Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective Fe(II)-fluorescence sensor with validated two-consecutive working range using N,S,I-GQDs associated with garlic extract as an auxiliary green chelating agent
Nipaporn Pimsina,
Chayanee Keawproma,
Yonrapach Areerobb,
Nunticha Limchoowong *c,
Phitchan Sricharoen
*c,
Phitchan Sricharoen *de,
Prawit Nuengmatcha
*de,
Prawit Nuengmatcha f,
Won-Chun Oh
f,
Won-Chun Oh g and
Saksit Chanthai
g and
Saksit Chanthai *a
*a
aMaterials Chemistry Research Center, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand. E-mail: sakcha2@kku.ac.th
bDepartment of Industrial Engineering, School of Engineering, King Mongkut's Institute of Technology Ladkrabang, Bangkok 10520, Thailand
cDepartment of Chemistry, Faculty of Science, Srinakharinwirot University, Bangkok 10110, Thailand. E-mail: nuntichoo@gmail.com
dDepartment of Premedical Science, Faculty of Medicine, Bangkokthonburi University, Thawi Watthana, Bangkok 10170, Thailand. E-mail: phitchan.s@gmail.com
eThailand Institute of Nuclear Technology (Public Organization), Ongkharak, Nakhon Nayok, 26120, Thailand
fCreative Innovation in Science and Technology and Nanomaterials Chemistry Research Unit, Department of Chemistry, Nakhon Si Thammarat Rajabhat University, Nakhon Si Thammarat 80280, Thailand
gDepartment of Advanced Materials Science and Engineering, Hanseo University, Seosan, Chungnam, Republic of Korea
First published on 12th May 2022
Abstract
The goal of this work was to use the pyrolysis process to synthesize graphene quantum dots doped with garlic extract (as N,S-GQDs) and simultaneously co-doped with iodine (as I-GQDs). XPS, HR-TEM, FE-SEM/EDX, FT-IR, fluorescence, and UV-visible absorption spectroscopy were used to characterize the N,S,I-GQDs and analyze their morphological images. The quantum yield of N,S,I-GQDs was found to be 45%, greater than that of undoped GQDs (31%). When stimulated at 363 nm, the N,S,I-GQDs display a strong fluorescence intensity at a maximum wavelength of 454 nm. Using N,S,I-GQDs as a fluorescence quenching sensor for screening tests with various metal ions, it was discovered that they are extremely selective towards Fe2+ over Fe3+ and other ions. Thus, solution pH, concentration of N,S,I-GQDs, quantity of garlic extract, EDTA and AgNO3 concentration as masking agents, reaction duration under ultrasonic aid, and tolerable limit of Fe3+ presence in the target analyte were all optimized for Fe2+ detection. A highly sensitive detection of Fe2+ was obtained using a linear curve with y = 141.34x + 5.5855, R2 = 0.9961, LOD = 0.11 mg L−1, and LOQ = 0.35 mg L−1. The method precision, given as RSDs, was determined to be satisfactory at 1.04% for intra-day analysis and 3.22% for inter-day analysis, respectively. As a result, the selective determination of trace amounts of Fe2+ in real water samples using such labile multi-element doped GQDs in conjunction with garlic extract as a green chelating agent to maintain its enhanced sensitivity was successfully applied with good recoveries ranging from 89.16 to 121.45%.
1. Introduction
Heavy metals are commonly discovered in high quantities in groundwater in industrially impacted regions such as the textile, metallurgical, agrochemical, and iron sectors. Their toxicity here stems from the fact that heavy metals pollute not only drinking water, but also the soil, where they accumulate in plants and animals, posing serious health risks to people. The most frequent and abundant transition metal ions in the human body are iron ions.1 Iron (Fe) is an important metal in biology, medicine, the environment, and industry. It is one of the most important trace elements in living bio systems, where it performs critical and flexible functions in a variety of physiological and pathological processes such as enzyme catalysis, oxygen transport, cellular metabolism, electron transfer, and DNA and RNA synthesis.2–6 Iron speciation is frequently linked to its role and has been discovered at a trace level. Depending on the physiological condition, it usually appears as ferrous (Fe2+) or ferric (Fe3+) ions.7 Iron ions in sufficient amounts are essential for optimal health. Excess iron ions in the human body can lead to major issues such as kidney and liver damage,8,9 neurological illnesses,10,11 malignancies, hemochromatosis, and crucial organ malfunction. As a result, determining iron characteristics is critical for the early detection and diagnosis of many disorders. Furthermore, measuring iron concentrations in water samples is critical for environmental safety.12 Solid phase extraction,13 high performance liquid chromatography (HPLC),14 inductively coupled plasma mass spectrometry (ICP-MS),15 fiber-optic chemosensor,16 inductively coupled plasma optical emission spectrometry (ICP-OES),17 and slotted quartz tube-flame atomic absorption spectrometry (SQT-FAAS)18,19 are currently used to determine Fe2+ and Fe3+ ions. Although these techniques are very sensitive and selective, they need time-consuming sample preparation and pre-concentration processes, as well as costly apparatus and experienced people. As a result, developing simple analytical procedures for the identification of Fe2+ and Fe3+ ions in natural materials remains difficult. Several fluorescence sensors have been developed in recent years for qualitative and quantitative studies due to their experimental speed, simplicity, and ability to give a selective and sensitive approach for iron speciation.Light-emitting quantum-sized graphene quantum dots (GQDs) have lately received a lot of interest as a replacement for quantum dots (QDs) in a number of applications.20–25 Because of their ease of manufacture, high quantum yield, sensitivity, selectivity, biocompatibility, adjustable emission, cheap cost, strong photo stability, and nontoxicity, GQDs are intriguing in the field of chemical sensing for the detection of metal ions.26 In addition to the qualities listed above, GQDs offer exceptional optical, electrical, and thermal properties.27–30 It is one of the most common solutions for detecting harmful metal ions when combined with sensors. As a result, GQDs must enhance the surface for high selectivity, strong sensitivity, different detection limits, easy-to-use sensors, and good stability.
Garlic (Allium sativum L.) has been regarded as a great spice with powerful therapeutic powers by people all over the world since ancient times.31 The major ingredient in garlic extract is allicin; garlic's therapeutic benefits rely on organosulfur compounds obtained mostly from alliin, which has an inhibitory impact on the development of many bacteria and fungus. Garlic has been used to treat a range of diseases, including heart disease, infections, and cancer prevention.32–34 Other sulfur-containing phytoconstituents found in garlic include ajoenes, vinyldithiins, and amino acids such as arginine, glutamic acid, aspartic acid, and leucine.35–37 We introduce a new fluorescence sensor probe and have developed a selective method for detecting Fe2+ in drinking water samples in this study. Preparation of fluorescent N,S,I co-doped GQDs, comprised of garlic extract doped GQDs and I-GQDs, utilizing a simple, green, and low-cost one-pot pyrolysis technique using garlic, citric acid, potassium iodide (KI), and potassium iodate (KIO3) as the precursor. However, nearly no data on such I-GQDs have been published, however iodine supplementation can affect the optical characteristics of the GQDs with great selectivity and stability for the detection of Fe2+. Furthermore, the best conditions for each garlic extract and N,S,I-GQDs content, pH of the solution, masking agent, and interfering ions were thoroughly examined. The devised approach was then used to determine the iron(II) ion in actual water samples.
2. Experimental
2.1. Materials and reagents
The chemicals utilized were all of analytical grade. Carlo Erba supplied the citric acid and sodium hydroxide (Italy). Sigma-Aldrich provided iron(II) sulfate heptahydrate, iron(III) chloride hexahydrate, silver nitrate, ethylenediaminetetraacetic acid (EDTA), and boric acid (H3BO3) (Germany). Acetic acid (Merck, Germany), potassium iodate (KIO3) (QRec, New Zealand), and phosphoric acid (H3PO4) (BDH, England) were also utilized. Ajex Finechem supplied the paraffin oil (Australia). Throughout the studies, deionized water (Simplicity Water Purification System, Model Simplicity 185, Millipore, USA) was utilized.2.2. Apparatus and instruments
The principal instrument was a spectrofluorophotometer (Shimadzu RF-5301PC, Japan) with excitation and emission slit widths of 5 nm. Agilent's UV-visible spectrophotometer model 8453 was used (Germany). A pH meter UB-10 UltraBasic (Denver, USA), an analytical balance (Model LX 220A, Precisa, Thailand), a quartz cell with a path length of 1 cm (Fisher Scientific, USA), and an ultrasonic cleaner (Model VGT-2300, GT SONIC, Hong Kong) were also utilized. On a TENSOR27 system Fourier transform infrared spectrometer, an attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopic observation was made (Bruker, Germany). The technique of high-resolution transmission electron microscopy (HR-TEM, Electron gun: Schottky field emission type electron gun) was employed. A HITACHI S-3000N scanning electron microscope was used to collect EDX spectra (SEM, Hitachi Co. Ltd, Japan). For the citric acid pyrolysis, a round bottom flask (Pyrex®, England) and a heated plate with a magnetic stirrer in conjunction with a paraffin oil bath were used.2.3. Synthesis and characterization of GQDs and N,S,I-GQDs
Pyrolysis was used to prepare garlic extract doped/iodine co-doped graphene quantum dots (N,S,I-GQDs). Citric acid (0.9 g) was put into a 100 mL round bottom flask. The flask was heated to 230 °C using a paraffin oil bath for 5 min. The citric acid was slowly liquated with its yellow color. Then garlic extract (1 mL), KI (0.15 g) and KIO3 (0.15 g) were added into the flask and thermally treated for 5 min. After that, NaOH solution (0.25 M, 50 mL) was mixed with the liquid at room temperature under continuous stirring for 30 min. The achieved N,S,I-GQDs solution was stored at 4 °C until use.2.4. Fluorescence measurement
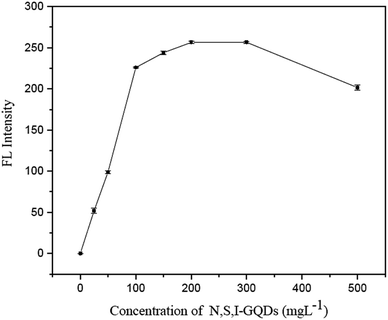
The N,S,I-GQDs fluorescence was measured in Britton–Robinson buffer solution at pH 8. In a 10 mL volumetric flask, 100 mgL−1 of N,S,I-GQDs solution was well mixed for the following tests. Then, at room temperature, different amounts of Fe2+ were added to an aliquot of the N,S,I-GQDs solution in a 10 mL final volume. The Fe2+ fluorescence sensor of each N,S,I-GQDs solution was instantly recorded at λex/λem = 365/455 nm. Their spectral data were utilized to generate a quenching calibration curve for Fe2+ in the presence of AgNO3 solution as a masking agent for Fe3+ in the sample solution, if necessary.2.5. Real sample analysis
The applicability of the suggested approach for actual water samples including tap water and drinking water was evaluated using an N,S,I-GQDs-based fluorescence sensor for Fe2+ detection concurrently in an artificial system. Tap water samples were obtained from the Khon Kaen region and placed in plastic bottles that had been prepared with 1% (v/v) dilute nitric acid. 1 mL of water and 1 mL of N,S,I-GQDs (100 mg L−1) solution were put into a 10.0 mL volumetric flask for this process. Prior to fluorescence measurement, each of the sample mixes was spiked with three concentration levels of Fe2+ standard solution (10, 25, and 50 mg L−1) as was done for its recovery investigation.3. Results and discussion
3.1. Characterization of the as-synthesized GQDs and N,S,I-GQDs
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
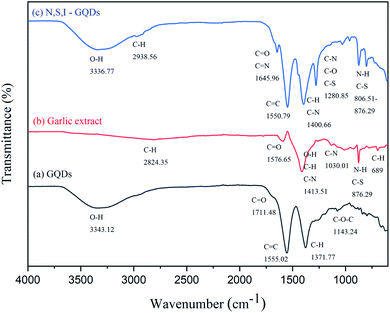
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and C–O bonds,38,39 and these results confirm that the garlic extract doped with I-GQDs were successfully synthesized by citric acid pyrolysis. Fig. 1c depicts the FTIR spectrum of N,S,I-GQDs, with the widened band at 800–1200 cm−1 corresponding to the stretching vibrations of C–S, C–N, and N–H.40
N, and C–O bonds,38,39 and these results confirm that the garlic extract doped with I-GQDs were successfully synthesized by citric acid pyrolysis. Fig. 1c depicts the FTIR spectrum of N,S,I-GQDs, with the widened band at 800–1200 cm−1 corresponding to the stretching vibrations of C–S, C–N, and N–H.40
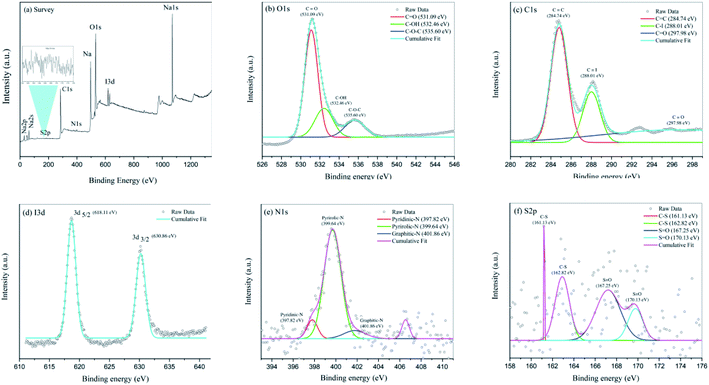
The elemental compositions, carbon bonding, oxygen bonding, and iodine bonding topologies of N,S,I-GQDs were determined using X-ray photoelectron spectroscopy (XPS). The survey spectrum of the N,S,I-GQDs is shown in Fig. 2a. Peaks at 282.56, 528.49, and 619.90 eV may correspond to the binding energies of carbon (C 1s), oxygen (O 1s), and iodine (I 3d), respectively. The XPS spectra of O 1s (Fig. 2b) reveals the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH, and C–O–C functional groups, with deconvoluted binding energies of 531.09, 532.46, and 535.60 eV, respectively.41 This clearly suggested that iodine was doped into GQDs effectively. The N,S,I-GQDs' high-resolution I3d spectra may be divided into two peaks at 618.65 eV (3d5/2) and 630.20 eV (3d3/2) (Fig. 2d),42 a substantial number of oxygen functional groups were detected in the C 1s high-resolution XPS spectra of N,S,I-GQDs (Fig. 2c), indicating inadequate carbonization during the pyrolysis of citric acid and iodine. Furthermore, two additional peaks were found in the C 1s spectra of N,S,I-GQDs at 284.74, 288.01, and 297.98 eV, corresponding to the C
O, C–OH, and C–O–C functional groups, with deconvoluted binding energies of 531.09, 532.46, and 535.60 eV, respectively.41 This clearly suggested that iodine was doped into GQDs effectively. The N,S,I-GQDs' high-resolution I3d spectra may be divided into two peaks at 618.65 eV (3d5/2) and 630.20 eV (3d3/2) (Fig. 2d),42 a substantial number of oxygen functional groups were detected in the C 1s high-resolution XPS spectra of N,S,I-GQDs (Fig. 2c), indicating inadequate carbonization during the pyrolysis of citric acid and iodine. Furthermore, two additional peaks were found in the C 1s spectra of N,S,I-GQDs at 284.74, 288.01, and 297.98 eV, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–I, and C
C, C–I, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively. The N,S,I-GQDs high-resolution N 1s spectra may be divided into three peaks at 397.80 eV (pyridinic-N), 399.66 eV (pyrrolic-N), and 406.56 eV (graphitic-N) (Fig. 2e). The S 2p spectra of N,S,I-GQDs (Fig. 2f) has two peaks centered at 161.1 and 169.82 eV, indicating that S exists in two forms. The former peak may be deconvoluted into two different components at 161.1 and 162.8 eV, which agree with –C–S–C– locations, while the later peak can be fit with two components at 167.3 and 169.82 eV, which agree with O
O bonds, respectively. The N,S,I-GQDs high-resolution N 1s spectra may be divided into three peaks at 397.80 eV (pyridinic-N), 399.66 eV (pyrrolic-N), and 406.56 eV (graphitic-N) (Fig. 2e). The S 2p spectra of N,S,I-GQDs (Fig. 2f) has two peaks centered at 161.1 and 169.82 eV, indicating that S exists in two forms. The former peak may be deconvoluted into two different components at 161.1 and 162.8 eV, which agree with –C–S–C– locations, while the later peak can be fit with two components at 167.3 and 169.82 eV, which agree with O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. According to XPS spectra, the N,S,I-GQDs contain 58.67% carbon, 38.67% oxygen, 1.41% nitrogen, 0.85% iodine, and 0.41% sulfur.
O. According to XPS spectra, the N,S,I-GQDs contain 58.67% carbon, 38.67% oxygen, 1.41% nitrogen, 0.85% iodine, and 0.41% sulfur.
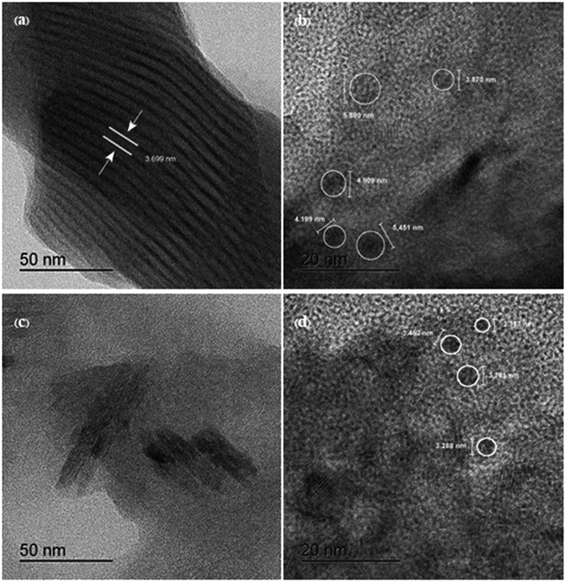 | ||
| Fig. 3 (a and b) High resolution transmission electron microscopy (HR-TEM) images of I-GQD and (c and d) HR-TEM image of N,S,I-GQDs. | ||
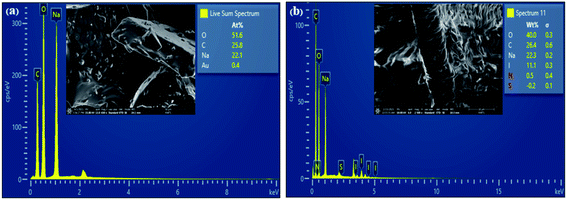
Energy-dispersive X-ray spectroscopy confirms the elemental compositions of GQDs and N,S,I-GQDs (EDX). The GQDs had the following elements: 51.6% O, 25.8% C, and 22.1% Na (Fig. 5a). For the N,S,I-GQDs, the compositional constituents were 40% O, 26.4% C, 22.3% Na, and 11.1% I (Fig. 5b). The Na peak in GQDs and N,S,I-GQDs is caused by the NaOH solution employed in the synthesis. According to the I-GQDs, C (0.28 keV) and O (0.53 keV) occurred while a faint signal of N (0.39 keV) overlapped in between C and O, resulting in a greater compositional element of C in N,S,I-GQDs than in GQDs.
3.2. Fluorescence and UV-visible absorption properties of N,S,I-GQDs
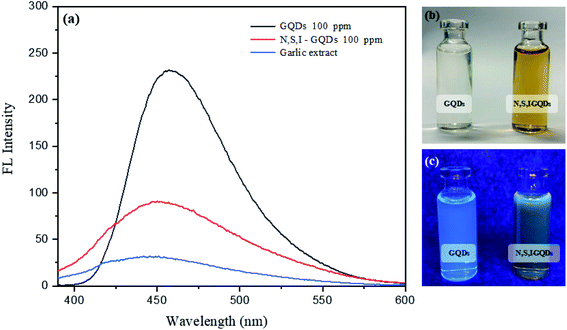
Fig. 6 shows the fluorescence spectra of I-GQDs, garlic extract, and N,S,I-GQDs, as well as a snapshot of both GQDs and N,S,I-GQDs solution under 365 nm UV light. The GQDs and N,S,I-GQDs aqueous solutions were compared under visible light (Fig. 6b) and emitted blue light under UV irradiation (Fig. 6c). As a result, the effective doping of garlic extract-I into GQDs was confirmed. The results demonstrated that the blue emission of the GQD solution was brighter than that of the N,S,I-GQDs solution.45 The UV-visible absorption spectra (dotted line) and fluorescence emission spectrum of N,S,I-GQDs (Fig. 7) reveal a comparable absorption band extending from 300 nm to 600 nm as reported previously for N-doped CDs.46 When stimulated at 365 nm, the N,S,I-GQDs exhibit a very strong fluorescence spectrum spanning the wavelength range of 375–600 nm, with a maximum wavelength of 455 nm (Fig. 7, red line). The N,S,I-GQDs fluorescence excitation spectrum shows a wide peak with a maximum wavelength of 365 nm (Fig. 7, dotted line).GQDs and garlic extract-(I-GQDs) quantum yields were estimated by comparing their integrated fluorescence intensities and absorbance values to those of quinine sulfate. The quantum yield of quinine standard solution in 0.1 M H2SO4 is 0.54. Table 1 summarizes the quantum yields of several doping materials. The fluorescence quantum yields of the GQDs and garlic extract-(I-GQDs) produced were found to be 31% and 45%, respectively. The quantum yield of garlic extract-(I-GQDs) is larger than that of undoped GQDs, showing that doping of both garlic extract and I on the GQDs surface resulted in a considerable increase in the fluorescence quantum yield of the GQDs. The quantum yield (Q) of N,S,I-GQDs was estimated using the equation: Q = QR × [m/mR] × [n2/nR2].47
| Material | Quantum yield (%) |
|---|---|
| Undoped GQDs | 31 |
| N,S,I-GQDs from 2% (v/v) garlic extract doped and 0.6% (w/v) KI/KIO3 doped | 45 |
| Quinine sulfate | 54 |
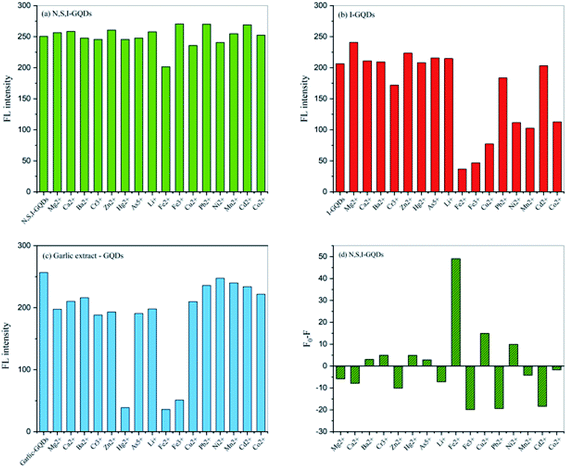
3.3. Effect of N,S,I-GQDs on the Fe2+–garlic extract complex
3.4. Effect of AgNO3 as selective masking agent for Fe3+
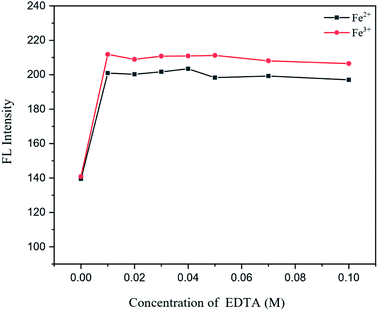
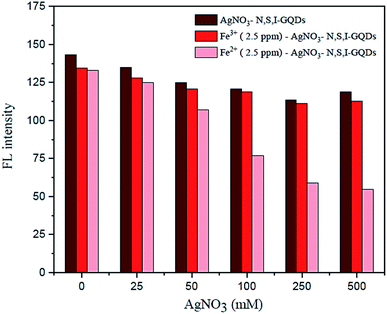
Under ideal conditions, the AgNO3 solution in the presence of garlic extract might be reduced to get a trace of silver nanoparticles, AgNPs. By adding AgNO3 to the sample solution, preferably to mask Fe3+, the fluorescence spectrum observations were utilized to plot the quenching calibration curve for Fe2+. According to the results, varying the quantity of AgNO3 from 100 to 500 M (Fig. 11) had no discernible effect on the fluorescence intensity of the reaction conditions. As a result, 100 M AgNO3 was utilized in subsequent studies.3.5. Effect of the garlic extract amount
Various concentrations of the garlic extract approximately spiked at 0.005, 0.01, 0.025, 0.05 and 0.1 mL were added into 100 mg L−1 of the N,S,I-GQDs 0.5 mgL−1 Fe2+ and 100 μM AgNO3. From the results (Fig. 12a and b), the fluorescence intensity and fluorescence spectra were recorded and plotted as F0–F were made comparison to suitable amount to induce the Fe2+–garlic extract (N,S–) complex formation on the surface of N,S,I-GQDs. The concentration of garlic extract was decided to be 0.01 mL based on the disclosed findings since Fig. 12b indicates the results of the experiment Fo-F at 0.01 mL greatest fluorescence intensity.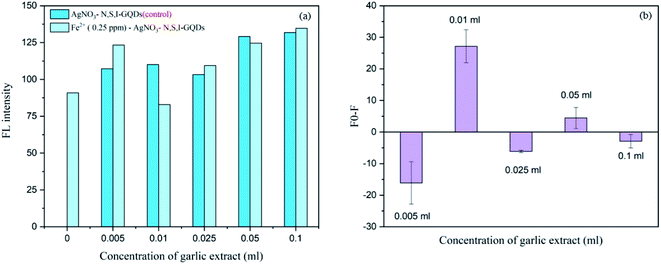 | ||
| Fig. 12 Effect of garlic extract amount on fluorescent intensity in the presence of 0.25 mg L−1 of Fe2+ containing 100 mgL−1 N,S,I-GQDs and 100 μM AgNO3 (at λex/λem = 365/455 nm slit width 5/5 nm). | ||
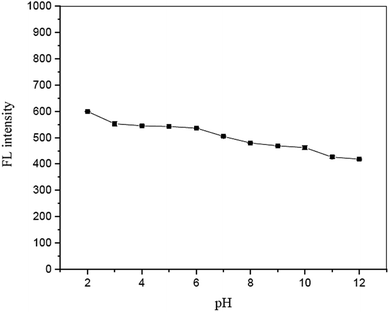
3.6. Effect of solution pH
The influence of solution pH on the fluorescence quenching of garlic extract-(I-GQDs) by Fe2+ was also investigated. The results show that a pH range of 8 to 10 (Fig. 13) had no effect on the fluorescence intensity of the N,S,I-GQDs. As a result, the pH 8 solution was chosen for future investigation.3.7. Effect of tolerant limit of Fe3+presence in the analysis of Fe2+
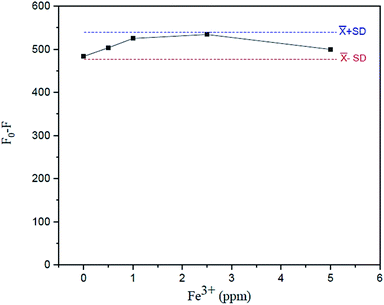
The effect of the Fe3+ tolerant limit on the measurement of Fe2+ in the sample solution using the suggested fluorescence quenching technique was investigated. The tolerating limit of Fe3+ is that to be regarded, for example, in drinking water (5 mg L−1 excess Fe3+ can be tolerated) for the measurement of Fe2+ was thoroughly explored by adding their known amounts (0–5 mgL−1 Fe3+), as shown in Fig. 14. It was discovered that their fluorescence signals constantly varied within an acceptable standard deviation of the mean values of F0–F versus Fe3+ concentrations.3.8. Analytical features of the proposed fluorescence quenching sensor
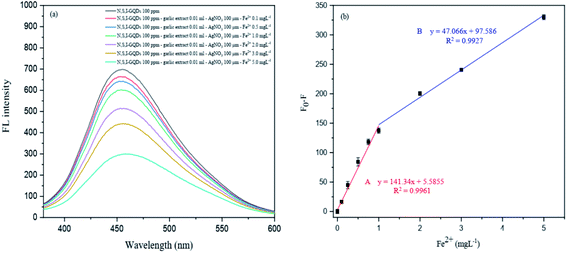
This created approach was tested for quantitative applications such as linearity, detection limit (LOD), quantification limit (LOQ), precision (% RSD), and accuracy to evaluate the fluorescence quenching sensor for Fe2+ detection (% recovery) (Fig. 15).| Linear curve | Linear equation | R2 | LOD (mg L−1) | LOQ (mg L−1) |
|---|---|---|---|---|
| A | y = 141.34x + 5.5855 | 0.9961 | 0.11 | 0.35 |
| B | y = 47.066x + 97.586 | 0.9954 | 0.32 | 1.07 |
| Material | Technique | LOD | Response time | Ref. |
|---|---|---|---|---|
| a LOD 0.11 mg L−1 = 0.4 μM, linear range 0.1–5 mg L−1 = 0.36–17.98 μM. | ||||
| HMFluNox, HMRhoNox | Fluorescence | 10 μM | 60 min | 52 |
| Dicyanomethylene-4H-pyran and N-oxide (DCM-Fe) | NIR-fluorescence | 4.5 μM | 15 min | 53 |
| Acylated hydroxylamine moiety into the naphthalimide fluorophore | Fluorescence | 0.5 μM | 15 min | 54 |
| Cou-T and Rh-T | Fluorescence | 0.75 μM | 30 min | 55 |
| RhoNox-1 | Fluorescence | 0.2 μM | 60 min | 56 |
| C20H21BrNP, HATU, DIPEA, DMF, RT | Fluorescence | 1.03 μM | — | 57 |
| N,S,I-GQDs | Fluorescence | a0.4 μM | 20 min | This work |
| Calibration no. | Intra-day analysis (n = 3) | Inter-day analysis (n = 5) | ||
|---|---|---|---|---|
| Linear | Linear | |||
| A | B | A | B | |
| RSD (%) | 1.04 | 1.05 | 3.23 | 2.72 |
| Parameter | Analytical data |
|---|---|
| pH value (ammonium buffer solution) | 8.0 |
| Amount of garlic extract doped in GQDs (%) | 2.0 |
| N,S,I-GQDs concentration (mg L−1) | 100 |
| Amount of garlic extract (μL) | 10 |
| AgNO3 concentration (μM) | 100 |
| Reaction time under ultrasound assisted (min) | 20 |
3.9. Real sample analysis
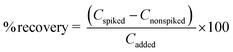
Under optimal conditions, the suggested fluorescence quenching sensor was used to determine the iron(II) ion in various sample matrices such as drinking water and tap water. The method's correctness was confirmed by calculating the recovery study in these water samples. Each sample was spiked with three different doses of the standard Fe2+ solution (10, 25, and 50 mg L−1). The relative recoveries were then computed as follows:where Cfound, Creal and Cadded denote the concentration of analyte in the real sample after addition of the known amount of standard, the concentration of an analyte in the real sample, and the concentration of the known amount of standard that was spiked in the real sample, respectively, as shown in Table 6.
| Sample | Fe2+ added (mg L−1) | Fe2+ found (mg L−1) | Recovery (%) |
|---|---|---|---|
| Drinking water 1 | — | 0.04 ± 11.25 | — |
| 10 | 10.01 ± 3.59 | 99.46 | |
| 25 | 22.34 ± 5.83 | 89.16 | |
| 50 | 51.00 ± 2.01 | 101.90 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Drinking water 2 | — | 0.22 ± 8.01 | — |
| 10 | 9.97 ± 10.46 | 101.96 | |
| 25 | 27.76 ± 10.28 | 111.94 | |
| 50 | 48.97 ± 8.27 | 98.38 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Drinking water 3 | — | 0.38 ± 8.01 | — |
| 10 | 9.96 ± 10.46 | 103.43 | |
| 25 | 29.50 ± 10.28 | 119.52 | |
| 50 | 48.31 ± 8.27 | 97.40 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Drinking water 4 | — | 0.30 ± 0.60 | — |
| 10 | 9.97 ± 8.26 | 102.76 | |
| 25 | 29.84 ± 4.19 | 121.45 | |
| 50 | 48.18 ± 2.85 | 97.68 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Drinking water 5 | — | 0.28 ± 4.73 | — |
| 10 | 9.97 ± 7.50 | 102.46 | |
| 25 | 29.85 ± 9.44 | 120.50 | |
| 50 | 48.19 ± 6.18 | 96.93 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Tap water 1 | — | 0.16 ± 3.28 | — |
| 10 | 9.98 ± 5.91 | 101.46 | |
| 25 | 29.93 ± 2.42 | 119.93 | |
| 50 | 48.09 ± 7.39 | 96.16 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Tap water 2 | — | 0.08 ± 9.99 | — |
| 10 | 9.99 ± 1.14 | 100.70 | |
| 25 | 29.50 ± 2.81 | 118.32 | |
| 50 | 48.31 ± 0.58 | 96.78 | |
4. Conclusion
The goal of this research was to use pyrolysis to create graphene quantum dots doped with garlic extract and concurrently co-doped with iodine. XPS, HR-TEM, FE-SEM/EDX, FT-IR, fluorescence, and UV-visible absorption spectroscopy were used to characterize and analyze the N,S,I-GQDs. When compared to undoped GQDs (31%), the quantum yield of N,S,I-GQDs was found to be 45%. When excited at 363 nm, the N,S,I-GQDs display strong fluorescence intensity at a maximum wavelength of 454 nm. Using N,S,I-GQDs as a fluorescence quenching sensor for screening tests with various metal ions, it was discovered that Fe2+ is extremely selective over Fe3+ and others. Thus, solution pH, concentration of N,S,I-GQDs, quantity of garlic extract, EDTA and AgNO3 concentration as masking agents, reaction duration under ultrasonic aided, and tolerable limit of Fe3+ presence in the target analyte were all optimized for Fe2+ detection. The suggested method's analytical properties were unquestionably proven, particularly with two successive working ranges of their calibration curves. The technique precision, given as RSDs, was found to be satisfactory, and therefore good accuracy was reached by utilizing spiked Fe2+ into drinking water and tap water samples in the recovery research. As a result, the selective measurement of trace Fe2+ in actual water samples employing N,S,I-GQDs linked with garlic extract as a green chelating agent to aid raise its improved sensitivity was effective.Conflicts of interest
The authors have declared no conflict of interest.Acknowledgements
The authors thank Materials Chemistry Research Center (MCRC), Department of Chemistry, Faculty of Science, and Center of Excellence for Innovation in Chemistry (PERCH-CIC), Khon Kaen University, Khon Kaen, Thailand for financial support. We are grateful for the support received from Thailand Institute of Nuclear Technology (Public Organization) and the FE-SEM center, School of Engineering, King Mongkut's Institute of Technology Ladkrabang, Bangkok.References
- A. A. Tariq, I. M. Mohamed, M. A. M. Gaber, A. Mohammed, M. H. H. Mahmoud, K. Tushar, S. Ahmed and M. A. El-Bindary, Anal. Chim. Acta, 2021, 1180, 331180 Search PubMed.
- H. W. Matthias, M. U. Martina, G. Bruno and C. Clara, Cell, 2010, 142, 24–38 CrossRef PubMed.
- C. Clara, N. Antonella and S. Laura, Haematologica, 2020, 105, 1–13 CrossRef PubMed.
- B. Halliwell and J. M. Gutteridge, FEBS Lett., 1992, 307, 108–112 CrossRef CAS PubMed.
- T. Samarina and M. Proskurnin, F1000Research, 2015, 4, 623 Search PubMed.
- S. V. Torti and F. M. Torti, Nat. Rev. Cancer, 2013, 13, 342–355 CrossRef CAS PubMed.
- E. C. Theil and D. J. Goss, Chem. Rev., 2099, 109, 4568–4579 CrossRef PubMed.
- J. P. Riley and R. Chester, Limnol. Oceanogr., 1971, 16, 1006–1007 Search PubMed.
- E. Madsen and J. D. Gitlin, Annu. Rev. Neurosci., 2007, 30, 317–337 CrossRef CAS PubMed.
- S. Toyokuni, Cancer Sci., 2009, 100, 9–16 CrossRef CAS PubMed.
- L. Kai and R. Heinz, J. Neural., 2016, 123, 389–399 Search PubMed.
- World Health Organization [WHO], Water safety plans in WHO Guidelines for Drinking Water Quality, 2011, retrieved December 8, 2021, from, http://www.whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf Search PubMed.
- Y. Chen, Y. Huang, S. Feng and D. Yuan, Anal. Methods, 2015, 7, 4971–4978 RSC.
- P. Bermejo, E. Pena, R. Dom inguez, A. Bermejo, J. M. Fraga and J. A. Cocho, Talanta, 2000, 50, 1211–1222 CrossRef CAS PubMed.
- X. P. Yan, M. J. Hendry and R. Kerrich, Anal. Chem., 2000, 72, 1879–1884 CrossRef CAS PubMed.
- Y. H. He, J. P. Lai, H. Sun, Z. M. Chen and S. Lan, Sens. Actuators, B, 2016, 225, 405–412 CrossRef CAS.
- M. J. Poursharifi and A. Moghimi, Asian J. Chem., 2009, 21, 2533–2540 CAS.
- G. Özzeybek, B. Alacakoç, M. Y. Kocabaş, E. G. Bakırdere, D. S. Chormey and S. Bakırdere, Environ. Monit. Assess., 2018, 190, 498 CrossRef PubMed.
- M. Fırat and E. G. Bakırdere, Environ. Monit. Assess., 2018, 191, 129 CrossRef PubMed.
- R. V. Khose, P. Bangde, M. P. Bondarde, P. S. Dhumal, M. A. Bhakare, G. Chakraborty, A. K. Ray, P. Dandekar and S. Some, Spectrochim. Acta, Part A, 2022, 266, 120453 CrossRef CAS PubMed.
- X. Yan, Y. Song, C. Zhu, J. Song, D. Du, X. Su and Y. Lin, ACS Appl. Mater. Interfaces, 2016, 8, 21990–21996 CrossRef CAS PubMed.
- R. V. Khose, G. Chakraborty, M. P. Bondarde, P. H. Wadekar, A. K. Ray and S. Some, New J. Chem., 2021, 45, 4617–4625 RSC.
- N. Pimsin, N. Kongsanan, C. Keawprom, P. Sricharoen, P. Nuengmatcha, W. C. Oh, Y. Areerob, S. Chanthai and N. Limchoowong, ACS Omega, 2021, 6, 14796–14805 CrossRef CAS PubMed.
- N. Kongsanan, N. Pimsin, C. Keawprom, P. Sricharoen, Y. Areerob, P. Nuengmatcha, W. C. Oh, S. Chanthai and N. Limchoowong, ACS Omega, 2021, 6, 14379–14393 CrossRef CAS PubMed.
- C. Sakaew, P. Sricharoen, N. Limchoowong, P. Nuengmatcha, C. Kukusamude, S. Kongsri and S. Chanthai, RSC Adv., 2020, 10, 20638–20645 RSC.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2015, 44, 362–381 RSC.
- A. C. Varun, K. Rajnish, K. Naveen, D. Akash, R. Changanamkandath and K. H. Kim, RSC Adv., 2018, 8, 11446–11454 RSC.
- Y. Lei, Y. Wu, Z. Jiang, Z. Ouyang, J. Hu, Y. Lin, P. Du and B. Zou, Mater. Sci. Semicond. Process., 2021, 128, 105740 CrossRef CAS.
- H. Tetsuka, A. Nagoya and S. I. Tamura, Nanoscale, 2016, 47, 19677–19683 RSC.
- G. Haider, P. Roy, C. W. Chiang, W. C. Tan, Y. R. Liou, H. T. Chang, C. T. Liang, W. H. Shih and Y. F. Chen, Adv. Funct. Mater., 2016, 4, 620–628 CrossRef.
- I. Malgorzata, K. Inga and W. Lidia, Environ. Mol. Mutagen., 2009, 50, 247–265 CrossRef PubMed.
- B. B. Aggarwal and S. Shishodia, Ann. N. Y. Acad. Sci., 2004, 1030, 434–441 CrossRef CAS PubMed.
- M. S. Butt, M. T. Sultan and J. Iqbal, Crit. Rev. Food Sci. Nutr., 2009, 49, 538–551 CrossRef CAS PubMed.
- H. J. Park, B. T. Jeon, H. C. Kim, G. S. Roh, J. H. Shin, N. J. Sung, J. Han and D. Kang, Acta Physiol., 2012, 205, 61–70 CrossRef CAS PubMed.
- G. E. S. Batiha, A. M. Beshbishy, L. G. Wasef, Y. H. A. Elewa, A. A. Sagan, M. E. A. El-Hack, A. E. Taha, Y. M. Abd-Elhakim and H. P. Devkota, Nutrients, 2020, 12, 872 CrossRef CAS PubMed.
- N. Ide and B. H. S. Lau, J. Nutr., 2001, 131, 1020S–1026S CrossRef CAS PubMed.
- J. H. Ryu, H. J. Park, Y. Y. Jeong, S. Han, J. H. Shin, S. J. Lee, M. J. Kang, N. J. Sung and D. Kang, J. Med. Food, 2015, 18, 439–445 CrossRef CAS PubMed.
- X. Wang, X. Sun, J. Lao, H. He, T. Cheng, M. Wang, S. Wang and F. Huang, Colloids Surf., B, 2014, 122, 638–644 CrossRef CAS PubMed.
- R. El-Hnayn, L. Canabady-Rochelle, C. Desmarets, L. Balan, H. Rinnert, O. Joubert, G. Medjahdi, H. B. Ouada and R. Schneider, Nanomaterials, 2020, 10, 104 CrossRef CAS PubMed.
- C. Yang, R. Ogaki, L. Hansen, J. Kjems and B. M. Teo, RSC Adv., 2015, 5, 97836 RSC.
- A. Aumber, A. T. Tanveer, J. B. Steve, M. L. Tuti and N. P. Anh, Sci. Rep., 2020, 10, 21262 CrossRef PubMed.
- H. Saswata, K. Amit, M. Noa and L. Uriel, J. Phys. Chem. C, 2021, 125, 15134–15144 CrossRef.
- M. Mojtaba and N. G. Jaber, Res. Chem. Intermed., 2018, 44, 3641–3657 CrossRef.
- S. Zhao, M. Lan, X. Zhu, H. Xue, T. Wai Ng, X. Meng, C. S. Lee, P. Wang and W. Zhang, ACS Appl. Mater. Interfaces, 2015, 31, 17054–17060 CrossRef PubMed.
- C. Kaewprom, P. Sricharoen, N. Limchoowong, P. Nuengmatcha and S. Chanthai, Spectrochim. Acta, Part A, 2019, 207, 79–87 CrossRef CAS.
- Z. L. Wua, P. Zhangb, M. X. Gaoa, C. F. Liua, W. Wangb, F. Lenga and C. Z. Huang, J. Mater. Chem. B, 2013, 22, 2868–2873 RSC.
- C. Kaewprom, Y. Areerob, W. C. Oh, K. L. Ameta and S. Chanthai, Arabian J. Chem., 2020, 13, 3714–3723 CrossRef CAS.
- P. Siyal, A. Nafady, S. juddin, R. Memon, S. T. H. Sherazi, J. Nisar, A. A. Siyal, M. R. Shah, S. A. Mahesar and S. Bhagat, , Spectrochim. Acta A., 2021, 254, 119645 CrossRef CAS PubMed.
- P. Sricharoen, N. Limchoowong, P. Nuengmatcha and S. Chanthai, Ultrason. Sonochem., 2020, 63, 104966 CrossRef CAS PubMed.
- O. A. Al-Khashman, H. M. Alnawafleh, A. M. A. Jrai and A. H. Al-Muhtaseb, Open J. Mod. Hydrol., 2017, 7, 331–349 CrossRef CAS.
- World Health Organization [WHO], Guidelines for Drinking-Water Quality, https://www.who.int/publications/i/item/9789241549950, accessed December 9, 2021 Search PubMed.
- M. Niwa, T. Hirayama, K. Okuda and H. Nagasawa, Org. Biomol. Chem., 2014, 12, 6590–6597 RSC.
- X. Yang, Y. Wang, R. Liu, Y. Zhang, J. Tang, E. B. Yang, D. Zhang, Y. Zhao and Y. Ye, Sens. Actuators, B, 2019, 288, 217–224 CrossRef CAS.
- W. Xuan, R. Pan, Y. Wei, Y. Cao, H. Li, F. S. Liang, K. J. Liu and W. Wang, Bioconjugate Chem., 2016, 27, 302–308 CrossRef CAS PubMed.
- S. Maiti, Z. Aydin, Y. Zhang and M. Guo, Dalton Trans., 2015, 44, 8942–8949 RSC.
- T. Hirayama, K. Okuda and H. Nagasawa, Chem. Sci., 2013, 4, 1250–1256 RSC.
- S. Khatun, S. Biswas, A. Binoy, A. Podder, N. Mishra and S. Bhuniya, J. Photochem. Photobiol., B, 2020, 209, 111943 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |


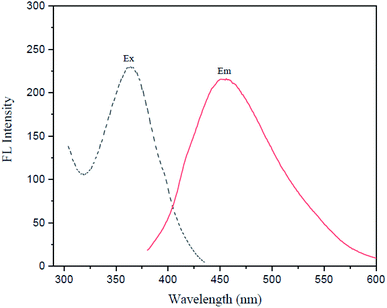
![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ) and fluorescence spectra (—) of N,S,I-GQDs;
) and fluorescence spectra (—) of N,S,I-GQDs;