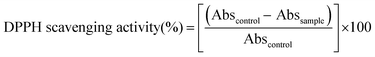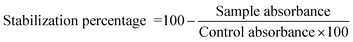Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
HPLC-DAD phenolic screening and in vitro assessment of antimicrobial, antioxidant and anti-inflammatory activities of Tanteboucht dates
Saliha Dassamiour ab,
Selsabil Meguellati
ab,
Selsabil Meguellati b,
Hdouda Lamraoui
b,
Hdouda Lamraoui b,
Mohamed Sabri Bensaad
b,
Mohamed Sabri Bensaad ac,
Rokayya Sami
ac,
Rokayya Sami *d,
Garsa Alshehry
*d,
Garsa Alshehry d,
Eman Hillal Althubaiti
d,
Eman Hillal Althubaiti e and
Areej Suliman Al-Meshal
e and
Areej Suliman Al-Meshal f
f
aLaboratory of Biotechnology of Bioactive Molecules and Cellular Physiopathology (LBMBPC), Department of Microbiology and Biochemistry, Faculty of Natural and Life Sciences, University Batna 2, Fesdis, Batna 05078, Algeria. E-mail: s.dassamiour@univ-batna2.dz; m.bensaad@univ-batna2.dz
bDepartment of Microbiology and Biochemistry, Faculty of Natural and Life Sciences, University Batna 2, Fesdis, Batna 05078, Algeria. E-mail: katrykorina@gmail.com; onzukaeikich87@yahoo.com
cLaboratory of Cellular and Molecular Physio-Toxicology-Pathology and Biomolecules (LPTPCMB), Faculty of Natural and Life Sciences, University Batna 2, Fesdis, Batna 05078, Algeria
dDepartment of Food Science and Nutrition, College of Sciences, Taif University, P.O. 11099, Taif, 21944, Saudi Arabia. E-mail: rokayya.d@tu.edu.sa; garsa.a@tu.edu.sa
eDepartment of Biotechnology, Faculty of Science, Taif University, P.O. 11099, Taif, 21944, Saudi Arabia. E-mail: i.althubaiti@tu.edu.sa
fDepartment of Biology, College of Science and Humanities in Al-Kharj, Prince Sattam bin Abdulaziz University, Al-Kharj, 11942, Saudi Arabia. E-mail: a.almashal@psau.edu.sa
First published on 4th May 2022
Abstract
The date palm (Phoenix dactylifera L.) is one of the most important crops in arid and semi-arid zones. Date fruit occupies a good place in traditional medicine among the Saharan residents, due to its therapeutic virtues; although there may be several therapeutic virtues yet to be discovered. The aim of this study was to investigate the phytochemical and pharmacological properties of the hexanic (EHx), chloroformic (ECh), ethyl acetate (EAc) and aqueous (EAq) extracts of Tanteboucht pulp. The phytochemical characterization and estimation of phenolic compounds were done based on an HPLC-DAD approach. The antioxidant activity was evaluated by a DPPH scavenging effect test. The sensitivity of 7 bacterial strains and Candida albicans to Tanteboucht extracts was tested using the diffusion disc on agar medium method. The membrane stabilization test was used to determine the in vitro anti-inflammatory effect of the fruit extracts. Fourteen phenolic compounds were detected in organic extracts and EAc was the richest followed by ECh and finally EHx which was very poor in these molecules. All extracts showed antioxidant, anti-inflammatory and antimicrobial properties which differ in rate. Indeed, ECh had the greatest scavenging effect on DPPH, followed by EAc and then EAq. EAc was the most potent inhibitor of microbial strains. EAc and ECh were more efficient at membrane stabilization followed by EAq and the three extracts had more anti-inflammatory capacity than the positive control acetyl salicylic acid. The obtained considerable activities were significantly correlated with flavonoid and tannin contents in the extracts.
1. Introduction
At the present time the majority of the inhabitants of the terrestrial globe use a lot of plants as remedies in traditional medicines. Crude plant extracts are gaining interest as a potential source of bioactive natural molecules. They are being used for the treatment of infectious diseases and the protection of food against oxidation.1The date palm (Phoenix dactylifera L.) is one of the most important crops in arid and semi-arid zones, and plays an important role in the economic and social life of the populations of these regions.2 It is one of the fruit species whose cultivation has existed since ancient times.3
The date is a fruit obtained from the date palm, has significant pharmaceutical, nutritional and commercial value. In addition to its high energy value4 due to its high carbohydrate content, date pulp is rich in phytochemicals such as phenolic compounds, especially anthocyanins, procyanidins, flavonoids and phenolic acids. These compounds are the subject of increasing interest5,6 besides their pharmacological properties. Indeed, several activities such as antioxidant, antimicrobial, anti-inflammatory and anti-tumour activities are attributed to these metabolites.7–9 The latter remain less defined in the case of dates in general and that of its common varieties in particular. Tanteboucht, which is one of these varieties, is the subject of this study where we (the authors) targeted its phytochemical composition and its related biological activities.
2. Experimental
2.1. Plant material
The used variety of date (Tanteboucht) is a semi-soft fruit one, growing in the south-eastern region (Ziban) of Algeria (Fig. 1). The fruits were harvested in October (Which year???); samples were sorted and stored in the refrigerator until the time of physiochemical analysis, in order to slow down chemical and physiological changes.2.2. Extracts preparation
The crude extract was prepared by macerating 1500 g of pitted and crushed samples into 4 L of the mixture of acetone/water (60/40). The mixture was stored in a dark environment for 24 h before it was filtered. The obtained filtrate was then concentrated by evaporation of acetone at 37 °C. The remaining aqueous phase was used for carrying out a liquid–liquid extraction with successively three organic solvents of increasing polarity, hexane, chloroform and ethyl acetate to ensure good fractionation of the molecules. Dry extracts, obtained after evaporation of the three solvents respectively, EHx, ECh and EAc were used, in addition to the raw aqueous extract (EAq), to carry out the assays and the tests of the biological activities.2.3. Microbial strains
The bacterial strains used in this study were: of Gram-positive bacteria: Staphylococcus aureus ATCC 25293, methicillin-resistant staphylococcus aureus (MRSA), Staphylococcus Mu50 ATCC 700699; and Gram-negative bacteria: Acenitobacter sp, Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, Enterobacter cancerogenus ATCC 35316, Bacillus polyma, Salmonella typhimurium ATCC 35316 besides a fungal strain ‘Candida albicans’.The microbial strains were obtained from the microbiology laboratory of the University of Batna 1-Algeria.
2.4. HPLC-DAD phenolics screening
HPLC analysis was carried out by an AGILENT TECHNOLOGY apparatus. The extracts (EHx, ECh and EAc) used in this assay were dissolved in extraction solvents and injected in a volume of 15 μL each, onto an RP-C18 column set at a temperature of 35 °C. The mobile phase consisted of a mixture of acetonitrile (ACN)/methanol/acidified water. Elution was carried out in a gradient at a flow rate of 0.5 mL min; spread over 70 min of time. The gradient program was started with 5% of ACN and 95% acidified H2O for the first 5 min and then increased gradually to 60% till 30 min for ACN before bringing it down again to 5%, but decreased gradually to 15% for acidified H2O. Whereas the methanol starting addition was of 10% at 30 min and increased to 80% at the end of analysis.Detection was performed at three different wavelengths, 250 nm, 280 nm and 340 nm.10
2.5. Tests of the biological activities of the extracts
where, Abscontrol: is the absorbance of control group, and Abssample: is the absorbance of plant sample.
In addition, the anti-free radical efficacy is calculated as follows: EA = 1/IC50
Where the IC50 is the concentration of extract required to obtain 50% of the reduced form of the DPPH radical.
2.6. Statistical analysis
The experiments were carried out in triplicate and Results were expressed as mean ± standard deviation (n = 3) for each case. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by multiple Tukey's comparison tests. Differences were considered significant at P <0.001.3. Results and discussion
3.1. Results of HPLC analysis
Identified phenolic acids in Tanteboucht extracts were gallic, caffeic, chlorogenic and 4-hydroxybenzoic acids. The presence of these six flavonoids was highlighted in the ECh and EAc extracts as: rutin, quercetin, luteolin, catechin, epicatechin and cyanidine chloride, besides the coumarin and vanillin compounds. Furthermore, tannic acid and procyanidin B2 were detected as hydrolysable and condensed tannins respectively (Table 1).| Phenolic compounds | Content (μg g−1) of EHx | Content (μg g−1) of ECh | Content (μg g−1) of EAc | En μg/100 g of FFw |
|---|---|---|---|---|
| a ‘—’: absent; FFW: fruit fresh weight. | ||||
| Gallic acid | — | 11.27 ± 0.35 | 8.91 ± 1.33 | 60.38 ± 5.72 |
| Caffeic acid | — | 12.89 ± 0.16 | 5.94 ± 0.79 | 53.58 ± 3.27 |
| Chlorogenic acid | — | 40.28 ± 2.79 | 32.47 ± 3.75 | 218.08 ± 20.55 |
| 4-Hydroxybenzoic acid | — | 26.87 ± 1.77 | 4051.10 ± 71.98 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 812.63 ± 266.4 812.63 ± 266.4 |
| Coumaric acid | — | — | — | |
| Trans-ferulic acid | — | — | ||
| Salicylic acid | — | — | — | |
| Vanillin | 1.83 ± 0.17 | 29.68 ± 1.39 | — | 95.16 ± 3.60 |
| Coumarin | 50.59 ± 5.74 | 22.58 ± 2.02 | — | 107.64 ± 10.42 |
| Rutin | — | 21.02 ± 1.60 | — | 52.13 ± 3.96 |
| Quercetin | — | — | 207.05 ± 25.55 | 753.66 ± 93.02 |
| Catechin | — | 19.69 ± 2.91 | 17.01 ± 2.62 | 110.75 ± 16.75 |
| Epicatechin | — | 14.65 ± 2.02 | — | 36.33 ± 5.02 |
| Luteolin | — | — | 12.69 ± 1.85 | 46.19 ± 6.74 |
| Cyanidine chloride | — | 20.02 ± 4.48 | 4.26 ± 1.75 | 65.16 ± 17.50 |
| Tannic acid | — | 7.25 ± 2.15 | 8.11 ± 1.61 | 47.5 ± 11.20 |
| Procyanidine B2 | — | 6.85 ± 0.78 | 4.20 ± 0.49 | 32.28 ± 3.73 |
These results are in agreement with those earlier found by Mansouri et al.11 for the same date variety; where they highlighted the presence of flavonoids and phenolic acids as caffeic acid and derivatives of cinnamic acid. It has been observed that Gallic acid was present, in large amount in several date varieties.14,15 Caffeic, ferulic, 4-hydroxybenzoic and chlorogenic acids are some of the compounds detected in many date varieties, besides different flavonoids and derivatives like: rutin, catechin, quercetin, luteolin, vanillin and coumarin,14,16 and these results are in agreement with ours.
3.2. Scavenger effect of extracts against the DPPH radical
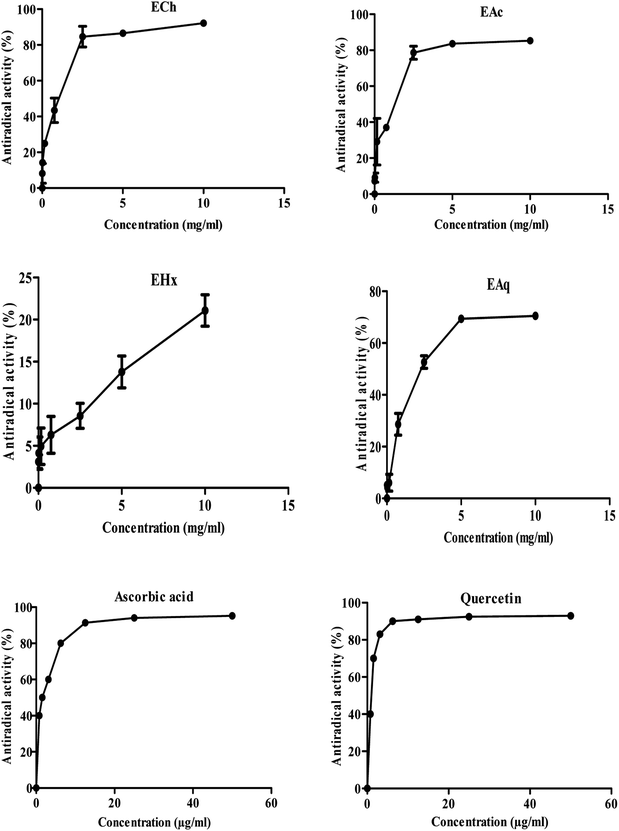
Fig. 2 represents the profiles of the anti-free radical activity obtained for the various extracts of Tanteboucht besides that of ascorbic acid and quercetin standards. The IC50 and AE of each of extracts and standards were also determined (Table 2).| Extracts/standards | IC50 (mg mL−1) | AE |
|---|---|---|
| a 1st letter: comparison (p <0.001) of all samples to quercetin standard.b 2nd letter: comparison (p <0.001) of all samples to ascorbic acid standard. | ||
| EAc | 1.19 ± 0.02ab | 0.83 ± 0.02ab |
| ECh | 0.86 ± 0.08ab | 1.16 ± 0.11ab |
| EAq | 2.84 ± 0.90ab | 0.37 ± 0.12ab |
| EHx | — | — |
| Quercetin | 0.07 ± 0.01 | 14.48 ± 0.32 |
| Ascorbic acid | 0.25 ± 0.02 | 4.01 ± 2.09 |
These results revealed that all the extracts tested had an anti-free radical activity at the maximum concentration (10 mg mL−1); ECh exhibited the highest anti-free radical activity (92.22%), followed by EAc (85.25%) and then EAq (70.5%). The IC50 of DPPH of EHx could not be really quantified because at the maximum concentrations tested, only a fraction of 21% of DPPH was captured, indicating a low concentration of antioxidants in this extract. Moreover, ECh was found to be the most active extract with an IC50 of 0.86 mg mL−1 and an AE estimated at 1.16 followed by EAc with an IC50 of around 1.19 mg mL−1 and an AE of 0.83. The EAq gave the lowest anti-free radical activity with an IC50 of 2.84 mg mL−1 and an AE of around 0.37.
For comparative purposes, ascorbic acid and quercetin were used as antioxidant standards; they showed an interesting anti-free radical activity with an IC50 of the order of 0.25 and 0.07 mg mL−1, therefore an AE of the order of 4.01 and 14.48 respectively.
In comparison with ascorbic acid and quercetin all the extracts tested were found to be less active. Noting that, these standards are pure while the extracts are of crude composition.
Ghiaba et al.17 found IC50 equal to: 10.83, 13.20, 16.77 mg L−1 for methanolic extracts of the varieties Deglet Nour, Ghars, and Degla Baidha, these values are higher than those of the variety Tanteboucht; which indicates an antiradicalaire efficiency lower than that of Tanteboucht extracts. Noting that, these are not only different species but also the combined action of the different compounds with anti-free radical activity that they may contain.
Antioxidant molecules such as ascorbic acid, tocopherol, flavonoids and tannins have been shown to reduce and discolor DPPH, due to their ability to transfer the hydrogen atom and single electron.18 The polyphenols in the date extracts are probably responsible for the antioxidant activity of these extracts. Indeed the phytochemical investigation on Tanteboucht revealed its considerable contents of several flavonoids and phenolic acids besides tannins which may contribute to the pharmacological properties of this fruit. Substantial linear coefficients of determination (R2) of 0.71, 0.68 and 0.73, were obtained between the anti-free radical efficacy (EA) and the total identified phenolics, phenolic acids and tannins contents respectively. Furthermore, a very significant coefficient of determination of 0.96 existed between AE and flavonoid content. It is evident therefore, that the high activity of the ECh and EAc extracts is attributed in large part to phenolic acids, flavonoids and tannins detected in Tanteboucht extracts.
Noting that, Alam et al.19 conclude that the antioxidant activity depends on the polymerization degree as well as the hydroxylation of phenolic compounds, when studying this activity in several date varieties. Phenolic acids such gallic and chlorogenic acids play an important role in antioxidant reactions; these compounds can increase levels of superoxide dismutase, glutathione and catalase, which enhance considerably antioxidant defense mechanisms.20,21 On the other hand, Sato et al.22 reported that antioxidant activity of caffeic acid is stronger than that of its precursor chlorogenic acid.
In the same way, Shim et al.23 found that p-hydroxybenzoic acid had strong scavenging activity of DPPH radical. It was mentioned that vanillin had potent neuroprotective effect against oxidative brain damage.24 Coumarins are aromatic compounds, having good antioxidant capacity; which was reported in several studies.
Khalil and Mustafa25 found that isolated coumarins from Granny Smith apple seeds had considerable free radicals scavenging capacity. They were able to enhance antioxidant defence and thus improve tomato plant tolerance to salinity in the study of Antonijević et al. (2021).26 Indeed, they were found to be good inhibitors, with flavonoids, of peroxyl radicals.27
Rutin and quercetin are considered from the most potent antioxidant agents and several studies28,29 reported that these flavonoids may considerably reduce oxidative stress and lipid peroxidation in brain, kidney and liver by enhancing the expression of key genes involved in antioxidant processes like glutathione S transferase α (GSTα), paraoxonase-1(PON-1) and glutamate-cysteine ligase (GCL). This process will considerably decrease the plasma level of malondialdehyde (MDA) and Glutathione (GSH) considered as the principal markers of oxidative stress.30
Otherwise, quercetin and catechin have protective effect against collagen fragmentation, premature skin cancer and skin photo-aging processes31,32 by significantly reducing and neutralizing free radical and oxygen-mediated damage in cells and also in extracellular matrix.33 The pre-treatment with cyanidin chloride provided a neuroprotective effect againt ROS in a study on the nematode Caenorhabditis elegans (C. elegans).34 Otherwise, a strong correlation was obtained between antioxidant properties and cyanidin and tannins content of blackberry.35 Luteolin was found to ameliorate the activities of antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase36 and thus showed a high activity in antioxidant process.
A research study highlighted antioxidant properties of this molecule against pro-oxidant effect at higher doses.37 These activities occurred by interacting with H2O2 and metal ions.
3.3. Antimicrobial activity
Fig. 3 shows the majority of effects of the aqueous and organic extracts on the microbial studied strains. The results showed that under these experimental conditions, all the extracts had an inhibiting effect on at least one of the microbial strains tested. Indeed, all organic extracts of Tanteboucht exhibited an antimicrobial activity which depend on the bioactive molecules they contain, essentially the extract of ethyl acetate which seems to have the greatest activity against Gram (+) and Gram (−) bacteria; knowing that the most sensitive bacteria were Staphylococcus aureus and P. aeruginosa with a diameter of 22 mm followed by E. coli with a diameter of 20 mm, Acinetobacter 19.75 mm and finally Enterobacter with 10.55 mm (Table 3). The inhibition effect of EAc against S. aureus, P. aeruginosa and E. coli was considerable and comparable to some synthetic antibiotics used as standards (Table 3).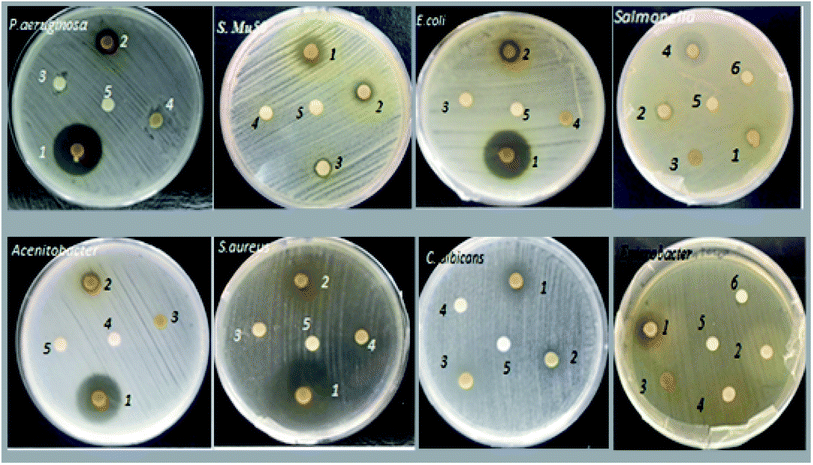 | ||
| Fig. 3 Major effects of Tanteboucht extracts on the sensitivity of the microbial studied strains; 1: EAc, 2: ECh, 3: EHx, 4: EAq, 5: DMSO, 6: Sterile distilled water. | ||
| Extract/strain | EAc | EHx | ECh | EAq | Standards |
|---|---|---|---|---|---|
| Enterobacter Cancerogenus | 10.55 ± 0.20 | — | 6.60 ± 0.35 | 6.55 ± 0.41 | — |
| MRSA | 7.75 ± 0.37 | — | 6.70 ± 0.43 | — | — |
| Staphylococcus Mu50 | 10.90 ± 0.33 | 8.00 ± 0.05 | 9.50 ± 0.11 | — | — |
| Salmonella typhimurium | 7.30 ± 0.21 | — | 9.20 ± 0.26 | 16.75 ± 0.30 | 19 (cefoxitine) |
| 22 (imipenem) | |||||
| Acinetobacter Sp | 19.75 ± 0.45 | — | 8.00 ± 0.15 | — | — |
| E. coli | 20.00 ± 0.32 | — | 8.50 ± 0.45 | — | 20 (amoxicilline) |
| 25(Gentamycine) | |||||
| Staphylococcus aureus | 22.00 ± 0.38 | 8.00 ± 0.5 | 9.00 ± 0.16 | — | 19 (oxacilline) |
| 19 (vancomycine) | |||||
| Bacillus polyma | 10.60 ± 0.15 | — | — | — | — |
| P. aeruginosa | 22.00 ± 0.37 | — | 9.00 ± 0.35 | — | 10 (tetracycline) |
| 20 (Gentamycine) | |||||
| C. albicans | 10.00 ± 0.18 | 8.50 ± 0.13 | 8.50 ± 0.18 | — | — |
ECh gave a lower antibacterial activity compared to EAc and whose most sensitive strains were Staphylococcus Mu50 and S. typhimurium with inhibition diameters of 9.5 and 9.2 mm respectively; followed by P. aeruginosa and Staphylococcus aureus which gave 9.00 mm and finally E. coli and Candida albicans with 8.5 mm of inhibition diameter. However, it did not give any activity against Bacillus polyma. Daas amiour et al.12 found that alcoholic extracts from three varieties of dates had minimal inhibitory effect against the Escherichia coli strain which agree with this result.
EHx has been shown to be active only against Candida albicans (8.5 mm), Staphylococcus aureus and Staphylococcus Mu50 both with 8 mm as the diameter of inhibition zone. This activity may be due to the richness of this extract in lipid compounds which can hinder microbial growth. Indeed according to Branen et al.38 long chain fatty acids have interesting antibacterial effects.
The aqueous extract gave a good zone of inhibition against Gram (−) strains mainly S. typhimurium (16.75 mm), however, it gave minimal effect with Enterobacter. This activity can be explained by the richness of this extract in tannins which are capable of combining with microbial enzymes and of chelating certain metals such as iron,39 thus stopping microbial growth.
The fungal strain C. albicans appears to be sensitive to all organic extracts with diameters of 10 mm, 8.5 mm, 8.5 mm for EAc, ECh and EHx respectively, it may be due to the presence of phenol acids and flavonoids in these extracts. Indeed, Branen et al.38 confirmed that phenolics have both antibacterial and antifungal activity. In contrast, no inhibition of Candida albicans was observed with the aqueous extract.
To ensure that the activity is intrinsic to the extracts, the activities of the solvents used for the extraction and that used for the dissolution (DMSO) of the organic extracts were tested and gave negative results, one concentration initially. To determine the minimum inhibitory concentration (MIC) of EAc extract which had the most potent antimicrobial effect with maximum diameter of inhibitory zone of 22 mm (Table 3), dilutions (50%, 25%, 12.5%, 6.25%, 3.12%) were tested and the results are mentioned in Table 4.
| Strain/dilution | 50 | 25 | 12.5 | 6.25 | 3.12 |
|---|---|---|---|---|---|
| S. aureus | 18.2 ± 0.1 | 12.9 ± 0.16 | 10.75 ± 0.08 | 10.00 ± 0.01 | 6.5 ± 0.01 |
| E. coli | 17.78 ± 0.47 | 14.22 ± 0.02 | 11.93 ± 0.10 | 10.53 ± 0.20 | 6.75 ± 0.02 |
| C. albicans | 15.55 ± 0.04 | 11.02 ± 0.02 | 06.73 ± 0.09 | — | — |
According to the results obtained by HPLC, the ECh and EAc extracts from Tanteboucht contain several flavonoids and phenolic acids, which have significant antibacterial activity.40 Indeed, this author signalled that these molecules could exert antibacterial effects since they are powerful in vitro as inhibitors of DNA gyrase. He added that they have antimicrobial activity attributed to their phenolic function, this activity is supposed to increase with number of hydroxyl, methoxyl or glucosyl substituents and according to Mansouri et al.11 these types of flavonoids are present in dates.
The mechanism of the antimicrobial effects of polyphenols is undoubtedly very complex, among the hypotheses put forward, we can cite: Inhibition of extracellular microbial enzymes, the sequestration of the substrate necessary for microbial growth, the chelation of metals such as iron and the inhibition of microbial metabolism.40
The correlation results between the content of phenolic compounds and the antimicrobial effect of the extracts showed that the inhibitory effect exerted on S. Mu50, C. albicans and Enterobacter cancerogenus by the hexane, chloroform and ethyl acetate extracts seems to be due to flavonoids, given that there is a strong correlation between the content of flavonoids and the inhibitory effect whose correlation coefficients are (R2 = 0.98), (R2 = 0.89), (R2 = 0.85), respectively. Tannic acid was found as inhibitor of both Gram-positive and Gram-negative bacteria, such as: Staphylococcus aureus, Escherichia coli, Streptococcus pyogenes, Enterococcus faecalis, Pseudomonas aeruginosa, Yersinia enterocolitica, Listeria innocua.41
Coumarin was found active on Escherichia coli 81nr.149 SKN541, Enterobacter aerogenes CIP 104 725, Salmonella typhimurium SKN533 and Salmonella infantis SKN 557 in the study carried out by Nitiema et al.,42 where minimum inhibitory concentration of this molecule was from 0.625 to 5.0 mg mL; however, the minimum bactericidal concentration was ≤5 mg mL−1. Additionally, Rutin from Peumus boldus extract had highest antibacterial activity, especially against E. coli and Bacillus cereus.43
On the other hand, it was signalled in the study of Gutiérrez-Venegas et al.44 that luteolin, quercetin and rutin were actives against Escherichia coli, staphylococcus aureus and Candida albicans while catechin inhibited only staphylococcus aureus. It was found also in the study of Tyagi et al.45 that gallic, caffeic and tannic acids besides catechin and quercetin have considerable inhibition of strains of Escherichia coli and Pseudomonas aeruginosa.
Furthermore, it was reported by Paz et al.46 that, identified phenolics in extracts of Hamelia patens were able to inhibit Escherichia coli, Staphylococcus aureus and Salmonella typhi growth. This is because, these phenolic compounds contain hydroxycinnamic acid, quercetin, procyanidin B2, catechin besides epicatechin; therefore, they concluded that the last compound was more implicated in this activity.
3.4. Membrane stabilizing effect
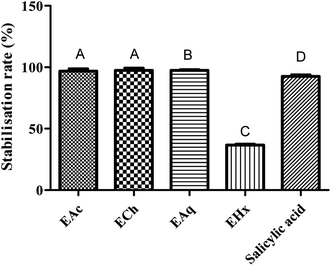
The results showed that all Tanteboucht extracts exhibited membrane stabilizing activity using concentration of 2 mg mL−1, essentially the ECh which had the greatest activity with a degree of 99.25 ± 0.08% of membrane stabilization, followed by the EAc with a percentage of 98.85 ± 0.12%, then the EAq with 98 ± 0.13% and finally the hexane extract giving 37 ± 0.19% as a stabilization rate (Fig. 4). The activity of the first three extracts is very important and significantly higher than that obtained with the positive control which gave an activity of 93%.The significant membrane stabilizing activity of extracts from the Tanteboucht date can be attributed to the identified flavonoids, in particular flavonols and flavanols, as well as tannins; indeed, examination of these results revealed a significant linear coefficient of determination (R2 = 0.64) between membrane stabilizing effect and flavonoid content and a very significant coefficient of determination (R2 = 0.99) between this activity and tannin content. According to Lavanya et al.47 all these compounds are endowed with anti-inflammatory power.
The obtained results allow us to conclude that Tanteboucht phenolics are involved in the membrane stabilization capacity of its extracts which decreases the possibility of installation of inflammation as it was indicated in several studies that inhibition of erythrocyte membrane damage is considered to be an index of anti-inflammatory activity.48,49
In the study of Yin et al.,50 Procyanidin B2 from grape seed showed an anti-inflammatory activity and thus provided protective effect against the damage of the diabetic pancreas. Lesjak and his collaborators51 carried out a study on antioxidant and anti-inflammatory activities of quercetin and its derivatives and concluded that, these compounds may act as effective anti-inflammatory and antioxidant agents. Soyocak et al.52 signalled that tannic acid may contribute to the treatment of inflammation by decreasing myeloperoxidase activity in rats against formalin-induced paw oedema. Hence, the p-hydroxybenzoic acid found abundantly in Tanteboucht extracts has potent biological activities, as previously confirmed in several studies. Indeed, this molecule reduced oxidative stress induced by hydrogen peroxide and contributed in inhibition of neurodegeneration process, which confirm its anti-inflammatory effect in the study conducted by Winter et al.53 On the other hand, Maleki et al.54 mentioned that flavonoids have beneficial properties in vitro in inflammatory diseases and they can inhibit enzymes or transcription factors involved in inflammation process.
4. Conclusion
Antioxidant, anti-inflammatory and anti-microbial studies were investigated in this work, and results showed important effects of Tanteboucht extracts, especially ethyl acetate and chloroformic extracts. Indeed, EAc and ECh were more potent in inhibition of microbial strains and EAc showed comparable efficiency to antibiotics. Furthermore, considerable scavenging activity was obtained with these extracts but it was less than the standards one. On the other hand, EAC, ECh and EAq exhibited very important rates of anti-inflammatory activity which was significantly higher than that of the positive control. The obtained considerable activities were significantly correlated with flavonoids and tannins contents in the extracts. Tanteboucht date must be valued, given its pharmacological properties and further investigations are needed to clearly understand the full potential of this fruit.Author contributions
Conceptualization, Saliha Dassamiour; data curation, Saliha Dassamiour, Selsabil Meguellati and Hdouda Lamraoui; Formal Analysis, Saliha Dassamiour and Mohamed Sabri Bensaad; Funding acquisition, Rokayya Sami; Investigation, Saliha Dassamiour and Selsabil Meguellati and Hdouda Lamraoui; Methodology, Saliha Dassamiour and Mohamed Sabri Bensaad; Project administration Saliha Dassamiour and Rokayya Sami; Resources, Saliha Dassamiour and Rokayya Sami; Software, Saliha Dassamiour; Supervision, Saliha Dassamiour and Rokayya Sami; Validation, Saliha Dassamiour, Mohamed Sabri Bensaad and Rokayya Sami; Visualization, Saliha Dassamiour, Mohamed Sabri Bensaad, Rokayya Sami, Garsa Alshehry, Eman Hillal Althubaiti and Areej Suliman Al-Meshal; Writing – original draft, Saliha Dassamiour, Selsabil Meguellati and Hdouda Lamraoui; Writing – review & editing, Saliha Dassamiour, Mohamed Sabri Bensaad, Rokayya Sami, Garsa Alshehry, Eman Hillal Althubaiti and Areej Suliman Al-Meshal.Conflicts of interest
The authors declare no conflicts of interest.Ethical statement
The authors of the publication declare that the patients/participants have been informed that their blood will be used for testing, provided their written informed consent to participate in this study and agreed to use the research results to prepare a scientific publication.Acknowledgements
The authors wish to express thanks to the Algerian ministry of higher education and scientific research (MESRS, DGRSDT). Taif University Researchers Supporting Project Number (TURSP-2020/140), Taif University, Taif, Saudi Arabia Also, the authors thank Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia for their scientific contributions.References
- A. Sofowora, E. Ogunbodede and A. Onayade, Afr. J. Tradit., Complementary Altern. Med., 2013, 10, 210–229 Search PubMed.
- F. Biglari, A. F. AlKarkhi and A. M. Easa, Food Chem., 2008, 107, 1636–1641 CrossRef CAS.
- P. Munier, Le pays de Dilmoun et la culture du palmier-dattier, Fruits, 1973, 28, 641–642 Search PubMed.
- Z. Sayah and M. D. Ould El Hadj, Ann.: Food Sci. Technol., 2010, 2, 87–92 Search PubMed.
- S. Daas Amiour and L. Hambaba, Postharvest Biol. Technol., 2016, 111, 77–82 CrossRef CAS.
- I. A. Ahmed, A. W. K. Ahmed and R. K. Robinson, Food Chem., 1995, 54, 305–309 CrossRef CAS.
- M. Takó, E. B. Kerekes, C. Zambrano, A. Kotogán, T. Papp, J. Krisch and C. Vágvölgyi, Antioxidants, 2020, 9, 165–185 CrossRef PubMed.
- B. R. Albuquerque, S. A. Heleno, M. B. Oliveira, L. Barros and I. C. Ferreira, Food Funct., 2021, 12, 14–29 RSC.
- S. L. Sampaio, S. A. Petropoulos, M. I. Dias, C. Pereira and R. C. Calhelha, Food Chem., 2021, 363, 130360–130367 CrossRef CAS PubMed.
- M. S. Bensaad, S. Dassamiour, L. Hambaba, C. Bensouici, K. Ouffroukh and M. A. Kahoul, Herba Pol., 2021, 67, 1–16 Search PubMed.
- A. Mansouri, G. Embarek, E. Kokkalou and P. Kefalas, Food Chem., 2005, 89, 411–420 CrossRef CAS.
- S. Daas Amiour, O. Alloui-Lombarkia, F. Bouhdjila, A. Ayachi and L. Hambaba, Phytotherapie, 2014, 12, 135–142 CrossRef CAS.
- U. A. Shinde, A. S. Phadke, A. M. Nair, A. A. Mungantiwar and V. J. Dikshit, Fitoterapia, 1999, 70, 251–257 CrossRef CAS.
- A. Alahyane, H. Harrak, J. Ayour, I. Elateri, A. Ait-Oubahou and M. S. Benichou, Afr. J. Bot., 2019, 121, 402–409 CrossRef CAS.
- S. A. Siddiqi, S. Rahman, M. M. Khan, S. Rafiq, A. Inayat, M. S. Khurram, T. Seerangurayar and F. Jamil, Sci. Total Environ., 2020, 748, 141234–141242 CrossRef CAS PubMed.
- M. S. Baliga, B. R. Baliga, S. M. Kandathil, H. P. Bhat and P. K. Vayalil, Int. Food Res. J., 2011, 44, 1812–1822 CrossRef CAS.
- A. H. Elhakem, N. Benajiba, M. Y. Koko, E. Khojah and A. Rok, Pakistan Journal of Agricultural Sciences, 2021, 24, 182–187 Search PubMed.
- N. F. Santos-Sánchez, R. Salas-Coronado, C. Villanueva-Cañongo and B. Hernández-Carlos, Antioxydants, 2019, 4, 1–28 Search PubMed.
- M. Z. Alam, M. S. Alhebsi, S. Ghnimi and A. Kamal-Eldin, Nutr. Food Sci., 2021, 22, 32–40 Search PubMed.
- N. Kumar and N. Goel, Biotechnol. Rep., 2019, 24, e00370–e00379 CrossRef PubMed.
- U. Sarker and S. Oba, Sci. Rep., 2020, 10, 18287–18297 CrossRef CAS PubMed.
- Y. Sato, S. Itagaki, T. Kurokawa, J. Ogura, M. Kobayashi, T. Hirano, M. Sugawara and K. Iseki, Int. J. Pharm., 2011, 403, 136–138 CrossRef CAS PubMed.
- S. Y. Shim, Y. E. Lee, H. Y. Song and M. Lee, Antioxidants, 2020, 9, 258–270 CrossRef CAS PubMed.
- V. F. Salau, O. L. Erukainure, C. U. Ibeji, T. A. Olasehinde, N. A. Koorbanally and M. S. Islam, Metab. Brain Dis., 2020, 35, 727–738 CrossRef CAS PubMed.
- R. R. Khalil and Y. F. Mustafa, Syst. Rev. Pharm., 2020, 11, 57–63 CAS.
- M. R. Antonijević, D. M. Simijonović, E. H. Avdović, A. Ćirić, Z. D. Petrović, J. D. Marković, V. Stepanić and Z. S. Marković, Antioxidants, 2021, 10, 1106–1123 CrossRef PubMed.
- E. Gerasimova, E. Gazizullina, E. Radosteva and A. Ivanova, Chemosensors, 2021, 9, 112–126 CrossRef CAS.
- K. Igarashi and M. Ohmuma, Biosci., Biotechnol., Biochem., 1995, 59, 595–601 CrossRef CAS PubMed.
- Q. Liu, R. Pan, L. Ding, F. Zhang, L. Hu, B. Ding, L. Zhu, Y. Xia and X. Dou, Int. Immunopharmacol., 2017, 49, 132–141 CrossRef CAS PubMed.
- S. S. Al-Rejaie, A. M. Aleisa, M. M. Sayed-Ahmed, O. A. Al-Shabanah, H. M. Abuohashish, M. M. Ahmed, K. A. Al-Hosaini and M. M. Hafez, BMC Complementary Med. Ther., 2013, 13, 136–144 CrossRef CAS PubMed.
- G. Petruk, R. Del Giudice, M. M. Rigano and D. M. Monti, Oxid. Med. Cell. Longevity, 2018, 2018, 1454936–1454946 Search PubMed.
- L. C. Cefali, J. A. Ataide, A. R. Fernandes, I. Sousa, F. Gonçalves, S. Eberlin, J. L. Dávila, A. F. Jozala, M. V. Chaud, E. Sanchez-Lopez, J. Marto, M. A. d'Ávila, H. M. Ribeiro, M. A. Foglio, E. B. Souto and P. G. Mazzola, Antioxidants, 2019, 8, 443–459 CrossRef CAS PubMed.
- N. Saewan and A. Jimtaisong, J. Cosmet. Dermatol., 2015, 14, 47–63 CrossRef PubMed.
- A. Nguyen, Biol. Smr. Flw., 2017, 42, 247–256 Search PubMed.
- J. Ivanovic, V. Tadic, S. Dimitrijevic, M. Stamenic, S. Petrovic and I. Zizovic, Ind. Crops Prod., 2014, 53, 274–281 CrossRef CAS.
- P. Ashokkumar and G. Sudhandiran, Biomed. Pharmacother., 2008, 62, 590–597 CrossRef CAS PubMed.
- K. Sakano, M. Mizutani, M. Murata, S. Oikawa, Y. Hiraku and S. Kawanishi, Free Radical Biol. Med., 2005, 39, 1041–1049 CrossRef CAS PubMed.
- A. L. Branen, P. M. Davidson and B. Katz, Food Technol., 1980, 34, 42–63 Search PubMed.
- A. K. Farha, Q. Yang, G. Kim, H. Li, F. Zhu, H. Liu, R. Gan and H. Corke, Food Biosci., 2020, 38, 100751–100764 CrossRef CAS.
- H. A. Milane, G. Ubeaud, T. F. Vandamme and L. Jung, Bioorg. Med. Chem., 2004, 12, 3627–3635 CrossRef CAS PubMed.
- B. Kaczmarek, Materials, 2020, 13, 3224–3236 CrossRef CAS PubMed.
- L. W. Nitiema, A. Savadogo, J. Simpore, D. Dianou and A. S. Traoré, Int. J. Microbiol. Res., 2013, 3, 183–187 Search PubMed.
- C. Ferrante, A. Chiavaroli, P. Angelini, R. Venanzoni, G. Angeles Flores, L. Brunetti, M. Petrucci, M. Politi, L. Menghini, S. Leone, L. Recinella, G. Zengin, G. Ak, M. Di Mascio, F. Bacchin and G. Orlando, Antibiotics, 2020, 9, 783–802 CrossRef CAS PubMed.
- G. Gutiérrez-Venegas, J. A. Gómez-Mora, M. A. Meraz-Rodríguez, M. A. Flores-Sánchez and L. F. Ortiz-Miranda, Heliyon, 2019, 5, e03013–e03018 CrossRef PubMed.
- B. Tyagi, A. Dubey, A. Verma and S. Tiwari, Int. J. Pharm. Sci. Rev. Res., 2015, 35, 16–18 CAS.
- J. Paz, C. R. Contreras, A. R. Munguía, C. N. Aguilar and M. Inungaray, Braz. J. Microbiol., 2018, 49, 656–661 CrossRef CAS PubMed.
- R. Lavanya, S. Maheshwari, G. Harish, J. B. Raj, S. Kamali, D. Hemamalani, J. B. Varma and C. U. Reddy, Res. J. Pharm., Biol. Chem. Sci., 2010, 1, 745–752 Search PubMed.
- H. M. Manukumar and S. Umesha, Acta Sci. Pol., Technol. Aliment., 2015, 14, 85–90 CrossRef PubMed.
- S. Yesmin, A. Paul, T. Naz, A. B. M. A. Akhtar, M. I. Wahed and S. A. Siddiqui, Clin. Phytosci., 2020, 6, 1–10 CrossRef.
- W. Yin, B. Li, X. Li, F. Yu, Q. Cai, Z. Zhang, M. Cheng and H. Gao, Food Funct., 2015, 6, 3065–3071 RSC.
- M. Lesjak, I. Beara, N. Simin, D. Pintać, T. Majkić, K. Bekvalac, D. Orčić and N. Mimica-Dukić, J. Funct. Foods, 2018, 40, 68–75 CrossRef CAS.
- A. Soyocak, H. Kurt, D. T. Cosan, F. Saydam, I. U. Calis, U. K. Kolac, Z. O. Koroglu, I. Degirmenci, F. S. Mutlu and H. V. Gunes, Hum. Exp. Toxicol., 2019, 38, 1296–1301 CrossRef CAS PubMed.
- A. N. Winter, M. C. Brenner, N. Punessen, M. Snodgrass, C. Byars, Y. Arora and D. A. Linseman, Oxid. Med. Cell. Longevity, 2017, 2017, 6297080–6297092 Search PubMed.
- S. J. Maleki, J. F. Crespo and B. Cabanillas, Food Chem., 2019, 299, 125124–125134 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |