decarbomethoxy chanofruticosinic acid | K. hainanensis stem and leaf | 7 |
| 207 | 11,12-Dimethoxydanuphylline | K. fruticosa aerial part | 3 |
| 208 | Flavisiamine A (prunifoline D) | K. arborea leaf, K. flavida leaf | 82–84 |
| 209 | Flavisiamine B | K. flavida leaf | 83 |
| 210 | Flavisiamine C | K. arborea leaf, K. flavida leaf | 83 and 84 |
| 211 | Flavisiamine D (prunifoline E) | K. arborea leaf and stem bark, K. flavida leaf | 10 and 82–84 |
| 212 | Flavisiamine E | K. flavida leaf | 41 |
| 213 | Flavisiamine F | K. flavida leaf | 41 |
| 214 | 12-Hydroxylprunifoline A | K. lancibracteolata stem | 85 |
| 215 | 12-Hydroxylprunifoline C | K. lancibracteolata stem | 85 |
| 216 | Kopreasin A | K. arborea leaf | 84 |
| 217 | Kopsia A (methyl chanofruticosinate) | K. dasyrachis leaf, K. hainanensis stem and leaf, K. officinalis leaf, twig, and stem, K. pauciflora leaf | 7, 13, 16, 22, 25, 30, 75 and 86 |
| 218 | Kopsia B (des-N-(methoxycarbonyl)chanofruticosin-methyleste) | K. officinalis leaf | 86 |
| 219 | Kopsia C (6,7-methylendioxychanofruticosin-methylester or methyl 11,12-methylenedioxychanofruticosinate) | K. arborea leaf and stem bark, K. dasyrachis leaf, K. officinalis stem and leaf, twig and leaf, K. pauciflora stem bark and leaf | 10, 16, 22, 30, 75, 84, 86 and 87 |
| 220 | Kopsihainanine A | K. hainanensis leaf and stem | 6 |
| 221 | Kopsihainanine B | K. hainanensis leaf and stem | 6 |
| 222 | 12-Methoxychanofruticosinic acid | K. officinalis leaf and twig | 16 |
| 223 | Methyl chanofruticosinate N(4)-oxide | K. hainanensis stem and leaf | 7 |
| 224 | Methyl 11,12-dimethoxychanofruticosinate | K. arborea leaf, K. flavida leaf, K. officinalis leaf | 13, 22, 25, 82, 88 and 89 |
| 225 | Methyl N1-decarbomethoxychanofruticosinate | K. arborea leaf and stem bark, K. dasyrachis leaf, K. flavida leaf, K. fruticosa leaf, K. hainanensis stem and leaf, K. officinalis twig, leaf and stem, K. pauciflora leaf | 7, 10, 16, 25, 30, 41, 42, 65, 75, 82–84 and 87 |
| 226 | Methyl N1-decarbomethoxy chanofruticosinate N(4)-oxide | K. hainanensis stem and leaf | 7 |
| 227 | Methyl 12-methoxy-N1-decarbomethoxychanofruticosinate | K. arborea leaf, K. flavida leaf | 83, 84, 88 and 89 |
| 228 | Methyl 12-methoxychanofruticosinate | K. arborea leaf and stem bark, K. flavida leaf, K. officinalis stem, twig and leaf, K. pauciflora leaf | 10, 16, 22, 75, 82, 84, 88 and 89 |
| 229 | Methyl 11,12-methylenedioxy-N1-decarbomethoxychanofruticosinate | K. arborea stem bark and leaf, K. dasyrachis leaf, K. flavida leaf, K. officinalis leaf, twig and stem, K. pauciflora leaf and stem bark | 10, 16, 22, 25, 30, 42, 75, 82–84 and 87–89 |
| 230 | Methyl 11,12-methylenedioxy-N1-decarbomethoxy-Δ14,15-chanofruticosinate | K. arborea stem bark and leaf, K. flavida leaf, K. hainanensis stem and leaf | 7, 10, 82–84 and 87 |
| 231 | Methyl (2β,11β,12β,19α)-6,7-didehydro-8,21-dioxo-11,21-cycloaspidospermidine-2-carboxylate | K. officinalis leaf | 13 |
| 232 | Methyl 3-oxo-12-methoxy-N1-decarbomethoxy-14,15-didehydrochanofruticosinate | K. flavida leaf | 89 |
| 233 | Methyl 3-oxo-11,12-methylenedioxy-N1-decarbomethoxy-14,15-didehydrochanofruticosinate | K. flavida leaf | 89 |
| 234 | Δ1,2-Methyldemethoxycarbonylchanofruticosinate | K. officinalis leaf | 25 |
| 235 | 11,12-Methylenedioxychanofruticosinic acid | K. officinalis leaf and twig | 16 |
| 236 | 3-Oxo-11,12-dimethoxy-N1-decarbomethoxy-14,15-didehydrochanofruticosinate | K. fruticosa aerial part | 3 |
| 237 | N(4)-Oxide prunifoline D | K. lancibracteolata stem | 85 |
| 238 | Prunifoline A | K. arborea leaf | 82 |
| 239 | Prunifoline B | K. arborea leaf | 82 and 84 |
| 240 | Prunifoline C | K. arborea leaf, K. fruticosa leaf | 41 and 82 |
| 241 | Prunifoline F | K. arborea leaf | 82 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Aspidospermines | |||
| 242 | Aspidospermine | K. pauciflora leaf | 22 |
| 243 | (+)-1,2-Dehydroaspidospermine | K. pauciflora leaf | 22 |
| 244 | Eburenine | K. arborea aerial part | 90 |
| 245 | Kopsiofficine G | K. officinalis stem | 11 |
| 246 | Kopsiyunnanine G | K. arborea aerial part | 90 |
| 247 | Vincadifformine | K. arborea twig and stem bark, K. officinalis stem and fruit | 10, 11, 14 and 21 |
| 248 | Vincadifformine N(4)-oxide | K. officinalis stem | 11 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Danuphyllines | |||
| 249 | Danuphylline | K. dasyrachis leaf | 30 and 91 |
| 250 | Danuphylline B | K. arborea leaf | 78 |
| 251 | 11,12-De(methylenedioxy)danuphylline | K. officinalis leaf | 13 |
| 252 | Kopsihainin A | K. hainanensis stem | 65 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Eburnamines | |||
| 253 | (−)-Demethylnorpleiomutine | K. dasyrachis stem, K. pauciflora stem | 18 and 19 |
| 254 | (+)-Eburnamenine | K. pauciflora stem and stem bark | 19 and 22 |
| 255 | (−)-Eburnamenine | K. arborea aerial part, K. hainanensis twig, stem bark and leaf, K. larutensis bark, K. officinalis fruit | 9, 14, 29, 90 and 70 |
| 256 | (+)-Eburnamine | K. hainanensis stem bark | 29 |
| 257 | (−)-Eburnamine | K. arborea aerial part, K. griffithii stem bark, K. hainanensis twig and leaf, K. larutensis leaf, stem and stem bark, K. officinalis root and stem, K. pauciflora stem and stem bark, K. singapurensis stem bark, K. terengganensis bark | 5, 9, 15, 19, 22, 23, 50, 51, 66, 68, 70, 90 and 92 |
| 258 | (−)-Eburnaminol | K. larutensis stem bark, K. terengganensis bark | 68 and 92 |
| 259 | (+)-Eburnamonine | K. arborea aerial part, K. dasyrachis stem, K. griffithii leaf, K. larutensis leaf and stem bark, K. officinalis leaf and twig, K. pauciflora stem | 5, 13, 15, 17–19, 42, 51, 68, 70, 90 and 93 |
| 260 | (+)-Eburnamonine N(4)-oxide | K. larutensis leaf and stem | 5 and 70 |
| 261 | (−)-Eburnamonine | K. jasminiflora stem bark | 24 |
| 262 | (−)-O-Ethyleburnamine | K. arborea aerial part, K. larutensis stem | 70 and 90 |
| 263 | (+)-Ethylisoeburnamine | K. arborea aerial part | 90 |
| 264 | 16α-Hydroxy-19-oxoeburnamine | K. officinalis leaf | 25 |
| 265 | 16β-Hydroxy-19-oxoeburnamine | K. officinalis leaf | 25 |
| 266 | (+)-19(R)-Hydroxyeburnamine | K. dasyrachis stem | 18 and 93 |
| 267 | 19-Hydroxy-(−)-eburnamonine | K. arborea twig, K. larutensis leaf, K. officinalis twig | 5, 7 and 42 |
| 268 | (−)-19(R)-Hydroxyisoeburnamine | K. dasyrachis stem, K. pauciflora stem and stem bark | 19, 22 and 93 |
| 269 | (+)-(19R)-19-Hydroxyeburnamine | K. officinalis leaf, K. pauciflora stem and stem bark | 13, 19 and 22 |
| 270 | (−)-19(R)-Hydroxyeburnamenine | K. pauciflora stem | 19 |
| 271 | (−)-(19R)-19-Hydroxyisoeburnamine | K. dasyrachis stem, K. officinalis leaf | 13 and 18 |
| 272 | (−)-19(R)-Hydroxy-O-ethylisoeburnamine | K. pauciflora stem | 19 |
| 273 | 19(S)-Hydroxy-Δ14-vicamone | K. jasminiflora stem bark | 24 |
| 274 | (+)-Isoeburnamine | K. arborea aerial part, K. dasyrachis stem, K. hainanensis stem bark, K. larutensis leaf, stem and stem bark, K. teoi stem bark and leaf, K. officinalis leaf, K. pauciflora stem and stem bark, K. terengganensis bark | 5, 13, 18, 19, 22, 33, 29, 51, 68, 70, 90, 92 and 93 |
| 275 | (−)-Isoeburnamine | K. officinalis root | 28 and 69 |
| 276 | 16-Isoeburnamine ((+)-methylisoeburnamine) | K. arborea aerial part, K. officinalis stem | 75 and 90 |
| 277 | (+)-Kopsoffine | K. hainanensis, K. officinalis root | 28 and 29 |
| 278 | Kopsiofficine H | K. officinalis stem | 75 |
| 279 | Kopsiofficine I | K. officinalis stem | 75 |
| 280 | Kopsiofficine J | K. officinalis stem | 75 |
| 281 | Kopsiofficine K | K. officinalis stem | 75 |
| 282 | Kopsoffinol | K. dasyrachis stem, K. pauciflora stem | 19 and 93 |
| 283 | (+)-Larutensine | K. larutensis stem bark | 68 |
| 284 | Larutenine | K. larutensis leaf and stem, K. officinalis leaf, K. pauciflora leaf, K. terengganensis bark | 5, 13, 22, 70 and 92 |
| 285 | Larutenine A | K. pauciflora stem, stem bark and leaf | 19 and 22 |
| 286 | Larutenine B | K. pauciflora stem and stem bark | 19 and 22 |
| 287 | Melohenine B | K. hainanensis twig and leaf | 9 |
| 288 | (−)-Methyleburnamine | K. arborea aerial part | 90 |
| 289 | (−)-Norpleiomutine | K. dasyrachis stem, K. macrophylla bark, K. pauciflora stem and stem bark | 18, 19, 22, 27 and 51 |
| 290 | (+)-O-Methyleburnamine | K. officinalis stem | 75 |
| 291 | (−)-O-Methylisoeburnamine (O-methylvincanol) | K. hainanensis twig and leaf, K. officinalis stem | 9 and 75 |
| 292 | (+)-19-Oxoeburnamine | K. pauciflora stem and stem bark | 19, 22 and 51 |
| 293 | 19-Oxo-(−)-eburnamonine | K. jasminiflora stem bark, K. officinalis twig | 24 and 42 |
| 294 | (−)-19-Oxoisoeburnamine | K. pauciflora stem | 19 |
| 295 | O-Methyl-16-epi-vincanol | K. hainanensis twig and leaf | 9 |
| 296 | 20-Oxo-eburnamenine | K. officinalis root, leaf and stem | 25, 50 and 75 |
| 297 | Phutdonginin | K. arborea twig | 21 |
| 298 | Terengganensine A | K. terengganensis bark | 92 |
| 299 | Terengganensine B | K. terengganensis bark | 92 |
| 300 | Δ14-Vicamone | K. jasminiflora stem bark | 24 |
| 301 | Yunnanoffine C | K. officinalis leaf | 25 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Akuammilines | |||
| 302 | Akuammidine | K. arborea stem bark, K. singapurensis root, stem bark and leaf | 10, 23, 32, 48 and 49 |
| 303 | Akuammiline | K. macrophylla bark, K. teoi stem and stem bark | 27, 34, 43, 45 and 47 |
| 304 | Akuammiline N(4)-oxide | K. griffithii stem bark | 15 |
| 305 | ψ-Akuammigine | K. fruticosa stem bark | 31 |
| 306 | Deacetylakuammiline (rhazimol) | K. deverrei stem bark, K. griffithii leaf and stem bark, K. macrophylla bark, K. singapurensis stem bark, K. teoi stem and stem bark | 15, 17, 23, 27, 34, 45, 47 and 94 |
| 307 | Dregamine | K. macrophylla bark | 27 |
| 308 | 16-epi-akuammiline | K. singapurensis leaf, stem bark and root, K. teoi stem bark | 23, 32, 36, 43 and 48 |
| 309 | 16-epi-deacetylakuammiline | K. deverrei stem bark, K. griffithii stem bark, K. fruticosa stem bark, K. singapurensis bark, stem bark and leaf, K. teoi stem and stem bark | 15, 23, 31, 32, 34, 36, 43, 48 and 94 |
| 310 | 16-epi-deacetylakuammiline-N(4)-oxide | K. griffithii stem bark, K. singapurensis bark | 15 and 36 |
| 311 | 16-Hydroxymethyl-pleiocarpamine | K. deverrei stem bark, K. fruticosa stem bark, K. singapurensis stem bark and bark, K. teoi stem bark | 23, 31, 43, 36 and 94 |
| 312 | N-Methylpleiocarpamine | K. singapurensis root | 36 |
| 313 | 5-Methoxystrictamine | K. hainanensis twig and leaf | 9 |
| 314 | Rhazimal | K. arborea stem bark | 10 |
| 315 | Rhazinaline N(4)-oxide | K. griffithii stem bark | 15 |
| 316 | Rhazinoline | K. arborea stem bark | 10 |
| 317 | Picralinal | K. hainanensis twig and leaf | 9 |
| 318 | Picramicine | K. fruticosa stem bark, K. singapurensis stem bark | 23 and 31 |
| 319 | Pleiocarpamine | K. dasyrachis stem, K. deverrei stem bark, K. fruticosa stem bark, K. singapurensis bark, K. teoi stem bark | 18, 31, 36, 43 and 94 |
| 320 | Pleiocarpamine methochloride | K. officinalis leaf and twig | 16 |
| 321 | Pleiomalicine | K. hainanensis twig and leaf | 9 |
| 322 | Singaporentinidine | K. singapurensis root | 35 and 36 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Sarpagines | |||
| 323 | 10-Hydroxy-vincadiffine | K. hainanensis twig and leaf | 9 |
| 324 | Perivine | K. officinalis root and stem | 50 |
| 325 | Tabernaemontanine | K. macrophylla bark | 27 |
| 326 | Vincadiffine | K. hainanensis twig and leaf | 9 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Aspidophyllines | |||
| 327 | Aspidodasycarpine | K. singapurensis root and stem bark, K. teoi stem and stem bark | 23, 32, 34, 36, 43, 48 and 49 |
| 328 | Aspidophylline A | K. singapurensis stem bark | 32 |
| 329 | Aspidophylline B | K. singapurensis stem bark | 48 |
| 330 | Lonicerine | K. fruticosa stem bark, K. singapurensis bark and stem bark, K. teoi stem, stem bark and leaf | 23, 31–34, 36, 43 and 48 |
| 331 | Vincophylline | K. singapurensis leaf | 32 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Strychnoses | |||
| 332 | Akuammicine | K. pauciflora leaf | 22 |
| 333 | Arbolodinine B | K. arborea stem bark | 8 |
| 334 | Arbolodinine C | K. arborea stem bark | 8 |
| 335 | (E)-Condylocarpine | K. arborea aerial part, K. pauciflora leaf | 22 and 95 |
| 336 | (E)-Condylocarpine N-oxide | K. arborea aerial part | 95 |
| 337 | 14α-Hydroxycondylocarpine | K. deverri stem bark, K. singapurensis stem bark | 23 and 94 |
| 338 | 14α-Hydroxy-N(4)-methylcondylocarpine | K. singapurensis root | 35 and 36 |
| 339 | 14(S)-Hydroxy-19(R)-methoxytubotaiwine | K. jasminiflora stem bark | 24 |
| 340 | Isocondylocarpine | K. arborea aerial part | 95 |
| 341 | Isocondylocarpine N-oxide | K. arborea aerial part | 95 |
| 342 | Kopsiyunnanine A | K. arborea aerial part, K. officinalis aerial part | 96 and 97 |
| 343 | Kopsiyunnanine I | K. arborea aerial part | 98 and 99 |
| 344 | Kopsiyunnanine J1 | K. arborea aerial part | 99 and 100 |
| 345 | Kopsiyunnanine J2 | K. arborea aerial part | 99 and 100 |
| 346 | Kopsiyunnanine L | K. arborea aerial part | 101 and 102 |
| 347 | Kopsiyunnanine M | K. arborea aerial part | 101 and 102 |
| 348 | Kopsiyunnanine F1 | K. arborea aerial part | 95 |
| 349 | Kopsiyunnanine F2 | K. arborea aerial part | 95 |
| 350 | Kopsiyunnanine F3 | K. arborea aerial part | 95 |
| 351 | Leuconicine B | K. arborea aerial part | 98 |
| 352 | 19(R)-Methoxytubotaiwine | K. arborea aerial part and stem bark, K. jasminiflora stem bark | 10, 24 and 95 |
| 353 | 19(S)-Methoxytubotaiwine | K. arborea aerial part and stem bark, K. hainanensis twig | 10, 12 and 95 |
| 354 | Mossambine | K. singapurensis stem bark | 23 |
| 355 | Precondylocarpine | K. pauciflora leaf | 22 |
| 356 | Tubotaiwine | K. arborea aerial part, K. hainanensis stem and stem bark | 29, 64 and 95 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Stemmadenine | |||
| 357 | Stemmadenine | K. pauciflora leaf | 22 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Mersinines | |||
| 358 | Mersidasine A | K. singapurensis leaf | 103 |
| 359 | Mersidasine B | K. singapurensis leaf | 103 |
| 360 | Mersidasine C | K. singapurensis leaf | 103 |
| 361 | Mersidasine D | K. singapurensis leaf | 103 |
| 362 | Mersidasine E | K. singapurensis leaf | 103 |
| 363 | Mersidasine F | K. singapurensis leaf | 103 |
| 364 | Mersidasine G | K. singapurensis leaf | 103 |
| 365 | Mersifoline A | K. singapurensis leaf | 103 |
| 366 | Mersifoline B | K. singapurensis leaf | 103 |
| 367 | Mersifoline C | K. singapurensis leaf | 103 |
| 368 | Mersilongine | K. singapurensis leaf | 23 and 104 |
| 369 | Mersiloscine | K. singapurensis leaf | 103 and 105 |
| 370 | Mersiloscine A | K. singapurensis leaf | 103 |
| 371 | Mersiloscine B | K. singapurensis leaf | 103 |
| 372 | Mersinaline | K. singapurensis leaf | 23 and 106 |
| 373 | Mersinine A | K. fruticosa leaf, K. singapurensis leaf | 103 and 105, 107 |
| 374 | Mersinine B | K. singapurensis leaf | 103 and 105 |
| 375 | Mersinine C | K. singapurensis leaf | 103 |
| 376 | Mersiphyllines A | K. singapurensis leaf | 108 |
| 377 | Mersiphyllines B | K. singapurensis leaf | 108 |
| 378 | Mersirachine | K. singapurensis leaf | 23 and 106 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Pauciflorines | |||
| 379 | 11,12-Demethoxy-16-deoxypauciflorine | K. officinalis stem and leaf | 109 |
| 380 | 20-Deoxykopsijasminilam | K. jasminiflora leaf | 40 |
| 381 | Kopsiarborines C | K. arborea aerial part | 56 |
