Open Access Article
Open Access ArticleRecent trends in chemistry, structure, and various applications of 1-acyl-3-substituted thioureas: a detailed review
Urage Zahraa,
Aamer Saeed *a,
Tanzeela Abdul Fattaha,
Ulrich Flörkeb and
Mauricio F. Erbenc
*a,
Tanzeela Abdul Fattaha,
Ulrich Flörkeb and
Mauricio F. Erbenc
aDepartment of Chemistry, Quaid-i-Azam University-45320, Islamabad, Pakistan. E-mail: asaeed@qau.edu.pk
bDepartment Chemie, Fakultät für Naturwissenschaften, Universität Paderborn, Warburgerstrasse 100, D-33098 Paderborn, Germany
cCEQUINOR (UNLP, CONICET-CCT La Plata), Departamento de Química, Facultad de Ciencias Exactas, Universidad Nacional de La Plata, Bv. 120 1465, La Plata 1900, Argentina
First published on 26th April 2022
Abstract
The interest in acyl thioureas has continually been escalating owing to their extensive applications in diverse fields, such as synthetic precursors of new heterocycles, pharmacological and materials science, and technology. These scaffolds exhibit a wide variety of biological activities such as antitumor, enzyme inhibitory, anti-bacterial, anti-fungal, and anti-malarial activities and find utilization as chemosensors, adhesives, flame retardants, thermal stabilizers, antioxidants, polymers and organocatalysts. In addition, the synthesis, and applications of coordination complexes of these ligands have also been overviewed. The current review is a continuation of our previous efforts in this area, focusing on the recent advancements during the period 2017 to present.
1. Introduction
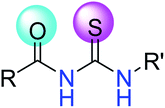
The thioureas comprise a broad family of compounds containing the >NC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SN< moiety, the general structure of 1-acyl/aroyl thioureas is shown in Fig. 1.
SN< moiety, the general structure of 1-acyl/aroyl thioureas is shown in Fig. 1.
Thioureas have gained marvelous attention in the last few decades because of their use in the synthesis of several important heterocyclic compounds. Due to the presence of sulfur and nitrogen atoms, which have a multitude of bonding possibilities, their coordination chemistry toward metal ions has become very significant. Their tremendously enhanced ligating properties resulted in the formation of transition metal complex compounds.1 Their abilities in complex formation and as heterocycle synthons have great significance in organic synthesis. These ligands have a variety of coordination modes and have wide applications in biological systems. Thiourea derivatives also act as organocatalysts and have been used in many reactions.2,3 Extensive studies showed they play a promising role in the fields of molecular recognition, materials science, agriculture, pharmaceuticals, and biological activities. Various articles have demonstrated the important biological activities of thioureas such as, herbicidal,4 insecticidal,5 antimicrobial,6 antitumor,7 antiviral,8 antiparasitic,9 antidiabetic,10 fungicidal, pesticidal,11,12 and urease inhibitory activities.13,14 The 1H-imidazol thiourea derivatives emerged as promising anti-HIV agents.15 Because of the high relevance of this prestigious family, different aspects deserved researchers' attention and the literature associated with N-substituted-N-aroyl(acyl)thioureas has been systematically reviewed. The first review on thiourea metal complexes was actualized in 2001 by Koch et al.16 which described the synthesis and coordination chemistry of N,N-alkyl-N′-acyl thioureas.16 Moreover, topics associated with chemical synthesis have been reviewed by Aly et al. in 2007 who focused on aroyl thioureas, their synthesis, and applications.17 The current review is the furtherance of our previous reviews and updates on these significant and versatile molecules in detail.
Our group published a review in 2014 that included the studies of thiourea metal complexes, their coordination chemistry, and interactions between the molecules,18 followed by another one in 2017, that discussed the synthetic schemes and reactivity of acyl/aroyl thiourea derivatives, their metal complexes along with biological activities,19 and further updated in 2019 by the same group, that described versatility of thioureas in heterocyclic syntheses and their multipurpose applications.20 In addition, some related advances included in 2019 review describing novel activities of these compounds.21 Another review by Lapasam et al. in 2020 was related to development of platinum metal complexes containing thiourea ligands and sulfur compound syntheses.22
The present review comprises the major advancements that have been taken place in the field of organic synthesis employing thioureas since 2017 when our last review appeared. Medical, synthetic, catalytic and many other fields have been progressed and benefited by these compounds. We have also described various applications of acyl-thioureas along with biological activities in detail which will fascinate the readers.
2. Synthesis
Various novel pathways have been introduced for the synthesis of thiourea derivatives including hybrid-molecule formation23 and optimization of one-pot syntheses procedures,24 but the most commonly used procedure is Douglas Dains' method,25 in which different amines are reacted with in situ generated acyl isothiocyanates in dry solvents at specific temperature.26 Some thioureas have also been synthesized by using ultrasound radiations.273. Heterocyclization reactions
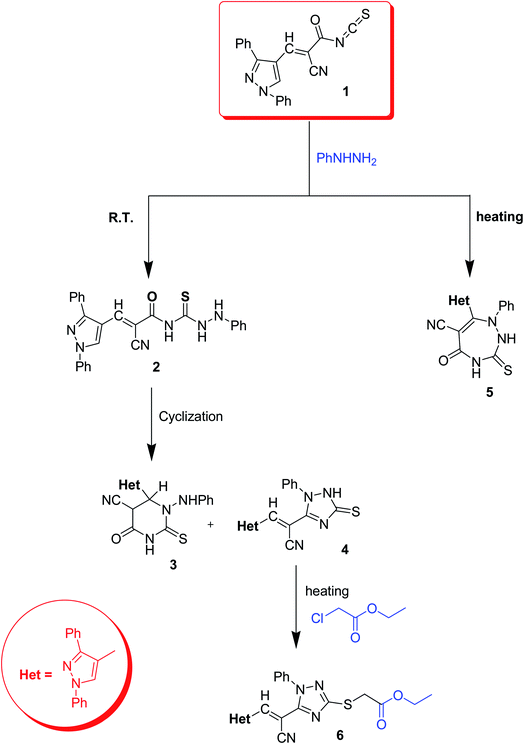
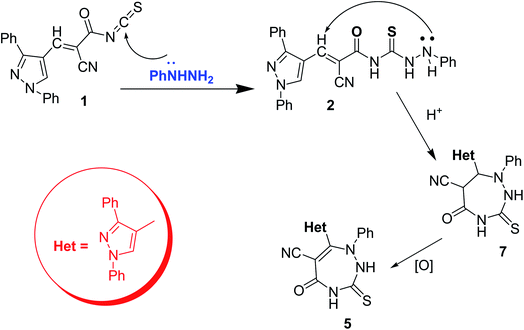
In this section, recent heterocyclization reactions of thioureas is described. In 2021 Badawy et al.28 synthesized a new series of pyrazole-based heterocycles from 2-cyano-3-pyrazolylpropenoyl isothiocyanate derivative 1 as an intermediate. The Scheme 1 depends on the reaction conditions, thus treatment of 2-cyano-3-pyrazolylpropenoyl isothiocyanate scaffold 1 with phenylhydrazine under room temperature yields a mixture of pyrimidine 3 and triazole compound 4 correspondingly.The reaction mechanism can be understood via the nucleophilic addition of amino group on the intermediate 1 to give compound 2 which undergoes heterocyclization in two different routes to yield 5 (Scheme 2). IR spectra of compound 3 retained the carbonyl group which is absent in 4. The 1H-NMR of compound 4 indicates one labile hydrogen which confirms the mercaptotriazole form rather thantriazolethione one. Alternatively, the reaction of 1 with phenylhydrazine under heating afforded the compound triazepine 5, its 1H-NMR shows two labile hydrogens. Lastly, alkylation of 4 with ethyl chloroacetate under heating in ethanol afforded derivative 6. The prepared compounds were then tested for antioxidant activities, compound 3 exhibits higher activity which may be due to extended conjugation and aromaticity which is absent in other compounds.
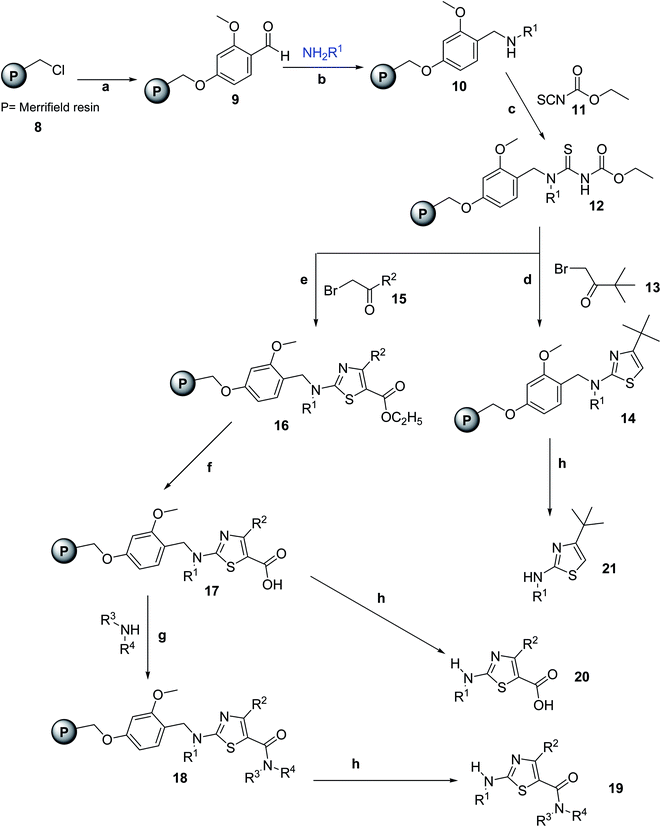
Heterocyclic compounds especially five-membered heterocycles displayed many promising pharmacological activities in the medicinal research area. Heterocyclization of thiourea is a good strategy to obtain these prestigious compounds. One such method was used by Kim et al.29 in 2019 on solid state as shown in Scheme 3. Kim developed a solid-phase synthetic method for the synthesis of 2-amino-5-carboxamide thiazoles, this method avoids the formation of undesired isomers. Various amines were introduced in solid-phase synthesis Scheme 4. Merrifield resin 8 was used as a starting compound and reacted with 4-hydroxy-2-methoxybenzaldehyde for 16 h to afford compound 9. Reductive amination of 9 with amines and NaBH(OAc)3 yields 10, which is attached with ethoxycarbonyl isothiocyanate 11 in DCM to give thiourea resin 12. Compound thiourea resin 12 then underwent dehydrative cyclization in the presence of DMF with α-bromoacetophenone 13 to afford compound 14. Moreover, following the hydrolysis of 16 with NaOH afforded 17. The resin 17 was cleaved from the polymer support using TFA at room temperature to yield the thiazole 20. Compound 17 then undergoes amide coupling reaction using EDC HCl, HOBt and amines to afford 18 shown in Scheme 4, resin 18 was cleaved from polymer support under the same conditions used to obtain 20, the thiazole 19 is obtained. In order to achieve the 4-tert-butylthiazole 21, resin 14 was cleaved at room temperature, all compounds were obtained in good yields.
4. Molecular and crystal structure
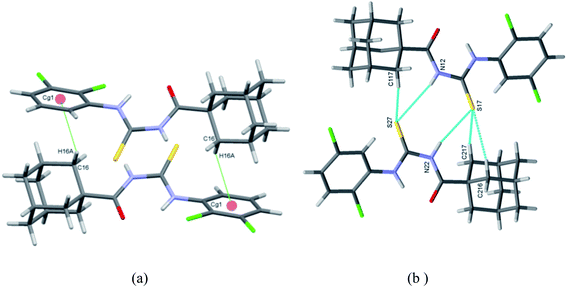
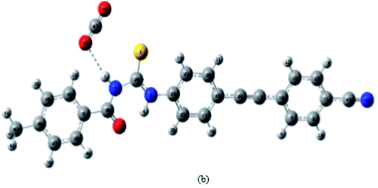
There has been much attention given to the conformational and structural properties of 1-(acyl/aroyl)-3-(mono-substituted) thioureas, the conformational flexibility depends upon the substitutions at nitrogen atom on thioureas, these attributes caused these compounds display remarkable activities30–32 and as fluorescent chemosensors for recognition of many ions.33 Many articles have been published on the conformational and structural aspects of thioureas.34Saeed et al.35 synthesized and completely characterized the two thiourea derivatives of adamantane-1-carbonyl isothiocyanate 22 using ammonium thiocyanate in very good yields. Their vibrational analysis revealed the intermolecular hydrogen bonds, as confirmed by the analysis of the single crystal molecular structure (Fig. 2). Compound 24 (a) crystallizes in triclinic system and 24 (b) has two molecules in the asymmetric unit of the orthorhombic unit cell. They exhibit planar structures due to N–H…O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C H-bonds that generate 6-membered rings. These intermolecular bonding increase the stability of the crystal structures (Scheme 5).
C H-bonds that generate 6-membered rings. These intermolecular bonding increase the stability of the crystal structures (Scheme 5).
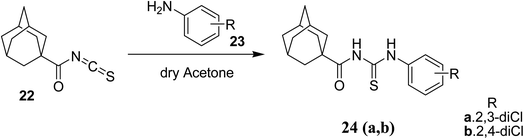
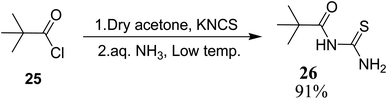
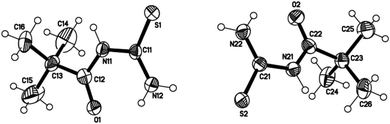
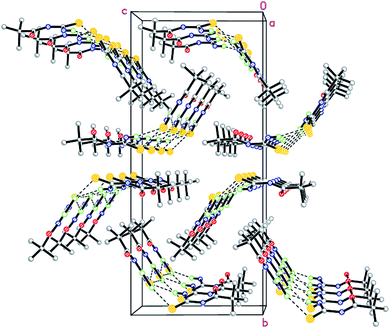
Novel acyl thiourea 26 was prepared and characterized through micro-elemental analysis by González et al.36 Thiourea 26 was prepared by using pivaloyl chloride 25, KNCS and aqueous ammonia in a 91% excellent yield Scheme 6. The chiral center contains two crystallographically independent molecules A and B with same chemical properties with numbering 1X and 2X (26) as shown in Fig. 3. Both molecules display equal bond lengths and angles, and geometric parameters for both showed no unanticipated features. Intermolecular N12–H12B⋯S2 and N22–H22B⋯S1 bonds connect A and B molecules into dimmers, forming pseudo 8 membered ring which are further connected with its neighboring molecules forming infinite chains as depicted in Fig. 4. Single crystal X-ray analysis showed the intermolecular H-bonding between N–H…C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O forming an additionally stabilized six-membered pseudo-ring. Hirshfeld analysis was also performed to confirm these findings.
O forming an additionally stabilized six-membered pseudo-ring. Hirshfeld analysis was also performed to confirm these findings.
 | ||
| Fig. 3 Molecular structure of compound 26 with anisotropic displacement ellipsoids, molecule 26 with 1X and 2X labeling. | ||
 | ||
| Fig. 4 Crystal packing with intermolecular hydrogen interactions with dotted lines, S, yellow, H, green, N, blue, O, red color. | ||
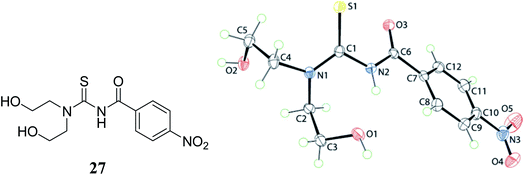
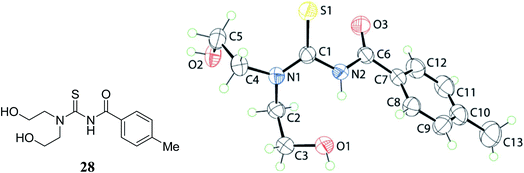
3,3-Bis(2-hydroxyethyl)-1-(4-methylbenzoyl)thiourea 28 and 3,3-bis(2-hydroxyethyl)-1-(4-nitrobenzoyl)thioureas 27 were prepared by using 4-methylbenzoyl chloride and 4-nitrobenzoyl chloride with ammonium thiocyanate in dry acetone, and the molecular structure of the compounds was evaluated which came out to be like the gas-phase structures Fig. 5 and 6. Hydrogen bonding in the molecular packing led to the supramolecular layer formations that were further confirmed by the Hirshfeld analysis. These interaction energies provided stability to the molecules. The dihedral and torsional angles of these compounds and spacial arrangements of the substituents were also studied by Tan et al.37,38
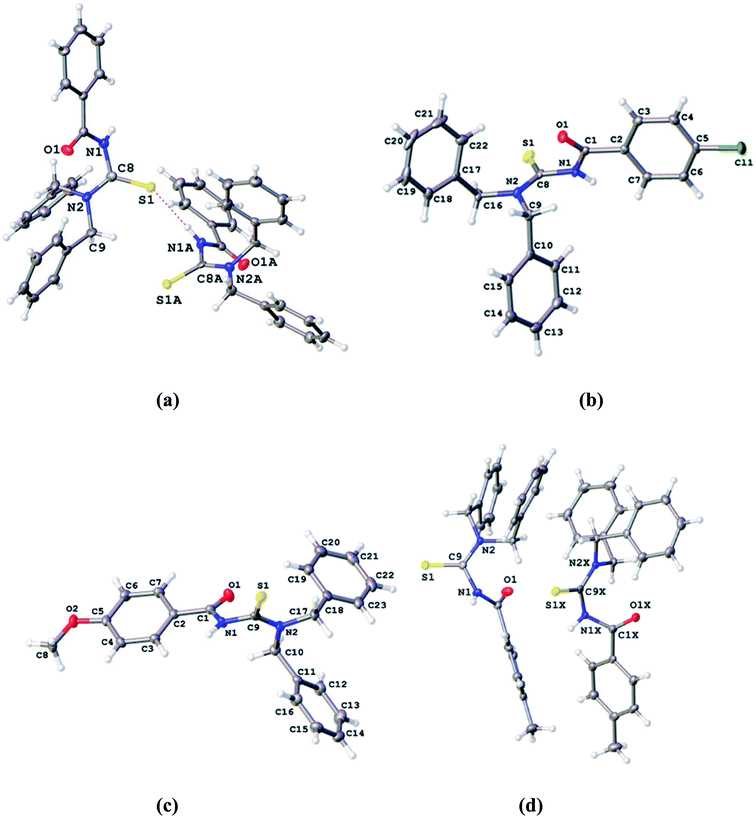
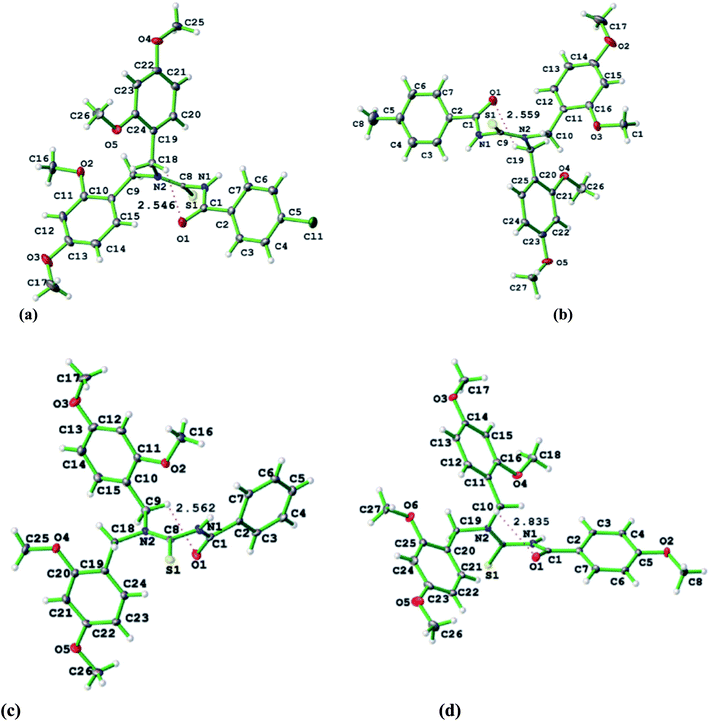
Gumus et al.39 synthesized and characterized a series of four closely related thiourea derivatives 31a–d and their crystal structures were characterized and studied to evaluate the bonding present in the compounds. 4-Substituted benzoyl chloride a–d was reacted with potassium thiocyanate and dibenzylamine 30 in dry acetone to afford the target thioureas 31a–d in good yields Scheme 7. Intermolecular interactions explained the network in terms of arranged supramolecular units through hydrogen bonding, π–π interactions contributing to the packing of species in the crystals (Fig. 7).
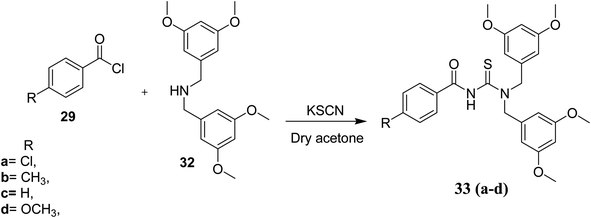
Gumus and coworkers40 also synthesized novel N-(bis(3,5-dimethoxybenzyl)carbamothioyl)-4-R-benzamide compounds 33a–d and these thioureas were characterized by spectroscopic methods and their crystal structures were also determined by single crystal XRD (Fig. 8). These novel derivatives were prepared in the same way as in Scheme 7 by Gumus39 only the amine is different which is bis(3,5-dimethoxybenzyl)amine 32 Scheme 8. The X-ray crystallography and Hirshfeld surfaces analysis of the novel derivatives 33a–d exhibits that hydrogen bonding and several weaker interactions e.g. N–H⋯S, and weak interactions such as C–H⋯O, C–H⋯S, C–H⋯π and C–H⋯N intermolecular interactions along with π–π stacking cooperative in the stabilization of supramolecular structures.
5. Metal complexes
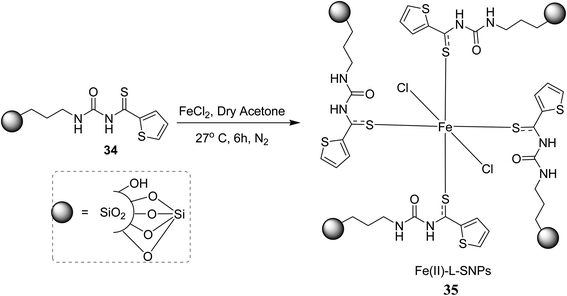
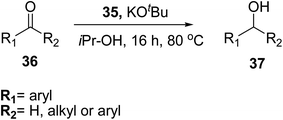
Thiourea derivatives are used as ligands in metal complexes, their fascinating coordination chemistry along with their derivatives finds a special place in the mind of researchers due to their vast potential applications in various fields. These are found to be excellent ligands because of their structure consisting of lone pair exhibiting sulfur and oxygen atoms that act as ligating centers. Because of the presence of sulphur and nitrogen atoms they act as valuable ligands and coordinate a variety of metal centers and form stable metal complexes.41 In this section, we will discuss the recent metal complexes of thioureas along with their crystal X-ray diffraction analysis.Sindhuja et al.42 prepared an Fe(II) catalyst containing acyl thiourea ligands 35 supported on silica nanoparticles, that can be used for the transfer hydrogenation of several carbonyl compounds as shown in Scheme 10. He studied the catalytic activity of 35 towards the transfer hydrogenation of ketones and different carbonyl compounds. The conversions occurred in a moderate to good yields and the catalyst can easily be regenerated from the reaction mixture by centrifugation for further use and can be utilized up to eight cycles without any activity loss. Compound Fe(II)-LSNPs 35 was synthesized by treating ligand 34 with FeCl2 at room temperature for six hours in dry acetone Scheme 9. In the next step 35 was stirred with carbonyl compound 36 in the presence of KOtBu Scheme 10 for transfer hydrogenation.
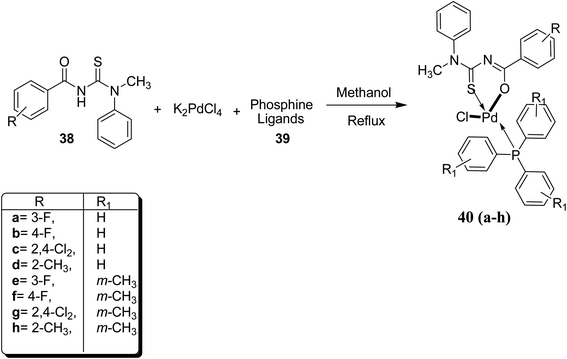
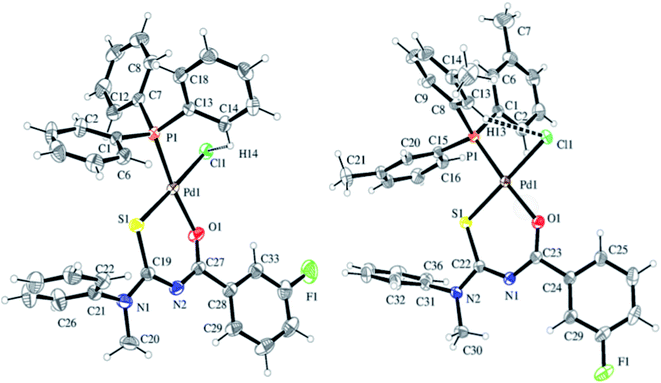
A series of Pd(II) complexes 40a–h with N-acyl-N,N′-(disubstituted) thioureas 38 and phosphine ligands 39 were synthesized by Khan et al.43 using K2PdCl4 Scheme 11. These complexes were further characterized by using spectroscopic as well as elemental techniques. FTIR, NMR and single crystal XRD have confirmed the structures of compound 40a and 40e (Fig. 9). The square planar geometry has shown the positions of the chelating agents and central atom as well. Various studies of these complexes revealed that they exhibit good antileishmanial activities.
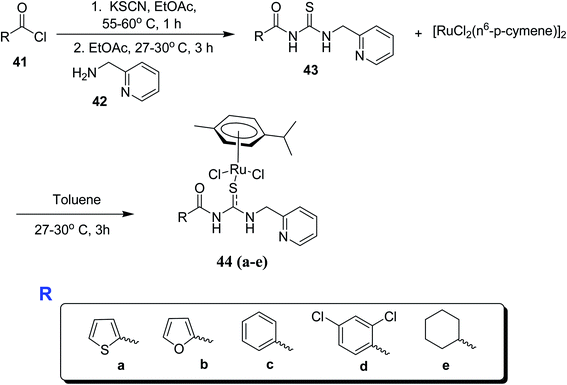
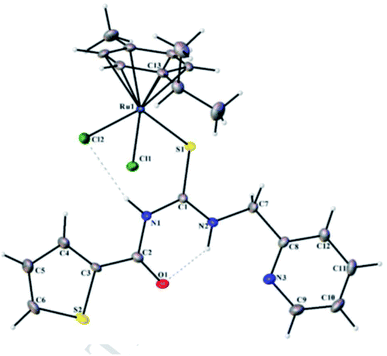
Similar work was conducted by Sathishkumar et al.,44 He synthesized Ru(II)(h6-p-cymene) complexes 44a–e with a new series of pyridine based acylthiourea ligands. These novel complexes were found to be useful as pre-catalysts for the transfer hydrogenation of carbonyl compounds utilizing 2-propanol as a source of hydrogen with KOH base and showing chemoselectivity towards the nitro group in the presence of carbonyl. Acyl chloride 41 was treated with KSCN and 2-(aminomethyl)pyridine 42 in the presence of ethyl acetate to afford acylthiourea ligands 43. A mixture of [RuCl2(h6-p-cymene)]2 and 43 was reacted at room temperature for 5 hours which ultimately afforded the target compounds 44a–e in good yields Scheme 12. The molecular structure of complex 44a was confirmed by single crystal XRD analysis Fig. 10, it crystallized in orthorhombic crystal system with inter and intra-molecular hydrogen bonds in the ligands and complex 44a. The catalysts were also compatible for many heterocycle conversions which include quinine, furfural and other heterocycles.
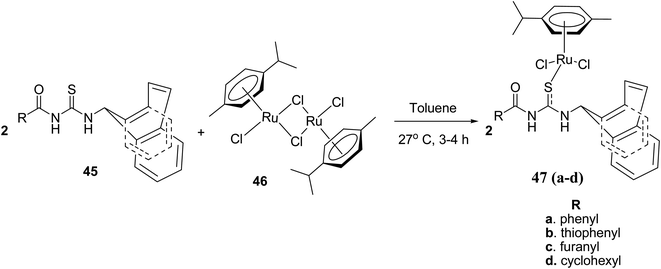
Ru(II)(η6-p-cymene) complexes 47a–d having monodentate dibenzosuberenyl substituted aroyl/acyl thiourea ligands have been synthesized and characterized by Rohini et al.45 The complexes 47a–d were synthesized by the reaction between 45 and 46 in dry toluene Scheme 13. As shown in Fig. 11, the single crystal X-ray analysis showed that the ligand is monodentate and sulfur atom coordinated with Ru(II) resulting in a “3-legged piano-stool” geometry. These catalysts are also used for the transfer hydrogenation (TH) of aldehydes and ketones, as Ru complexes put less steric effects around the [Ru–H] scaffold that initiates the process of TH. These complexes have an exceptional applicability in catalytic transfer hydrogenation. They also have a cytotoxic activity against human cervical, breast, and lung cancer cell lines while maintaining a low toxicity against non-cancerous cells.
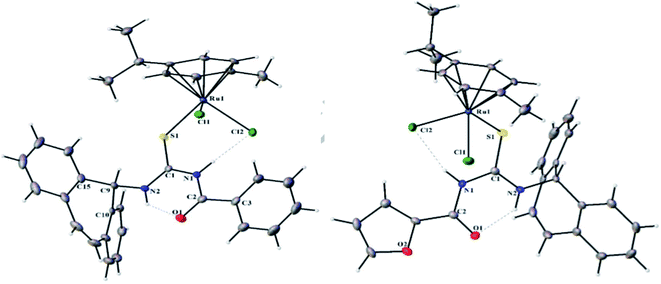 | ||
| Fig. 11 Molecular structure (50% probability ellipsoids) of 47(a) and 47(c) with atomic labeling scheme. | ||
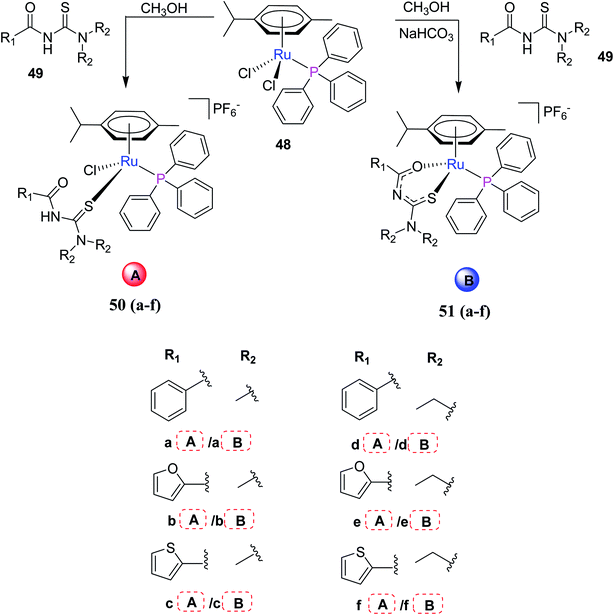
Cunha et al.46 synthesized half-sandwich Ru(II) complexes containing acyl thiourea ligands. The reaction of starting material 48 with acyl thioureas 49 in methanol afforded twelve new complexes 50, 51(a–f) Scheme 14. They carried out their spectroscopic characterization and cytotoxicity evaluation. Different synthetic routes resulted in differently coordinated complexes. These complexes showed inhibition in the growth of breast and lung cancer cells. Further studies were carried out on the complexes with (e) A/B and (f) A/B. These complexes inhibit the migration and formation of colony and induced morphology changes.
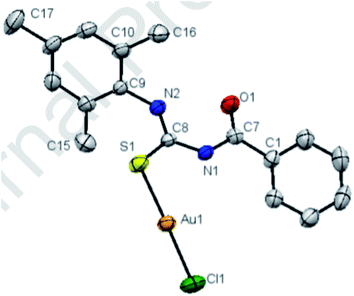
Gold(I), silver(I) and copper(I) complexes with 2,4,6-trimethylphenyl-3-benzoylthiourea ligand were synthesized by Khan et al.47 The ligand 53 was prepared by reacting aroyl chloride 41c with 52 Scheme 15, in the next step treatment of 53 with HAuCl4, AgNO3 and CuI in CH3CN yields their complexes 54–56. Their structures were confirmed by X-ray single crystal diffraction, showing the sulfur monodentate coordination mode towards the Au cation (Fig. 12). Complexes with Au/Ag were in the form of fine crystals. The cytotoxicity of these complexes exhibited antibacterial, antifungal, antioxidant, and DNA binding properties.
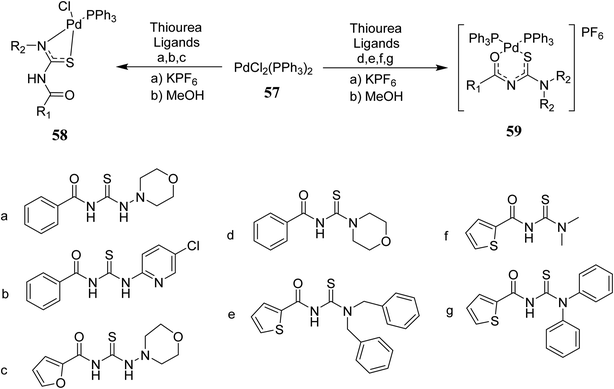
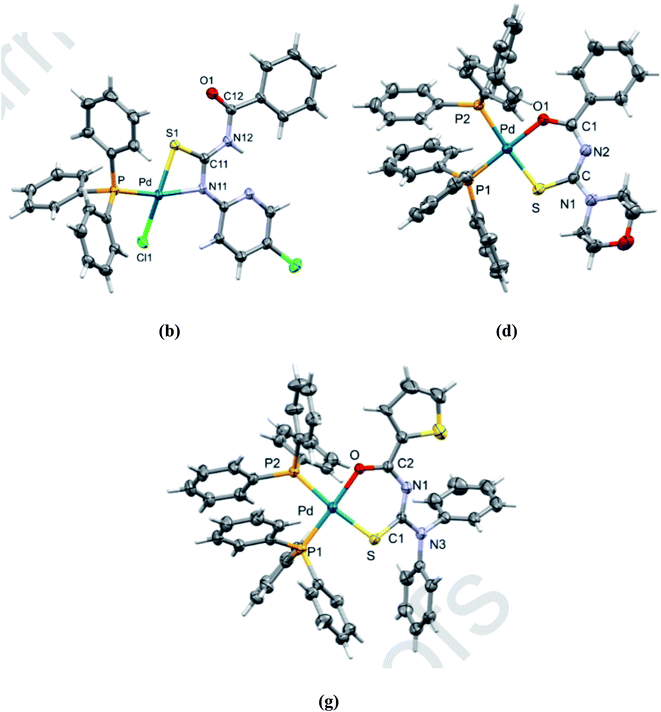
Plutín et al.48 synthesized seven novel thiourea complexes of Pd(II) and then characterized them by elemental analysis and spectroscopic methods. The ligands a–g were combined with Pd(II) by reacting them with 57 in methanol to afford the complexes 58, 59(a–g) Scheme 16. Interestingly, monosubstituted thioureas formed N–S coordinated neutral complex, whereas disubstituted one formed the O–S bidentate cationic complex, as shown in Fig. 13. These complexes showed cytotoxicity against tumor cells while the ligands were not cytotoxic. These findings indicated that metal–ligand bindings increase the antitumor activity.
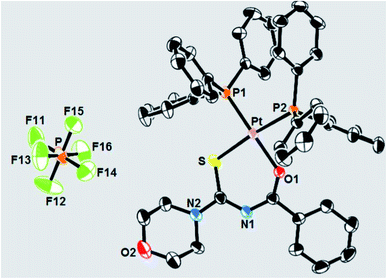
Pérez et al.49 synthesized a novel Pt(II)–metal complex 60 with thiourea ligand a bearing morpholine group and characterized it using elemental analysis and spectroscopic methods. Ligand a was treated with [PtCl2(PPh3)2] in the presence of methanol and KPF6 which afforded the target complex 60 in 80% yield (Scheme 17). Its single-crystal X-ray structure is shown in Fig. 14, displaying almost great square-planar coordination geometry. Rare C–H⋯Pt interactions are detected while the crystal is stabilized by weak hydrogen bonds, C–H⋯F, and C–H···pi stacking interactions. The complex exhibited activity against tumor cells and Mycobacterium tuberculosis.
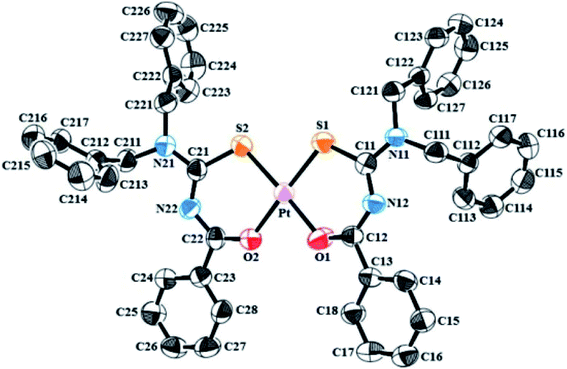
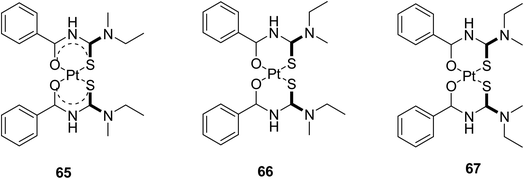
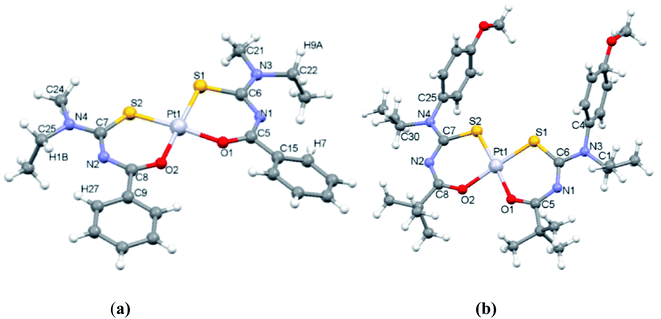
Correa et al.50 synthesized and characterized a ligand N-benzoyl-N′,N′-dibenzyl thiourea and then prepared its Pt(II) complex by reacting it with potassium tetrachloroplatinate(II). He evaluated its interaction with human (HAS) and bovine (BSA) serum albumin. The single crystal X-ray structure provided the spatial arrangements of atoms and geometry (Fig. 15) and Hirshfeld analysis explained the interactions responsible for protein-complex bindings. The X-ray diffraction concludes that Pt atom is coordinated with two chelated thiourea ligands in a square-planar geometry by two sulfur and two oxygen atoms, Fig. 16 shows that molecules in the whole crystal are connected by weak C–H⋯C and C–H⋯S along with hydrogen-bonding interactions.
 | ||
| Fig. 16 Molecules in the complex linked by C–H⋯S (black), C–H⋯C(red) and hydrogen bond dashed lines. | ||
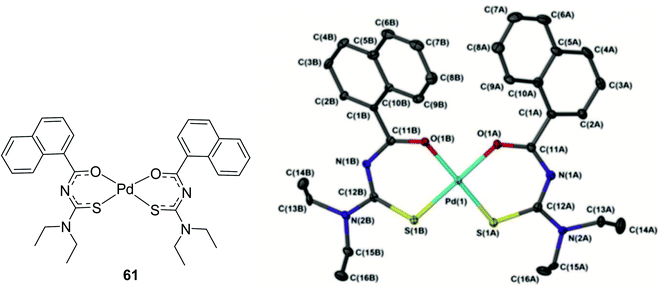
The phenomenon of reversible photo-isomerism using the Pd–thiourea ligand complex 61 was studied and the effect of different solvents on thermal isomerization was explained by Nkabyo et al.51 They also characterized the synthesized cis-bis(N,N-diethyl-N′-naphthoylthioureato)–palladium(II) complex using NMR and X-ray analysis (Fig. 17).
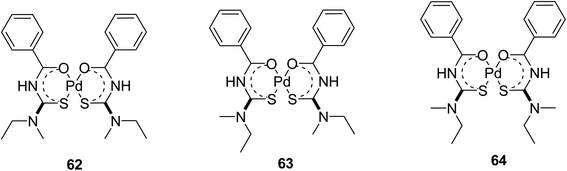
Nkabyo et al.52 also synthesized and characterized Pd(II) complex cis-bis(N,N-methyl-ethyl-N′-benzoylthioureato)-palladium(II) from an asymmetrically di-substituted ligand N,N-methylethyl-N′-benzoylthiourea. This ligand exists in chloroform as a mixture of 62, 63 and 64 isomers Scheme 18. The research group also characterized all three isomers using 1H, 13C, HMBC and 1D NOE NMR spectroscopy and single crystal X-ray studies, from these only one crystal of 62 was isolated and studies Fig. 18. These complexes were evaluated based on photo-induced isomerism and its effect on the configurational isomers of the prepared complexes.
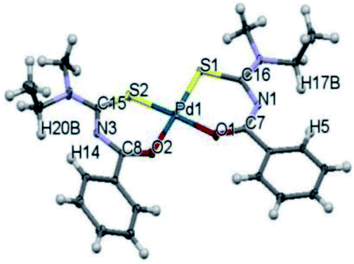 | ||
| Fig. 18 Molecular structure from single-crystal X-ray diffraction of 62 isomer of cis-bis(N,N-methyl-ethyl-N′-benzoylthioureato)–palladium(II). | ||
In a similar way Nkabyo et al.53 also synthesized Pt(II) complexes Scheme 19 with asymmetrical thiourea ligand N,N-methylethyl-N′-benzoylthiourea in the same manner as described in previous Scheme 18. The Pt(II) complexes exist in three configurational isomers in chloroform namely cis-EE-[Pt(L-κS,O)2], cis-ZZ-[Pt(L-κS,O)2], and cis-ZE-[Pt(L-κS,O)2]. These complexes were characterized by using 1H, 13C, HMBC and 1D NOESY NMR spectroscopy and X-ray diffraction (Fig. 19). According to these studies, long-chain and bulky N-alkyl substituents showed superior relative distribution of cis-ZZ-[Pt(L-κS,O)2] isomer than smaller ones and also separated different configurational isomers.
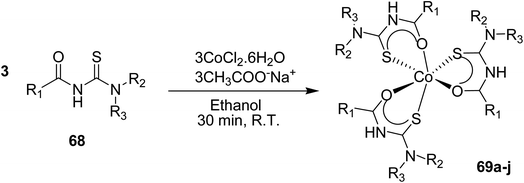
Octahedral diamagnetic complexes 69a–j of substituted thiourea ligands and cobalt metal were synthesized by Barnard et al.54 The synthesis of complexes 69a–j involves the reaction between ligand 68 and CoCl2·6H2O in ethanol at room temperature Scheme 20. These complexes were completely characterized by using 1H, 13C and 59Co NMR into configurational isomers, and the research group also reported the crystal structures of some of the isolated forms.
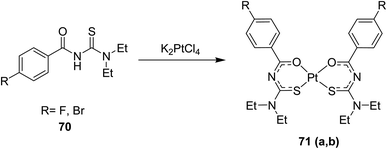
Thiourea derivatives 70 and their platinum complexes 71(a, b) were synthesized (Scheme 21) and characterized by Keskin et al.55 using all the spectroscopic techniques and their structures were also confirmed by single crystal XRD (Fig. 20). The C–H…pi and pi…pi interactions played important roles in the supra-molecular structures of the complexes.
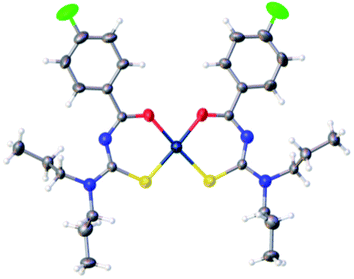
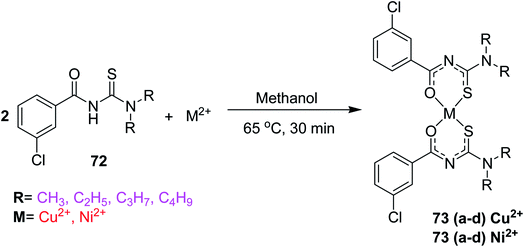
Binzet et al.56 synthesized four new derivatives of acyl thioureas 72 and their complexes with nickel(II) and copper(II) metals 73a–d and characterized them using different spectroscopic and elemental analysis techniques. The complexes 73a–d were prepared by reacting N,N-dialkyl-N,-3-chlorobenzoylthiourea 72 with metal (II) acetate in methanol Scheme 22. The thiourea ligands 72 coordinate with Cu(II), Ni(II) atoms through sulfur and oxygen atoms, two sulfur and two oxygen atoms are mutually cis to each other in both complexes. The Hirshfeld analysis and other studies of the prepared compounds have shown the intermolecular contacts and their anti-microbial activities against some strains of bacteria. The molecular structure of the 73d copper complex is shown in Fig. 21.
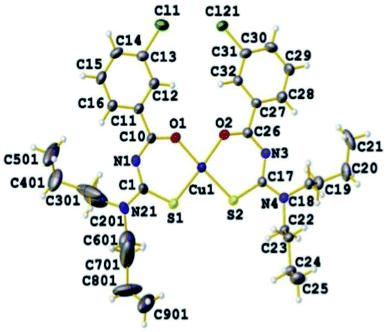 | ||
| Fig. 21 Molecular structure of bis(N,N-di-n-buthyl-N,-3-chlorobenzoilthioureato) copper(II) complex 73d. | ||
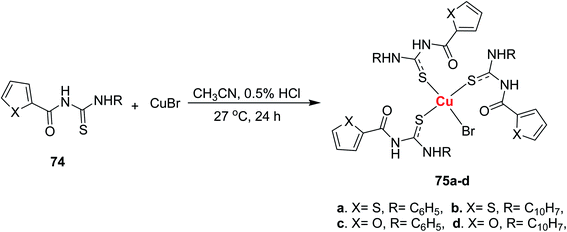
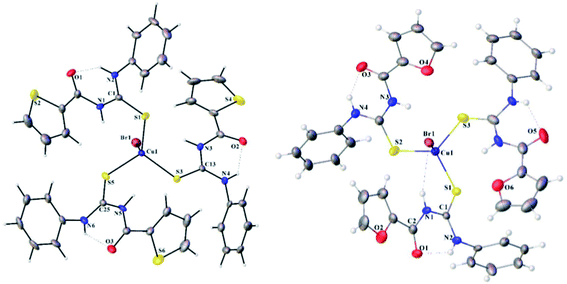
Jeyalakshmi et al.57 synthesized new copper complexes 75a–d by reacting aroyl thiourea ligands 74 and copper(I) bromide in acidic media Scheme 23. The molecular structures and geometry of the complexes 75a, and 75c were confirmed by X-ray analysis (Fig. 22). The crystal structure showed tetrahedral geometry of Cu(I) complex, in which the three coordination sites were engaged by sulfur atom from the ligands and the Br occupied the fourth one. The X-ray analysis also confirmed the presence of intramolecular hydrogen bonding between N–H and carbonyl and H.Br bonding between N–H and Br− ion. The compounds were also examined for their cytotoxic activities against cancer cell lines, compound 75a, and 75b exhibited potent activity against HeLa cells.
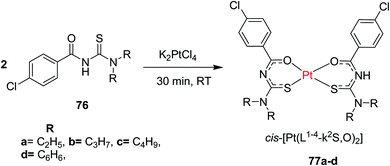
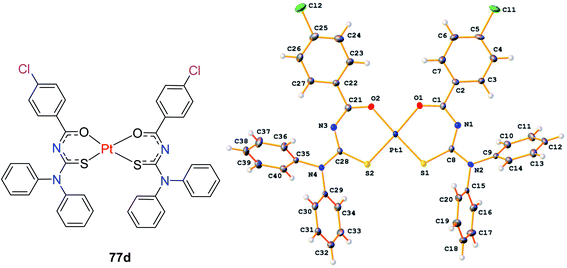
N,N-Disubstituted-4-chlorobenzoyl thiourea ligands 76 were combined with platinum(II) metal to form complexes 77a–d by Solmaz et al.58 Scheme 24. The complexes were completely characterized by spectroscopic techniques and X-ray analysis. The crystal structure of 77d (Fig. 23) showed that a square-planar coordination geometry is occurred around the Pt(II) atom by two oxygen and two sulfur atoms from the ligands having cis configuration. Their in vitro studies had revealed the antibacterial and antifungal activities associated with the complexes 77a–d.
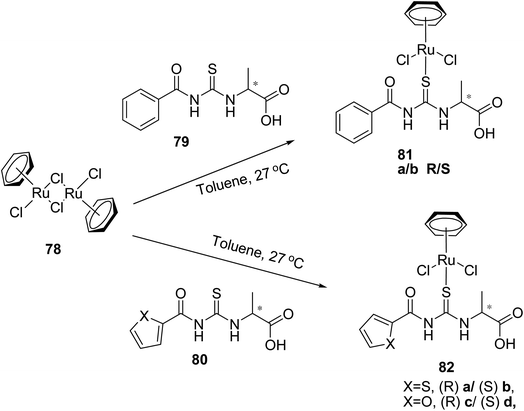
Sheeba et al.59 prepared novel water-soluble half-sandwich chiral Ru(II)-benzene complexes 81(a, b) and 82(a–d) using chiral aroyl thiourea ligands 79, 80 derived D/L-alanine and [RuCl2(η6-C6H6)]278 in toluene as shown in Scheme 25. These complexes were characterized, and their solid-state structures were confirmed. The X-ray analysis showed monodentate sulfur coordination of thiourea ligands in the complexes, and Ru(II) showed half-sandwich “3-legged piano-stool” geometry Fig. 24. These water-soluble complexes showed enhanced catalytic activity for the enantioselective reduction of ketones.
The novel chiral thiourea ligands 79, 80 derived from unprotected D/L-alanine were combined with Ru(II)-p-cymene 78 to form Ru(II)-complexes 81 and 82 by Sheeba et al.60 in the same manner as described in Scheme 25. Their structures were confirmed by using spectral and analytical methods. The synthesized compounds catalyzed the asymmetric transfer hydrogenation of ketones to secondary alcohols with high enantiomeric access.
6. Applications
6.1 Pharmacological aspects
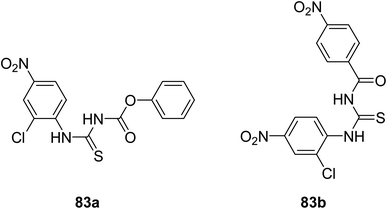
In last few decades, great interest has been given to the most prominent class of organic compounds that are thioureas. Many articles have been published on the biological activities of 1-(acyl/aroyl)-3-(substituted)thiourea derivatives. Nowadays it's the most mesmerizing area for most scientists, thousands of new derivatives are created by the alteration of structural topographies to get vast biological activities.19,61 In this section, we will discuss the pharmacological properties of thioureas and their analogs.Pandey and coworkers62 synthesized two new thiourea derivatives 83a–b and characterized them. The compounds were tested for their in vitro anticancer activities against seven human cancer cells; colorectal (HT29 and HCT116), ovarian carcinoma (A2780, A2780/CP and IGROV-1) and cervical (2008 and C13*) with compound 83(a) showed significant results against ovarian cancer cell lines and compound 83b demonstrated good results against cervical and colorectal cancer cells (Scheme 26).
Two other thioureas 84a–b were synthesized by the condensation reaction between substituted isothiocyanate with diphenylamine by Pandey and coworkers63 Scheme 27. The synthesized compounds were screened for their anti-cancer activities against five human cancer cell lines: ovarian carcinoma (A2780, A2780/CP and IGROV-1) and cervical (2008 and C13*). Both compounds have shown cytotoxic activities against IGROV-1 and cervical cancer cells compared to the other two. The IC50 values showed that thiourea 84b is more potent than 84a which may be attributed to the presence of Cl group at the para position of phenyl group, thus strong electronegative group at para position enhanced the lipophilicity and is the reason of increased cytotoxicity.
In another paper in 2020 Pandey et al.64 reported a new series of four thiourea compounds 85a–d compounds (Scheme 28) and their structures were confirmed by spectroscopic and single-crystal X-ray studies. Hirshfeld analysis and associated 2D fingerprint plots showed their intermolecular connections. The prepared compounds were screened for cytotoxic activities against seven human cancer cell lines, among them 85b and 85d demonstrated strong activities than other two compounds which again is due to the presence of strong electron-withdrawing groups (nitro and chloro) at the phenyl ring which are responsible for strong lipophilicity and cytotoxicity of compounds.
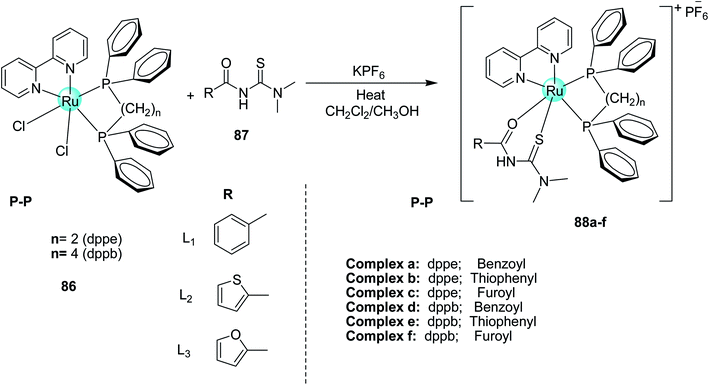
Five new ruthenium complexes 88 were synthesized by Oliveira et al.65 Using three different N–N-disubstituted acyl thiourea ligands 87 and two different diphenylphosphine ligands namely dppe and dppb 86 Scheme 29. All six complexes showed cytotoxic activity against lungs and breast tumor cells and caused alterations in their structures to inhibit the migration of cells, induce cell cycle in the Sub-G1 phase, and cell death by apoptosis. The compounds 88 showed higher selectivity indexes for breast cancer cells for the complex with dppb ligand. Complex 88c, and 88f cause structural alterations in the triple-negative breast cancer.
Acyl thiourea derivatives 89a–b attached to histone deacetylase inhibitors were synthesized and characterized by Amily et al.66 These compounds act as ligands toward zinc cation via the thiourea binding group and the complexes showed growth inhibition of human colon adenocarcinoma that have lower toxic effects on normal breast cells. Their mode of binding and effects has been studied by using docking studies and the results demonstrated them as good histone deacetylase inhibitors. Both the compounds exhibited good inhibitory potential against cancer cells greater than the normal cells. Compound 89b was found to be more active than 89a against HC-04 cell with IC50 values 21.44 μM and 24.12 μM (Scheme 30).
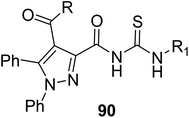
Koca et al.67 prepared new pyrazolyl acyl thioureas containing sulfa drug molecules 90, the molecular docking studies were performed to confirm the bonding interactions and the results revealed that these compounds exhibit potential anti-cancer properties (Scheme 31).
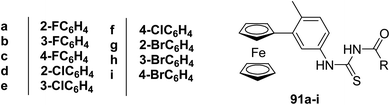
Asghar and coworkers69 synthesized a series of ferrocene-based thioureas 91a–i and characterized them by spectroscopic techniques. It was found that the incorporation of the ferrocenyl group into thioureas is responsible for their enhanced lipophilic character. Compounds were than tested for antifungal activities. The fluoro compounds 91a–c exhibited more potent activities than chloro and bromo derivatives 91d–i. In addition, substituent's at ortho position in compounds 91a, d, g were more biologically active than para and meta substituted compounds which may be attributed to the outstanding effect of an electronegative halo group at ortho position than at para and meta (the inductive effect is valid up to three or four bonds). The electron-withdrawing effect reduces the basicity of N–H and enhances the lipophilic character of the compounds Scheme 32.
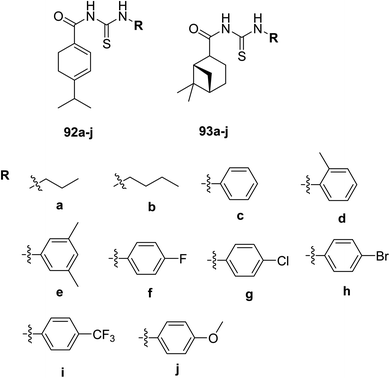
Gao and coworkers70 have synthesized two series of terpene-based acyl thiourea derivatives 92a–j, 93a–j (rosin and turpentine derivatives) Scheme 33 to confirm the antifungal activities of naturally occurring products. Using the growth rate method, their inhibitory activities have been assessed. Insertion of acyl-thiourea unit into terpene framework results in higher antifungal activity and lower toxicity. SAR and QSAR have revealed that electronic and steric effects markedly affect antifungal activities. Among all the compounds 93i exhibited prominent activity having IC50 lower than control drug, all 93a–j derivatives showed same antifungal activity compared to control drug carbendazim. Derivatives with electron-donating substituents and aliphatic chains exhibited low antifungal activities, while majority of the compounds in 92a–j and 93a–j with electron-withdrawing atoms like F, Cl, Br and –CF3 displayed much higher activities against control drug (92f–i > 92c–e > 92a–b > 92j; 93f–i > 93c–e > 93a–b > 93j). Depending upon the studies, these compounds could be used as replacements for leads and currently used fungicides with less toxic effects.
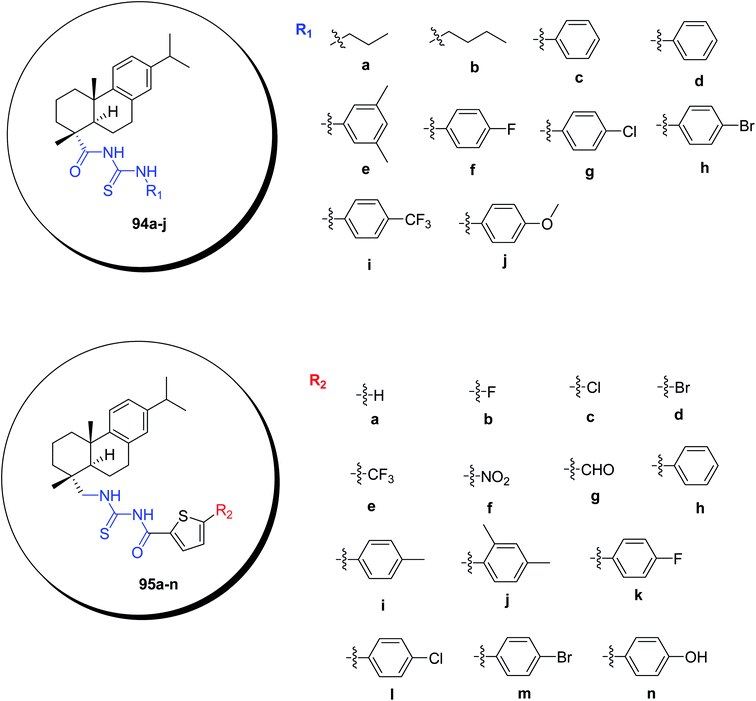
Rosin-based two series of acyl thiourea derivatives 94a–j, 95a–n were designed and synthesized by Wu et al.71 Scheme 34. One of them is thiophene heterocyclic group containing and the other one is without heterocyclic group. Thiophene containing derivatives showed mild to strong anti-fungal activities depending upon the electronic and steric effects and the energy differences indicated by the SAR and QSAR studies. Heterocycle containing derivatives 95a–n displayed much enhanced antifungal activity than 94a–j. The compounds 95b–e have similar activities. Studies revealed that electron-withdrawing substituents put more prominent effects than electron-donating groups (95j, 95k, 95l, 95m > 95i, 95n; 94f, 94g, 94h, 94i > 94d, 94e, 94j). Thus, these compounds could be used as potential fungicides.
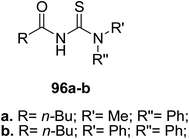
Two thiourea derivatives 96a–b were prepared and characterized by Ghazal and coworkers72 Scheme 35. These compounds have shown activities against three strains of fungi. Compound 96a exhibited strong antifungal activity against F. soloni and A. fumigatus strains.
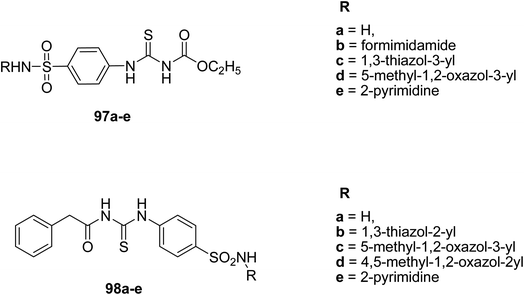
El-Gaby et al.75 synthesized two series of sulfonamide acyl-thiourea derivatives 97a–e, and 98a–e by using the ethyl carbamate and N-substituted 4-aminobenzene sulfonamide derivatives Scheme 36. The in vitro antibacterial activities of the compounds were tested for four Gram-positive bacteria namely Streptococcus pyogenes, Bacillus subtillis, methicillin-resistant Staphylococcus aureus, and Staphylococcus aureus, and three Gram-negative bacteria. The results indicated that compounds 98b and 97d displayed significant activities against both Gram-positive and Gram-negative bacteria, then 98a, 98c, and 98e. Compound 97d was found to be sensitive one. Different studies have referred towards their anti-bacterial activities and the final products involving these acyl-thiourea derivatives are useful in molecular docking studies.
A series of thioureas 99a–e using 4-nitro-2-cyano aniline with different carboxylic acids as starting materials was synthesized by Saeed et al.76 Scheme 37. All these compounds were obtained in very good yields and were evaluated for antibacterial, antifungal, and α-amylase activity. The compounds were tested against Enterobacter aerogenes, Escherichia coli, Micrococcus luteus, and Staphylococcus aureus bacterial strains. All compounds 99a–e displayed good to prominent antibacterial activity compared to standard drug kanamycin. Compound 99e was more potent among all and displayed very prominent activity in the series, which may be attributed to the presence of two aryl groups connected with thiourea part. Compound 99d exhibited low activity against bacterial strains because it contains acetyl attached with thiourea. Their molecular docking studies were also carried out to explain the enzyme inhibition activity.
A new series of thiourea and guanidine derivatives 100a–j were prepared by Saeed and coworkers.77 The compounds 100a–j were tested for antibacterial activity against Gram-positive, Gram-negative, and several isolates from patients with cystic fibrosis. The compound 100g showed good antibacterial activity against tested bacterial strains. The activities exhibited by several derivatives, highlight the importance of halo-phenyl group in the guanidine moiety Scheme 38.
Maalik et al.78 prepared a series of new 1-benzoyl, 3-phenyl-thiourea derivatives 101a–j with systematic substitution on the phenyl ring and evaluated their biological activity as antibacterial compounds to find their importance in the medicinal field Scheme 39. The research group evaluated the prepared compounds using molecular docking studies. Antibacterial activity was carried out against ten bacterial strains, according to the findings, compounds 101(a, c and i) with –Cl, and –OMe substituents displayed prominent activity against all bacterial strains, whereas rest of the derivatives exhibited moderate or no activity, these findings are confirmed the previous papers that halo and methoxy groups on aryl ring displayed excellent antibacterial activities.
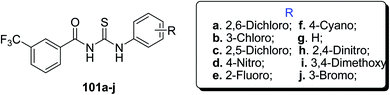
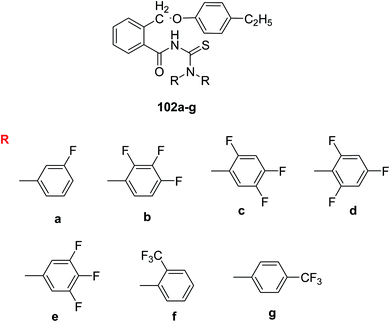
Similar halo-benzoyl thiourea derivatives with more than one fluorine atom and trifluoromethyl group as substituents were prepared and characterized by Limban et al.79 Scheme 40. The information from molecular docking studies had shown their affinity to bond with E. coli DNA and have exceptional antimicrobial activity. The compounds with fluorine atoms exhibited the best anti-bacterial effects whereas those with more than one fluorine atoms had shown antifungal activity. The compound 102a showed the highest spectrum of antibacterial activity, being active against both Gram-positive and negative bacteria which may be attributed to the presence of fluorine on the phenyl ring. Multiple fluorine atoms in the derivatives 102(b, c, d and e) did not affect the antibacterial activity. The compounds with trifluoromethyl group 101e and 102g were active on E. coli.
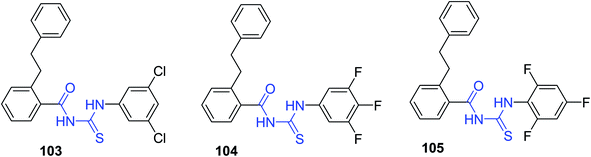
Noroc et al.82 designed and prepared three new N-(2-phenethylbenzoyl) thioureas 103–105 Scheme 41. The synthesized compounds were tested on both clinical and standard isolated strains of Mycobacterium tuberculosis and the acute toxicity and minimum inhibitory amounts were checked in this study. All the compounds have no toxicity in acute tests and are promising derivatives for the design of medicines for the treatment of tuberculosis. The phenethyl group in the derivatives was utilized as bioisoster of the isopentyl group and halogens were incorporated to enhance the lipophilicity. The results revealed that all 103–105 derivatives showed no toxicity in mice and tuberculostatic effects with a lower concentration of 10 μg mL−1 on both strains of Mycobacterium tuberculosis were seen. The halo substitutions and their position have little effect on tuberculostatic effect.
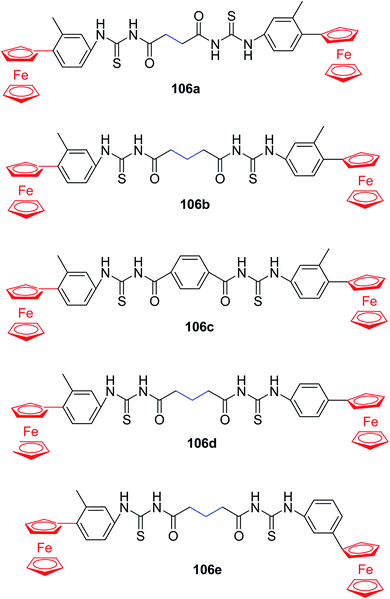
Patujo et al.84 synthesized a new series of bisferrocenylbisthiourea derivatives 106a–e in good yields Scheme 42 and evaluated their antidiabetic activities to check the pharmacological strength of the prepared compounds. For this purpose, the compounds 106a–e were explored for the enzyme scavenging potential against enzyme alpha-amylase. The results revealed that derivatives 106(e, b, c, a) showed scavenging potential having IC50 values, 22.8, 209.2, 112.0 and 251.5 μg m−1, showing only 106a active inhibitor of enzyme. The derivatives were then tested for scavenging potential against α-glucosidase enzyme. The results indicated that 106(e, d) inhibit enzyme, and 106e is much better than 106d which is due to the presence of ferrocenyl group at different position. Thus, these compounds can be used as potential drugs in the medicinal field.
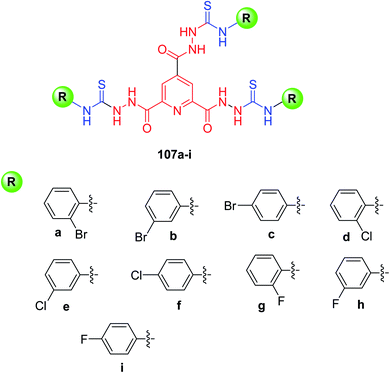
Rehman and coworkers85 synthesized a new series of pyridine-2,4,6-tricarbohydrazide thiourea derivatives 107a–i in good yields (63–92%) Scheme 43. In order to evaluate the pharmacological importance of newly synthesized compounds, they were tested against α- and β-glucosidases enzymes as these enzymes are responsible for treating type-2 diabetes mellitus (T2DM). The results revealed that among all the compounds 107a–i, derivative 107i was the more potent in the series with IC50 value 25.49 ± 0.67 μM and it was found even more potent than reference drug acarbose having IC50 = 38.22 ± 0.12 μM. The SAR studies suggested this activity in 107i is due to the presence of fluoro group at the C-4 position of aryl ring which offers a suitable environment for interaction with the active site of enzyme. The second active in the series is 107f (IC50 28.91 ± 0.43 μM), then 107h (IC50 = 30.66 ± 0.52 μM), and lastly 107e (IC50 = 35.01 ± 0.45 μM) offers good inhibition against α-glucosidase enzyme (107i > 107f > 107h > 107e > 107g > 107d). Thus, it can be concluded that halo substitution at the C-4 position delivers good inhibition against the enzyme.
Our research group86 synthesized a series of 1-acetyl-3-aryl thioureas 108a–o and characterized them using spectroscopic techniques and X-ray diffraction studies Scheme 44. These compounds were subjected to computational studies and their cholinesterase inhibition activities were evaluated. All these derivatives showed selective inhibition against acetylcholinesterase except 108i and 108o. Compound 108b showed exceptional potent inhibition activity against acetylcholinesterase whereas 108i has potential activity against butyrylcholinesterase. Molecular docking studies further supported the experimental results. Acetyl cholinesterase inhibitors perform important role in the treatment of Alzheimer's disease.
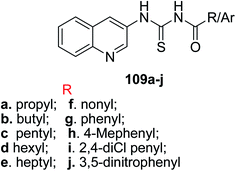
Saeed and our research group87 synthesized a series of quinoline-based thiourea derivatives 109a–j in excellent yields Scheme 45. These thioureas were evaluated to be efficient in radical scavenging activities and in tyrosinase inhibition. According to docking and kinetic studies, the compound 109c inhibited the enzyme non-competitively with IC50 = 0.0070 ± 0.0098 μM and it could also be identified as potent lead compound to design the effective tyrosinase inhibitors and can be used in food and medicine. Tyrosinase enzyme also play crucial role in melanin biosynthesis and browning of vegetables and fruits.
Our research group88 designed and synthesized a new series of acyl/aryl thiourea derivatives 110a–j in good yields Scheme 46 by utilizing the free amino group of sulfadiazine drug. In order to get the medicinal properties of sulfadiazine drug its new derivatives are prepared which have potential medicinal properties. The compounds 110a–j were tested for calf intestinal alkaline phosphatase (CIAP) activity. All the compounds displayed better inhibition capacity as compared to the reference drug, compound 110c was found to be active in the series with IC50 0.251 ± 0.012 μM. Their pharmacological studies have shown that all compounds obey Lipinski's rule. They also exhibit lead-like properties with much lesser toxicity. Due to non-mutagenic and irritant behavior, these compounds can be used as drug templates.
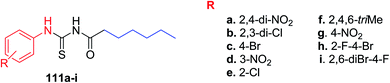
The newly developed 1-heptanoyl-3arylthioureas 111a–i that can be used as potential inhibitors of urease enzyme were synthesized by our group89 Scheme 47. These compounds were obtained in very good yield and characterized through spectroscopy and elemental analysis. All the synthesized compounds show drug likeliness according to the Lipinski's rule. Their kinetic and docking studies confirmed the mode of activity and binding affinity. Especially compounds 111a and 111c can act as lead molecules in 4D (drug designing discovery and development).
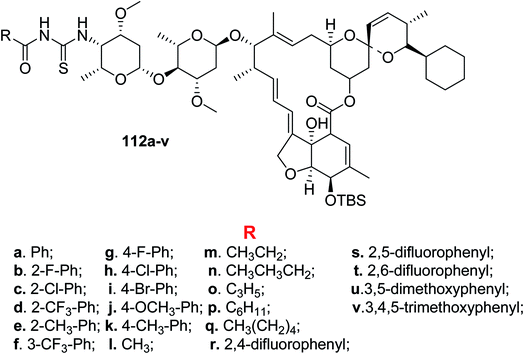
Zhang et al.90 synthesized and characterized a novel series of acyl thiourea derivatives of doramectin 112a–v Scheme 48. Their biological assay and molecular docking studies showed them to be used as potential insecticides. The presence of hydrogen bond groups in their structures might enhance the insecticidal activity.
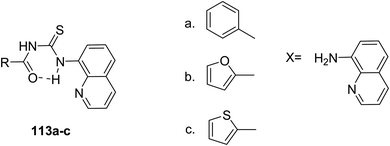
Acyl thiourea derivatives 113a–c were synthesized and characterized using spectroscopy and elemental analysis by Kalaiyarasi et al.91 Scheme 49. Their molecular structures were also determined, and molecular docking studies showed them to exhibit anti-malarial and anti-inflammatory activities. These compounds also possessed antioxidant activity.
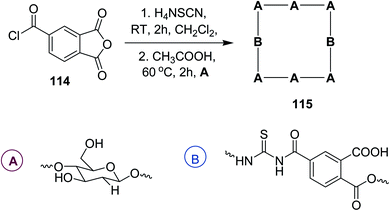
Cross-linked chitosan hydrogels 115 using novel trimellitic anhydride isothiocyanate were synthesized and characterized by spectroscopic techniques by Mohamed et al.92 Scheme 50. Their swell abilities and antimicrobial activities were found to be dependent on the cross-linking moiety contents. It has been found out that chitosan combined with functionalized groups provided the adequate systems and promising candidates in biomedical fields.
Many strategies have been developed for new bioactive agrochemicals for crop protection, and acyl thiourea derivatives have been reported as attractive compounds in this field.
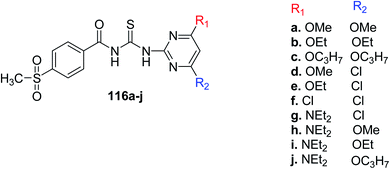
Li et al.4 synthesized a new series of thioureas containing substituted pyrimidines 116a–j (Scheme 51). The newly prepared compounds were tested for herbicidal activity, and the preliminary results indicated some derivatives had good activity against Amaranthus retroflexus, and Digitaria adscendens, particularly compound 116d and 116f displayed inhibitory potential on D. adscendens. Moreover, compounds 116d and 116f had higher comparative herbicidal activity on Echinochloa crus-galli than the commercial herbicides. The SAR studies showed that when the spatial volume of pyrimidine substituents is small like (Cl, OMe and Me) these derivatives have higher activities because these can easily fit into the active sites of enzyme.
6.2 As chemosensors
Several thiourea derivatives have been prepared and used for the naked eye detection of many metal ions, this activity of thiourea is due to the presence of nitrogen, sulfur and oxygen atoms which makes interactions with metal ions.61Carbon dioxide (CO2) is one of the important greenhouse gas in atmosphere mostly caused by fossil fuels and deforestation, which have quickly enhanced the concentration of CO2 in earth's atmosphere, causing global warming. Thus, global researchers are developing cheap, low power and miniature in size carbon dioxide sensors with high sensitivity.93 Daud et al.94 synthesized new ethynylated-thiourea derivatives of 4-ethylbenzoyl-3-(4-ethynylbenzonitrile-phenyl)-thiourea 117a, and 4-methylbenzoyl-3-(4-ethynylbenzonitrile-phenyl)-thiourea 117b Scheme 52. These can be used for the detection of CO2 gas as sensing layers. These sensors do not show any decline in response after recurring usage making them good indicators of significant reproducibility and low relative standard deviation. The sensing capacity is related with the presence of –NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety which makes H-bonding interaction with CO2 analyte Fig. 25.
O moiety which makes H-bonding interaction with CO2 analyte Fig. 25.
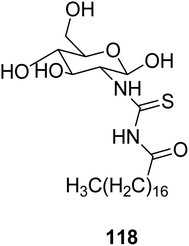
A new stearoyl thiourea derivative of chitosan 118 that can be used for the preconcentration of uranium from sulfate solution was synthesized. Its optimal conditions for elution and adsorption have also been studied. The adsorption is an exothermic process and follows a pseudo-first order kinetics. It has a high tolerance towards diverse ions. This method is applicable for uranium determination in various reference samples as reported by Orabi et al.95 (Scheme 53).
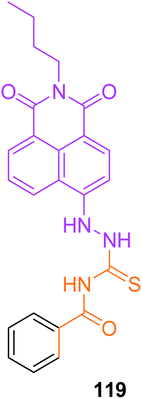
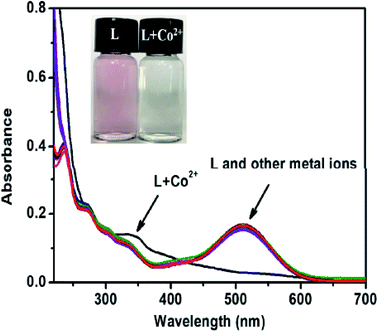
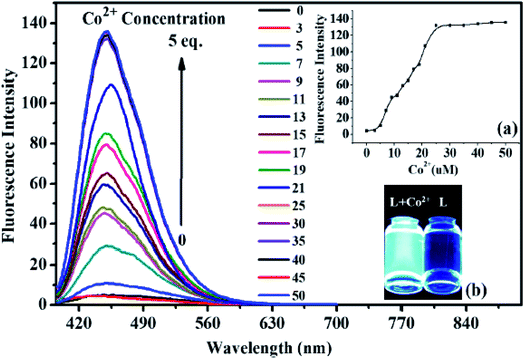
Liu et al.96 synthesized a fluorescent chemosensorN-(2-(2-butyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)hydrazine-1-carbonothioyl)benzamide 119 and characterized it by spectroscopic methods Scheme 54. This chemosensor was designed for the selective detection of Co2+ ions Scheme 55. The colour of chemosensor 119 is pink it detected the cobalt ions by a colour change from original pink to colourless and also a significant increase of the fluorescence intensity in CH3CN solution. This chemosensor is much effective in that it detects the Co2+ ions to a lower concentration of 0.26 μM. The analysis of Co2+ ions can be achieved via colour change and spectral behavior. Fig. 26 shows the selectivity of the sensor in many existing ions, pink color and a sharp peak at 510 nm is due to intramolecular charge transfer (ICT) transitions by adding 5 equivalents of cobalt ions this band vanish because of sensor and Co2+ complex (Scheme 55) and cause color change from pink to colourless which can be easily seen by naked eye. It is realized that 119 emits weak fluorescence due to a phenomenon called photo induced electron transfer (PET) from the imino nitrogen to the naphthalimide. Fig. 27 shows the fluorescence titration of 119 with cobalt ions, upon titration with cobalt ions, the intensity of fluorescence spectrum increases up to 3 equiv. of cobalt ion and then no change recorded up to 5 equiv. of cobalt ions at 450 nm. The sensor was only selective for Co2+ in a large number of other metal ions. Moreover, chemosensor 119 can be used as fluorescent sensor for detecting the Co2+ in various biological systems which shows its low toxicity to organisms and better cell permeability in living cells.
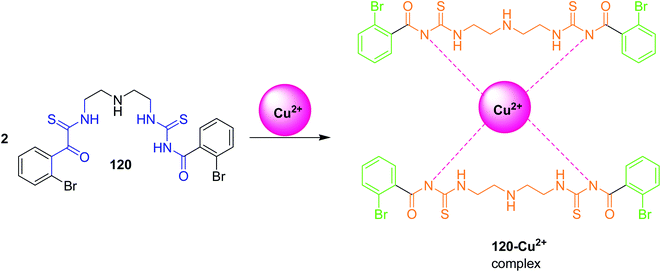
Hamedan et al.97 synthesized symmetrical benzoyl thiourea derivative 120 and used it as a colorimetric sensor for the naked-eye detection of Cu2+ ions. This sensor like the previous example displayed selectivity towards Cu2+ ions in ACN/H2O binary solutions, thus benzoylthiourea 120 plays a vital role in colorimetric recognition. Studies indicated that sensor 120 recognized the Cu2+ ions by creating a stable 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sensor-Cu2+ complex as shown in Scheme 56. When Cu2+ was added to sensor, colour change occurred from yellow to green while the presence of other cations such as Co2+, Fe2+, Ni2+, Mn2+, Cr3+, Zn2+ did not interfere in the detection process Fig. 28. In addition, the sensor detection limit for the Cu2+ was 1.15 × 10−5 M. Job's plot studies Fig. 29 indicated the complex formation and colour change between sensor 120–Cu2+ in 2
1 sensor-Cu2+ complex as shown in Scheme 56. When Cu2+ was added to sensor, colour change occurred from yellow to green while the presence of other cations such as Co2+, Fe2+, Ni2+, Mn2+, Cr3+, Zn2+ did not interfere in the detection process Fig. 28. In addition, the sensor detection limit for the Cu2+ was 1.15 × 10−5 M. Job's plot studies Fig. 29 indicated the complex formation and colour change between sensor 120–Cu2+ in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stable ratio. The detection process was monitored by the IR spectra titration method. The results revealed that when sensor formed a complex with Cu2+ the C
1 stable ratio. The detection process was monitored by the IR spectra titration method. The results revealed that when sensor formed a complex with Cu2+ the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption peak shifted to higher while thiourea C
O absorption peak shifted to higher while thiourea C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S peak remains same which indicated that sensor formed complex with Cu2+ through Cu2+–N bond which is near to carbonyl as in Scheme 56 that's why carbonyl absorption peak shifted to a higher number.
S peak remains same which indicated that sensor formed complex with Cu2+ through Cu2+–N bond which is near to carbonyl as in Scheme 56 that's why carbonyl absorption peak shifted to a higher number.
 | ||
| Fig. 28 Colour change by addition of several ions to the sensor 120 free sensor, Co2+, Fe2+, Ni2+, Mn2+, Cr3+, Zn2+, Cu2+ (left to right). | ||
6.3. Heterogenous catalysts
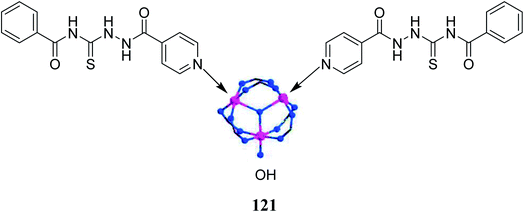
Due to efficient hydrogen-bond donating ability, thioureas have been used as organic ligands in the metal–organic frameworks (MOFs) that serve the purpose of heterogenous catalyst.98 These kinds of catalysts ensure the efficient conversion of desired compounds into high-value-added chemicals without any solvent, extreme conditions and even co-catalyst.99Mohammadian et al.100 changed a thiourea-bearing metal–organic framework (MOF) by the introduction of post-synthetic modification done by the complexation of MIL-101(Cr) with pyridine containing thiourea ligand 121 Scheme 57. The research group prepared three different thiourea ligands but the most efficient catalyst was found to be 121 with higher yields. The resultant complex acted as a heterogenous catalyst which prevents the hydrogen bond donating (HBD) organocatalyst to undergo a self-quenching phenomenon. Hence, improving the overall activity of the catalyst. The complexation of MIL-101(Cr) and pyridine bearing thioureas resulted in an activity better to that of parent MOF or ligand performing alone in Biginelli reactions and Friedel–Crafts alkylation.
7. Conclusions
The recent advances have proved thioureas to be crucial reagents for organic synthesis. The extensive use of these compounds as modified ligands in metal complex formation as well as bioactive templates in pharmaceuticals is increasing. The structural modifications have enabled them to be incorporated as chemosensors for many cations and molecules as well as assisted in the synthesis of efficient dugs with lower side-effects and toxicity. Long-chain polymer formation and their use as adhesives has increased their importance manifolds. Molecular docking studies have shown the potent activity against different strains of microorganisms. The modifications and advancements in the structures of these scaffolds is an active area of research. We hope that this review is an incentive for any further advancement in the discussed area.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
MFE is member of the Carrera del Investigador of CONICET (Argentina). The Argentinean author thanks the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and the Facultad de Ciencias Exactas, Universidad Nacional de La Plata (11/X794) for financial support.References
- K. Hollmann, A. Oppermann, M. Witte, S. Li, M. Amen, U. Flörke, H. Egold, G. Henkel and S. Herres-Pawlis, Copper (I) complexes with thiourea derivatives as ligands: revealing secrets of their bonding scheme, Eur. J. Inorg. Chem., 2017, 9, 1266–1279 CrossRef.
- J. M. Andrés, A. Maestro, M. Valle and R. Pedrosa, Chiral Bifunctional Thioureas and Squaramides and Their Copolymers as Recoverable Organocatalysts. Stereoselective Synthesis of 2-Substituted 4-Amino-3-nitrobenzopyrans and 3-Functionalized 3, 4-Diamino-4 H-Chromenes, J. Org. Chem., 2018, 83, 5546–5557 CrossRef PubMed.
- T. Parvin, R. Yadav and L. H. Choudhury, Recent applications of thiourea-based organocatalysts in asymmetric multicomponent reactions (AMCRs), Org. Biomol. Chem., 2020, 18, 5513–5532 RSC.
- J.-h. Li, Y. Wang, Y.-p. Wu, R.-h. Li, S. Liang, J. Zhang, Y.-g. Zhu and B.-j. Xie, Synthesis, herbicidal activity study and molecular docking of novel pyrimidine thiourea, Pestic. Biochem. Physiol., 2021, 172, 104766 CrossRef CAS PubMed.
- W. T. Lambert, M. E. Goldsmith and T. C. Sparks, Insecticidal activity of novel thioureas and isothioureas, Pest Manage. Sci., 2017, 73, 743–751 CrossRef CAS PubMed.
- A. Maalik, H. Rahim, M. Saleem, N. Fatima, A. Rauf, A. Wadood, M. I. Malik, A. Ahmed, H. Rafique and M. N. Zafar, Synthesis, antimicrobial, antioxidant, cytotoxic, antiurease and molecular docking studies of N-(3-trifluoromethyl) benzoyl-N′-aryl thiourea derivatives, Bioorg. Chem., 2019, 88, 102946 CrossRef CAS PubMed.
- Ji. J. Bai, Q. Huang and W. Wei, Synthesis and evaluation of new thiourea derivatives as antitumor and antiangiogenic agents, Tetrahedron Lett., 2020, 61, 152366 CrossRef.
- N. Kulabaş, Ö. B. Özakpınar, D. Özsavcı, P. Leyssen, J. Neyts and İ. Küçükgüzel, Synthesis, characterization and biological evaluation of thioureas, acylthioureas and 4-thiazolidinones as anticancer and antiviral agents, Marmara Pharm. J., 2017, 21, 371–384 CrossRef.
- G. M. Viana, D. C. Soares, M. V. Santana, L. H. do Amaral, P. W. Meireles, R. P. Nunes, L. C. R. P. da Silva, L. C. de Sequeira Aguiar, C. R. Rodrigues and V. P. de Sousa, Antileishmanial thioureas: synthesis, biological activity and in silico evaluations of new promising derivatives, Chem. Pharm. Bull., 2017, 65, 911–919 CrossRef CAS PubMed.
- M. Khan, J. Patujo, I. Mushtaq, A. Ishtiaq, M. N. Tahir, S. Bibi, M. S. Khan, G. Mustafa, B. Mirza and A. Badshah, Anti-diabetic potential, crystal structure, molecular docking, DFT, and optical-electrochemical studies of new dimethyl and diethyl carbamoyl-N,N′-disubstituted based thioureas, J. Mol. Struct., 2022, 1253, 132207 CrossRef CAS.
- H. Wang, Z.-W. Zhai, Y.-X. Shi, C.-X. Tan, J.-Q. Weng, L. Han, B.-J. Li and X.-H. Liu, Novel trifluoromethylpyrazole acyl thiourea derivatives: synthesis, antifungal activity and docking study, Lett. Drug Des. Discovery, 2019, 16, 785–791 CrossRef CAS.
- M. Irani, R. Ranjbar-Karimi and H. Izadi, The synthesis of some new benzoyl thioureas and investigation of pesticide activity, 2014, vol. 21 Search PubMed.
- T. A. Fattah, A. Saeed, Z. Ashraf, Q. Abbas, P. A. Channar, F. A. Larik and M. Hassan, 4-Aminocoumarin based aroyl thioureas as potential jack bean urease inhibitors; synthesis, enzyme inhibitory kinetics and docking studies, Med. Chem., 2020, 16, 229–243 CrossRef CAS PubMed.
- T. A. Fattah, A. Saeed, P. A. Channar, Z. Ashraf, Q. Abbas, M. Hassan and F. A. Larik, Synthesis, enzyme inhibitory kinetics, and computational studies of novel 1-(2-(4-isobutylphenyl) propanoyl)-3-arylthioureas as Jack bean urease inhibitors, Chem. Biol. Drug Des., 2018, 91, 434–447 CrossRef PubMed.
- R. Singh and S. Ganguly, Design, Synthesis and Evaluation of Some Novel 1-phenyl-3-(5-phenyl-1H-imidazol-1-yl) Thiourea Derivatives as Anti-HIV Agents, Indian J. Pharm. Educ. Res., 2018, 52, 655–665 CrossRef CAS.
- K. R. Koch, New chemistry with old ligands: N-alkyl-and N,N-dialkyl-N′-acyl (aroyl) thioureas in co-ordination, analytical and process chemistry of the platinum group metals, Coord. Chem. Rev., 2001, 216, 473–488 CrossRef.
- A. A. Aly, E. K. Ahmed, K. M. El-Mokadem and M. E. A. F. Hegazy, Update survey on aroyl substituted thioureas and their applications, J. Sulfur Chem., 2007, 28, 73–93 CrossRef CAS.
- A. Saeed, U. Flörke and M. F. Erben, A review on the chemistry, coordination, structure and biological properties of 1-(acyl/aroyl)-3-(substituted) thioureas, J. Sulfur Chem., 2014, 35, 318–355 CrossRef CAS.
- A. Saeed, R. Qamar, T. A. Fattah, U. Flörke and M. F Erben, Recent developments in chemistry, coordination, structure and biological aspects of 1-(acyl/aroyl)-3-(substituted) thioureas, Res. Chem. Intermed., 2017, 43, 3053–3093 CrossRef CAS.
- A. Saeed, M. N. Mustafa, M. Zain-ul-Abideen, G. Shabir, M. F. Erben and U. Flörke, Current developments in chemistry, coordination, structure and biological aspects of 1-(acyl/aroyl)-3-(substituted) thioureas: advances Continue, J. Sulfur Chem., 2019, 40, 312–350 CrossRef CAS.
- R. K. Mohapatra, P. K. Das, M. K. Pradhan, M. M. El-Ajaily, D. Das, H. F. Salem and M. K. E-Zahan, Recent advances in urea-and thiourea-based metal complexes: biological, sensor, optical, and corroson inhibition studies, Comments Inorg. Chem., 2019, 39, 127–187 CrossRef CAS.
- A. Lapasam and M. R. Kollipara, A survey of crystal structures and biological activities of platinum group metal complexes containing N-acylthiourea ligands, Phosphorus, Sulfur Silicon Relat. Elem., 2020, 1–26 Search PubMed.
- Z. Ngaini, F. Rasin, W. S. H. Wan Zullkiplee and A. N. Abd Halim, Synthesis and molecular design of mono aspirinate thiourea-azo hybrid molecules as potential antibacterial agents, Phosphorus, Sulfur Silicon Relat. Elem., 2020, 196, 275–282 CrossRef.
- X. H. Liu, C. X. Tan and J. Q. Weng, Phase Transfer–Catalyzed, One-Pot Synthesis of Some Novel N-Pyrimidinyl-N′-nicotinyl Thiourea Derivatives, Phosphorus, Sulfur Silicon Relat. Elem., 2011, 186, 552–557 CrossRef CAS.
- I. B. Douglass and F. Dains, The Preparation and Hydrolysis of Mono-and Disubstituted Benzoylthioureas1, J. Am. Chem. Soc., 1934, 56, 1408–1409 CrossRef CAS.
- A. K. Mukerjee and R. Ashare, Isothiocyanates in the chemistry of heterocycles, Chem. Rev., 1991, 91, 1–24 CrossRef CAS.
- F. Odame, E. C. Hosten, K. Lobb and Z. Tshentu, Ultrasound promoted synthesis, characterization and computational studies of some thiourea derivatives, J. Mol. Struct., 2020, 128302 CrossRef CAS.
- A. A. El-Badawy, A. S. Elgubbi and E. A. El-Helw, Acryloyl isothiocyanate skeleton as a precursor for synthesis of some novel pyrimidine, triazole, triazepine, thiadiazolopyrimidine and acylthiourea derivatives as antioxidant agents, J. Sulfur Chem., 2021, 42, 295–307 CrossRef CAS.
- Y.-J. Kim, H.-J. Kwon, S.-Y. Han and Y.-D. Gong, Synthesis of 2-Amino-5-Carboxamide Thiazole Derivatives via Dehydrative Cyclization of Thiourea Intermediate Resin on Solid Phase, ACS Comb. Sci., 2019, 21, 380–388 CrossRef CAS PubMed.
- M. Divya, P. Malliga, P. Sagayaraj and A. Joseph Arul Pragasam, Optical based electrical properties of thiourea borate NLO crystal for electro-optic Q switches, J. Electron. Mater., 2019, 48, 5632–5639 CrossRef CAS.
- T. Parvin, R. Yadav and L. H. Choudhury, Recent applications of thiourea-based organocatalysts in asymmetric multicomponent reactions (AMCRs), Org. Biomol. Chem., 2020, 18, 5513–5532 RSC.
- R. Eivazzadeh-Keihan, N. Bahrami, F. Radinekiyan, A. Maleki and M. Mahdavi, Palladium-coated thiourea core-shell nanocomposite as a new, efficient, and magnetic responsive nanocatalyst for the Suzuki-Miyaura coupling reactions, Mater. Res. Express, 2021, 8, 026102 CrossRef CAS.
- J. Mishra, H. Kaur, A. K. Ganguli and N. Kaur, Fluorescent chemosensor based on urea/thiourea moiety for sensing of Hg(II) ions in an aqueous medium with high sensitivity and selectivity: A comparative account on effect of molecular architecture on chemosensing, J. Mol. Struct., 2018, 1161, 34–43 CrossRef CAS.
- T. Pooventhiran, N. Al-Zaqri, A. Alsalme, U. Bhattacharyya and R. Thomas, Structural aspects, conformational preference and other physico-chemical properties of Artesunate and the formation of self-assembly with graphene quantum dots: A first principle analysis and surface enhancement of Raman activity investigation, J. Mol. Liq., 2021, 325, 114810 CrossRef CAS.
- A. Saeed, Z. Ashraf, M. F. Erben and J. Simpson, Vibrational spectra and molecular structure of isomeric 1-(adamantan-1-ylcarbonyl)-3-(dichlorophenyl)thioureas, J. Mol. Struct., 2017, 1129, 283–291 CrossRef CAS.
- D. L. N. González, A. Saeed, G. Shabir, U. Flörke and M. F. Erben, Conformational and crystal structure of acyl thiourea compounds: The case of the simple (2, 2-dimethyl-propionyl) thiourea derivative, J. Mol. Struct., 2020, 128227 CrossRef.
- S. L. Tan, M. M. Jotani and E. R. Tiekink, 3, 3-Bis (2-hydroxyethyl)-1-(4-nitrobenzoyl) thiourea: crystal structure, Hirshfeld surface analysis and computational study, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2020, 76, 155–161 CrossRef CAS PubMed.
- S. L. Tan, A. H. S. Azizan, M. M. Jotani and E. R. Tiekink, 3, 3-Bis (2-hydroxyethyl)-1-(4-methylbenzoyl) thiourea: crystal structure, Hirshfeld surface analysis and computational study, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2019, 75, 1472–1478 CrossRef CAS PubMed.
- I. Gumus, U. Solmaz, G. Binzet, E. Keskin, B. Arslan and H. Arslan, Hirshfeld surface analyses and crystal structures of supramolecular self-assembly thiourea derivatives directed by non-covalent interactions, J. Mol. Struct., 2018, 1157, 78–88 CrossRef CAS.
- I. Gumus, U. Solmaz, G. Binzet, E. Keskin, B. Arslan and H. Arslan, Supramolecular self-assembly of new thiourea derivatives directed by intermolecular hydrogen bonds and weak interactions: crystal structures and Hirshfeld surface analysis, Res. Chem. Intermed., 2019, 45, 169–198 CrossRef CAS.
- A. Mumtaz, J. Arshad, A. Saeed, M. A. H. Nawaz and J. Iqbal, Synthesis, characterization and urease inhibition studies of transition metal complexes of thioureas bearing ibuprofen moiety, J. Chil. Chem. Soc., 2018, 63, 3934–3940 CrossRef CAS.
- D. Sindhuja, P. Vasanthakumar, N. S. Bhuvanesh and R. Karvembu, An Acylthiourea Ligated Fe(II) Complex on Silica Nanoparticles for Transfer Hydrogenation of Carbonyl Compounds, Ind. Eng. Chem. Res., 2018, 57, 14386–14393 CrossRef CAS.
- M. R. Khan, S. Zaib, A. Khan, A. Badshah, M. K. Rauf, M. N. Tahir and J. Iqbal, Pd(II)-based heteroleptic complexes with N-(acyl)-N′, N′-(disubstituted) thioureas and phosphine ligands: Synthesis, characterization and cytotoxic studies against lung squamous, breast adenocarcinoma and Leishmania tropica, Inorg. Chim. Acta, 2018, 479, 189–196 CrossRef CAS.
- P. N. Sathishkumar, N. Raveendran, N. S. Bhuvanesh and R. Karvembu, Chemoselective transfer hydrogenation of nitroarenes, ketones and aldehydes using acylthiourea based Ru (II)(p-cymene) complexes as precatalysts, J. Organomet. Chem., 2018, 876, 57–65 CrossRef CAS.
- G. Rohini, J. Haribabu, K. N. Aneesrahman, N. S. Bhuvanesh, K. Ramaiah, R. Karvembu and A. Sreekanth, Half-sandwich Ru (II)(η6-p-cymene) complexes bearing N-dibenzosuberenyl appended thiourea for catalytic transfer hydrogenation and in vitro anticancer activity, Polyhedron, 2018, 152, 147–154 CrossRef CAS.
- B. N. Cunha, L. Luna-Dulcey, A. M. Plutin, R. G. Silveira, J. Honorato, R. R. Cairo and A. A. Batista, Selective Coordination Mode of Acylthiourea Ligands in Half-Sandwich Ru (II) Complexes and Their Cytotoxic Evaluation, Inorg. Chem., 2020, 59(7), 5072–5085 CrossRef CAS PubMed.
- U. A. Khan, A. Badshah, M. N. Tahir and E. Khan, Gold (I), silver (I) and copper (I) complexes of 2, 4, 6-trimethylphenyl-3-benzoylthiourea; synthesis and biological applications, Polyhedron, 2020, 114485 CrossRef CAS.
- A. M. Plutín, R. Ramos, R. Mocelo, A. Alvarez, E. E. Castellano, M. R. Cominetti and A. A. Batista, Antitumor activity of Pd (II) complexes with N, S or O, S coordination modes of acylthiourea ligands, Polyhedron, 2020, 114543 CrossRef.
- H. Pérez, R. Ramos, A. M. Plutín, R. Mocelo, M. F. Erben, E. E. Castellano and A. A. Batista, A Mixed Ligand Platinum (II) Complex: Spectral Analysis, Crystal Structure, Steric Demand of the Ligand, and Bioactivity of cis-[Pt (PPh3) 2 (L1-O, S)] PF6 (L1-O, S=N, N-Morpholine-N′-benzoylthiourea), Eur. J. Inorg. Chem., 2019, 21, 2583–2590 CrossRef.
- R. S. Correa, K. M. Oliveira, H. Perez, A. M. Plutín, R. Ramos, R. Mocelo and A. A. Batista, cis-bis (N-benzoyl-N′, N′-dibenzylthioureido) platinum (II): Synthesis, molecular structure and its interaction with human and bovine serum albumin, Arabian J. Chem., 2019, 12, 3454–3462 CrossRef CAS.
- H. A. Nkabyo, B. Procacci, S. B. Duckett and K. R. Koch, Reversible photoisomerization of cis-[Pd (L-κ S, O) 2](HL= N, N-diethyl-N′-1-naphthoylthiourea) to trans-[Pd (L-κ S, O) 2] and the unprecedented formation of trans-[Pd (L-κ S, N) 2] in solution, Dalton Trans., 2019, 48, 17241–17251 RSC.
- H. A. Nkabyo and K. R. Koch, Configurational E/Z and photo-induced cis-transisomerism in the Pd (II) complex of asymmetrical N, N-methyl-ethyl-N′-benzoylthiourea, J. Mol. Struct., 2019, 1190, 47–53 CrossRef CAS.
- H. A. Nkabyo, G. W. Bosman, R. C. Luckay and K. R. Koch, New E., Z platinum (II) complexes of asymmetrically di-substituted-acyl (aroyl) thioureas: Synthesis, characterization, photo-induced isomerism, Inorg. Chim. Acta, 2020, 119644 CrossRef CAS.
- I. Barnard and K. R. Koch, 59Co NMR, a facile tool to demonstrate EEE, EEZ, EZZ and ZZZ configurational isomerism in fac-[Co (L-κS, O) 3] complexes derived from asymmetrically substituted N, N-dialkyl-N′-aroylthioureas, Inorg. Chim. Acta, 2019, 495, 119019 CrossRef CAS.
- E. Keskin, U. Solmaz, G. Binzet, I. Gumus and H. Arslan, Synthesis, characterization and crystal structure of platinum (II) complexes with thiourea derivative ligands, Eur. J. Chem., 2018, 9(4), 360–368 CrossRef CAS.
- G. Binzet, I. Gumus, A. Dogen, U. Flörke, N. Kulcu and H. Arslan, Nickel (II) and copper (II) complexes of N, N-dialkyl-N′-3-chlorobenzoylthiourea: Synthesis, characterization, crystal structures, Hirshfeld surfaces and antimicrobial activity, J. Mol. Struct., 2018, 1161, 519–529 CrossRef CAS.
- K. Jeyalakshmi, J. Haribabu, C. Balachandran, E. Narmatha, N. S. Bhuvanesh, S. Aoki and R. Karvembu, Highly active copper (i) complexes of aroylthiourea ligands against cancer cells–synthetic and biological studies, New J. Chem., 2019, 43, 3188–3198 RSC.
- U. Solmaz, I. Gumus, G. Binzet, O. Celik, G. K. Balci, A. Dogen and H. Arslan, Synthesis, characterization, crystal structure, and antimicrobial studies of novel thiourea derivative ligands and their platinum complexes, J. Coord. Chem., 2018, 71, 200–218 CrossRef CAS.
- M. M. Sheeba, N. S. Bhuvanesh and R. Karvembu, Piano-stool Ru (II)-benzene complexes bearing D/L-alanine derived chiral aroylthiourea ligands for asymmetric transfer hydrogenation of ketones in water, J. Chem. Sci., 2018, 130, 163 CrossRef.
- M. M. Sheeba, M. M. Tamizh, N. S. Bhuvanesh and R. Karvembu, Water soluble Ru (II)–p-cymene complexes of chiral aroylthiourea ligands derived from unprotected D/L-alanine as proficient catalysts for asymmetric transfer hydrogenation of ketones, Appl. Organomet. Chem., 2019, 33, e4667 Search PubMed.
- E. Khan, S. Khan, Z. Gul and M. Muhammad, Medicinal importance, coordination chemistry with selected metals (Cu, Ag, Au) and Chemosensing of Thiourea derivatives. A review, Crit. Rev. Anal. Chem., 2021, 51, 812–834 CAS.
- S. K. Pandey, S. Pratap, M. K. Tiwari, G. Marverti and J. P. Jasinski, Experimental and theoretical exploration of molecular structure and anticancer properties of two N, N′–disubstituted thiocarbamide derivatives, J. Mol. Struct., 2019, 1175, 963–970 CrossRef CAS.
- S. K. Pandey, S. Pratap, S. K. Rai, G. Marverti, M. Kaur and J. P. Jasinski, Synthesis, characterization, Hirshfeld surface, cytotoxicity, DNA damage and cell cycle arrest studies of N, N-diphenyl-N'-(biphenyl-4-carbonyl/4-chlorobenzoyl) thiocarbamides, J. Mol. Struct., 2019, 1186, 333–344 CrossRef CAS.
- S. K. Pandey, S. Pratap, S. K. Rai, G. Marverti, M. Kaur and J. P. Jasinski, Synthesis, characterisation, Hirshfeld surface and in vitro cytotoxicity evaluation of new N-aryl-N′-Alkoxycarbonyl thiocarbamide derivatives, J. Mol. Struct., 2020, 1202, 127269 CrossRef CAS.
- T. D. de Oliveira, A. M. Plutín, L. Luna-Dulcey, E. E. Castellano, M. R. Cominetti and A. A. Batista, Cytotoxicity of ruthenium-N, N-disubstituted-N′-acylthioureas complexes, Mater. Sci. Eng., C, 2020, 111106 CrossRef CAS PubMed.
- D. H. Al-Amily and M. Hassan Mohammed, Design, Synthesis, and Docking Study of Acyl Thiourea Derivatives as Possible Histone Deacetylase Inhibitors with a Novel Zinc Binding Group, Sci. Pharm., 2019, 87, 28 CrossRef CAS.
- İ. Koca, S. Yiğitcan, M. Gümüş, H. Gökce and Y. Sert, A new series of sulfa drugs containing pyrazolyl acylthiourea moiety: Synthesis, experimental and theoretical spectral characterization and molecular docking studies, J. Mol. Struct., 2020, 1204, 127479 CrossRef.
- X. Yu, P. Teng, Y.-L. Zhang, Z.-J. Xu, M.-Z. Zhang and W.-H. Zhang, Design, synthesis and antifungal activity evaluation of coumarin-3-carboxamide derivatives, Fitoterapia, 2018, 127, 387–395 CrossRef CAS PubMed.
- F. Asghar, S. Rana, S. Fatima, A. Badshah, B. Lal and I. S. Butler, Biologically active halo-substituted ferrocenyl thioureas: synthesis, spectroscopic characterization, and DFT calculations, New J. Chem., 2018, 42, 7154–7165 RSC.
- Y. Gao, Y. Wang, J. Li, S. Shang and Z. Song, Improved application of natural forest product terpene for discovery of potential botanical fungicide, Ind. Crops Prod., 2018, 126, 103–112 CrossRef CAS.
- C. Wu, P. Tao, J. Li, Y. Gao, S. Shang and Z. Song, Antifungal application of pine derived products for sustainable forest resource exploitation, Ind. Crops Prod., 2020, 143, 111892 CrossRef CAS.
- K. Ghazal, S. Shoaib, M. Khan, S. Khan, M. K. Rauf, N. Khan and I. Ali, Synthesis, characterization, X-ray diffraction study, in vitro cytotoxicity, antibacterial and antifungal activities of nickel (II) and copper (II) complexes with acyl thiourea ligand, J. Mol. Struct., 2019, 1177, 124–130 CrossRef CAS.
- S. Hussain, Imtiaz-ud-Din, A. Raheel, S. Hussain, M. N. Tahir and I. Hussain, New bioactive Cu (I) thiourea derivatives with triphenylphosphine; synthesis, structure and molecular docking studies, J. Coord. Chem., 2020, 73, 1191–1207 CrossRef CAS.
- A. S. Faihan, S. A. Al-Jibori, M. R. Hatshan and A. S. Al-Janabi, Antibacterial, spectroscopic and X-ray crystallography of newly prepared heterocyclic thiourea dianion platinum (II) complexes with tertiary phosphine ligands, Polyhedron, 2022, 212, 115602 CrossRef CAS.
- M. S. El-Gaby, M. F. Hussein, M. I. Hassan, A. M. Ali, Y. A. M. M. Elshaier, A. S. Gebril and F. A. Faraghally, New sulfonamide hybrids: synthesis, in vitro antimicrobial activity and docking study of some novel sulfonamide derivatives bearing carbamate/acyl-thiourea scaffolds, Mediterr. J. Chem., 2018, 7, 370 CrossRef CAS.
- F. A. Larik, A. Saeed, M. Faisal, P. A. Channar, S. S. Azam, H. Ismail and B. Mirza, Synthesis, molecular docking and comparative efficacy of various alkyl/aryl thioureas as antibacterial, antifungal and α-amylase inhibitors, Comput. Biol. Chem., 2018, 77, 193–198 CrossRef CAS PubMed.
- A. Saeed, A. Bosch, M. Bettiol, D. L. N. González, M. F. Erben and Y. Lamberti, Novel Guanidine Compound against Multidrug-Resistant Cystic Fibrosis-Associated Bacterial Species, Molecules, 2018, 23, 1158 CrossRef PubMed.
- A. Maalik, H. Rahim, M. Saleem, N. Fatima, A. Rauf, A. Wadood and M. Riaz, Synthesis, antimicrobial, antioxidant, cytotoxic, antiurease and molecular docking studies of N-(3-trifluoromethyl) benzoyl-N′-aryl thiourea derivatives, Bioorg. Chem., 2019, 88, 102946 CrossRef CAS PubMed.
- C. Limban, M. C. Chifiriuc, M. T. Caproiu, F. Dumitrascu, M. Ferbinteanu, L. Pintilie and D. C. Nuta, New Substituted Benzoylthiourea Derivatives: From Design to Antimicrobial Applications, Molecules, 2020, 25, 1478 CrossRef CAS PubMed.
- T. S. Shim and K.-W. Jo, Medical treatment of pulmonary multidrug-resistant tuberculosis, Infect. Chemother., 2013, 45, 367–374 CrossRef CAS PubMed.
- B. Phetsuksiri, M. Jackson, H. Scherman, M. McNeil, G. S. Besra, A. R. Baulard, R. A. Slayden, A. E. DeBarber, C. E. Barry and M. S. Baird, Unique mechanism of action of the thiourea drug isoxyl on Mycobacterium tuberculosis, J. Biol. Chem., 2003, 278, 53123–53130 CrossRef CAS PubMed.
- S. E. Noroc, N. Ţurcan, V. Crudu, E. Romancenco, T. Cotelea, G.-M. Niţulescu, C. Chiriţă and L. Morusceag, Biological evaluation of new 2-phenethylbenzoyl thiourea derivatives as antituberculosis agents, Farmacia, 2018, 66, 97–106 Search PubMed.
- (a) G. Roglic, WHO Global report on diabetes: A summary, Int. J. Non-commun. Dis., 2016, 1, 3 CrossRef; (b) American Diabetes Association, 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019, Diabetes Care, 2019, 42(suppl 1), S90–S102 CrossRef PubMed.
- J. Patujo, M. Azeem, H. Muhammad, A. Raheel, S. Fatima, B. Mirza, Z. Hussain and A. Badshah, Assessing the biological potential of new symmetrical ferrocene based bisthiourea analogues, Bioorg. Chem., 2021, 106, 104180 CrossRef CAS PubMed.
- T. U. Rehman, S. Riaz, I. U. Khan, M. Ashraf, M. Bajda, A. Gawalska and M. Yar, Novel pyridine-2, 4, 6-tricarbohydrazide thiourea compounds as small key organic molecules for the potential treatment of type-2 diabetes mellitus: In vitro studies against yeast α-and β-glucosidase and in silico molecular modeling, Arch. Pharm., 2018, 351, 1700236 CrossRef PubMed.
- A. Saeed, M. S. Shah, F. A. Larik, S. U. Khan, P. A. Channar, U. Flörke and J. Iqbal, Synthesis, computational studies and biological evaluation of new 1-acetyl-3-aryl thiourea derivatives as potent cholinesterase inhibitors, Med. Chem. Res., 2017, 26, 1635–1646 CrossRef CAS.
- M. N. Mustafa, A. Saeed, P. A. Channar, F. A. Larik, G. Shabir, Q. Abbas and S. Y. Seo, Synthesis, molecular docking and kinetic studies of novel quinolinyl based acyl thioureas as mushroom tyrosinase inhibitors and free radical scavengers, Bioorg. Chem., 2019, 90, 103063 CrossRef CAS PubMed.
- S. Rehman, A. Saeed, G. Saddique, P. A. Channar and F. A. Larik, Synthesis of sulfadiazinyl acyl/aryl thiourea derivatives as calf intestinal alkaline phosphatase inhibitors, pharmacokinetic properties, lead optimization, Lineweaver-Burk plot evaluation and binding analysis, Bioorg. Med. Chem., 2018, 26, 3707–3715 CrossRef PubMed.
- F. A. Larik, M. Faisal, A. Saeed, P. A. Channar, J. Korabecny, F. Jabeen and G. S. Khan, Investigation on the effect of alkyl chain linked mono-thioureas as Jack bean urease inhibitors, SAR, pharmacokinetics ADMET parameters and molecular docking studies, Bioorg. Chem., 2019, 86, 473–481 CrossRef CAS PubMed.
- Q. Zhang, Y. Cheng, C. Zheng, P. Bai, J. J. Yang and X. Lu, Design, Synthesis, and Insecticidal Activity of Novel Doramectin Derivatives Containing Acylurea and Acylthiourea Based on Hydrogen Bonding, J. Agric. Food Chem., 2020, 68, 5806–5815 CrossRef CAS PubMed.
- A. Kalaiyarasi, J. Haribabu, D. Gayathri, K. Gomathi, N. S. P. Bhuvanesh, R. Karvembu and V. M. Biju, Chemosensing, molecular docking and antioxidant studies of 8-aminoquinoline appended acylthiourea derivatives, J. Mol. Struct., 2019, 1185, 450–460 CrossRef CAS.
- N. A. Mohamed, N. F. Al-Harby and M. S. Almarshed, Synthesis and characterization of novel trimellitic anhydride isothiocyanate-cross linked chitosan hydrogels modified with multi-walled carbon nanotubes for enhancement of antimicrobial activity, Int. J. Biol. Macromol., 2019, 132, 416–428 CrossRef CAS PubMed.
- J. Petersen, J. Kristensen, H. Elarga, R. Andersen and A. Midtstraum, in Accuracy and Air Temperature Dependency of Commercial Low-cost NDIR CO2 Sensors: An Experimental Investigation, Proceedings of the International Conference on Building Energy and Environment of COBEE2018, Melbourne, Australia, 2018, pp. 5–9 Search PubMed.
- A. I. Daud, K. A. A. Wahid and W. M. Khairul, Room-temperature operated cyano-terminated ethynylated-thiourea as a resistive-type carbon dioxide (CO2) gas sensor, Org. Electron., 2019, 70, 32–41 CrossRef CAS.
- A. Orabi, M. Atrees and H. Salem, Selective preconcentration of uranium on chitosan stearoyl thiourea prior to its spectrophotometric determination, Sep. Sci. Technol., 2018, 53, 2267–2283 CrossRef CAS.
- Y.-L. Liu, L. Li, L. Yang, Y.-Q. Guo, X.-X. Pang, P. Li, F. Ye and Y. Fu, A new fluorescent chemosensor for cobalt (II) ions in living cells based on 1, 8-naphthalimide, Molecules, 2019, 24, 3093 CrossRef CAS PubMed.
- N. Hamedan, S. Hasan, H. Zaki and N. Alias, In Colorimetric chemosensor of symmetrical benzoylthiourea derivatives as for detection of Cu2+ in aqueous solution, IOP Conference Series: Mater. Sci. Eng. IOP Publishing: 2017, p. 012038 Search PubMed.
- Z. Chang, R. B. Lin, Y. Ye, C. Duan and B. Chen, Construction of a thiourea-based metal–organic framework with open Ag+ sites for the separation of propene/propane mixtures, J. Mater. Chem. A, 2019, 7, 25567–25572 RSC.
- T. Dong, Y. J. Zheng, G. W. Yang, Y. Y. Zhang, B. Li and G. P. Wu, Cross linked Resin-Supported Bifunctional Organocatalyst for Conversion of CO2 into Cyclic Carbonates, ChemSusChem, 2020, 13, 4121–4127 CrossRef CAS PubMed.
- R. Mohammadian, M. M. Amini and A. Shaabani, Thiourea-functionalized MIL-101 (Cr) metal-organic framework as a hydrogen-bond-donating heterogeneous organocatalyst for the Friedel-Crafts alkylation and Biginelli reactions, Catal. Commun., 2020, 136, 105905 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |