Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Direct sialic acid 4-OAc substitution by nitrogen, sulfur and carbon nucleophiles with retention of stereochemistry†
Tiago Bozzola,
Ulf J. Nilsson and
Ulf Ellervik
and
Ulf Ellervik *
*
Centre for Analysis and Synthesis, Department of Chemistry, Lund University, P.O. Box 124, SE-221 00 Lund, Sweden. E-mail: ulf.ellervik@chem.lu.se
First published on 19th April 2022
Abstract
A direct one-step nucleophilic substitution of the 4-OAc of acetyl protected Neu5Ac is presented. Previously published methods for direct substitution of the 4-OAc are limited to cyclic secondary amines. Here we present conditions that allow for a much wider range of nitrogen nucleophiles as well as thiols and cyanide, to be used. The present investigation significantly broadens the scope of 4-aminations and allows for the introduction of a wide variety of different nucleophiles.
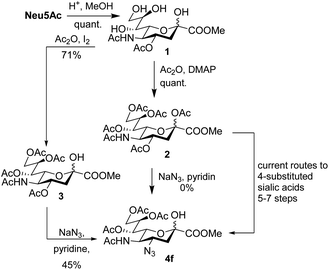
5-Acetylneuraminic acid (Neu5Ac) is the most abundant sialic acid, i.e. a member of a family of acidic 9-carbon sugars.1 Sialic acids are often found in the terminal positions of cell surface glycans and thus play crucial roles in both physiological and pathological conditions.2,3 Sialic acids are consequently important drug candidates and it is crucial to develop tools to functionalize and study unnatural sialic acid derivatives.4 As of now, modification of position 4 of sialic acid is complicated and examples in the literature are scarce.5–7 The introduction of an azido functionality has been achieved in the synthesis of the influenza drug Zanamivir, but such compounds still require long synthetic routes in the case of sialic acid derivatives other than glycals (Scheme 1).8–12
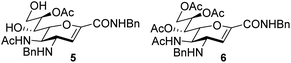
An elegant and convenient one-step amination was presented by Ye et al. with direct substitution of the 4-OAc of peracetylated Neu5Ac (2), by cyclic secondary amines with retention of stereochemistry.13 Compound 2 was stirred with 10 equivalents of the cyclic amine in pyridine for up to 48 hours and the stereochemical retention was hypothesized to result from neighbouring group participation by the 5-NHAc. However, the reported scope of the reaction remained limited to five- and six-membered secondary cyclic amines, such as morpholine and pyrrolidine. Acyclic, aromatic and hindered amines were reported as unreactive by the authors. Interestingly, Ye et al. showed that the reaction proceeded well using the hemiacetal 3. We hypothesized that the efficiency of the 4-amination reactions are proportional to the nucleophilicity of the amine, as cyclic amines are better nucleophiles compared to primary and acyclic secondary ones.14 Benzylamine, N-methylbenzylamine, aniline, and N-methylaniline were thus selected in an initial attempt to test this hypothesis. However, under the reported reaction conditions, the reaction with benzylamine led to a multitude of side products, including the partly deacetylated glycal 5 (Chart 1).
To prevent the observed side products, the reaction was instead conducted at −5 °C with 3 equivalents of benzylamine. Similar side products, such as 6 (Chart 1), were still formed, but we could also isolate 4a in 25% yield (Table 1). The reaction with N-methylbenzylamine yielded the desired 4b in 39% yield without further optimization. The reaction with aniline, as expected by the reduced nucleophilicity of the aromatic nitrogen, led to extended reaction time and formation of a mixture of side products. The reaction with N-methylaniline did not yield any product, neither through extended reaction time (>30 days), nor increased temperature (14 h at 80 °C, microwave reactor).
| Compound | X | R | Time | Temperature | Yield |
|---|---|---|---|---|---|
| a Reaction conditions: 3–10 eq of nucleophile in pyridine at room temperature until disappearance of starting material or appearance of side products.b 3 eq of t-BuOK are additionally present.c 3 eq of Cs2CO3 are additionally present. | |||||
| 4a | 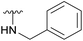 |
Ac | 3 days | −5 °C | 25% |
| H | 20 h | −10 °C | 34% | ||
| 4b | 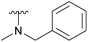 |
Ac | 7 days | RT | 39% |
| H | — | — | Not tested | ||
| 4c |  |
Ac | 30 days | RT | 34% |
| H | 17 days | RT | 39% | ||
| 4d |  |
Ac | 30 days | RT | 66% |
| H | — | — | Not tested | ||
| 4e | 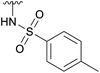 |
Ac | 3 daysb | RT | 20% |
| H | 2 hc | RT | 25% | ||
| 4f | 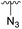 |
Ac | >30 days | RT | 0% |
| H | 7 days | RT | 45% | ||
| 4g | 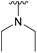 |
Ac | >30 days | RT | 0% |
| H | 4 h | RT | 41% | ||
| 4h | 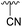 |
Ac | 7 days | RT | 21% |
| H | 2 days | RT | 38% | ||
| 4i | 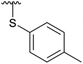 |
Ac | — | — | Not tested |
| H | 4 hours | RT | 89% | ||
| 4l | 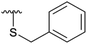 |
Ac | — | — | Not tested |
| H | 4 hours | RT | 63% | ||
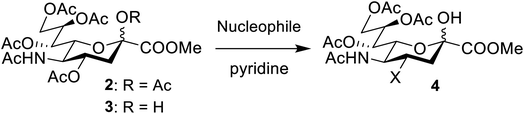
To further investigate the scope of the reaction, other nitrogen containing nucleophiles such as acyclic amines, sulfonamide, as well as azide and cyanide ions were included (Table 1).
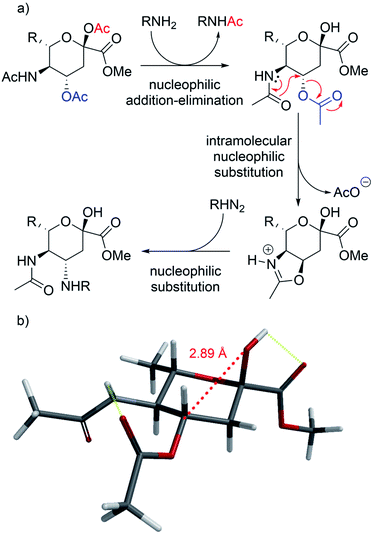
While the reaction with p-toluenesulfonamide and potassium cyanide proceeded to give 20% yield after 3 and 7 days respectively (Table 1, entries e and g), azide, and some acyclic amines did not result in product formation. Ye et al. proposed a reaction mechanism in which the 5-acetamide first reacts in an intramolecular nucleophilic substitution, with the 4-acetate as leaving group, to give the oxazolinium intermediate. This intermediate was supposed to be stabilized by the oxygen atom of the 2-OAc. The cyclic secondary amines then supposedly reacted with the activated 2-OAc to give the 2-OH. Finally, a second molecule of the amine reacted in an SN2-fashion with the oxazolinium moiety to give the product.
We instead argue that the reaction starts with a nucleophilic addition–elimination reaction that results in deacetylation of the 2-OAc and that this is the rate-limiting step (Scheme 2a). While some nitrogen nucleophiles, such as cyclic secondary amines, are capable both of nucleophilic addition–elimination and nucleophilic substitution, others, such as diethyl amine and azide ions are limited to the latter reaction. To test this hypothesis, we synthesized compound 315 and re-examined the reaction. To our delight, the reaction with diethyl amine proceeded smoothly with full consumption of starting material, and 41% yield, in 4 hours (Table 1, entry g).
Subsequently, we tested the reactions previously attempted with 2. Although the yields remained moderate, the reactions proceeded fast and gave products even with azide, where no reaction was observed with 2. The possibility to insert an azide group at C4 of sialic acid opens up for a multitude of reactions, such as CuAAC Huisgen cycloadditions, whereas the cyanide addition selectively creates a carbon–carbon bond at C4 and facilitates insertion of methylene bridged amines and the formation of various heterocycles such as tetrazoles. At this point, we also included thiocresol and benzyl mercaptan to establish if the array of reacting nucleophiles also could include thiols. The reactions with thiols proceeded fast and in good yields to the desired products. This opens up for a multitude of new chemical entities, since the sulfides can be selectively oxidized to sulfoxides and sulfones.
We argue that the 2-OH, in contrast to the electron poor 2-OAc, enables the reaction by through–space interactions as previously reported by Miljković et al. (Scheme 2b).16 The 2-OH is positioned at a 2.87 Å from C4, the same distance previously reported between the substituent at C4 and the oxacarbenium ion in the case of D-galactopyranosides.16 The electron density on the 2-OH of compound 3 is accentuated by an internal hydrogen bond between the proton of the 2-OH and the carbonyl at C1 (Scheme 2b). Through space, the 2-OH is then able to donate into the antibonding orbital (σ*) of the C4–O4 bond, thus weakening the σ bond. Subsequentially, the HOMO from the carbonyl oxygen of the acetamide is capable of forming the oxazolinium ring, to later allow for the final SN2. Interestingly, the corresponding 2-OMe compound was unreactive versus cyanide ions (data not shown). An alternative explanation would be a reaction via the open-chain form, which is only accessible with the hemiacetal.
Conclusions
Sialic acids are complex monosaccharide structures typically found at the terminal position of cell surface glycans and are important drug candidates. In order to functionalize and study unnatural sialic acid derivatives, access to efficient methods for derivatization of complex and poly-functional sialic acids is crucial.As of now, synthesis of 4-amino sialic acid derivatives requires long synthetic routes. In our research for routes to 4-aminated sialic acid analogues, we investigated a convenient one-step amination with direct substitution of the 4-OAc of peracetylated Neu5Ac. To expand the scope of the reaction, which was limited to secondary cyclic amines, we investigated several nitrogen nucleophiles under various conditions and observed that, by using a 2-OH derivative as starting material, the scope was expanded to involve not only acyclic and aromatic amines, but also nucleophiles such as thiols, azide and cyanide ions.
Sulfide, azido and cyano functionalities allow for further derivatisation and are intensively used in drug discovery programs. The present investigation thus significantly broadens the scope of 4-substitutions and represents the shortest route to 4-aminated sialic acids, and opens up for efficient syntheses of other sialic acid derivatives via installation of and subsequent reaction of 4-N3,4-CN, or 4-SR groups.
Experimental
General information
All moisture-and air-sensitive reactions were carried out under an atmosphere of dry nitrogen using oven-dried glassware. All solvents were dried using an MBRAUN SPS-800 Solvent purification system before use unless otherwise stated. Purchased reagents were used without further purification. Thin-layer chromatography was performed on precoated TLC glass plates with silica gel 60 F254 0.25 mm (Merck). Spots were visualized with UV light or by charring with 10% H2SO4 in ethanol. Biotage Isolute phase separators were used for drying of combined organic layers. Preparative chromatography was performed on a Biotage Isolera One flash purification system using Biotage SNAP Sfär HD silica cartridges. Optical rotationswere measured on a Bellingham and Stanley model ADP450 polarimeter and are reported as [α]DT (c = g/100 mL), where D indicates the sodium D line (589 nm) and T indicates the temperature. NMR spectra were recorded at ambient temperatures on a Bruker Avance II at 400 MHz (1H) and 100 MHz (13C) or a Bruker Ascend at 500 MHz (1H) and 125 MHz (13C) and assigned using 2D methods (COSY, HMQC). Chemical shifts are reported in ppm, with reference to residual solvent peaks (δH CHCl3 = 7.26 ppm, CD3OH = 3.31 ppm) and solvent signals (δC CDCl3 = 77.0 ppm, CD3OD = 49.0 ppm). Coupling constant values are given in Hz. Mass spectra were recorded on Waters XEVO G2 (positive ESI). Infrared spectroscopy was recorded on a Bruker α II FT-IR spectrometer. IR was only used to confirm structural features, and only the peak of interest was reported.General procedure for 4-amination
Starting material 2 or 3 was dissolved in dry pyridine, the mixture was allowed to attain the designated temperature, and the desired amine (3–10 eq.) was added. The reaction was monitored via TLC and LCMS until either full conversion, or appearance of side products. The reaction mixture was then evaporated, and the residues dissolved in EtOAc, washed with distilled water and brine, dried and purified by flash chromatography.Methyl 5-acetamido-4-azido-7,8,9-tri-O-acetyl-3,4,5-trideoxy-D-glycero-β-D-galacto-non-2-ulopyranosidonate (4f) starting material 3 (1 g, 2.03 mmol) was reacted with sodium azide (0.396 g, 6.09 mmol). The reaction was completed after 7 days and the reaction mixture evaporated and flash chromatography performed directly (Hep![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 60
EtOAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 → 20
40 → 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 over 6 CV, then isocratic). The purification afforded 4f as an amorphous white solid (438 mg, 45%). [α]D20 + 18 (c 0.5, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ 5.52 (d, J = 9.4 Hz, 1H, AcN-H), 5.32 (dd, J = 6.5, 2.0 Hz, 1H, H-7), 5.24 (td, J = 6.6, 2.4 Hz, 1H, H-8), 4.41 (dd, J = 12.4, 2.3 Hz, 1H, H-9), 4.30 (dd, J = 10.5, 2.0 Hz, 1H, H-6), 4.05 (m, 2H, H-9, H-4), 3.87 (s, 3H), 3.80–3.66 (m, 1H, H-5), 2.21 (dd, J = 13.1, 4.9 Hz, 1H, H-3eq), 2.15–1.99 (m, 13H, H-3ax, OCOCH3 x 3, HNCOCH3). 13C NMR (126 MHz, CDCl3) δ 171.3, 170.9, 170.6, 169.2, 94.5, 70.5, 70.0, 68.0, 62.7, 58.0, 53.8, 51.1, 36.2, 23.6, 21.2, 21.1, 21.0, 20.9. ESI-MS (m/z): Calcd. for C18H26N4O11 [M + Na]+, 497.1496; found, 497.1490. IR: 2106 cm−1 (N3).
80 over 6 CV, then isocratic). The purification afforded 4f as an amorphous white solid (438 mg, 45%). [α]D20 + 18 (c 0.5, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ 5.52 (d, J = 9.4 Hz, 1H, AcN-H), 5.32 (dd, J = 6.5, 2.0 Hz, 1H, H-7), 5.24 (td, J = 6.6, 2.4 Hz, 1H, H-8), 4.41 (dd, J = 12.4, 2.3 Hz, 1H, H-9), 4.30 (dd, J = 10.5, 2.0 Hz, 1H, H-6), 4.05 (m, 2H, H-9, H-4), 3.87 (s, 3H), 3.80–3.66 (m, 1H, H-5), 2.21 (dd, J = 13.1, 4.9 Hz, 1H, H-3eq), 2.15–1.99 (m, 13H, H-3ax, OCOCH3 x 3, HNCOCH3). 13C NMR (126 MHz, CDCl3) δ 171.3, 170.9, 170.6, 169.2, 94.5, 70.5, 70.0, 68.0, 62.7, 58.0, 53.8, 51.1, 36.2, 23.6, 21.2, 21.1, 21.0, 20.9. ESI-MS (m/z): Calcd. for C18H26N4O11 [M + Na]+, 497.1496; found, 497.1490. IR: 2106 cm−1 (N3).
Author contributions
Tiago Bozzola – conceptualization, investigation, visualization, writing – original draft, writing – review & editing. Ulf Nilsson – conceptualization, funding acquisition, supervision, visualization, writing – original draft, writing – review & editing. Ulf Ellervik – conceptualization, funding acquisition, supervision, visualization, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the European Joint Doctorate educational training network in Glyco-Drug Discovery and Development (PhD4GlycoDrug, Marie Skłodowska-Curie grant agreement No 765581), and Lund University.Notes and references
- A. Angata and T. Varki, Chem. Rev., 2002, 102, 439–469 CrossRef PubMed.
- N. M. Varki and A. Varki, Lab. Invest., 2007, 87, 851–857 CrossRef CAS PubMed.
- H. M. Bauer and J. Osborn, Future Med. Chem., 2015, 7, 2131–2141 CrossRef PubMed.
- B. A. H. Smith and C. R. Bertozzi, Nat. Rev. Drug Discovery, 2021, 20, 217–243 CrossRef CAS PubMed.
- S. Buda and D. Crich, J. Am. Chem. Soc., 2016, 138, 1084–1092 CrossRef CAS PubMed.
- D. R. Groves and M. Von Itzstein, J. Chem. Soc., Perkin Trans., 1996, 2817–2821 RSC.
- M. Hartmann, R. Christian and E. Zbiral, Monatsh. Chem., 1991, 125, 111–125 CrossRef.
- M. Chandler, M. J. Bamford, R. Conroy, B. Lamont, B. Patel, V. K. Patel, I. P. Steeples, R. Storer, N. G. Weir, M. Wright and C. Williamson, J. Chem. Soc., Perkin Trans., 1995, 1, 1173–1180 RSC.
- S. Kelm, P. Madge, T. Islam, R. Bennett, H. Koliwer-Brandl, M. Waespy, M. von Itzstein and T. Haselhorst, Angew. Chem., Int. Ed., 2013, 52, 3616–3620 CrossRef CAS PubMed.
- M. von Itzstein, W. Y. Wu and B. Jin, Carbohydr. Res., 1994, 259, 301–305 CrossRef CAS PubMed.
- B. P. Bandgar, S. V Patil and E. Zbiral, Carbohydr. Res., 1995, 276, 337–345 CrossRef CAS.
- S. Ciccotosto and M. von Itzstein, Tetrahedron Lett., 1995, 36, 5405–5408 CAS.
- D. Ye, J. Li, J. Zhang, H. Jiang and R. N. Neuac, Tetrahedron Lett., 2007, 48, 4023–4027 CrossRef CAS.
- F. Brotzel, C. C. Ying and H. Mayr, J. Org. Chem., 2007, 72, 3679–3688 CrossRef CAS PubMed.
- K. P. R. Kartha and R. A. Field, Tetrahedron, 1997, 53, 11753–11766 CrossRef CAS.
- M. Miljković, D. Yeagley, P. Deslongchamps and Y. L. Dory, J. Org. Chem., 1997, 62, 7597–7604 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01576e |
| This journal is © The Royal Society of Chemistry 2022 |