| 382 | Kopsijasminilam | K. jasminiflora leaf | 40 |
| 383 | Δ14-Kopsijasminilam | K. jasminiflora leaf | 40 |
| 384 | Kopsioffine A | K. officinalis stem and leaf | 109 |
| 385 | Kopsioffine B | K. officinalis stem and leaf | 109 |
| 386 | Kopsioffine C | K. officinalis stem and leaf | 109 |
| 387 | Pauciflorine A | K. pauciflora leaf | 110 |
| 388 | Pauciflorine B | K. pauciflora leaf | 110 |
| 389 | Pauciflorine C | K. pauciflora leaf | 22 |
| 390 | Paucifoline | K. pauciflora leaf | 22 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Skytanthines | |||
| 391 | Kinabalurine A (kinabalurine) | K. pauciflora leaf | 111 and 112 |
| 392 | Kinabalurine B | K. pauciflora leaf | 112 |
| 393 | Kinabalurine C | K. pauciflora leaf | 112 |
| 394 | Kinabalurine D | K. pauciflora leaf | 112 |
| 395 | Kinabalurine E | K. pauciflora leaf | 112 |
| 396 | Kinabalurine F | K. pauciflora leaf | 112 |
| 397 | Kinabalurine G | K. dasyrachis leaf | 30 |
| 398 | Kopsilactone | K. macrophylla bark | 27 |
| 399 | Kopsirachine | K. dasyrachis leaf | 30 and 113 |
| 400 | Kopsone | K. macrophylla bark | 27 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Rhazinilams | |||
| 401 | 5,21-Dihydrorhazinilam | K. arborea stem bark, K. singapurensis stem bark and leaf | 10, 23 and 48 |
| 402 | Kopsiyunnanine C1 | K. arborea aerial part, K. officinalis aerial part | 96 and 114 |
| 403 | Kopsiyunnanine C2 | K. arborea aerial part, K. officinalis aerial part | 96 and 114 |
| 404 | Kopsiyunnanine C3 | K. arborea aerial part, K. officinalis aerial part | 96 and 114 |
| 405 | Leuconolam | K. griffithii leaf and stem bark, K. hainanensis twigs, stems and leaves, K. officinalis leaf, K. pauciflora leaf, K. singapurensis stem bark | 7, 9, 12, 15, 17, 22, 23, 25 and 32 |
| 406 | O-Methylleuconolam | K. arborea stem bark, K. hainanensis twig, K. officinalis stem | 10, 12 and 87 |
| 407 | Rhazinal | K. dasyrachis stem | 32 |
| 408 | Rhazinicine | K. arborea stem bark, K. dasyrachis stem, K. singapurensis root | 10, 18, 49 and 60 |
| 409 | Rhazinilam | K. arborea aerial part and stem bark, K. officinalis leaves and twigs, K. pauciflora leaves and stem bark, K. singapurensis leaf, bark and stem bark, K. teoi stem, stem bark and leaf | 16, 13, 22, 23, 25, 32–34, 36, 45, 47, 48 and 114 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Lundurines | |||
| 410 | Epilapidilectinol | K. lapidilecta stem and bark | 81 |
| 411 | Grandilodine A | K. grandifolia stem bark | 72 |
| 412 | Grandilodine B | K. grandifolia stem bark | 72 |
| 413 | Grandilodine C | K. grandifolia leaf | 72 |
| 414 | Isolapidilectine A | K. grandifolia leaf, K. lapidilecta stem and bark | 72 and 81 |
| 415 | Lapidilectam | K. grandifolia stem bark, K. lapidilecta stem and bark | 72 and 81 |
| 416 | Lapidilectine A | K. grandifolia stem bark, K. lapidilecta bark, stem and leaf | 72 and 115 |
| 417 | Lapidilectine B | K. grandifolia stem bark, K. lapidilecta bark, stem and leaf | 72 and 115 |
| 418 | Lapidilectinol | K. lapidilecta stem and bark | 81 |
| 419 | Lundurine A | K. tenuis leaf | 71 |
| 420 | Lundurine B | K. tenuis leaf | 71 |
| 421 | Lundurine C | K. tenuis leaf | 71 |
| 422 | Lundurine D | K. tenuis leaf | 71 |
| 423 | Tenuisine A | K. tenuis leaf | 116 and 117 |
| 424 | Tenuisine B | K. tenuis leaf | 71, 116 and 117 |
| 425 | Tenuisine C | K. tenuis leaf | 71, 116 and 117 |
| 426 | Tenuiphylline | K. tenuis leaf | 71 and 117 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Aspidospermas | |||
| 427 | Buchtienine | K. griffithii leaf and stem bark | 15 and 17 |
| 428 | Corynantheol | K. hainanensis twig and leaf | 9 |
| 429 | 19,20-Dihydroisositsirikine | K. officinalis stem | 75 |
| 430 | Dihydrocorynantheol | K. hainanensis twig and leaf | 9 |
| 431 | 16(R)-19,20-E-Isositsirikine | K. griffithii leaf, K. pauciflora leaf | 15, 17 and 22 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Catharinensines | |||
| 432 | Catharinensine | K. pauciflora leaf | 22 |
| 433 | Kopsirensine A | K. pauciflora leaf | 22 |
| 434 | Kopsirensine B | K. pauciflora leaf | 22 |
| 435 | Kopsirensine C | K. pauciflora leaf | 22 |
| 436 | Kopsiyunnanine B | K. arborea aerial part, K. officinalis aerial part | 96 and 97 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Leuconoxines | |||
| 437 | Arboloscine | K. arborea stem bark | 10 and 118 |
| 438 | Arboloscine A | K. pauciflora leaf | 22 |
| 439 | Leuconodine D | K. officinalis stem | 75 |
| 440 | Leuconodine F (6-oxoleuconoxine) | K. griffithii leaf, K. pauciflora leaf | 22 and 43 |
| 441 | Leuconoxine | K. arborea stem bark, K. griffithii leaf and stem bark, K. pauciflora stem, stem bark and leaf, K. singapurensis stem bark, K. teoi stem bark | 15, 17, 19, 22, 23 and 43 |
| 442 | Melodinine E | K. arborea twig | 21 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Pericines | |||
| 443 | Pericidine | K. arborea stem bark | 10 and 118 |
| 444 | Pericine | K. arborea stem bark | 10 |
| 445 | Pericine N-oxide | K. arborea stem bark | 10 |
| 446 | Valparicine | K. arborea stem bark | 119 and 120 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Alstonines | |||
| 447 | Oxayohimban-16-carboxy acid | K. officinalis stem | 75 |
| 448 | (−)-Tetrahydroalstonine | K. arborea stem bark, K. dasyrachis stem, K. griffithii leaf, K. officinalis root, twigs and leaves, K. larutensis stem bark and leaf, K. pauciflora stem, stem bark and leaf, K. singapurensis stem bark; K. teoi stem bark | 10, 15, 17–19, 23, 25, 32, 42, 43, 66 and 69 |
| 449 | Tetrahydroalstonine pseudoindoxyl | K. pauciflora leaf | 22 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Quebrachamines | |||
| 450 | Kopsiyunnanine D | K. arborea aerial part | 114 |
| 451 | Kopsiyunnanine H | K. arborea aerial part | 90 |
| 452 | (−)-Quebrachamine | K. arborea aerial part, K. hainanensis twig and leaf, K. officinalis root, K. pauciflora leaf | 9, 22, 69 and 114 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Arbophyllinines | |||
| 453 | Arbophyllinine A | K. arborea bark | 59 |
| 454 | Arbophyllinine B | K. arborea bark | 59 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Arboflorines | |||
| 455 | Arboflorine | K. arborea stem bark | 10 |
| 456 | Kopsiyunnanine E | K. arborea aerial part, K. officinalis aerial part | 96, 99 and 121 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Andrasinines | |||
| 457 | Andransinine | K. pauciflora leaf | 22 |
| 458 | Andransinine A | K. pauciflora leaf | 22 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Corynantheines | |||
| 459 | Arboricine | K. arborea leaf and stem bark | 10 and 120 |
| 460 | Arboricinine | K. arborea leaf and stem bark | 10 and 120 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Carbolines | |||
| 461 | Harmane | K. griffithii leaf and stem | 15 and 17 |
| 462 | Harmicine | K. griffithii leaf | 15 and 17 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Arbophyllidine | |||
| 463 | Arbophyllidine | K. arborea stem bark | 59 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Mersicarpine | |||
| 464 | Mersicarpine | K. arborea stem bark, K. pauciflora leaf, K. singapurensis stem bark | 10, 22 and 23 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Azepane-fused tetrahydro-β-carboline | |||
| 465 | Kopsiyunnanine K | K. arborea aerial part | 102 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Andranginine | |||
| 466 | Andranginine | K. arborea aerial part | 102 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Triterpenoids and sterols | |||
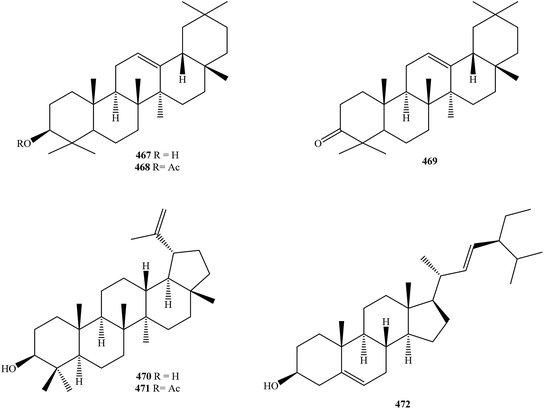
| 467 | β-Amyrin | K. singapurensis leaf and bark | 122 |
| 468 | β-Amyrin acetate | K. singapurensis leaf and bark | 122 |
| 469 | β-Amyrone | K. singapurensis leaf and bark | 122 |
| 470 | Lupeol | K. singapurensis leaf and bark | 122 |
| 471 | Lupeol acetate | K. singapurensis leaf and bark | 122 |
| 472 | Stigmasterol | K. singapurensis leaf and bark | 122 |
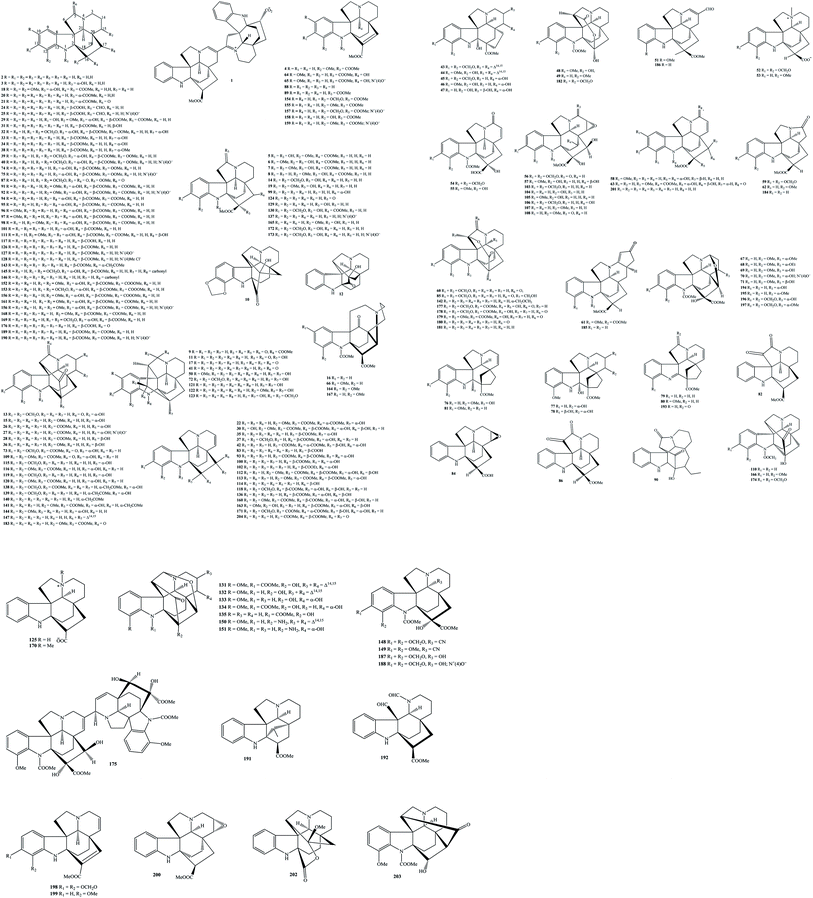
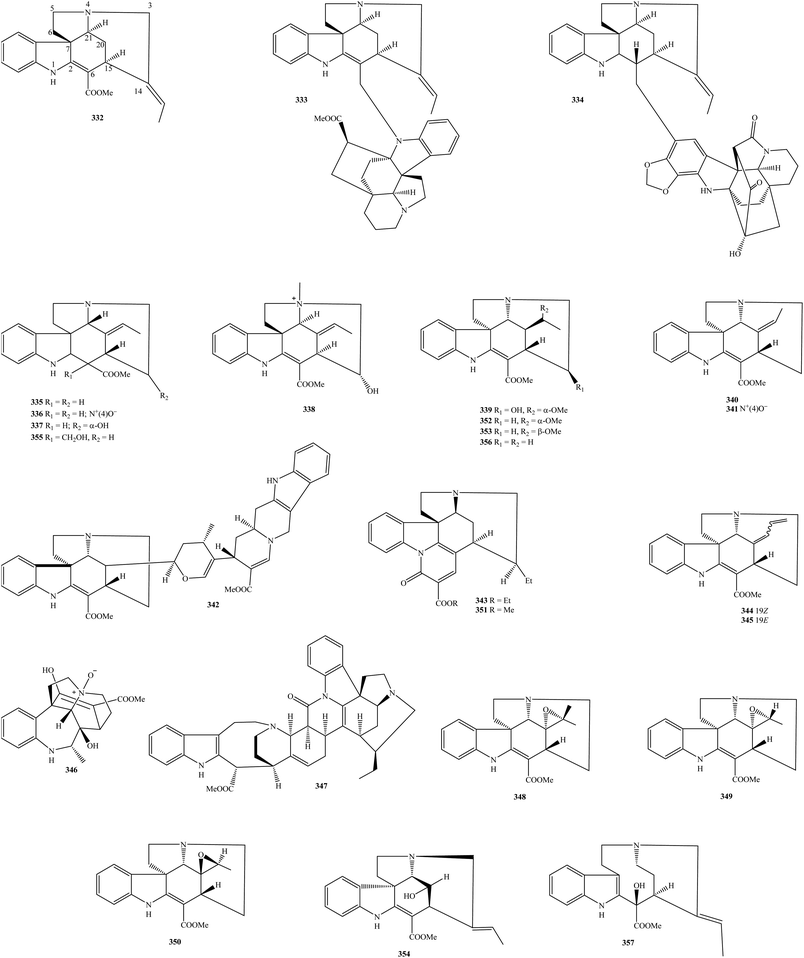
2.1. Aspidofractinines
Aspidofractinines are the largest phytochemical class of isolated alkaloids from the genus Kopsia. As shown in Table 1, more than two hundred aspidofractinines have been isolated to date, and they derive from various parts of K. arborea, K. dasyrachis, K. fruticosa, K. grandifolia, K. griffithii, K. hainanensis, K. hainanensis, K. jasminiflora, K. larutensis, K. macrophylla, K. officinalis, K. pauciflora, K. profunda, K. singapurensis, and K. teoi.4,7–59,61–79,81 From Fig. 1, Kopsia aspidofractinines 1–204 occurred in both monomer and dimer forms, but they did not bind to sugar units. Aspidofractinines 1–204 have been generally associated with the esterification at nitrogen N-1 and carbon C-16, carbonylation at carbon C-5, expoxydation at carbons C-11 and C-12, and hydroxylation, or methoxylation at carbons C-11, C-12, C-16, C-17, and C-18. It was found that 5,22-dioxokopsane (17), kopsamine (39), kopsamine N-oxide (40), kopsanone (41), kopsifine (73), kopsilongine (91), kopsininic acid (117), kopsinilam (124), kopsinine (126), kopsinine-N(4)-oxide (127), pleiocarpine (189), and (−)-venalstonine (201) might be seen as characteristic metabolites in the group of Kopsia aspidofractinines. For instance, compound 126 was recorded to appear in K. arborea twig and stem bark, K. dasyrachis stem, K. fruticosa stem bark, K. jasminiflora stem bark, K. grandifolia stem bark, K. griffithii leaf and stem bark, K. hainanensis leaf, stem, stem bark and twig, K. larutensis stem, stem bark and leaf, K. officinalis root, stem, twig, leaf and fruit, K. singapurensis stem bark and leaf, K. pauciflora stem, stem bark and leaf, and K. teoi stem bark, whereas its N(4)-oxide (127) presented in K. dasyrachis stem, K. griffithii stem bark, K. hainanensis stem and leaf, K. officinalis fruit and leaf, K. pauciflora stem, and K. singapurensis bark.5–7,9–11,13–19,21–25,28,29,32,36,42,43,48,51,65,66,68–72Taking phytochemical studies into account, a new bisindole alkaloid arbolodinine A (1) was isolated from K. arborea stem bark.8 Based on NMR, MS, and ECD data, compound 1 was a product by the combination of two apidofractinine units, and its biosynthetic pathway was structurally formulated from precursor 126. Aspidofractinine (2) can be found in K. arborea stem bark, K. hainanensis twigs and leaves, and K. officinalis stem,9–11 but aspidofractinine-1,3-dicarboxylic acid (4) was only detected in K. officinalis stem.11 (2β,5β)-Aspidofractinin-16-ol (3) was a new 16-alcohol derivative found in K. officinalis leaves for the first time, and then was detected in K. hainanensis twigs and leaves.9,12,13 Compounds 5–9 have shared the same feature of carbomethoxylation at nitrogen N-1,14–19 in which N-carbomethoxy-11-hydroxy-12-methoxykopsinaline (5) and N-carbomethoxy-11-methoxy-12-hydroxykopsinaline (6) were two new metabolites in nature.14–16 Dasyrachine (10) containing isokopsine skeleton was one of the new metabolites present in the 95% EtOH extract of K. dasyrachis stem.18 In contrast to compounds 5–9, the next compounds decarbomethoxykopsine (11), decarbomethoxyisokopsine (12), decarbomethoxykopsifine (13), N(1)-decarbomethoxykopsamine (14), Na-demethoxycarbonyl-12-methoxykopsine (15), and 10-demethoxykopsidasinine (16) are associated with the decarbomethoxylation at nitrogen N-1.10,11,16,18–26 Among them, compounds 13, 15, and 16 were new in nature. 11,12-Dimethoxykopsamine (18) was a known metabolite found in K. dasyrachis leaves, but 11,12-dimethoxykopsinaline (19) was a new one in the stem bark of K. pauciflora stem bark.22,30 Similarly, 16-epi-kopsinine (20), 16-epi-kopsinilam (21), 16-epi-17α-hydroxy-Δ14,15-kopsinine (22), 14,15-β-epoxykopsingine (23), N(1)-formylkopsininic acid (24), N(1)-formylkopsininic acid-N(4)-oxide (25), fruticosamine (26), fruticosiamine A (27), and fruticosine (28) were new aspidofractinines, and found in genus Kopsia for the first time.11,20,24,31–37,39–42 The known metabolite 11-hydroxykopsilongine (29) has been detected in both the fruit and leaf of K. officinalis,13,25 while 11-hydroxykopsingine (30), 5β-hydroxykopsinine (31), and 15-hydroxykopsamine (32) were first isolated from polar extracts of K. teoi leaf, K. jasminiflora stem bark, and K. singapurensis root, respectively.24,34,35 Two known compounds 33 and 34 were products of 15α and 17α-hydroxylation of kopsinine, respectively (Fig. 1). In the meantime, the structure of the new metabolite 35 is closely related to kopsinine by 17α-OH and olefinic double bond at carbons C-14 and C-15.44 For a long time, Ruangrungsi et al. (1987) successfully isolated two new aspidofractinines, named jasminiflorine (36) and kopsijasmine (89), from the MeOH extract of K. jasminiflora leaves, whereas kopsamidines A–B (37–38) were separated from the acidic EtOH extract of K. arborea stem bark.10,40
To search for bioactive metabolites from Kopsia plants, Long et al. (2018) isolated five new aspidofractinines kopsiafrutines A–E (43–47) from the 80% EtOH extract of K. fruticosa aerial part.52 Eleven new analogs, kopsiahainanins A–F (48–53) and kopsiahainins A–E (54–58) were among the new compounds found in the 80% EtOH extract of K. hainanensis twigs and leaves.53,54 In another approach, chromatographic separation of the 95% EtOH extract of K. officinalis aerial part can lead to the isolation of three new metabolites (59–61), which named kopsiaofficines A–C.55 From K. arborea aerial part, the new compound kopsiarborines A (62) was isolated.56 Three new metabolites, kopsidasine (64), kopsidasine-N-oxide (65), and kopsidasinine (66) were separated from K. dasyrachis leaves and structurally confirmed by the NMR analysis and Hofmann reaction.57 Thirteen previously undescribed metabolites kopsidines A–D (67–69 and 71), kopsinitarines B–D (132–134), mersingines A–B (150–151), 11-methoxykopsingine (160), 11-methoxy-12-hydroxy-kopsinol (163), 11,12-methylenedioxykopsaporine (171), and nitaphylline (175) have further been observed in K. teoi leaf, while its stem bark also contained seven other new compounds kopsinganol (111), kopsinginine (113), kopsinginol (114), kopsinol (136), kopsinitarine E (135), kopsinol (136), and kopsonoline (146).33,34,43–45,73,74,80 Kopsidarine (63), kopsidine C N-oxide (70), and singaporentine A (193) were three new compounds existed in K. singapurensis leaf, whereas its bark encompassed four new others singapurensines A–D (194–197)36,48,58,79 In two years 2007 and 2008, primarily based on CC approach, Subramaniam et al. successfully isolated nineteen new aspidofractinines, including kopsilongine-N-oxide (92), kopsiloscines A–J (93–102), kopsinalines A–F (103–108), kopsinicine (118), and kopsofinone (145) from K. singapurensis leaf or stem bark (Table 1 and Fig. 1).23,32,48 Kopsiflorine (74) is now available in the genus Kopsia, but its N(4)-oxide (75) and kopsinarine (109) were new in nature and were found in K. dasyrachis stem.18 Six indole alkaloidal constituents kopsifolines A–F (76–81) with unprecedented hexacyclic carbon skeleton were detected in the acidic EtOH extract of K. fruticosa leaves.62,63 Kopsifoline G (82) and kopsihainins B–F (83–87) were purified as new alkaloids from the stem or twig extracts of K. hainanensis.12,64,65 Among the isolated compounds, kopsijasminine (88) and kopsilarutensinine (90) were also identified to be two new aspidofractinines derived from the stem bark of K. teoi and K. larutensis, respectively.43,66 The earliest report by Guggisberg et al. (1963) identified that kopsine (110) was a new and major component of K. fruticosa leaves, and it was then isolated frequently.18,20,38,39,41,67 In a phytochemical research on the acidic EtOH extract of K. arborea stem bark, five new aspidofractinines, kopsinidines A–B (115–116), kopsinidines A–B (119–120), and paucidactine C (179) were isolated.10
Phytochemical analysis aided by NMR structural elucidation on the CHCl3 and n-BuOH extracts of K. officinalis leaf and twig has resulted in the isolation of eight new compounds kopsinidines C–E (121–123), N(1)-methoxycarbonyl-11,12-methoxylenedioxykopsinaline (153), N(1)-methoxycarbonyl-12-methoxykopsinaline (156), N(4)-methylkopsininate (170), (−)-11,12-methylenedioxykopsinaline (172), and 5-oxokopsinic acid (176), in addition to seven known compounds kopsinilam (124), kopsinine (126), kopsinine methochloride (128), kopsinine B (129), (−)-kopsinoline (137), (−)-12-methoxykopsinaline (165), and 11,12-methylenedioxykopsinaline N(4)-oxide (173).16 Among the isolates from K. hainanensis stem and leaves, the new compound kopisininate (125) itself displayed an interesting feature since it contained a carboxylate group (δC 181.6 ppm in CD3OD).7 Besides known compounds, the application of NMR and MS tools would take a good advance in the natural product chemistry field, by which the chemical structures of seven new aspidofractinines kopsiofficines A–F and L (138–144) from K. officinalis stem and three new analogs yunnanoffines A–C (202–204) from K. officinalis leaf have been determined.11,25,75 Aspidofractinines were further observed in other Kopsia plants. For instance, apart from known compounds, five new derivatives N(1)-methoxycarbonyl-11,12-methylenedioxy-Δ16,17-kopsinine (154), N(1)-methoxycarbonyl-12-methoxy-Δ16,17-kopsinine (155), N(1)-methoxycarbonyl-11,12-methylenedioxy-Δ16,17-kopsinine N(4) oxide (157), N(1)-methoxycarbonyl-12-hydroxy-Δ16,17-kopsinine (158), and N(1)-methoxycarbonyl-12-methoxy-Δ16,17-kopsinine N(4) oxide (159) were characteristics of K. profunda,4,77 or lahadinines A–B (145–146), 12-methoxy-10-demethoxykopsidasinine (167), paucidactines D–E (180–181), paucidactinine (182), paucidisine (183), paucidirinine (184), paucidirisine (185), pauciduridine (186), paucifinine (187), and paucifinine-N-oxide (188) were new metabolites isolated from the parts of K. pauciflora.19,51,76
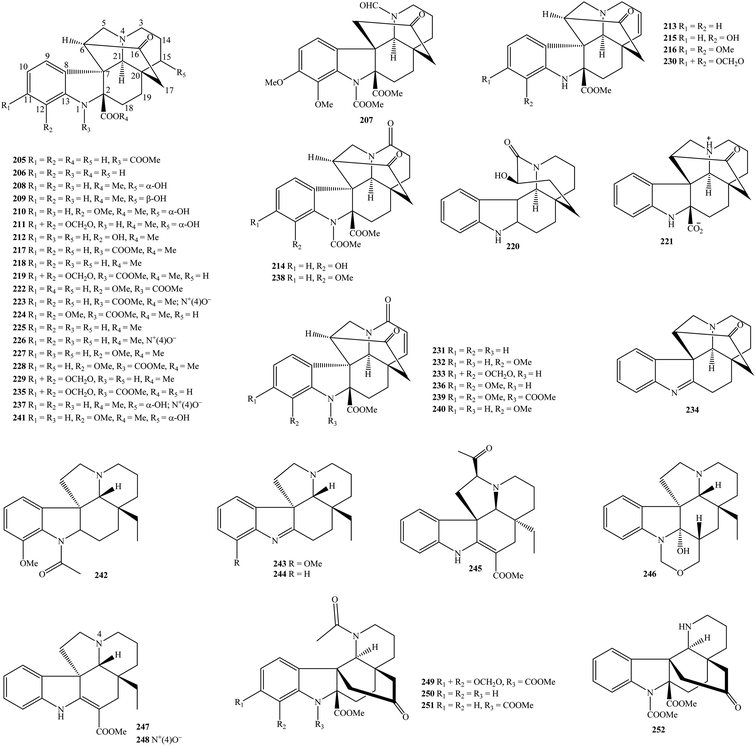
2.2. Chanofruticosinates, aspidospermines, and danuphyllines
In general, Kopsia chanofruticosinate derivatives 205–241 have established a similarity in the chemical structural skeleton with aspidofractinines (Table 1 and Fig. 2). However, fragment C-2–C-16–C-17–C-20 in aspidofractinines was replaced by a carbon bridge between C-6 and C-20 in chanofruticosinates. To date, these phytochemicals occurred in K. arborea, K. dasyrachis, K. fruticosa, K. flavida, K. hainanensis, K. lancibracteolata, K. officinalis, and K. pauciflora.3,6,7,10,13,16,22,25,30,41,42,65,75,82–89 From Table 1, kopsia A (217), kopsia C (219), methyl 11,12-dimethoxychanofruticosinate (224), methyl N1-decarbomethoxychanofruticosinate (225), methyl 12-methoxychanofruticosinate (228), methyl 11,12-methylenedioxy-N1-decarbomethoxychanofruticosinate (229), and methyl 11,12-methylenedioxy-N1-decarbomethoxy-Δ14,15-chanofruticosinate (230) were major components in the group of Kopsia chanofruticosinates. Analyzing chemical composition further, the rich alkaloid fraction of K. officinalis leaf and twig have also contained five new derivatives, chanofruticosinic acid (205), kopsias A–C (217–219), 12-methoxychanofruticosinic acid (222), and methyl (2β,11β,12β,19α)-6,7-didehydro-8,21-dioxo-11,21-cycloaspidospermidine-2-carboxylate (231).13,16,86 According to the phytochemical report of Chen and partners, N1-decarbomethoxy chanofruticosinic acid (206), kopsihainanines A–B (220–221), methyl chanofruticosinate N(4)-oxide (223), and methyl N1-decarbomethoxy chanofruticosinate N(4)-oxide (226) were previously unrecorded compounds and found in K. hainanensis stem and leaf for the first time.6,7 The application of HPLC chromatographic procedure to the 70% EtOH extract of K. fruticosa aerial part has resulted in the isolation of two new substances, 11,12-dimethoxydanuphylline (207) and 3-oxo-11,12-dimethoxy-N1-decarbomethoxy-14,15-didehydrochanofruticosinate (236).3 The MeOH extract of K. flavida leaf consisted of serial new alkaloids type chanofruticosinates flavisiamines A–F (208–213).41,83 Besides known compounds, the chromatographic isolation of the alcoholic extracts of K. arborea leaves has allowed to identify the appearance of seven new methyl chanofruticosinate alkaloids, kopreasin A (216), and prunifolines A–F (208, 211, and 238–241).82,84 Finally, three new derivatives 12-hydroxylprunifoline A (214), 12-hydroxylprunifoline A (215), and N(4)-oxide prunifoline D (3) were purified from the 70% EtOH extract of K. lancibracteolata stem.85Regarding aspidospermines, the acidic EtOH extract of K. pauciflora leaf contained aspidospermine (242), and its (+)-1,2-dehydro derivatives (243).22 A phytochemical report conducted by Wu et al. (2010) revealed that the MeOH extract of K. arborea aerial part was characterized by the presence of the new aspidospermine kopsiyunnanine G (246), and known compound eburenine (244).90 Similarly, new compound kopsiofficine G (245), together with two known ones, vincadifformine (247) and vincadifformine N(4)-oxide (248) represented for K. officinalis stem.11
Only four indole alkaloids danuphyllines 249–252 were found in Kopsia plants, in which danuphylline (249), danuphylline B (250), 11,12-de(methylenedioxy)danuphylline (251), and kopsihainin A (252) were separated from K. dasyrachis leaf, K. arborea leaf, K. officinalis leaf, and K. hainanensis stem, respectively (Table 1 and Fig. 2).13,30,65,78,91 All these isolates were new in nature. Similar to aspidofractinine derivatives, chanofruticosinates, aspidospermines, and danuphyllines were unique chemical classes found in the family Apocynaceae. Especially, danuphylline derivatives were only detected in Kopsia, thereby they can be used as chemical markers to recognize this genus.
2.3. Eburnamines
As can be seen from Table 1 and Fig. 3, eburnamines are also a crucial phytochemical class of the genus Kopsia. Forty-nine compounds 253–301 were isolated to date, and they were mainly derived from K. arborea, K. dasyrachis, K. griffithii, K. hainanensis, K. hainanensis, K. jasminiflora, K. larutensis, K. macrophylla, K. officinalis, K. pauciflora, K. singapurensis, K. teoi, and K. terengganensis.5,9,13–15,17–19,21–25,27–29,33,42,50,51,66,68–70,75,90,92,93 Kopsia eburnamines appeared in both monomer and dimer forms, but not to have connected with sugar units. (−)-Eburnamenine (255), (−)-eburnamine (257), (+)-eburnamonine (259), (+)-isoeburnamine (274), and larutenine (284) were isolated frequently, e.g., compound 274 was detected in K. arborea aerial part, K. dasyrachis stem, K. hainanensis stem bark, K. larutensis leaf, stem and stem bark, K. teoi stem bark and leaf, K. officinalis leaf, K. pauciflora stem and stem bark, and K. terengganensis bark.5,13,18,19,22,29,33,51,68,70,90,92,93(−)-Demethylnorpleiomutine (253), (−)-eburnaminol (258), (−)-O-ethyleburnamine (262), 19-hydroxy-(−)-eburnamonine (267), (−)-19(R)-hydroxyisoeburnamine (268), (+)-(19R)-19-hydroxyeburnamine (269), (−)-(19R)-19-hydroxyisoeburnamine (271), (+)-kopsoffine (277), kopsoffinol (282), (−)-norpleiomutine (289), (−)-O-methylisoeburnamine (291), and 19-oxo-(−)-eburnamonine (293) were found in two or three Kopsia plants (Table 1). (+)-Eburnamenine (254), (+)-eburnamine (256), (−)-eburnamonine (261), (+)-ethylisoeburnamine (263), 16α-hydroxy-19-oxoeburnamine (264), 16β-hydroxy-19-oxoeburnamine (265), melohenine B (287), (−)-methyleburnamine (288), (+)-O-methyleburnamine (290), and O-methyl-16-epi-vincanol (295), and Δ14-vicamone (300) have never been observed in genus Kopsia before. Especially, (−)-eburnaminol (258), (+)-eburnamonine N(4)-oxide (260), (+)-19(R)-hydroxyeburnamine (266), (−)-19(R)-hydroxyisoeburnamine (268), (−)-19(R)-hydroxyeburnamenine (270), (−)-(19R)-19-hydroxyisoeburnamine (271), (−)-19(R)-hydroxy-O-ethylisoeburnamine (272), (−)-isoeburnamine (275), kopsiofficines H–K (278–281), (+)-larutensine (283), larutenine (284), larutenines A–B (285–286), (−)-norpleiomutine (289), (+)-19-oxoeburnamine (292), (−)-19-oxoisoeburnamine (294), 20-oxo-eburnamenine (296), phutdonginin (297), terengganensines A–B (298–299), and yunnanoffine C (301) were new in literature and isolated from genus Kopsia for the first time. Eburnamines is now abundant in genus Kopsia, but this chemical class was only found in the family Apocynaceae.
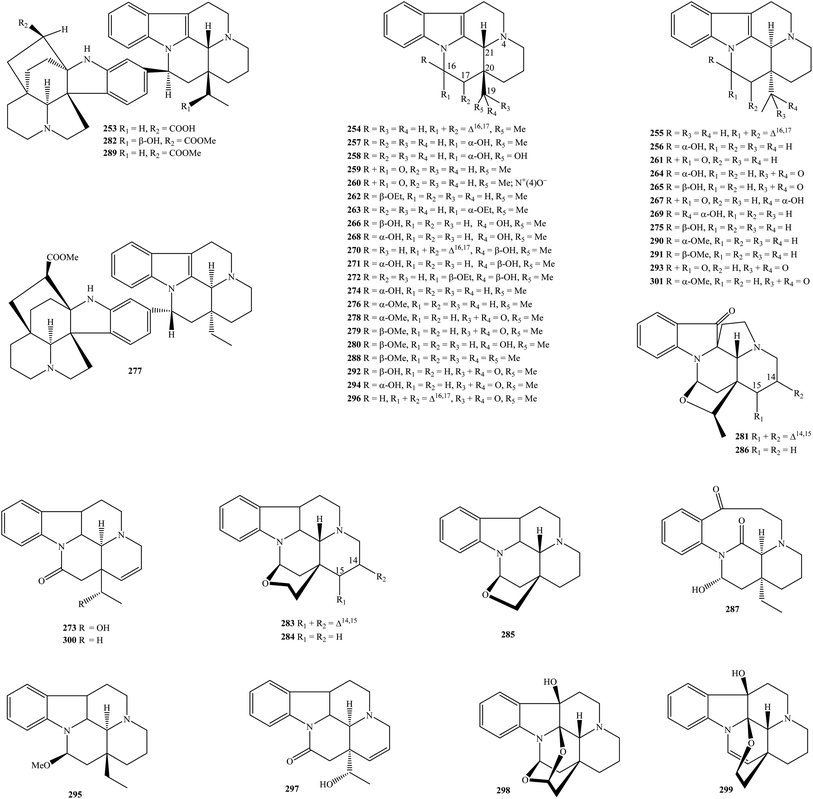
2.4. Akuammilines, sarpagines, and aspidophyllines
A total of twenty-one akuammilines 302–322 have been outlined in Table 1 and Fig. 4. K. arborea, K. dasyrachis, K. deverrei, K. fruticosa, K. griffithii, K. hainanensis, K. macrophylla, K. officinalis, K. singapurensis, and K. teoi were main resource of these phyto-constituents.9,10,15–17,23,27,31,32,34–36,43,45,47–49,94 Previous studies revealed that deacetylakuammiline (306), 16-epi-deacetylakuammiline (309), 16-hydroxymethyl-pleiocarpamine (311), and pleiocarpamine (319) were likely to be major akuammilines in genus Kopsia.The first compound akuammidine (302) was originated from K. arborea stem bark, K. singapurensis root, stem bark, and leaves, while akuammiline (303) presented in the aerial part of K. macrophylla and K. teoi.10,23,27,32,34,43,45,47–49 Akuammiline N(4)-oxide (304) and 16-epi-deacetylakuammiline-N(4)-oxide (310) were reported to be two new derivatives, which were separated from the rich alkaloidal fraction of K. griffithii stem bark.15 ψ-Akuammigine (305), dregamine (307), N-methylpleiocarpamine (312), 5-methoxystrictamine (313), rhazimal (314), rhazinaline N(4)-oxide (315), picralinal (317), pleiocarpamine methochloride (320), and pleiomalicine (321) were isolated from genus Kopsia for the first time.9,10,15,16,27,31,36 Lastly, two new metabolites, rhazinoline (316) and singaporentinidine (322), were purified from the extracts of K. arborea stem bark, K. singapurensis root, respectively.10,35
A list of four alkaloidal sarpagines 323–326 has been updated in Table 1 and Fig. 4.9,27,50 Vincadiffine (326) was a well-known metabolite, but its 10-hydroxy derivative (323) was a new compound in the literature, and both of them were isolated from the MeOH extract of K. hainanensis.9 Perivine (324) and tabernaemontanine (325) were two known sarpagines derived from K. officinalis root and stem and K. macrophylla bark, respectively.27,50
Resemble sarpagines, aspidophylline derivatives are not available in genus Kopsia. A total of five isolates 327–331 were summarized in Table 1 and Fig. 4.23,31–34,36,43,48,49 Aspidodasycarpine (327) was recorded by various authors and was detected in K. singapurensis root and stem bark, K. teoi stem, and stem bark.23,32,34,36,43,48,49 Two new phyto constituents aspidophyllines A–B (328–329), were determined to exist in K. singapurensis stem bark, while the new analog vincophylline (331) was found in its leaves.32,48 It can be concluded that lonicerine (330) was a major component in the group of aspidophyllines because it has occurred in various Kopsia plants such as K. fruticosa stem bark, K. singapurensis bark and stem bark, and K. teoi stem, stem bark and leaf.23,31–34,36,43,48
2.5. Strychnoses
Compounds 332–357 have been fallen into the group of alkaloidal strychnos derivatives (Table 1 and Fig. 5). Similar to aspidofractinines and eburnamines, Kopsia strychnoses were presented in both mono-or dimer forms, and they were mainly sourced from K. deverri, K. hainanensis, K. jasminiflora, K. officinalis, K. pauciflora, K. singapurensis, especially K. arborea.8,10,12,22–24,29,35,36,64,94–102 Significantly, except for akuammicine (332), (E)-condylocarpine (335), (E)-condylocarpine N-oxide (336), leuconicine B (351), precondylocarpine (355), and tubotaiwine (356), the remaining compounds were new in nature.By the analysis of NMR, MS, and CD data, two isolated dimeric compounds, arbolodinines B–C (333–334), were elucidated as bulk novel strychnoses, which were derived from K. arborea stem bark.8 Compound 335 is a known compound,22,95 but its 14α-hydroxy and 14(S)-hydroxy-19(R)-methoxy derivatives 337–338 were new in the literature and first were isolated from K. deverri stem bark and K. singapurensis root, respectively.35,94 Mossambine (354) was another new strychnos found in K. singapurensis stem bark.23 K. arborea aerial part has so far distributed thirteen new compounds, isocondylocarpine (340), isocondylocarpine N-oxide (341), kopsiyunnanines A, I, J1–J2, L, M, and F1–F3 (342–350), 19(R)-methoxytubotaiwine (352), and 19(S)-methoxytubotaiwine (353).10,95–98,100,101 The well-known compound tubotaiwine (356) was characteristic of K. arborea aerial part, K. hainanensis stem and stem bark, but its 14(S)-hydroxy-19(R)-methoxy derivative 339 isolated from the MeOH extract of K. jasminiflora stem bark has been determined as a new metabolite.24,29,64,95 Stemmadenine (357) from K. pauciflora leaves was the only stemmadenine detected in the genus Kopsia.22
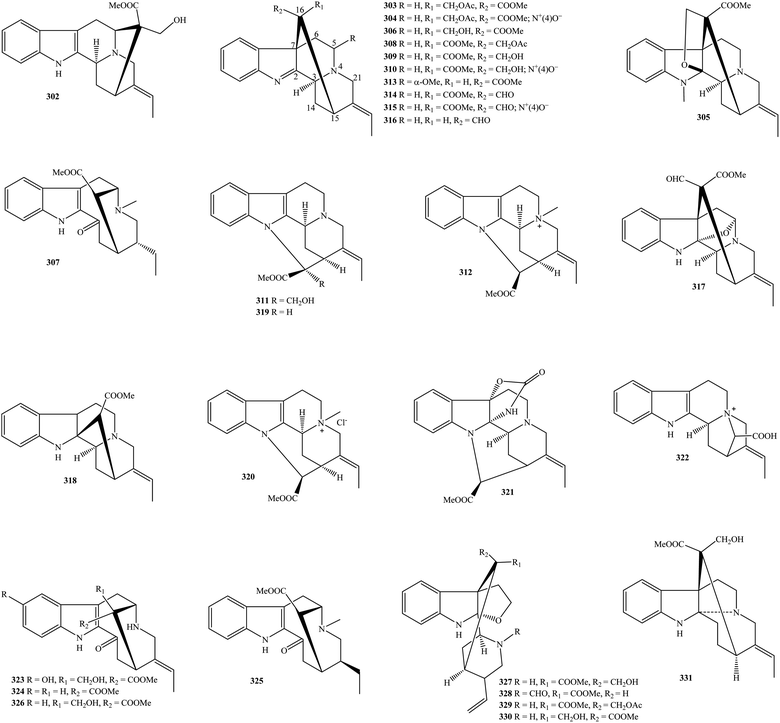
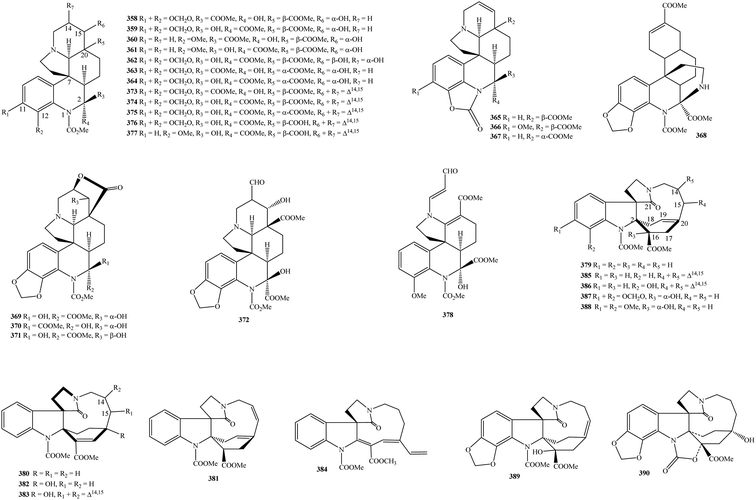
2.6. Mersinines and pauciflorines
Mersinines with tetracyclic quinolinic skeleton are a new subclass of monoterpenoid indole alkaloids, which were only found in the plants genus Kopsia. Kopsia mersinines 358–378 were only detected in K. singapurensis leaves and occasionally in K. fruticosa leaves (Table 1 and Fig. 6).23,103–108 Of particular interest, all these isolates were novel compounds in literature. Searching for cytotoxic agents from plants, sixteen novel mersinines, comprising of mersidasines A–G (358–364), mersifolines A–C (365–367), mersiloscine (369), mersiloscines A–B (370–371), and mersinines A–C (373–375) were isolated from the acidic EtOH extract of K. singapurensis leaf.103 Their stereochemistry was confirmed by NMR, IR, UV, and X-ray analysis. K. singapurensis leaf has further been shown to contain five novel congeners, mersilongine (368), mersinaline (372), mersiphyllines A–B (376–377), and mersirachine (378).23,106,108It is similar to mersinines, Kopsia pauciflorines 379–390 have induced interest since all isolates were novel in the literature, except for 11,12-demethoxy-16-deoxypauciflorine (379). K. arborea, K. jasminiflora, K. officinalis, and K. pauciflora might be a reservoir of this chemical class.22,40,56,109,110
Besides aspidofractinines, the MeOH extract of K. jasminiflora leaf has associated with the presence of three novel pauciflorines 20-deoxykopsijasminilam (380), kopsijasminilam (382), and Δ14-kopsijasminilam (383).40 In addition to known compound 379, three novel derivatives, kopsioffines A–C (384–386) were arisen from the 95% EtOH extract of K. officinalis dried stem and leaves.109 Pauciflorines A–B (387–388) reached 0.22 and 0.03 g kg−1 in K. pauciflora leaf.110 In the meantime, two other novel compounds, pauciflorine C (389) and paucifoline (390), were minor components in the acidic EtOH extract of K. pauciflora leaves.22 It is possible to conclude that mersinines and pauciflorines could be used as chemical indicators to distinguish the genus Kopsia and other genera of the family Apocynaceae.
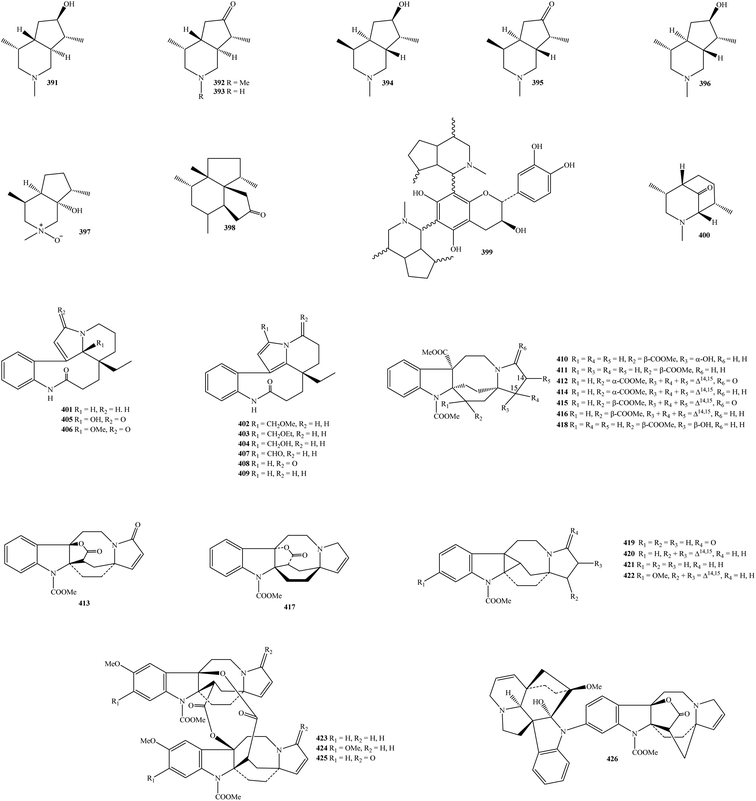
2.7. Skytanthines, rhazinilams, and lundurines
It is recognized that the unique chemical class of skytanthines can be arranged as a new group of alkaloids. These phytochemicals were isolated from Apocynaceae Skytanthus acutus for the first time in 1960.123 From Table 1 and Fig. 7, ten new skytanthines 391–400 have been summarized. The extracts of K. dasyrachis and K. macrophylla, especially K. pauciflora, are accompanied by the presence of this type.27,30,112,113 Two publications in 1996 and 1997 by Kam and partners successfully reported the structures of serial new skytanthines kinabalurines A–F (391–396) from K. pauciflora leaves.111,112 while their following congener kinabalurine G (397) was derived from K. dasyrachis leaf.30 Significantly, the novel alkaloidal kopsirachine (399) isolated from K. dasyrachis leaves was determined to be a hybrid compound by the combination of catechin and skytanthine.113 After being run Sephadex LH-20 and silica gel CC, a new monoterpene alkaloids containing a lactone ring, kopsilactone (398), and other new monoterpene alkaloids possessing 2-azabicyclo[3.3.1] backbone, kopsone (400), were isolated from the MeOH extract of K. macrophylla bark.27 Based on these findings, skytanthines can be seen as chemical evidence to determine the close relationship among Apocynaceae plants, especially between genera Skytanthus and Kopsia.Rhazinilam (409) is an alkaloid discovered in the Apocynaceae plant Melodinus australis in 1965.124 It was then isolated from the shrub of the other Apocynaceae plant Rhazya stricta as well as other organisms.125 This compound was established as a main component in the group of Kopsia rhazinilams since it was found in K. arborea aerial parts and stem bark, K. officinalis leaf and twig, K. pauciflora leaf and stem bark, K. singapurensis leaf, bark and stem bark, and K. teoi stem, stem bark and leaf.13,16,22,23,25,32–34,36,45,47,48,114 Leuconolam (405) can be also seen as another main component because of its occurrence in K. griffithii leaves and stem bark, K. hainanensis twig, stem and leaf, K. officinalis leaf, K. pauciflora leaves, and K. singapurensis stem bark.7,9,12,15,17,22,23,25,32 As shown in Table 1, known compound 5,21-dihydrorhazinilam (401) existed in K. arborea stem bark and K. singapurensis stem bark and leaves.10,23,48 From Fig. 7, three new compounds, kopsiyunnanines C1–C3 (402–404), which were isolated from the aerial part of K. arborea and K. officinalis, established the same backbone with rhazinilam (409).96,114 O-Methylleuconolam (406) and rhazinal (407) were two well-known compounds, but their congener rhazinicine (408) separated from K. arborea stem bark, K. dasyrachis stem, and K. singapurensis root was a new derivative.10,12,18,32,49,60,87 To the best of our knowledge, rhazinilams were only observed in the family Apocynaceae, as well as the plants of three genus Melodinus, Rhazya, and Kopsia being the main resources.
Kopsia lundurines 410–426 have generally been formed by the combination of an indole ring and a lactam ring through an eight-ring member (Fig. 7). Notably, all of these seventeen compounds were novel in nature, and the three plants, K. lapidilecta, K. grandifolia, and K. tenuis, are the main reservoirs (Table 1).
Awang and partners also isolated and identified six novel pauciflorines, epilapidilectinol (410), isolapidilectine A (414), lapidilectam (415), lapidilectines A–B (416–417), and lapidilectinol (418) from aerial part of K. lapidilecta.81,115 Three novel indole alkaloids, grandilodines A–C (411–413) were extracted from the EtOH extract of K. grandifolia stem bark or leaves with the yield ranging from 0.07 to 3.18%, and their chemical structures were proved by NMR, MS, and X-ray spectral data.72 The eight remainders, including lundurines A–B (419–422), tenuisine A–C (423–425), and tenuiphylline (426), were novel lundurines presented in the K. tenuis leaf.71,116,117 In which compounds 423–425 were unprecedented dimers, while compound 426 is unique due to the incorporation between aspidofractinine and lundurine units. As of a consequence, Kopsia lundurines, especially compounds 423–426, could be seen as significant chemotaxonomic agents.
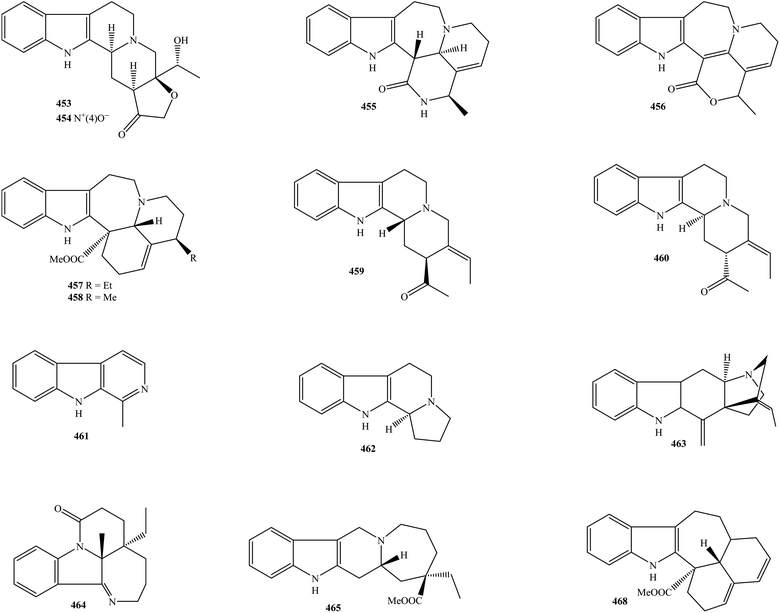
2.8. Aspidospermas, catharinensines, leuconoxines, pericines, alstonines, and quebrachamines
Alkaloid type aspidospermas were named following the name of the genus Aspidospermas (family Apocynaceae). With regard to genus Kopsia, five known isolates 427–431 were summarized in Table 1 and Fig. 8. It turns out that buchtienine (427) was presented in either the leaf or stem of K. griffithii.15,17 The MeOH extract of K. hainanensis twig and leaf consisted of two aspidospermas, corynantheol (428) and dihydrocorynantheol (430).9 Only K. officinalis stem was found to contain 19,20-dihydroisositsirikine (429), while its congener 16(R)-19,20-E-isositsirikine (431) has been observed in the leaf of both K. griffithii and K. pauciflora.15,17,22 Therefore, alkaloidal aspidospermas are usefully chemotaxonomic agents to confirm the close relationship between the genus Kopsia and other genera in the family Apocynaceae.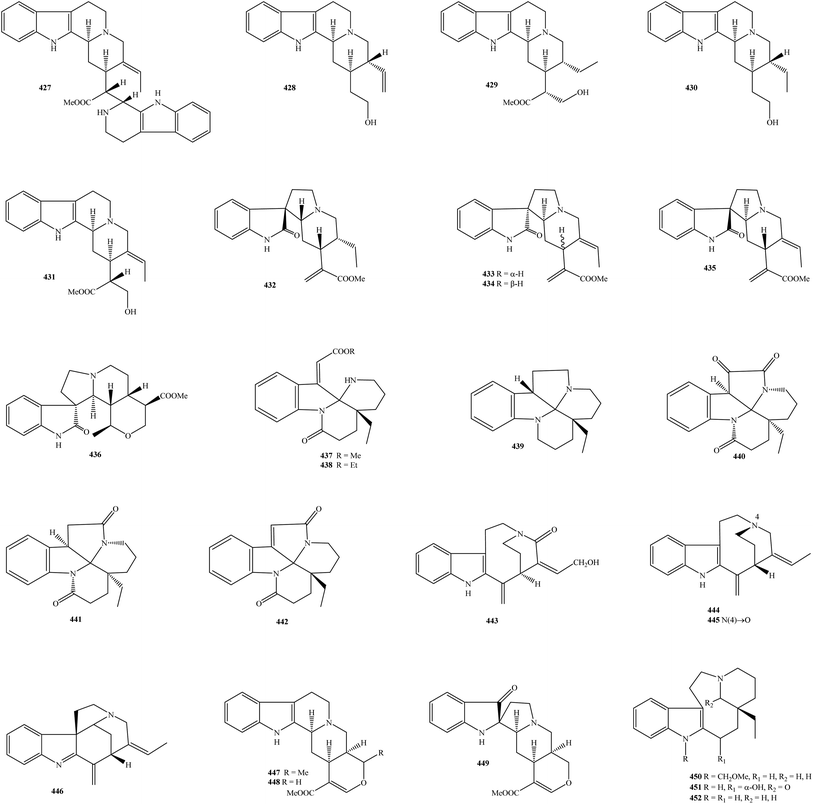 | ||
| Fig. 8 Aspidospermas, catharinensines, leuconoxines, pericines, alstonines and quebrachamines from genus Kopsia. | ||
Catharinensines, which belong to the group of oxindole alkaloids, can be found in several higher plants, such as Peschiera catharinensis.126 In Kopsia plants, five catharinensines 432–436 were detected (Table 1 and Fig. 8). Phytochemical research conducted by Gan and partners revealed that the use of mobile phase CHCl3–MeOH is appropriate to isolate alkaloidal catharinensines.22 By this approach, three new compounds, kopsirensines A–C (433–435), together with known analog catharinensine (432), have been successfully purified from the acidic EtOH extract of K. pauciflora leaves.22 New catharinensine kopsiyunnanine B (436) was first collected as a light yellow solid from the alcoholic extract of K. officinalis aerial part, and then was detected in the K. arborea aerial part.96,97
Phytochemical studies on Kopsia plants have also led to the isolation of alkaloid leuconoxines 437–442, and their structures were compiled in Fig. 8. Leuconoxine (441) was described as a major component since it occurred in K. arborea stem bark, K. griffithii leaf and stem bark, K. pauciflora stem, stem bark and leaf, K. singapurensis stem bark, K. teoi stem bark.15,17,19,22,23,43 Arboloscine (437) was one of the new compounds in K. arborea stem bark, while melodinine E (442) was a known metabolite extracted from its twigs.10,21,118 New compound arboloscine A (438) isolated from K. pauciflora leaf has a similarity in structural feature with compound 437, but the methyl group of 437 was replaced by the ethyl group in 438.22 In the genus Kopsia, leuconodine D (439) was only detected in K. officinalis stems, whereas leuconodine F (440) was characteristic of K. griffithii leaves and K. pauciflora leaves.22,43,75
To find bioactive molecules from medicinal plants, four alkaloids type pericines, including two new compounds pericidine (443) and pericine N-oxide (445) and two known analogs pericine (444) and valparicine (446) were isolated (Table 1 and Fig. 8). All of these isolates originated from K. arborea stem bark.10,118,119
To the best of our knowledge, only three compounds 447–449 were classified as alkaloid alstonines (Table 1 and Fig. 8). Oxayohimban-16-carboxy acid (447) derived from K. officinalis stem has never been isolated from the genus Kopsia before.75 The major component (−)-tetrahydroalstonine (448) appeared in K. arborea stem bark, K. dasyrachis stem, K. griffithii leaf, K. officinalis root, twig and leaf, K. larutensis stem bark and leaf, K. pauciflora stem, stem bark and leaf, K. singapurensis stem bark; K. teoi stem bark.10,15,17–19,22,23,25,32,42,43,66 Compound 449, a pseudoindoxyl derivative of compound 448, was identified to be a new constituent from the acidic EtOH extract of K. pauciflora leaves.22
In the same manner, there are only three quebrachamines from the genus Kopsia till now (Table 1 and Fig. 8). (−)-Quebrachamine (452) is now abundant in nature and can be found in K. arborea aerial parts, K. hainanensis twigs and leaves, K. officinalis roots, and K. pauciflora leaves.9,22,69,114 However, kopsiyunnanines D and H (450–451) from K. arborea aerial part were confirmed to be two new analogs.90,114
2.9. Others indole alkaloids and non-alkaloids
Phytochemical studies on Kopsia plants also recorded the appearance of other alkaloidal types (Table 1 and Fig. 9). Chromatographic procedure on the acidic MeOH extract of K. arborea bark has resulted in the isolation of three new metabolites, arbophyllinines A–B (453–454) and arbophyllidine (463).59 Arboflorine (455) from K. arborea stem bark was a known alkaloid type arboflorine, but its new analog kopsiyunnanine E (456) was detected in the aerial part of K. arborea and K. officinalis.10,96,99,121 Besides the main constituents, the EtOH extract of K. pauciflora leaves has composed of a new component, andransinine A (458), along with a known one andransinine (457).22 New corynantheines arboricine (459) and arboricine (460) were found in both the leaves and stem of K. arborea.10,120 The new carboline harmane (461) was presented in both leaves and stem of K. griffithii, but the new congener harmicine (462) was only detected in its leaves.15,17 To find bioactive compounds from plants, mersicarpine (464) was first isolated from K. arborea stem bark.10 It was then further found in K. pauciflora leaves and K. singapurensis stem bark.22,23 Two final alkaloids, a new alkaloid type, azepane-fused tetrahydro-β-carboline kopsiyunnanine K (465) and a known alkaloid type andranginine (466), were constituents of K. arborea aerial part.102To date, there have not been many results on the separation of non-alkaloidal constituents from the plants of the genus Kopsia. A phytochemical report from Shan and partner (2017) identified that the n-hexane extract of K. singapurensis dried leaf and bark has accompanied with the existence of five triterpenoids β-amyrin (467), β-amyrin acetate (468), β-amyrone (469), lupeol (470), lupeol acetate (471), and one sterol stigmasterol (472) (Table 1 and Fig. 10).122 This is the first time to observe these compounds in the genus Kopsia.
Taken together, despite the fact that there have been preliminary chemotaxonomic and synthetic reviews.127,128 This is the first time that we provide fully information on phytochemical separation, a detailed list of almost isolated compounds, chemical classification, botanical resource, and the great value of Kopisa monoterpene alkaloids in botanical and chemical relationship.
3. Pharmacological activities
Cytotoxic, antimicrobial, anti-inflammatory, anti-diabetic, cardiovascular, vasorelaxant, and other positive properties have been studied utilizing Kopsia secondary metabolites and extracts in pharmacological research. In Table 2, a summary of prior pharmacological appraisals on Kopsia plant materials is presented in detail.| Compounds | Models | Effect | Positive control | Effect | References |
|---|---|---|---|---|---|
| Anti-cancer activity | |||||
| 39 | In vitro | CD50 > 60 μg mL−1/NIH/3T3 and HeLa cells | Vincristine | CD50 > 60 μg mL−1/NIH/3T3 cells | 49 |
| CD50 = 6.9 μg mL−1/HL-60 cells | CD50 = 1.8 μg mL−1/HL-60 cells | ||||
| CD50 = 0.4 μg mL−1/HeLa cells | |||||
| 40 | In vitro | CD50 > 60 μg mL−1/NIH/3T3, HL-60 and HeLa cells | Vincristine | CD50 > 60 μg mL−1/NIH/3T3 cells | 49 |
| CD50 = 1.8 μg mL−1/HL-60 cells | |||||
| CD50 = 0.4 μg mL−1/HeLa cells | |||||
| 43 | In vitro | IC50 = 33.7 μM/HS-1 cells | Adiamycin | IC50 = 17.8 μM/HS-1 cells | 52 |
| IC50 = 28.4 μM/HS-4 cells | IC50 = 24.7 μM/HS-4 cells | ||||
| IC50 = 32.4 μM/SCL-1 cells | IC50 = 21.8 μM/SCL-1 cells | ||||
| IC50 = 29.7 μM/A-431 cells | IC50 = 33.7 μM/A-431 cells | ||||
| IC50 = 30.9 μM/BGC-823 cells | IC50 = 28.4 μM/BGC-823 cells | ||||
| IC50 = 27.1 μM/MCF-7 cells | IC50 = 37.6 μM/MCF-7 cells | ||||
| IC50 = 31.2 μM/W-480 cells | IC50 = 14.1 μM/W-480 cells | ||||
| 44 | In vitro | IC50 = 34.9 μM/HS-1 cells | Adiamycin | IC50 = 17.8 μM/HS-1 cells | 52 |
| IC50 = 29.9 μM/HS-4 cells | IC50 = 24.7 μM/HS-4 cells | ||||
| IC50 = 33.1 μM/SCL-1 cells | IC50 = 21.8 μM/SCL-1 cells | ||||
| IC50 = 30.1 μM/A-431 cells | IC50 = 33.7 μM/A-431 cells | ||||
| IC50 = 35.5 μM/BGC-823 cells | IC50 = 28.4 μM/BGC-823 cells | ||||
| IC50 = 31.2 μM/MCF-7 cells | IC50 = 37.6 μM/MCF-7 cells | ||||
| IC50 = 32.6 μM/W-480 cells | IC50 = 14.1 μM/W-480 cells | ||||
| 45 | In vitro | IC50 = 12.4 μM/HS-1 cells | Adiamycin | IC50 = 17.8 μM/HS-1 cells | 52 |
| IC50 = 12.3 μM/HS-4 and BGC-823 cells | IC50 = 24.7 μM/HS-4 cells | ||||
| IC50 = 12.9 μM/SCL-1 cells | IC50 = 21.8 μM/SCL-1 cells | ||||
| IC50 = 11.8 μM/A-431 cells | IC50 = 33.7 μM/A-431 cells | ||||
| IC50 = 12.6 μM/MCF-7 cells | IC50 = 28.4 μM/BGC-823 cells | ||||
| IC50 = 13.8 μM/W-480 cells | IC50 = 37.6 μM/MCF-7 cells | ||||
| IC50 = 14.1 μM/W-480 cells | |||||
| 46 | In vitro | IC50 = 11.6 μM/HS-1 cells | Adiamycin | IC50 = 17.8 μM/HS-1 cells | 52 |
| IC50 = 11.4 μM/HS-4 cells | IC50 = 24.7 μM/HS-4 cells | ||||
| IC50 = 12.1 μM/SCL-1 cells | IC50 = 21.8 μM/SCL-1 cells | ||||
| IC50 = 10.3 μM/A-431 cells | IC50 = 33.7 μM/A-431 cells | ||||
| IC50 = 11.7 μM/BGC-823 cells | IC50 = 28.4 μM/BGC-823 cells | ||||
| IC50 = 10.4 μM/MCF7 cells | IC50 = 37.6 μM/MCF-7 cells | ||||
| IC50 = 12.5 μM/W-480 cells | IC50 = 14.1 μM/W-480 cells | ||||
| 47 | In vitro | IC50 = 7.3 μM/HS-1 cells | Adiamycin | IC50 = 17.8 μM/HS-1 cells | 52 |
| IC50 = 8.6 μM/HS-4 and MCF-7 cells | IC50 = 24.7 μM/HS-4 cells | ||||
| IC50 = 8.2 μM/SCL-1 cells | IC50 = 21.8 μM/SCL-1 cells | ||||
| IC50 = 9.5 μM/A431 cells | IC50 = 33.7 μM/A-431 cells | ||||
| IC50 = 8.9 μM/BGC-823 cells | IC50 = 28.4 μM/BGC-823 cells | ||||
| IC50 = 9.2 μM/W-480 cells | IC50 = 37.6 μM/MCF-7 cells | ||||
| IC50 = 14.1 μM/W-480 cells | |||||
| 48 | In vitro | IC50 = 11.3 μM/A-549 cells | Doxorubicin | IC50 = 0.02 μM/A-549, HepG-2 and W-480 cells | 53 |
| IC50 = 9.4 μM/BGC-823 cells | IC50 = 0.01 μM/BGC-823 cells | ||||
| IC50 = 10.1 μM/HepG-2 cells | IC50 = 0.03 μM/HL-60 cells | ||||
| IC50 = 11.1 μM/HL-60 cells | IC50 = 0.04 μM/SMMC-7721 cells | ||||
| IC50 = 10.4 μM/MCF-7 cells | |||||
| IC50 = 9.7 μM/SMMC-7721 cells | |||||
| IC50 = 11.7 μM/W-480 cells | |||||
| 49 | In vitro | IC50 = 12.7 μM/A-549 cells | Doxorubicin | IC50 = 0.02 μM/A-549, HepG-2 and W-480 cells | 53 |
| IC50 = 12.2 μM/BGC-823 cells | IC50 = 0.01 μM/BGC-823 cells | ||||
| IC50 = 12.8 μM/HepG-2 cells | IC50 = 0.03 μM/HL-60 cells | ||||
| IC50 = 13.8 μM/HL-60 cells | IC50 = 0.04 μM/SMMC-7721 cells | ||||
| IC50 = 14.3 μM/MCF-7 and SMMC-7721 cells | |||||
| IC50 = 15.9 μM/W-480 cells | |||||
| 50 | In vitro | IC50 = 31.9 μM/A-549 cells | Doxorubicin | IC50 = 0.02 μM/A-549, HepG-2 and W-480 cells | 53 |
| IC50 = 31.2 μM/BGC-823 cells | IC50 = 0.01 μM/BGC-823 cells | ||||
| IC50 = 30.7 μM/HepG-2 cells | IC50 = 0.03 μM/HL-60 cells | ||||
| IC50 = 32.2 μM/HL-60 cells | IC50 = 0.04 μM/SMMC-7721 cells | ||||
| IC50 = 28.1 μM/MCF-7 cells | |||||
| IC50 = 29.9 μM/SMMC-7721 cells | |||||
| IC50 = 27.6 μM/W-480 cells | |||||
| 51 | In vitro | IC50 = 29.7 μM/A-549 cells | Doxorubicin | IC50 = 0.02 μM/A-549, HepG-2 and W-480 cells | 53 |
| IC50 = 29.6 μM/BGC-823 cells | IC50 = 0.01 μM/BGC-823 cells | ||||
| IC50 = 29.4 μM/HepG-2 and HL-60 cells | IC50 = 0.03 μM/HL-60 cells | ||||
| IC50 = 27.1 μM/MCF-7 cells | IC50 = 0.04 μM/SMMC-7721 cells | ||||
| IC50 = 30.1 μM/SMMC-7721 cells | |||||
| IC50 = 24.9 μM/W-480 cells | |||||
| 52 | In vitro | IC50 = 76.3 μM/A-549 cells | Doxorubicin | IC50 = 0.02 μM/A-549, HepG-2 and W-480 cells | 53 |
| IC50 = 68.7 μM/BGC-823 cells | IC50 = 0.01 μM/BGC-823 cells | ||||
| IC50 = 66.8 μM/HepG-2 cells | IC50 = 0.03 μM/HL-60 cells | ||||
| IC50 = 72.3 μM/HL-60 cells | IC50 = 0.04 μM/SMMC-7721 cells | ||||
| IC50 = 76.2 μM/MCF-7 cells | |||||
| IC50 = 70.8 μM/SMMC-7721 cells | |||||
| IC50 = 69.4 μM/W-480 cells | |||||
| 53 | In vitro | IC50 = 80.2 μM/A-549 cells | Doxorubicin | IC50 = 0.02 μM/A-549, HepG-2 and W-480 cells | 53 |
| IC50 = 78.8 μM/BGC-823 cells | IC50 = 0.01 μM/BGC-823 cells | ||||
| IC50 = 79.4 μM/HepG-2 cells | IC50 = 0.03 μM/HL-60 cells | ||||
| IC50 = 80.3 μM/HL-60 cells | IC50 = 0.04 μM/SMMC-7721 cells | ||||
| IC50 = 80.5 μM/MCF-7 cells | |||||
| IC50 = 81.6 μM/SMMC-7721 cells | |||||
| IC50 = 81.8 μM/W-480 cells | |||||
| 54 | In vitro | IC50 = 15.8 μM/BGC-823 cells | Doxorubicin | IC50 = 0.02 μM/BGC-823 cells | 54 |
| IC50 = 16.8 μM/HepG-2 cells | IC50 = 0.01 μM/HepG-2 and SK-OV-3 cells | ||||
| IC50 = 16.5 μM/MCF-7 cells | IC50 = 0.06 μM/MCF-7 cells | ||||
| IC50 = 18.7 μM/SGC-7901 cells | IC50 = 0.05 μM/SGC-7901 cells | ||||
| IC50 = 19.7 μM/SK-MEL-2 cells | IC50 = 0.03 μM/SK-MEL-2 cells | ||||
| IC50 = 17.6 μM/SK-OV-3 cells | |||||
| 55 | In vitro | IC50 = 13.8 μM/BGC-823 cells | Doxorubicin | IC50 = 0.02 μM/BGC-823 cells | 54 |
| IC50 = 12.4 μM/HepG-2 cells | IC50 = 0.01 μM/HepG-2 and SK-OV-3 cells | ||||
| IC50 = 14.8 μM/MCF-7 cells | IC50 = 0.06 μM/MCF-7 cells | ||||
| IC50 = 13.9 μM/SGC-7901 and SK-OV-3 cells | IC50 = 0.05 μM/SGC-7901 cells | ||||
| IC50 = 12.6 μM/SK-MEL-2 cells | IC50 = 0.03 μM/SK-MEL-2 cells | ||||
| 56 | In vitro | IC50 = 7.3 μM/BGC-823 cells | Doxorubicin | IC50 = 0.02 μM/BGC-823 cells | 54 |
| IC50 = 8.6 μM/HepG-2 cells | IC50 = 0.01 μM/HepG-2 and SK-OV-3 cells | ||||
| IC50 = 8.2 μM/MCF-7 cells | IC50 = 0.06 μM/MCF-7 cells | ||||
| IC50 = 9.5 μM/SGC-7901 cells | IC50 = 0.05 μM/SGC-7901 cells | ||||
| IC50 = 8.9 μM/SK-MEL-2 cells | IC50 = 0.03 μM/SK-MEL-2 cells | ||||
| IC50 = 8.6 μM/SK-OV-3 cells | |||||
| 57 | In vitro | IC50 = 9.5 μM/BGC-823 cells | Doxorubicin | IC50 = 0.02 μM/BGC-823 cells | 54 |
| IC50 = 10.6 μM/HepG-2 cells | IC50 = 0.01 μM/HepG-2 and SK-OV-3 cells | ||||
| IC50 = 9.3 μM/MCF-7 cells | IC50 = 0.06 μM/MCF-7 cells | ||||
| IC50 = 10.4 μM/SGC-7901 cells | IC50 = 0.05 μM/SGC-7901 cells | ||||
| IC50 = 9.2 μM/SK-MEL-2 cells | IC50 = 0.03 μM/SK-MEL-2 cells | ||||
| IC50 = 10.3 μM/SK-OV-3 cells | |||||
| 58 | In vitro | IC50 = 33.1 μM/BGC-823 cells | Doxorubicin | IC50 = 0.02 μM/BGC-823 cells | 54 |
| IC50 = 32.4 μM/HepG-2 cells | IC50 = 0.01 μM/HepG-2 and SK-OV-3 cells | ||||
| IC50 = 29.7 μM/MCF-7 cells | IC50 = 0.06 μM/MCF-7 cells | ||||
| IC50 = 30.9 μM/SGC-7901 cells | IC50 = 0.05 μM/SGC-7901 cells | ||||
| IC50 = 27.1 μM/SK-MEL-2 cells | IC50 = 0.03 μM/SK-MEL-2 cells | ||||
| IC50 = 30.1 μM/SK-OV-3 cells | |||||
| 59 | In vitro | IC50 = 12.9 μM/95-D cells | Doxorubicin | IC50 = 24.7 μM/95-D cells | 55 |
| IC50 = 12.4 μM/A-549 cells | IC50 = 21.8 μM/A-549 cells | ||||
| IC50 = 13.8 μM/ATCC cells | IC50 = 33.7 μM/ATCC cells | ||||
| IC50 = 14.8 μM/H-446 cells | IC50 = 22.3 μM/H-446 cells | ||||
| IC50 = 13.3 μM/H-460 cells | IC50 = 14.1 μM/H-460 cells | ||||
| IC50 = 12.6 μM/H-292 cells | IC50 = 13.7 μM/H-292 cells | ||||
| IC50 = 13.9 μM/SPCA-1 cells | IC50 = 14.1 μM/SPCA-1 cells | ||||
| 60 | In vitro | IC50 = 46.8 μM/95-D cells | Doxorubicin | IC50 = 24.7 μM/95-D cells | 55 |
| IC50 = 47.1 μM/ATCC cells | IC50 = 33.7 μM/ATCC cells | ||||
| IC50 = 46.6 μM/H-446 cells | IC50 = 22.3 μM/H-446 cells | ||||
| IC50 = 45.9 μM/H-292 cells | IC50 = 13.7 μM/H-292 cells | ||||
| 61 | In vitro | IC50 = 9.5 μM/95-D cells | Doxorubicin | IC50 = 24.7 μM/95-D cells | 55 |
| IC50 = 8.6 μM/A-549 cells | IC50 = 21.8 μM/A-549 cells | ||||
| IC50 = 9.3 μM/ATCC and H-292 cells | IC50 = 33.7 μM/ATCC cells | ||||
| IC50 = 9.4 μM/H-446 cells | IC50 = 22.3 μM/H-446 cells | ||||
| IC50 = 9.2 μM/H-460 cells | IC50 = 14.1 μM/H-460 cells | ||||
| IC50 = 9.7 μM/SPCA-1 cells | IC50 = 13.7 μM/H-292 cells | ||||
| IC50 = 14.1 μM/SPCA-1 cells | |||||
| 73 | In vitro | CD50 = 20.7 μg mL−1/NIH/3T3 cells | Vincristine | CD50 > 60 μg mL−1/NIH/3T3 cells | 49 |
| CD50 = 0.9 μg mL−1/HL-60 cells | CD50 = 1.8 μg mL−1/HL-60 cells | ||||
| CD50 = 36.5 μg mL−1/HeLa cells | CD50 = 0.4 μg mL−1/HeLa cells | ||||
| 74 | In vitro | To suppress the bound of [3H]azidopine to P-glycoprotein | 61 | ||
| 76 | In vitro | IC50 = 67.3 μM/HS-4 cells | Adiamycin | IC50 = 24.7 μM/HS-4 cells | 52 |
| IC50 = 74.2 μM/A-431 cells | IC50 = 33.7 μM/A-431 cells | ||||
| IC50 = 66.2 μM/W-480 cells | IC50 = 14.1 μM/W-480 cells | ||||
| 88 | In vitro | IC50 = 38.7 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 43 |
| 93 | In vitro | IC50 = 19.5 μg mL−1/KB cells | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 32 |
| IC50 = 18.0 μg mL−1/KB (VJ300) cells | |||||
| IC50 = 3.80 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | |||||
| 102 | In vitro | IC50 = 15.0 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 23 |
| 103 | In vitro | IC50 = 3.9 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 23 |
| 104 | In vitro | IC50 = 13.0 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 23 |
| 105 | In vitro | IC50 = 18.2 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 23 |
| 106 | In vitro | IC50 = 9.2 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 23 |
| 107 | In vitro | IC50 = 18.0 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 23 |
| 214 | In vitro | IC50 = 29.7 μM/BGC-823 cells | Doxorubicin | IC50 = 29.7 μM/BGC-823 cells | 85 |
| IC50 = 37.6 μM/HepG-2 cells | IC50 = 37.6 μM/HepG-2 cells | ||||
| IC50 = 35.8 μM/MCF-7 cells | IC50 = 35.8 μM/MCF-7 cells | ||||
| IC50 = 36.8 μM/SGC-7901 cells | IC50 = 36.8 μM/SGC-7901 cells | ||||
| IC50 = 36.5 μM/SK-MEL-2 cells | IC50 = 36.5 μM/SK-MEL-2 cells | ||||
| 215 | In vitro | IC50 = 32.1 μM/BGC-823 cells | Doxorubicin | IC50 = 29.7 μM/BGC-823 cells | 85 |
| IC50 = 29.8 μM/HepG-2 cells | IC50 = 37.6 μM/HepG-2 cells | ||||
| IC50 = 31.9 μM/MCF-7 cells | IC50 = 35.8 μM/MCF-7 cells | ||||
| IC50 = 27.9 μM/SGC-7901 cells | IC50 = 36.8 μM/SGC-7901 cells | ||||
| IC50 = 33.3 μM/SK-MEL-2 cells | IC50 = 36.5 μM/SK-MEL-2 cells | ||||
| 237 | In vitro | IC50 = 8.6 μM/BGC-823 cells | Doxorubicin | IC50 = 29.7 μM/BGC-823 cells | 85 |
| IC50 = 7.2 μM/HepG-2 cells | IC50 = 37.6 μM/HepG-2 cells | ||||
| IC50 = 8.3 μM/MCF-7 cells | IC50 = 35.8 μM/MCF-7 cells | ||||
| IC50 = 8.2 μM/SGC-7901 cells | IC50 = 36.8 μM/SGC-7901 cells | ||||
| IC50 = 8.9 μM/SK-MEL-2 cells | IC50 = 36.5 μM/SK-MEL-2 cells | ||||
| 282 | In vitro | IC50 = 9.7 μg mL−1/PC-3 cells | Cisplatin | IC50 = 1.5 μg mL−1/PC-3 cells | 19 |
| IC50 = 15.9 μg mL−1/HCT-116 cells | IC50 = 3.2 μg mL−1/HCT-116 cells | ||||
| IC50 = 14.1 μg mL−1/MCF-7 cells | IC50 = 4.2 μg mL−1/MCF-7 cells | ||||
| IC50 > 25 μg mL−1/A-549 and KB (VJ300) cells | IC50 = 4.3 μg mL−1/A-549 cells | ||||
| IC50 = 8.6 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Verapamil | IC50 = 4.7 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | |||
| 289 | In vitro | IC50 = 7.1 μg mL−1/PC-3 cells | Cisplatin | IC50 = 1.5 μg mL−1/PC-3 cells | 19 |
| IC50 = 7.6 μg mL−1/HCT-116 cells | IC50 = 3.2 μg mL−1/HCT-116 cells | ||||
| IC50 = 9.7 μg mL−1/MCF-7 cells | IC50 = 4.2 μg mL−1/MCF-7 cells | ||||
| IC50 = 20.4 μg mL−1/A-549 cells | IC50 = 4.3 μg mL−1/A-549 cells | ||||
| IC50 = 23 μg mL−1/KB (VJ300) cells | |||||
| IC50 = 4.80 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Verapamil | IC50 = 4.7 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | |||
| 302 | In vitro | CD50 > 60 μg mL−1/NIH/3T3 cells | Vincristine | CD50 > 60 μg mL−1/NIH/3T3 cells | 49 |
| CD50 = 30.2 μg mL−1/HL-60 cells | CD50 = 1.8 μg mL−1/HL-60 cells | ||||
| CD50 = 2.8 μg mL−1/HeLa cells | CD50 = 0.4 μg mL−1/HeLa cells | ||||
| 327 | In vitro | CD50 = 6.4 μg mL−1/NIH/3T3 cells | Vincristine | CD50 > 60 μg mL−1/NIH/3T3 cells | 49 |
| CD50 > 60 μg mL−1/HL-60 cells | CD50 = 1.8 μg mL−1/HL-60 cells | ||||
| CD50 = 7.5 μg mL−1/HeLa cells | CD50 = 0.4 μg mL−1/HeLa cells | ||||
| 333 | In vitro | IC50 = 1.3 μg mL−1/HT-29 cells | Cisplatin | IC50 = 8.8 μg mL−1/HT-29 cells | 8 |
| IC50 = 4.9 μg mL−1/MCF-7 cells | IC50 = 6.6 μg mL−1/MCF-7 cells | ||||
| IC50 = 4.7 μg mL−1/PC-3 cells | IC50 = 4.2 μg mL−1/PC-3 cells | ||||
| IC50 = 7.0 μg mL−1/MDA-MB -231 cells | IC50 = 2.1 μg mL−1/MDA-MB -231 cells | ||||
| IC50 = 7.3 μg mL−1/HCT-116 cells | IC50 = 4.6 μg mL−1/HCT-116 cells | ||||
| IC50 = 9.6 μg mL−1/A-549 cells | IC50 = 5.4 μg mL−1/A-549 cells | ||||
| IC50 = 3.0 μg mL−1/KB (VJ300) cells | Vincristine | IC50 = 0.8 μg mL−1/KB (VJ300) cells | |||
| 366 | In vitro | IC50 = 3.70 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 103 |
| 367 | In vitro | IC50 = 7.0 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 103 |
| 373 | In vitro | IC50 = 4.1 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 103 |
| 374 | In vitro | IC50 = 3.2 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 103 |
| 375 | In vitro | IC50 = 11.2 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 103 |
| 402 | In vitro | IC50 = 5.38 μM/A-549 cells | Docetaxel | IC50 = 4.95 × 10−4 μM/A-549 cells | 114 |
| IC50 = 4.67 μM/HT-29 cells | IC50 = 3.34 × 10−4 μM/HT-29 cells | ||||
| 403 | In vitro | IC50 = 7.44 μM/A-549 cells | Docetaxel | IC50 = 4.95 × 10−4 μM/A-549 cells | 114 |
| IC50 = 6.39 μM/HT-29 cells | IC50 = 3.34 × 10−4 μM/HT-29 cells | ||||
| 404 | In vitro | IC50 = 8.21 μM/A-549 cells | Docetaxel | IC50 = 4.95 × 10−4 μM/A-549 cells | 114 |
| IC50 = 8.89 μM/HT-29 cells | IC50 = 3.34 × 10−4 μM/HT-29 cells | ||||
| 407 | In vitro | IC50 = 0.24 μg mL−1/KB cells | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 32 |
| IC50 = 0.25 μg mL−1/KB (VJ300) cells | |||||
| IC50 = 0.30 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | |||||
| 408 | In vitro | CD50 = 20.8 μg mL−1/NIH/3T3 cells | Vincristine | CD50 > 60 μg mL−1/NIH/3T3 cells | 49 |
| CD50 > 60 μg mL−1/HL-60 cells | CD50 = 1.8 μg mL−1/HL-60 cells | ||||
| CD50 = 2.9 μg mL−1/HeLa cells | CD50 = 0.4 μg mL−1/HeLa cells | ||||
| IC50 = 0.19 μg mL−1/KB cells | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 32 | ||
| IC50 = 0.25 μg mL−1/KB (VJ300) cells | |||||
| IC50 = 0.34 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | |||||
| 409 | In vitro | IC50 = 0.35 μM/A-549 and HT-29 cells | Docetaxel | IC50 = 4.95 × 10−4 μM/A-549 cells | 114 |
| IC50 = 1.25 μg mL−1/KB cells | IC50 = 3.34 × 10−4 μM/HT-29 cells | ||||
| IC50 = 2.50 μg mL−1/KB (VJ300) cells | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 32 | ||
| IC50 = 1.85 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | |||||
| 411 | In vitro | IC50 = 4.35 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 72 |
| 413 | In vitro | IC50 = 4.11 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 72 |
| 417 | In vitro | IC50 = 0.39 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 72 |
| 434 | In vitro | IC50 = 21.8 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 22 |
| 437 | In vitro | IC50 = 15.0 μg mL−1/KB cells | Vincadifformine | IC50 = 10.2 μg mL−1/KB cells | 10 |
| IC50 = 11.0 μg mL−1/KB (VJ300) cells | IC50 = 6.3 μg mL−1/KB (VJ300) cells | ||||
| IC50 = 3.8 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | IC50 = 4.5 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | ||||
| 438 | In vitro | IC50 = 6.4 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | Vincristine | IC50 = 1.0 μg mL−1/KB (VJ300) | 22 |
| 446 | In vitro | IC50 = 0.25 μg mL−1/Jurkat cells | Vincadifformine | IC50 = 21.8 μg mL−1/Jurkat cells | 10 |
| IC50 = 3.6 μg mL−1/KB cells | IC50 = 10.2 μg mL−1/KB cells | ||||
| IC50 = 0.75 μg mL−1/KB (VJ300) cells | IC50 = 6.3 μg mL−1/KB (VJ300) cells | ||||
| IC50 = 0.46 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | IC50 = 4.5 μg mL−1/KB (VJ300) + 0.1 μg mL−1 vincristine | ||||
| 450 and 452 | In vitro | IC50 > 30 μM/A-549 cells | Docetaxel | IC50 = 4.95 × 10−4 μM/A-549 cells | 114 |
| IC50 = 30 μM/HT-29 cells | IC50 = 3.34 × 10−4 μM/HT-29 cells | ||||
| 463 | In vitro | IC50 = 6.2 μM/HT-29 cells | 59 | ||
| 467 | In vitro | IC50 = 15.5 μg mL−1/MCF-7 cells | 122 | ||
| 468 | In vitro | IC50 = 22.5 μg mL−1/MCF-7 cells | 122 | ||
| 469 | In vitro | IC50 = 21.5 μg mL−1/MCF-7 cells | 122 | ||
| 470 | In vitro | IC50 = 17 μg mL−1/MCF-7 cells | 122 | ||
| 471 | In vitro | IC50 = 26 μg mL−1/MCF-7 cells | 122 | ||
| 472 | In vitro | IC50 = 14.5 μg mL−1/MCF-7 cells | 122 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Anti-microbial activity | |||||
| 14 | In vitro | MIC = 31.3 μg mL−1/E. coli, E. carotovra, B. subtilis, B. cereus, and S. aureus | Ampicillin | MIC = 100 μg mL−1/E. coli and E. carotovra | 7 |
| MIC = 15.5 μg mL−1/E. carotovra | MIC = 12.5 μg mL−1/B. subtilis | ||||
| MIC = 25.0 μg mL−1/B. cereus and S. aureus | |||||
| EC50 = 33.3 μg mL−1/R. solani | Mildothane | EC50 = 17.0 μg mL−1/R. solani | |||
| EC50 = 29.2 μg mL−1/P. italicum | EC50 = 7.8 μg mL−1/P. italicum | ||||
| EC50 = 16.3 μg mL−1/F. oxysporum f. sp. Cubense | EC50 = 57.0 μg mL−1/F. oxysporum f. sp. Cubense | ||||
| EC50 = 31.8 μg mL−1/F. oxysporum f. sp. Niveum | EC50 = 101.0 μg mL−1/F. oxysporum f. sp. Niveum | ||||
| 43 | In vitro | IZ = 11 mm/K. pneumoniae | Sanguinarine | IZ = 25 mm/S. mutans and S. viridans | 52 |
| IZ = 10 mm/E. coli, S. aureus and S. viridans | Netilmicin | IZ = 21 mm/S. aureus | |||
| IZ = 9 mm/C. glabrata, E. cloacae and S. mutans | IZ = 8 mm/S. epidermidis and K. pneumoniae | ||||
| IZ = 8 mm/S. epidermidis and S. dysenteriae | IZ = 24 mm/E. coli | ||||
| IZ = 7 mm/C. albicans, C. tropicalis and P. aeruginosa | IZ = 22 mm/E. cloacae | ||||
| IZ = 23 mm/P. aeruginosa and S. dysenteriae | |||||
| 44 | In vitro | IZ = 12 mm/P. aeruginosa and S. mutans | Sanguinarine | IZ = 25 mm/S. mutans and S. viridans | 52 |
| IZ = 11 mm/E. coli | Netilmicin | IZ = 21 mm/S. aureus | |||
| IZ = 10 mm/C. glabrata | IZ = 8 mm/S. epidermidis and K. pneumoniae | ||||
| IZ = 9 mm/E. cloacae, S. aureus and S. dysenteriae | IZ = 24 mm/E. coli | ||||
| IZ = 8 mm/C. albicans, K. pneumoniae and S. epidermidis | IZ = 22 mm/E. cloacae | ||||
| IZ = 7 mm/C. tropicalis and S. viridans | IZ = 23 mm/P. aeruginosa and S. dysenteriae | ||||
| 45 | In vitro | IZ = 18 mm and MIC = 0.77 mM/K. pneumoniae | Sanguinarine | IZ = 25 mm/S. mutans and S. viridans | 52 |
| IZ = 18 mm and MIC = 0.87 mM/S. viridans | Netilmicin | IZ = 21 mm/S. aureus | |||
| IZ = 17 mm and MIC = 0.89 mM/E. coli | IZ = 8 mm/S. epidermidis and K. pneumoniae | ||||
| IZ = 18 mm and MIC = 0.97 mM/S. aureus and S. epidermidis | IZ = 24 mm/E. coli | ||||
| IZ = 18 mm and MIC = 0.97 mM/E. cloacae | IZ = 22 mm/E. cloacae | ||||
| IZ = 19 mm and MIC = 1.01 mM/P. aeruginosa | IZ = 23 mm/P. aeruginosa and S. dysenteriae | ||||
| IZ = 18 mm and MIC = 1.13 mM/S. mutans | |||||
| IZ = 19 mm and MIC = 1.18 mM/C. tropicalis | |||||
| IZ = 18 mm and MIC = 2.68 mM/S. dysenteriae | |||||
| IZ = 17 mm and MIC = 2.87 mM/C. albicans | |||||
| IZ = 17 mm and MIC = 3.09 mM/C. glabrata | |||||
| 46 | In vitro | IZ = 20 mm and MIC = 0.72 mM/E. coli | Sanguinarine | IZ = 25 mm/S. mutans and S. viridans | 52 |
| IZ = 20 mm and MIC = 0.82 mM/S. mutans | |||||
| IZ = 20 mm and MIC = 0.91 mM/S. epidermidis | |||||
| IZ = 20 mm and MIC = 1.03 mM/S. dysenteriae | |||||
| IZ = 20 mm and MIC = 1.11 mM/S. viridans | |||||
| IZ = 20 mm and MIC = 1.18 mM/P. aeruginosa | Netilmicin | IZ = 21 mm/S. aureus | |||
| IZ = 19 mm and MIC = 1.20 mM/E. cloacae | IZ = 8 mm/S. epidermidis and K. pneumoniae | ||||
| IZ = 20 mm and MIC = 1.23 mM/C. tropicalis and S. aureus | IZ = 24 mm/E. coli | ||||
| IZ = 17 mm and MIC = 1.32 mM/C. glabrata | IZ = 22 mm/E. cloacae | ||||
| IZ = 21 mm and MIC = 1.37 mM/K. pneumoniae | IZ = 23 mm/P. aeruginosa and S. dysenteriae | ||||
| IZ = 17 mm and MIC = 2.87 mM/C. albicans | |||||
| 47 | In vitro | IZ = 24 mm and MIC = 0.15 mM/E. coli | Sanguinarine | IZ = 25 mm/S. mutans and S. viridans | 52 |
| IZ = 24 mm and MIC = 0.20 mM/S. epidermidis | |||||
| IZ = 23 mm and MIC = 0.22 mM/C. glabrata | |||||
| IZ = 23 mm and MIC = 0.30 mM/C. tropicalis | |||||
| IZ = 24 mm and MIC = 0.30 mM/S. dysenteriae and C. albicans | |||||
| IZ = 24 mm and MIC = 0.25 mM/S. aureus | Netilmicin | IZ = 21 mm/S. aureus | |||
| IZ = 24 mm and MIC = 0.27 mM/E. cloacae | IZ = 8 mm/S. epidermidis and K. pneumoniae | ||||
| IZ = 24 mm and MIC = 0.32 mM/P. aeruginosa | IZ = 24 mm/E. coli | ||||
| IZ = 23 mm and MIC = 0.37 mM/K. pneumoniae | IZ = 22 mm/E. cloacae | ||||
| IZ = 23 mm and MIC = 0.87 mM/S. viridans | IZ = 23 mm/P. aeruginosa and S. dysenteriae | ||||
| IZ = 24 mm and MIC = 1.14 mM/S. mutans | |||||
| 48 | In vitro | IZ = 23 mm and MIC = 0.12 mM/K. pneumoniae | Netilmicin | IZ = 25 mm and MIC = 0.009 mM/K. pneumoniae | 53 |
| IZ = 24 mm and MIC = 0.12 mM/S. dysenteriae | IZ = 23 mm and MIC = 0.011 mM/S. dysenteriae | ||||
| IZ = 24 mm and MIC = 0.13 mM/P. aeruginosa | IZ = 23 mm and MIC = 0.015 mM/P. aeruginosa | ||||
| IZ = 23 mm and MIC = 0.15 mM/E. cloacae | IZ = 22 mm and MIC = 0.01 mM/E. cloacae | ||||
| IZ = 23 mm and MIC = 0.16 mM/S. epidermidis | IZ = 25 mm and MIC = 0.004 mM/S. epidermidis | ||||
| IZ = 24 mm and MIC = 0.18 mM/S. aureus | IZ = 21 mm and MIC = 0.005 mM/S. aureus | ||||
| IZ = 24 mm and MIC = 0.23 mM/E. coli | IZ = 24 mm and MIC = 0.015 mM/E. coli | ||||
| 49 | In vitro | IZ = 24 mm and MIC = 0.14 mM/K. pneumoniae | Netilmicin | IZ = 25 mm and MIC = 0.009 mM/K. pneumoniae | 53 |
| IZ = 23 mm and MIC = 0.16 mM/P. aeruginosa | IZ = 23 mm and MIC = 0.015 mM/P. aeruginosa | ||||
| IZ = 24 mm and MIC = 0.17 mM/S. aureus | IZ = 21 mm and MIC = 0.005 mM/S. aureus | ||||
| IZ = 22 mm and MIC = 0.18 mM/S. dysenteriae | IZ = 22 mm and MIC = 0.01 mM/E. cloacae | ||||
| IZ = 24 mm and MIC = 0.19 mM/E. cloacae | IZ = 25 mm and MIC = 0.004 mM/S. epidermidis | ||||
| IZ = 23 mm and MIC = 0.19 mM/S. epidermidis | IZ = 24 mm and MIC = 0.015 mM/E. coli | ||||
| IZ = 24 mm and MIC = 0.26 mM/E. coli | |||||
| 50 | In vitro | IZ = 18 mm and MIC = 0.94 mM/P. aeruginosa | Netilmicin | IZ = 23 mm and MIC = 0.015 mM/P. aeruginosa | 53 |
| IZ = 17 mm and MIC = 1.10 mM/E. cloacae | IZ = 22 mm and MIC = 0.01 mM/E. cloacae | ||||
| IZ = 17 mm and MIC = 1.12 mM/K. pneumoniae and S. dysenteriae | IZ = 25 mm and MIC = 0.009 mM/K. pneumoniae | ||||
| IZ = 18 mm and MIC = 1.20 mM/S. aureus | IZ = 23 mm and MIC = 0.011 mM/S. dysenteriae | ||||
| IZ = 19 mm and MIC = 1.23 mM/S. epidermidis | IZ = 21 mm and MIC = 0.005 mM/S. aureus | ||||
| IZ = 18 mm and MIC = 1.32 mM/E. coli | IZ = 25 mm and MIC = 0.004 mM/S. epidermidis | ||||
| IZ = 24 mm and MIC = 0.015 mM/E. coli | |||||
| 51 | In vitro | IZ = 17 mm and MIC = 0.92 mM/P. aeruginosa | Netilmicin | IZ = 23 mm and MIC = 0.015 mM/P. aeruginosa | 53 |
| IZ = 18 mm and MIC = 1.01 mM/E. cloacae | IZ = 22 mm and MIC = 0.01 mM/E. cloacae | ||||
| IZ = 19 mm and MIC = 1.02 mM/S. dysenteriae | IZ = 23 mm and MIC = 0.011 mM/S. dysenteriae | ||||
| IZ = 18 mm and MIC = 1.09 mM/K. pneumoniae | IZ = 25 mm and MIC = 0.009 mM/K. pneumoniae | ||||
| IZ = 19 mm and MIC = 1.15 mM/S. epidermidis | IZ = 25 mm and MIC = 0.004 mM/S. epidermidis | ||||
| IZ = 20 mm and MIC = 1.18 mM/S. aureus | IZ = 21 mm and MIC = 0.005 mM/S. aureus | ||||
| IZ = 17 mm and MIC = 1.24 mM/E. coli | IZ = 24 mm and MIC = 0.015 mM/E. coli | ||||
| 52 | In vitro | IZ = 17 mm and MIC = 1.19 mM/K. pneumoniae | Netilmicin | IZ = 25 mm and MIC = 0.009 mM/K. pneumoniae | 53 |
| IZ = 18 mm and MIC = 1.21 mM/E. coli | IZ = 24 mm and MIC = 0.015 mM/E. coli | ||||
| IZ = 17 mm and MIC = 1.21 mM/P. aeruginosa | IZ = 23 mm and MIC = 0.015 mM/P. aeruginosa | ||||
| IZ = 17 mm and MIC = 1.31 mM/E. cloacae | IZ = 22 mm and MIC = 0.01 mM/E. cloacae | ||||
| IZ = 15 mm and MIC = 1.31 mM/S. dysenteriae | IZ = 23 mm and MIC = 0.011 mM/S. dysenteriae | ||||
| 53 | In vitro | IZ = 16 mm and MIC = 0.99 mM/K. pneumoniae | Netilmicin | IZ = 25 mm and MIC = 0.009 mM/K. pneumoniae | 53 |
| IZ = 18 mm and MIC = 1.01 mM/S. dysenteriae | IZ = 23 mm and MIC = 0.011 mM/S. dysenteriae | ||||
| IZ = 17 mm and MIC = 1.24 mM/P. aeruginosa | IZ = 23 mm and MIC = 0.015 mM/P. aeruginosa | ||||
| IZ = 15 mm and MIC = 1.31 mM/E. coli | IZ = 24 mm and MIC = 0.015 mM/E. coli | ||||
| IZ = 17 mm and MIC = 1.32 mM/E. cloacae | IZ = 22 mm and MIC = 0.01 mM/E. cloacae | ||||
| 74 | In vitro | IZ = 9.7 mm/S. aureus | Kanamycin sulfate | IZ = 24.7 mm/S. aureus | 12 |
| 76 | In vitro | IZ = 13 mm/S. aureus | Kanamycin sulfate | IZ = 24.7 mm/S. aureus | 12 |
| IZ = 12 mm/S. epidermidis | |||||
| IZ = 9 mm/ C. albicans and C. glabrata | |||||
| IZ = 8 mm/C. tropicalis, S. mutans and S. dysenteriae | |||||
| IZ = 7 mm/E. coli and K. pneumoniae | |||||
| 85 | In vitro | IZ = 11.2 mm/S. aureus | Kanamycin sulfate | IZ = 24.7 mm/S. aureus | 12 |
| 86 | In vitro | IZ = 9.1 mm/S. aureus | Kanamycin sulfate | IZ = 24.7 mm/S. aureus | 12 |
| 87 | In vitro | IZ = 10.3 mm/S. aureus | Kanamycin sulfate | IZ = 24.7 mm/S. aureus | 12 |
| 206 | In vitro | MIC = 15.5 μg mL−1/E. coli, Erwinia carotovra, Bacillus subtilis, B. cereus, and S. aureus | Ampicillin | MIC = 100 μg mL−1/E. coli and E. carotovra | 7 |
| MIC = 7.8 μg mL−1/E. carotovra | |||||
| EC50 = 21.9 μg mL−1/R. solani | Mildothane | MIC = 12.5 μg mL−1/B. subtilis | |||
| EC50 = 19.4 μg mL−1/P. italicum | MIC = 25.0 μg mL−1/B. cereus and S. aureus | ||||
| EC50 = 15.2 μg mL−1/F. oxysporum f. sp. Cubense | EC50 = 17.0 μg mL−1/R. solani | ||||
| EC50 = 43.8 μg mL−1/F. oxysporum f. sp. Niveum | EC50 = 7.8 μg mL−1/P. italicum | ||||
| EC50 = 57.0 μg mL−1/F. oxysporum f. sp. Cubense | |||||
| EC50 = 101.0 μg mL−1/F. oxysporum f. sp. Niveum | |||||
| 267 and 297 | In vitro | MIC = 32 μg mL−1/E. coli | 21 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Anti-inflammatory activity | |||||
| 11 | In vitro | IC50 = 25.4 μM/T cell inhibition | 16 | ||
| 170 | In vitro | IC50 = 21.6 μM/T cell inhibition | 16 | ||
| 222 | In vitro | IC50 = 27.8 μM/T cell inhibition | 16 | ||
| 409 | In vitro | IC50 = 1.0 μM/T cell inhibition | 16 | ||
| To arrest the G2/M phase of the T cell cycle | |||||
| To decrease IL-6 and IL-17 levels in T cells | |||||
| 219, 225, 228, 279–280, 291, and 439 | In vitro | The inhibitory effects on IL-1β and TNF-α, and PGE2 were comparable with positive control dexamethasone | 75 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Anti-allergic activity | |||||
| 90 | In vitro | IC10 = 3.73 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | Ketotifen fumarate | IC10 = 1.37 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | 66 |
| 126 | In vitro | IC10 = 7.06 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | Ketotifen fumarate | IC10 = 1.37 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | 66 |
| 257 | In vitro | IC10 = 5.51 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | Ketotifen fumarate | IC10 = 1.37 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | 66 |
| 448 | In vitro | IC10 = 11.78 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | Ketotifen fumarate | IC10 = 1.37 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | 66 |
| The MeOH extract of K. larutensis bark | In vitro | IC10 = 2.17 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | Ketotifen fumarate | IC10 = 1.37 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | 66 |
| The MeOH extract of K. arborea bark | In vitro | IC10 = 3.82 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | Ketotifen fumarate | IC10 = 1.37 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | 129 |
| The MeOH extract of K. larutensis leaf | In vitro | IC10 = 3.01 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | Ketotifen fumarate | IC10 = 1.37 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | 66 |
| The MeOH extract of K. arborea leaf | In vitro | IC10 = 2.58 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | Ketotifen fumarate | IC10 = 1.37 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | 129 |
| The MeOH extract of K. larutensis root | In vitro | IC10 = 1.61 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | Ketotifen fumarate | IC10 = 1.37 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | 66 |
| The MeOH extract of K. arborea root | In vitro | IC10 = 4.32 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | Ketotifen fumarate | IC10 = 1.37 μg mL−1/histamine and β-hexosaminidase inhibition in RBL-2H3 cell | 129 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Anti-diabetic activity | |||||
| 29 | In vitro | EC50 = 24.5 μM/glucose-evoked podocyte injury inhibition | Astragaloside IV | EC50 = 15.4 μM/glucose-evoked podocyte injury inhibition | 25 |
| 126 | In vitro | EC50 = 3.0 μM/glucose-evoked podocyte injury inhibition | Astragaloside IV | EC50 = 15.4 μM/glucose-evoked podocyte injury inhibition | 25 |
| 224 | In vitro | EC50 = 10.2 μM/glucose-evoked podocyte injury inhibition | Astragaloside IV | EC50 = 15.4 μM/glucose-evoked podocyte injury inhibition | 25 |
| 264 | In vitro | EC50 = 12.0 μM/glucose-evoked podocyte injury inhibition | Astragaloside IV | EC50 = 15.4 μM/glucose-evoked podocyte injury inhibition | 25 |
| 405 | In vitro | EC50 = 3.80 μM/glucose-evoked podocyte injury inhibition | Astragaloside IV | EC50 = 15.4 μM/glucose-evoked podocyte injury inhibition | 25 |
| 379 and 384–386 | In vitro | IC50 > 50 μM/α-glucosidase inhibition | 109 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| AChE inhibitory activity | |||||
| 39 | In vitro | MIR = 12.5 μg/AChE inhibition | Galanthamine | MIR = 0.004 μg/AChE inhibition | 21 |
| 220 | In vitro | IC50 = 12.5 μg/AChE inhibition | 6 | ||
| 221 | In vitro | IC50 = 12.5 μg/AChE inhibition | 6 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Anti-manic activity | |||||
| 165 | In vitro | IC50 = 12.5 mg mL−1/anti-manic activity in Drosophila | 13 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Anti-tussive activity | |||||
| 126 | In vivo | 88% Cough inhibition/citric acid activated Guinea pig cough model | 65 | ||
| Interaction to δ-opioid receptor | |||||
| 250 | In vivo | 76% Cough inhibition/citric acid activated Guinea pig cough model | 65 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Anti-nociceptive activity | |||||
| The alkaloidal extract of K. macrophylla | In vivo | To decrease in the number of contortion and stretching via peripheral mechanism | 130 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Cardiovascular and vasorelaxant activities | |||||
| 112 | In vivo | To decrease arterial blood pressure and heart rate | 131 | ||
| 208 | In vivo | 13% Relaxation occurred rat aorta ring | 84 | ||
| 210 | In vivo | 24% Relaxation occurred rat aorta ring | 84 | ||
| 211 | In vivo | 26% Relaxation occurred rat aorta ring | 84 | ||
| 216 | In vivo | 28% Relaxation occurred rat aorta ring | 84 | ||
| 219 | In vivo | 40% Relaxation occurred rat aorta ring | 84 | ||
| 225 | In vivo | 41% Relaxation occurred rat aorta ring | 84 | ||
| 227 | In vivo | 15% Relaxation occurred rat aorta ring | 84 | ||
| 228 | In vivo | 37% Relaxation occurred rat aorta ring | 84 | ||
| 229 | In vivo | 19% Relaxation occurred rat aorta ring | 84 | ||
| 230 | In vivo | 19% Relaxation occurred rat aorta ring | 84 | ||
| 239 | In vivo | 23% Relaxation occurred rat aorta ring | 84 | ||
3.1. Cytotoxic activity
It is obvious to the view that monoterpene alkaloids are the major phytochemicals in Kopsia plants so that cytotoxic experiments using Kopsia constituents may be thought of as a big content in pharmacological development. Six alkaloidal constituents 39–40, 73, 302, 327, and 408 from K. singapurensis root were submitted to cytotoxic assay against NIH/3T3, HL-60, and HeLa cells.49 Among them, kopsifine (73) induced the lowest CD50 value of 0.9 μg mL−1 against HL-60 cells in referencing with the positive control vincristine (CD50 1.8 μg mL−1).49Kopsiafrutine E (47) possessing hydroxyl groups at carbons C-14 and C-15 demonstrated as the most bioactive compound against HS-1, HS-4, SCL-1, A-431, BGC-823, MCF-7, and W-480 with the IC50 values of 7.3–9.5 μM.52 Meanwhile, its congeners kopsiafrutines C–D (45–46) containing a hydroxyl group at carbon C-15 have shown to associate with the respective IC50 values of 10.3–12.5 and 11.8–13.8 μM, but kopsiafrutines A–B (43–44) and kopsifoline A (76) did not inhibit cancer cell growth (IC50 > 20 μM).52 In the same way, the following new aspidofractinines kopsiahainanins A–B (48–49) with a lactone bridge have induced the respective IC50 values of 9.4–11.7 and 12.2–15.9 μM against A-549, BGC-823, HepG-2, HL-60, MCF-7, SMMC-7721, and W-480 cells.53 However, four new analogous kopsiahainanins C–F (50–53) accompanied by the IC50 values of >20 μM.53
From Table 2, new aspidofractines kopsiahainins A–E (54–58) were also further examined by cytotoxic test towards BGC-823, HepG-2, MCF-7, SGC-7901, SK-MEL-2, and SK-OV-3 cancer cells. It evidenced that compounds 56–57 demonstrated strong activity with IC50 values of ≤10 μM.54 Similarly, in the N(4)-oxide group, new alkaloid 237 possessed the IC50 values from 7.2 to 8.9 μM to inhibit BGC-823, HepG-2, MCF-7, SGC-7901, and SK-MEL-2 cells, but new metabolites 214–215 was inactive (IC50 > 20 μM).85
The new metabolite kopsiaofficines C (61) showed the IC50 values of <10 μM towards cancer cell lines 95-D, A-549, ATCC, H-446, H-460, H-292, and SPCA-1, and was better than its analogs 59 (10 < IC50 ≤ 20 μM) and 60 (IC50 > 20 μM).55 The bulk dimeric molecule arbolodinine B (333) successfully controlled the growth of HT-29, MCF-7, PC-3, KB (VJ300), MDA-MB-231, HCT-116, and A-549 with the IC50 values ranging from 1.3 to 9.6 μg mL−1, while arbolodinines A and C (1 and 334) failed to do so.8
Rhazinilam (409) itself displayed the potential application in cancer treatments because its strong inhibitory capacity to A-549 and HT-29 cells (IC50 0.35 μM), kopsiyunnanines A–C (402–404) indicated moderate activities (IC50 4.67–8.89 μM), but both kopsiyunnanine D (450) and (−)-quebrachamine (452) were inactive (>30 μM).114 Novel alkaloidal arbophyllidine (463) suppressed HT-29 cell growth with the IC50 value of 6.2 μM, but the novel metabolite arbophyllinine A (453) failed to inhibit.59 Six non-alkaloidal constituents 467–472 were also subjected to cytotoxic assay, in which their IC50 values ranged from 14.5 to 22.5 μg mL−1.122
Vincristine, a renowned chemotherapy medication, is usually used in combining with other drugs to treat many types of cancers.132 In this scenario, experiments using a combination of Kopsia alkaloids and vincristine for anticancer treatments also bring out significant results. In VJ300 cells, kopsiflorine 74 (10 μg mL−1) showed reversal of multiple drug resistance (MRD) by suppressing the bound of [3H]azidopine to P-glycoprotein.61 Alkaloidal compounds 88, 102–107, 411, 413, 417, 434, and 438 exhibited no appreciable cytotoxic activity against KB (VJ300) cells.22,23,43,72 However, they possessed IC50 values of 0.39–38.7 μg mL−1 against KB (VJ300) cells in the presence of 0.1 μg mL−1 vincristine. Subramaniam et al. (2007) reported that kopsiloscine A (93), rhazinilam (409), especially two alkaloids rhazinal (407) and rhazinicine (408), showed inhibition to both KB, KB (VJ300), and KB (VJ300) + 0.1 μg mL−1 vincristine.32
Dimeric alkaloid norpleiomutine (282) exhibited cytotoxicity to PC-3, HCT-116, MCF-7, A-549, KB (VJ300), especially in terms of KB (VJ300) + 0.1 μg mL−1 vincristine, better than its analogous dimer kopsoffinol (289).19 This can be explained by the functionality of OH group at carbon C-19. Most Kopsia mersinines seem not to be anticancer agents. However, novel compounds 366–367 and 373–375 also established the significant cytotoxicity to reserve MDR in drug-resistant KB (VJ300) with the IC50 values of 3.2–11.2 μg mL−1.103 Valparicine (446) would be superior to the positive control vincadifformine in a cytotoxic assay against Jurkat cell growth.10 In addition, this compound and arboloscine (437) showed positive signals to resist the growth of KB (VJ300) and KB (VJ300) + 0.1 μg mL−1 vincristine (Table 2).10
3.2. Anti-microbial activity
Nowadays, microbial resistance to well-known antibiotics has caused major concern about the treatment of infectious diseases. A vast amount of studies has recently been conducted to determine possible answers. Phytochemicals have been shown to exhibit antibacterial activity against sensitive and resistant infections through various approaches. To have a look at the IZ (inhibitory zone) and MIC values of Kopsia constituents (Table 2), compounds 43–47, 48–53, and 76 are not only potential anticancer molecules but also useful antimicrobial agents.52,53 Especially, kopsiafrutine E (47) with the MIC values of 0.15–1.14 mM established a remarkable antimicrobial effect against twelve pathogenic microorganisms, including two Gram positive bacteria Staphylococcus aureus and S. epidermidis, five Gram negative bacteria Escherichia coli, Enterobacter cloacae, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Shigella dysenteriae, three fungi Candida albicans, C. tropicalis, and C. glabrata, and two oral pathogens Streptococcus mutans and S. viridans.52 Likewise, compounds 48–49 showed strong antimicrobial activity with MIC values of less than 0.3 mM against seven bacteria E. cloacae, E. coli, K. pneumoniae, P. aeruginosa, S. aureus, S. dysenteriae, and S. epidermidis.53In another assessment, kopsiflorine (74) and kopsihainins D–F (85–87) showed suppression towards the Gram positive bacterium Staphylococcus aureus with IZ values ranging from 9.7 to 11.2 mm, but compounds 3, 17, 73, 109, 124, 405, and 406 were inactive.12 In an antimicrobial assay against E. coli, Erwinia carotovra, Bacillus subtilis, B. cereus, and S. aureus, two best agents N-decarbomethoxykopsamine (14) and N1-decarbomethoxy chanofruticosinic acid (206) were associated with the MIC values of 7.8–15.5 and 15.5–31.3 μg mL−1, respectively.7 These two molecules further showed antifungal activity against Rhizoctonia solani, Penicillium italicum, Fusarium oxysporum f. sp. Cubense, and F. oxysporum f. sp. Niveum (Table 2).7 Lastly, two eburnamines 19-hydroxy-(−)-eburnamonine (267) and phutdonginin (297) showed moderate activity against the growth of E. coli with the same MIC value of 32 μg mL−1.21
3.3. Anti-inflammatory activity
Inflammation is a part of the complicated biological reaction of living bodies to harmful stimuli such as irradiation, physical injury, metabolic stress, and infection.133–135 K. officinalis constituents are such useful agents to treat autoimmune diseases due to their inhibition of human T cell proliferation and proinflammatory cytokines.16 Indeed, K. officinalis constituents decarbomethoxykopsine (11), N(4)-methylkopsininate (170), 12-methoxychanofruticosinic acid (222), and rhazinilam (409) inhibited T cell growth with the IC50 values of 25.4, 21.6, 27.8, and 1.0 μM, respectively.16 The best molecule 409 also responded to the arrest in the G2/M phase of the T cell cycle and caused a decrease in IL-6 and IL-17 levels in activated T cells.16The secretion of cytokines IL-1β and TNF-α or PGE2 levels has mainly caused inflammatory reactions. When LPS-stimulated RAW 264.7 cells, at the concentration of 5 μg mL−1, kopsia C (219), methyl N1-decarbomethoxychanofruticosinate (225), methyl 12-methoxychanofruticosinate (228), kopsiofficines I–J (279–280), (+)-O-methyleburnamine (290), (−)-O-methylisoeburnamine (291), and leuconodine D (439) have remarkable anti-inflammatory effects on IL-1β and TNF-α, and PGE2, and comparable with positive control dexamethasone at the concentration of 10 μg mL−1.75
3.4. Anti-allergic and antidiabetic activities
Naturally occurring compounds have been recognized as potential antiallergic agents. In an experiment against histamine and β-hexosaminidase in RBL-2H3 cells, the IC10 values of 3.73–11.78 μg mL−1 were assigned to four alkaloids kopsilarutensinine (90), kopsinine (126), (−)-eburnamine (257), and (−)-tetrahydroalstonine (448).66 In the same model against histamine and β-hexosaminidase in RBL-2H3 cells, in contrast to the MeOH extract of K. arborea leaves, the MeOH extracts of K. larutensis bark and root were found better than those of K. arborea bark and root (Table 2).66,129For antidiabetic activity, among tested compounds for the high glucose-evoked podocyte injury inhibition, the EC50 values were orderly run as kopsinine 126 (3.0 μM) > leuconolam 405 (3.8 μM) > methyl 11,12-dimethoxychanofruticosinate 224 (10.2 μM) > 16α-hydroxy-19-oxoeburnamine 264 (12.0 μM) > reference compound astragaloside IV (15.4 μM) > 11-hydroxykopsilongine 29 (24.5 μM).25 However, four pauciflorine derivatives 11,12-demethoxy-16-deoxypauciflorine (379) and kopsioffines A–C (384–386) failed to suppress enzyme α-glucosidase (IC50 > 50 μM).109
3.5. AChE inhibitory, anti-manic, anti-tussive, and anti-nociceptive activities
In Alzheimer's disease treatment based AChE inhibitory examination, kopsamine (39) has the minimum inhibitory requirement (MIR) value of 12.5 μg, as compared with that of the reference compound galanthamine (MIR 0.004 μg).21 Meanwhile, two novel chanofruticosinates, kopsihainanines A–B (220–221), displayed weak AChE inhibitory activity with the respective IC50 values of 38.5 and 50.6 μM.6 (−)-12-Methoxykopsinaline (165) with the IC50 value of 12.5 mg mL−1, showed anti-manic activity in Drosophila.13Kopsinine 126 (70 mg kg−1, i.p.) and methyl N1-decarbomethoxychanofruticosinate 225 (250 mg kg−1, i.p.) exhibited 88 and 76% cough inhibition in the antitussive assays when citric acid activated guinea pig cough model.65 In addition, anti-tussive effect of compound 126 was due to its interaction with δ-opioid receptors.65
The alkaloidal extract of K. macrophylla (400 mg kg−1, p.o.) was responsible for a decrease in the number of contortions and stretching via the peripheral mechanism in anti-nociceptive assays when acetic acid stimulated pain in mice, but it has no effect in anti-pyretic assay.130
3.6. Cardiovascular and vasorelaxant activities
Cardiovascular disease (CVD) refers to a group of illnesses affecting the heart and blood arteries. CVD is the largest cause of death worldwide with 17.9 million deaths (32.1%) in 2015.136 Drug discovery for CVD started from the 18th century at least.137 To consider Kopsia constituents for cardiovascular treatment, at doses of 0.2–10.0 mg kg−1 intravenous injection, kopsingine (112) caused decreases in arterial blood pressure and heart rate when hypertensive mice were anesthetized.123 However, kopsaporine (42) was reasonable for blood pressure increase, and kopsidine A (67) with the deletion of the methoxy group did not alter the responsible hypotension.123Vasodilators can be used for cerebral vasospasm and hypertension treatments, as well as to enhance peripheral circulation.138,139 Flavisiamines A, C, and D (208 and 210–211), kopreasin A (216), methyl 11,12-methylenedioxychanofruticosinate (219), methyl N1-decarbomethoxychanofruticosinate (225), methyl 12-methoxy-N1-decarbomethoxychanofruticosinate (227), methyl 12-methoxychanofruticosinate (228), methyl 11,12-methylenedioxy-N1-decarbomethoxychanofruticosinate (229), methyl 11,12-methylenedioxy-N1-decarbomethoxy-Δ14,15-chanofruticosinate (230), and prunifoline B (239) at the concentration of 3 × 10−5 M showed a moderate vasorelaxant effect of 14–41% when phenylephrine (3 × 10−7 M) precontracted rat aortic rings.84
4. Conclusion and future perspectives
To a certain extent, our comprehensive review establishes a panel of useful information on phytochemistry and pharmacology of the genus Kopsia. Since the 1950s, about nineteen Kopsia plants were used in phytochemical investigations, and more than four hundred seventy secondary metabolites have been isolated. Among 472 isolated compounds, monoterpene alkaloids (466 compounds) accounted for 98.73%. Kopsia monoterpene alkaloids have been fallen into about 30 structural skeletons, but aspidofractinines (204 compounds), eburnamines (48 compounds), and chanofruticosinates (37 compounds) predominated over. Various compounds were isolated from Kopsia plants for the first time. Many chemical classes of isolated compounds, such as mersinines and pauciflorines, can be seen as newly alkaloidal classes and were useful for chemotaxonomy. Some metabolites, such as kopsamine (39), kopsinine (126), (−)-eburnamine (257), (+)-isoeburnamine (274), rhazinilam (409), and (−)-tetrahydroalstonine (448), are characteristic metabolites of genus Kopsia. It also evidenced that Kopsia plant extracts and isolated compounds have induced a variety of pharmacological results, e.g., antimicrobial, anti-inflammatory, anti-diabetic, cardiovascular, vasorelaxant activities, especially cytotoxicity. With the great cytotoxic values, monoterpene alkaloids derived from Kopsia plants are promising anticancer agents in drug development programmes. However, studies on in vivo apoptotic mechanism, bioavailability, and metabolic approaches seem not available. To this end, no research was carried out to determine toxic effects of Kopsia plant extracts and their constituents. Therefore, it is necessary to deal with the extensive clinical studies to confirm the effects of Kopsia constituents on humans.This review will be especially useful in offering fundamental insights into the medicinal usefulness of Kopsia plants. Furthermore, this evaluation can be used as a reference for clinical medication, long-term development, and plant consumption.
Abbreviations
| HPLC | High performance liquid chromatography |
| MS | Mass spectrum |
| CC | Column chromatography |
| IC50 | Half-maximal inhibitory concentration |
| IZ | Inhibitory zone |
| MDR | Multidrug resistance |
| MIR | Minimum inhibitory requirement |
| MIC | Minimum inhibitory concentration |
| LPS | lipopolysaccharide |
| AChE | Acetylcholinesterase |
| NIH/3T3 | Normal mouse fibroblast cells |
| HL-60 | Human promyelocytic cells |
| HeLa | Human cervical cancer cells |
| HS-1, HS-4, SCL-1, and A-431 | Dermatoma cells |
| BGC-823 | Human gastric carcinoma cells |
| MCF-7 | Human breast cancer cells |
| W-480 | Colon cancer cells |
| HepG-2 | Human hepatocellular carcinoma cells; SMMC-7721 cells |
| SGC-7901 | Human gastric adenocarcinoma cells |
| SK-MEL-2 | Human skin cancer cells |
| SK-OV-3 | Ovarian cancer cells |
| A-549, 95-D, ATCC, H-446, H-460 and H-292, and SPCA-1 | Lung cancer cells |
| HT-29 and HCT-116 | Colorectal cancer cells |
| PC-3 | Human prostate cancer cells |
| Jurkat | Human T lymphocyte cells |
| KB | Epidermoid carcinoma cells |
Conflicts of interest
The authors declare no conflict of interest, financial or otherwise.References
- D. A. Williams, W. O. Foye and T. L. Lemke, Natural products. Foye's principles of medicinal chemistry, Lippincott Williams Wilkins, Philadelphia, 5th edn, 2002, ch. 1 Search PubMed.
- J. W. Kadereit and V. Bittrich, Flowering Plants. Eudicots, Springer, 2018 Search PubMed.
- J. Hu, X. Mao, L. Zhang, N. Jin, S. Yin, T. Peng and J. Shi, Chem. Nat. Compd., 2019, 55, 502–505 CrossRef CAS.
- T. S. Kam and O. S. Tan, Phytochemistry, 1990, 29, 2321–2322 CrossRef CAS.
- T. S. Kam, P. S. Tan and C. H. Chuah, Phytochemistry, 1992, 31, 2936–2938 CrossRef CAS.
- J. Chen, J. J. Chen, X. Yao and K. Gao, Org. Biomol. Chem., 2011, 9, 5334–5536 RSC.
- J. Chen, M. L. Yang, J. Zeng and K. Gao, Phytochem. Lett., 2014, 7, 156–160 CrossRef CAS.
- S. K. Wong, J. S. Y. Yeap, C. H. Tan, K. S. Sim, S. H. Lim, Y. Y. Low and T. S. Kam, Tetrahedron, 2021, 78, 131802 CrossRef CAS.
- T. He, Y. D. Wang, F. R. Li, S. Y. He, Q. M. Cui, Y. P. Liu, T. R. Zhao and G. G. Cheng, Biochem. Syst. Ecol., 2020, 93, 104159 CrossRef CAS.
- K. H. Lim, O. Hiraku, K. Komiyama, T. M. Koyano, M. Hayashi and T. S. Kam, J. Nat. Prod., 2007, 70, 1302–1307 CrossRef CAS PubMed.
- T. Z. Xie, Y. L. Zhao, W. G. Ma, Y. F. Wang, H. F. Yu, B. W. Wang, H. Z. Xin, P. F. Zhu, Y. P. Liu and X. D. Luo, Chin. J. Org. Chem., 2020, 40, 679–687 CrossRef CAS.
- Y. Yang, W. J. Zuo, Y. X. Zhao, W. H. Dong, W. L. Mei and H. F. Dai, Planta Med., 2012, 78, 1881–1884 CrossRef CAS PubMed.
- H. Zhou, H. P. He, N. C. Kong, Y. H. Wang, X. D. Liu and X. J. Hao, Helv. Chim. Acta, 2006, 89, 515–519 CrossRef CAS.
- J. J. Zheng, Y. L. Zhou and Z. H. Huang, Acta Chim. Sin., 1989, 2, 168–175 CrossRef.
- T. S. Kam, K. M. Sim, T. Koyano and K. Komiyama, Phytochemistry, 1999, 50, 75–79 CrossRef CAS.
- T. Zeng, X. Y. Wu, S. X. Yang, W. C. Lai, S. D. Shi, Q. Zou, Y. Liu and L. M. Li, J. Nat. Prod., 2017, 80, 864–871 CrossRef CAS PubMed.
- T. S. Kam and K. M. Sim, Phytochemistry, 1998, 47, 145–147 CrossRef CAS.
- T. S. Kam, G. Subramaniam and W. Chen, Phytochemistry, 1999, 51, 159–169 CrossRef CAS.
- W. S. Yap, C. Y. Gan, K. S. Sim, S. H. Lim, Y. Y. Low and T. S. Kam, J. Nat. Prod., 2016, 79, 230–239 CrossRef CAS PubMed.
- A. Guggisberg, T. R. Govindachari, K. Nagarajan and H. Schmid, Helv. Chim. Acta, 1963, 46, 679–683 CrossRef CAS.
- S. Cheenprachaa, A. Raksat, T. Ritthiwigrom and S. Laphookhieo, Nat. Prod. Commun., 2014, 9, 1441–1443 CrossRef.
- C. Y. Gan, K. Yoganathan, K. S. Sim, Y. Y. Low, S. H. Lim and T. S. Kam, Phytochemistry, 2014, 108, 234–242 CrossRef CAS PubMed.
- G. Subramaniam, O. Hiraku, M. Hayashi, T. Koyano, K. Komiyama and T. S. Kam, J. Nat. Prod., 2008, 71, 53–57 CrossRef CAS PubMed.
- M. Kitajima, M. Anbe, N. Kogure, S. Wongseripipatana and H. Takayama, Tetrahedron, 2014, 70, 9099–9106 CrossRef CAS.
- Z. W. Wang, C. R. Guo, Y. L. Lin, H. J. Yan, Y. Mu, Y. L. Geng, W. Liua and X. Wang, Fitoterapia, 2019, 137, 104258 CrossRef CAS PubMed.
- M. O. Hamburger, G. A. Cordell, K. Likhitwitayawuid and N. Ruangrungsi, Phytochemistry, 1988, 27, 2719–2723 CrossRef CAS.
- C. Kan-Fan, T. Sevenet, H. A. Hadi, M. Bonin, J. C. Quirion and H. P. Husson, Nat. Prod. Lett., 1995, 7, 283–290 CrossRef CAS.
- X. Z. Feng, C. Kan, H. P. Husson, P. Potie, S. K. Kan and M. Lounasmaa, J. Nat. Prod., 1984, 47, 117–122 CrossRef CAS.
- J. Zhu, A. Guggisberg and M. Hesse, Planta Med., 1986, 52, 63–64 CrossRef.
- T. S. Kam, Y. M. Choo, W. Chen and J. X. Yao, Phytochemistry, 1999, 52, 959–963 CrossRef CAS.
- T. S. Kam and Y. M. Choo, Phytochemistry, 2004, 65, 2119–2122 CrossRef CAS PubMed.
- G. Subramaniam, O. Hiraku, M. Hayashi, T. Koyano, K. Komiyama and T. S. Kam, J. Nat. Prod., 2007, 70, 1783–1789 CrossRef CAS PubMed.
- T. Varea, C. Kan, F. Remy, T. Sevene, J. C. Quirion, H. P. Husson and H. A. Hadi, J. Nat. Prod., 1993, 56, 2166–2169 CrossRef CAS.
- T. S. Kam, K. Yoganathan and S. L. Mok, Phytochemistry, 1997, 45, 789–792 CrossRef.
- K. Ahmad, Y. Hirasawa, A. E. Nugroho, A. H. A. Hadi and H. Morita, Heterocycles, 2012, 86, 1611–1619 CrossRef CAS.
- K. Ahmad, Y. Hirasawa, A. E. Nugroho, A. H. A. Hadi, K. Takeya, N. F. Thomas, K. Awang, H. Morita, T. S. Ping and M. A. Nafiah, Open Conf. Proc. J., 2013, 4, 75–82 CrossRef CAS.
- A. R. Battersby, J. C. Byrne, H. Gregory and S. P. Popli, J. Chem. Soc. C, 1967, 9, 813–819 RSC.
- A. R. Battersby and H. Gregor, J. Am. Chem. Soc., 1963, 16, 22–32 RSC.
- R. P. Glover, K. Yoganathan and M. S. Butler, Magn. Reson. Chem., 2005, 43, 483–485 CrossRef CAS PubMed.
- N. Ruangrungsi, K. Likhitwitayawuid, V. Jongbunprasert, D. Ponglux, N. Aimi, K. Ogata, M. Yasuoka, J. Haginiwa and S. Sakai, Tetrahedron Lett., 1987, 28, 3679–3682 CrossRef CAS.
- M. Sekiguchi, Y. Hirasawa, K. Zaima, T. C. Hoe, K. L. Chan and H. Morita, Heterocycles, 2008, 76, 867–874 CrossRef CAS.
- C. Q. Yang, Y. F. Ma and Y. G. Chen, Chem. Nat. Compd., 2017, 53, 595–597 CrossRef CAS.
- S. H. Lim, K. M. Sim, Z. Abdullah, O. Hiraku, M. Hayashi, K. Komiyama and T. S. Kam, J. Nat. Prod., 2007, 70, 1380–1383 CrossRef CAS PubMed.
- T. S. Kam, K. Yoganathan, C. H. Chuah and C. Wei, Phytochemistry, 1993, 32, 1343–1346 CrossRef CAS.
- T. S. Kam, K. Yoganathan and C. H. Chuah, Tetrahedron Lett., 1993, 34, 1819–1822 CrossRef CAS.
- T. S. Kam, T. M. Lim, G. Subramaniam, Y. M. Tee and K. Yoganathan, Phytochemistry, 1990, 50, 171–175 CrossRef.
- T. S. Kam and K. Yoganathan, Phytochemistry, 1996, 42, 539–541 CrossRef CAS.
- G. Subramaniam and T. S. Kam, Helv. Chim. Acta, 2008, 91, 930–937 CrossRef CAS.
- Halimatussakdiah, U. Amna, S. P. Tan, K. Awang, A. M. Ali, M. A. Nafiah and K. Ahmad, Int. J. Pharm. Sci. Rev. Res., 2015, 31, 89–95 CAS.
- Y. L. Zhou, Z. H. Huang, L. Y. Huang, J. P. Zhu, C. M. Li and G. L. Wu, Acta Chim. Sin., 1985, 1, 82–83 Search PubMed.
- T. S. Kam, L. Arasu and K. Yoganathan, Phytochemistry, 1996, 43, 1385–1387 CrossRef CAS.
- S. Y. Long, C. L. Li, J. Hu, Q. J. Zhao and D. Chen, Fitoterapia, 2018, 129, 145–149 CrossRef CAS PubMed.
- W. Q. Chia, Y. H. Jianga, J. Hub and J. Pan, Fitoterapia, 2018, 130, 259–264 CrossRef PubMed.
- Y. Wanga, L. Hang, L. Jiaoc, H. Liud and F. Li, Fitoterapia, 2017, 121, 53–57 CrossRef PubMed.
- T. Liu, J. Hu, J. X. Li and M. W. Chen, J. Asian Nat. Prod. Res., 2020, 22, 724–731 CrossRef CAS PubMed.
- X. D. Chen, J. Hu, J. X. Li and F. S. Chi, J. Asian Nat. Prod. Res., 2020, 22, 1024–1030 CrossRef CAS PubMed.
- K. Homberger and M. Hesse, Helv. Chim. Acta, 1982, 65, 2548–2557 CrossRef CAS.
- T. S. Kam, K. Yoganathan and K. H. Chuah, Phytochemistry, 1997, 45, 623–625 CrossRef CAS.
- S. K. Wong, S. P. Wong, K. S. Sim, S. H. Lim, Y. Y. Low and T. S. Kam, J. Nat. Prod., 2019, 82, 1902–1907 CrossRef CAS PubMed.
- T. S. Kam and G. Subramaniam, Nat. Prod. Lett., 1998, 11, 131–136 CrossRef CAS.
- M. C. Rho, M. Toyoshima, M. Hayashi, T. Koyano, G. Subramaniam, T. S. Kam and K. Komiyama, Planta Med., 1999, 65, 307–310 CrossRef CAS PubMed.
- T. S. Kam and Y. M. Choo, Tetrahedron Lett., 2003, 44, 1317–1319 CrossRef CAS.
- T. S. Kam and Y. M. Choo, Helv. Chim. Acta, 2004, 78, 991–998 CrossRef.
- J. Chen, X. Li, N. Li, J. Lu, X. Xu, H. Duan and L. Qin, Chem. Nat. Compd., 2012, 48, 834–835 CrossRef CAS.
- M. J. Tan, C. Yin, C. P. Tang, C. Q. Ke, G. Lin and Y. Ye, Planta Med., 2011, 77, 939–944 CrossRef CAS PubMed.
- M. S. Shahari, A. F. Ismail, E. Kumolosasi, N. F. Rajab and K. Husain, J. Innovations Pharm. Biol. Sci., 2017, 4, 80–86 CAS.
- A. Bhattacharya, A. Chatterjee and P. B. Bose, J. Am. Chem. Soc., 1949, 71, 3370–3372 CrossRef.
- K. Awang, M. Pais, T. Sevenet, H. Schaller, A. M. Nasir and A. H. A. Hadi, Phytochemistry, 1991, 30, 3164–3167 CrossRef CAS.
- X. Z. Feng, C. Kan, P. Potier, S. K. Kan and M. Lounasmaa, J. Med. Plants Res., 1983, 84, 280–282 CrossRef PubMed.
- T. S. Kam, P. S. Tan and C. Wei, Phytochemistry, 1993, 33, 921–924 CrossRef CAS.
- T. S. Kam, K. H. Lim, K. Yoganathan, M. Hayashi and K. Komiyama, Tetrahedron, 2004, 60, 10739–10745 CrossRef CAS.
- W. S. Yap, C. Y. Gan, Y. Y. Low, Y. M. Choo, T. Etoh, M. Hayashi, K. Komiyama and T. S. Kam, J. Nat. Prod., 2011, 74, 1309–1312 CrossRef CAS PubMed.
- T. S. Kam, K. Yoganathan and C. H. Chuah, Tetrahedron Lett., 1994, 35, 4457–4460 CrossRef CAS.
- T. S. Kam, K. Yoganathan and C. Wei, J. Nat. Prod., 1996, 59, 1109–1112 CrossRef CAS.
- T. Z. Xie, Y. L. Zhao, J. J. He, L. X. Zhao, X. Wei, Y. P. Liub and X. D. Luo, Fitoterapia, 2020, 143, 104547 CrossRef CAS PubMed.
- T. S. Kam and K. Yoganathan, Phytochemistry, 1997, 46, 785–787 CrossRef CAS.
- T. S. Kam and P. S. Tan, Phytochemistry, 1995, 39, 469–471 CrossRef CAS.
- K. H. Lim, Y. Y. Low, G. H. Tan, T. M. Lim and T. S. Kam, Helv. Chim. Acta, 2008, 91, 1559–1566 CrossRef CAS.
- K. Awang, O. Thoison, A. H. A Hadi, M. Païs and T. Sévenet, Nat. Prod. Lett., 1993, 3, 283–289 CrossRef CAS.
- T. S. Kam and K. Yoganathan, Nat. Prod. Lett., 1997, 10, 69–74 CrossRef CAS.
- K. Awang, T. Sévenet, M. Païs and A. H. A. Hadi, J. Nat. Prod., 1993, 56, 1134–1139 CrossRef CAS.
- K. H. Lim and T. S. Kam, Phytochemistry, 2008, 69, 558–561 CrossRef CAS PubMed.
- M. Sekiguchi, Y. Hirasawa, K. Zaima, T. H. Hoe, K. L. Chan and H. Morita, Heterocycles, 2008, 75, 2283–2288 CrossRef CAS.
- K. Zaima, Y. Matsuno, Y. Hirasawa, A. Rahman, G. Indrayanto, N. C. Zaini and H. Morita, Heterocycles, 2008, 75, 2535–2540 CrossRef CAS.
- J. F. Wang, Y. Wang, H. L. Wang, C. B. Xu and H. T. Wang, J. Asian Nat. Prod. Res., 2018, 21, 227–233 CrossRef PubMed.
- W. S. Chen, S. H. Li, A. Kirfel, G. Win and E. Breitmaier, Liebigs Ann. Chem., 1981, 1981, 1886–1892 CrossRef.
- T. S. Kam, P. S. Tan, P. Y. Hoong and C. H. Chuah, Phytochemistry, 1993, 32, 489–491 CrossRef CAS.
- K. Husain, I. Jantan, N. Kamaruddin, I. M. Said, N. Aimia and H. Takayama, Phytochemistry, 2001, 57, 603–606 CrossRef CAS PubMed.
- K. Husain, I. Jantan, I. M. Said, N. Aimi and H. Takayama, J. Asian Nat. Prod. Res., 2003, 5, 63–67 CrossRef CAS PubMed.
- Y. Wu, M. Kitajima, N. Kogure, Y. Wang, R. Zhang and H. Takayama, Chem. Pharm. Bull., 2010, 58, 961–963 CrossRef CAS PubMed.
- T. S. Kam, T. M. Lim, Y. M. Choo and G. Subramaniam, Tetrahedron Lett., 1998, 39, 5823–5826 CrossRef CAS.
- S. Uzir, A. M. Mustapha, A. H. A. Hadi, K. Awang, C. Wiart, J. F. Gallard and M. Pais, Tetrahedron Lett., 1997, 38, 1571–1574 CrossRef CAS.
- T. S. Kam, G. Subramaniam and C. Wei, Nat. Prod. Lett., 1998, 12, 293–298 CrossRef CAS.
- C. Kan, J. R. Deverre, T. Sevenet, J. C. Quirion and H. P. Husson, Nat. Prod. Lett., 1995, 7, 275–281 CrossRef CAS.
- Y. Wu, M. Kitajima, N. Kogure, Y. Wang, R. Zhang and H. Takayama, J. Nat. Med., 2009, 63, 283–289 CrossRef CAS PubMed.
- Y. Wu, M. Suehiro, N. Takahashi, M. Kitajima, N. Kogure, R. Zhang and H. Takayama, Chem. Nat. Prod., 2008, 50, 47–52 Search PubMed.
- Y. Wu, M. Kitajima, N. Kogure, R. Zhang and H. Takayama, Tetrahedron Lett., 2008, 49, 5935–5938 CrossRef CAS.
- N. Kogure, Y. Suzuki, Y. Wu, M. Kitajima, R. Zhang and H. Takayama, Tetrahedron Lett., 2012, 53, 6523–6526 CrossRef CAS.
- Y. Murakami, T. Koyama, Y. Suzuki, N. Takahashi, Y. Wu, N. Kogure, R. Zhang, M. Kitajima and H. Takayama, Chem. Nat. Prod., 2018, 56, 327–332 Search PubMed.
- M. Kitajima, T. Koyama, Y. Wu, N. Kogure, R. Zhan and H. Takayama, Nat. Prod. Commun., 2015, 10, 49–51 CrossRef PubMed.
- M. Kitajima, M. Nakazawa, Y. Wu, N. Kogure, R. P. Zhang and H. Takayama, Tetrahedron, 2016, 72, 6692–6696 CrossRef CAS.
- N. Kogure, R. Tokuda, S. Tooriyama, M. Nakazawa, T. Koyama, Y. Okamoto, Y. Mimori, Y. Wu, R. P. Zhang, M. Kitajima and H. Takayama, Chem. Nat. Prod., 2017, 24, 139–144 Search PubMed.
- G. Subramaniam, Y. M. Choo, O. Hiraku, K. Komiyama and T. S. Kam, Tetrahedron, 2008, 64, 1397–1408 CrossRef CAS.
- T. S. Kam and G. Subramaniam, Tetrahedron Lett., 2004, 45, 3521–3524 CrossRef CAS.
- T. S. Kam, G. Subramaniam and T. M. Lim, Tetrahedron Lett., 2001, 42, 5977–5980 CrossRef CAS.
- G. Subramaniam and T. S. Kam, Tetrahedron Lett., 2007, 48, 6677–6680 CrossRef CAS.
- G. Subramaniam, T. S. Kam and S. W. Ng, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2003, 59, o555–o557 CrossRef CAS.
- Y. Y. Low, G. Subramaniam, K. H. Lim, R. C. S. W. Wong, T. Robinson and T. S. Kam, Tetrahedron, 2009, 65, 6873–6876 CrossRef CAS.
- Z. W. Wang, X. J. Shi, Y. Mu, L. Fang, Y. Y. Chen and L. Lin, Fitoterapia, 2017, 119, 8–11 CrossRef CAS PubMed.
- T. S. Kam, K. Yoganathan, T. Koyano and K. Komiyama, Tetrahedron Lett., 1996, 37, 5765–5768 CrossRef CAS.
- T. S. Kam, K. Yoganathan and C. Wei, Nat. Prod. Lett., 1996, 8, 231–235 CrossRef CAS.
- T. S. Kam, K. Yoganathan and C. Wei, J. Nat. Prod., 1997, 60, 673–676 CrossRef CAS.
- K. Homherger and M. Hesse, Helv. Chim. Acta, 1984, 67, 237–248 CrossRef.
- Y. Wu, M. Suehiro, M. Kitajima, T. Matsuzaki, S. Hashimoto, M. Nagaoka, R. Zhang and H. Takayama, J. Nat. Prod., 2009, 72, 204–209 CrossRef CAS PubMed.
- K. Awang, T. Sévenet, A. H. A. Hadi, B. David and M. Païs, Tetrahedron Lett., 1992, 33, 2493–2496 CrossRef CAS.
- T. S. Kam, K. Yoganathan and H. Y. Li, Tetrahedron Lett., 1996, 37, 8811–8814 CrossRef CAS.
- T. S. Kam, K. Yoganathan, K. H. Y. Li and N. Harada, Tetrahedron, 1997, 53, 12661–12670 CrossRef CAS.
- K. H. Lim and T. S. Kam, Helv. Chim. Acta, 2007, 90, 31–35 CrossRef CAS.
- K. H. Lim, Y. Y. Low and T. S. Kam, Tetrahedron Lett., 2006, 47, 5037–5039 CrossRef CAS.
- K. H. Lim, K. Komiyama and T. S. Kam, Tetrahedron Lett., 2007, 48, 1143–1145 CrossRef CAS.
- M. Kitajima, Y. Murakami, N. Takahashi, Y. Wu, N. Kogure, R. P. Zhang and H. Takayama, Org. Lett., 2014, 16, 5000–5003 CrossRef CAS PubMed.
- L. Y. Shan, T. C. Thing, T. S. Ping, K. Awang, N. M. Hashim, M. A. Nafiah and K. Ahmad, J. Chem. Pharm. Res., 2014, 6, 815–822 Search PubMed.
- G. B. Marini-Bettòlo, Ann. Ist. Super. Sanita, 1968, 4, 489–500 Search PubMed.
- J. H. Jiang, W. D. Zhang and Y. G. Chen, Trop. J. Pharm. Res., 2015, 14, 2325–2344 CrossRef CAS.
- K. Jewers, D. F. G. Pusey, S. R. Shama and Y. Ahmad, Planta Med., 1980, 38, 359–362 CrossRef CAS.
- A. R. Araujo, C. Kascheres, F. Fujiwara and A. J. Marsaioli, Phytochemistry, 1984, 23, 2359–2363 CrossRef CAS.
- T. Sevenet, L. Allorge, B. David, K. Awang, A. Hamid, C. Kan-Fan, J. C. Quirion, F. Remy, H. Schaller and L. E. Teo, J. Ethnopharmacol., 1994, 41, 1471–1483 CrossRef.
- B. Qin, Y. Wang, X. Wang and Y. Jia, Org. Chem. Front., 2021, 8, 369–383 RSC.
- M. S. Shahari, K. Husain, E. Kumolosasi and N. F. Rajab, Nat. Prod.: Indian J., 2017, 13, 1–7 Search PubMed.
- W. Reanmongkol, S. Subhadhirasakul, S. Thienmontree, K. Thanyapanit, J. Kalnaowakul and S. Sengsui, Songklanakarin J. Sci. Technol., 2005, 27, 509–516 Search PubMed.
- S. L. Mok, K. Yoganathan, T. M. Lim and T. S. Kam, J. Nat. Prod., 1998, 61, 328–332 CrossRef CAS PubMed.
- G. Z. Li, Y. H. Hu, D. Y. Li, Y. Zhang, H. L. Guo, Y. M. Li, F. Chen and J. Xu, Neurotoxicology, 2020, 81, 161–171 CrossRef CAS PubMed.
- N. T. Son, L. T. Anh, D. T. T. Thuy, N. D. Luyen, T. T. Tuyen, H. T. M. Duong and N. M. Ha, Nat. Prod. Commun., 2021, 16, 1–6 CrossRef.
- N. T. Son, L. T. Anh, D. T. T. Thuy, N. D. Luyen, T. T. Tuyen and P. T. Hai, Z. Naturforsch., C: J. Biosci., 2022, 77, 207–218 Search PubMed.
- N. T. T. Linh, N. T. T. Ha, N. T. Tra, L. T. T. Anh, N. V. Tuyen and N. T. Son, Mini-Rev. Med. Chem., 2021, 21, 273–287 CrossRef PubMed.
- H. Wang, M. Naghavi, C. Allen, R. M. Barber, Z. A. Bhutta and A. Carter, et al., Lancet, 2016, 388, 1459–1544 CrossRef.
- F. B. Alberti, Bull. Roy. Coll. Surg. Engl., 2013, 95, 168–169 CrossRef.
- S. Saponara, M. Durante, O. Spiga, P. Mugnai, G. Sgaragli, T. T Huong, P. N. Khanh, N. T. Son, N. M. Cuong and F. Fusi, Br. J. Pharmacol., 2016, 173, 292–304 CrossRef CAS PubMed.
- F. Fusi, M. Durante, G. Sgaragli, P. N. Khanh, N. T. Son, T. T. Huong and N. M. Cuong, Planta Med., 2015, 81, 298–304 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |