Open Access Article
Open Access ArticleRecent advances in the application of magnetic bio-polymers as catalysts in multicomponent reactions
Zohreh Kheilkordi a,
Ghodsi Mohammadi Ziarani
a,
Ghodsi Mohammadi Ziarani *a,
Fatemeh mohajer
*a,
Fatemeh mohajer a,
Alireaza Badiei
a,
Alireaza Badiei b and
Mika Sillanpääcde
b and
Mika Sillanpääcde
aDepartment of Chemistry, Faculty of Physics and Chemistry, Alzahra University, Tehran, Iran 1993893979. E-mail: gmohammadi@alzahra.ac.ir; Fax: +98 2188613937; Tel: +98 2188613937
bSchool of Chemistry, College of Science, University of Tehran, Tehran, Iran
cDepartment of Chemical Engineering, School of Mining, Metallurgy and Chemical Engineering, University of Johannesburg, P. O. Box 17011, Doornfontein 2028, South Africa. E-mail: Mika.Sillanpaa@uef.fi
dDepartment of Applied Physics, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia
eInternational Research Centre of Nanotechnology for Himalayan Sustainability (IRCNHS), Shoolini University, Solan, 173212, Himachal Pradesh, India
First published on 26th April 2022
Abstract
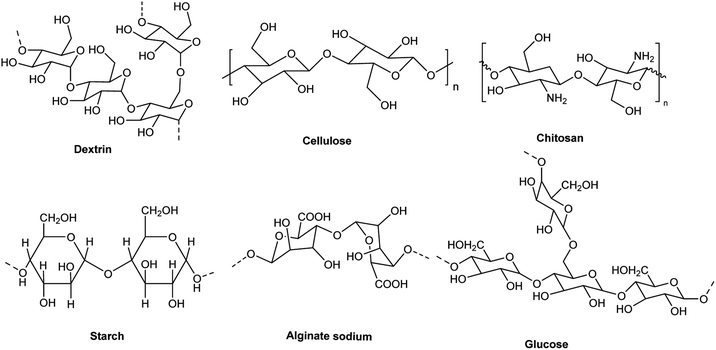
Magnetic nanoparticles have attracted significant attention due to their high surface area and superparamagnetic properties. Bio-polymers composed of polysaccharides including alginate, cellulose, glucose, dextrin, chitosan, and starch can be immobilized on magnetic nanoparticles. Bio-polymers can be obtained from natural sources, such as plants, tunicates, algae, and bacteria. Bio-polymers obtained from natural sources have attracted attention due to their various properties including efficient functional groups, non-toxicity, low cost, availability, and biocompatibility. According to the targets of “green chemistry”, the application of bio-polymers is effective in reducing pollution. Furthermore, they are excellent agents for the functionalization of magnetic nanoparticles to yield nanomagnetic bio-polymers, which can be applied as recoverable and eco-friendly catalysts in multicomponent reactions.
1. Introduction
Among the various magnetic nanoparticles, nano magnetic iron oxide (Fe3O4) is very important because of its low cost, easy synthesis, and high magnetic ability. Recently, magnetic nanoparticles have been extensively applied in various fields including drug delivery, sensing, water treatment, removal of heavy metals, and catalysis. However, they are unstable in alkaline and acidic media due to the easy oxidation of their surface area.1–8 These drawbacks can be alleviated via the modification of Fe3O4 nanoparticles with materials such as silanes,9,10 activated carbon,11 and biocompatible polymers.12,13 Another class of magnetic nanoparticles is ferrite magnetic nanomaterials and hexaferrite (M-type). Ferrite magnetic nanomaterials and hexaferrite have various applications including high chemical strength materials, home appliances, supercapacitors, loudspeakers, electromagnetic wave absorption, and permanent magnets.14–18 Overall, due to the properties of ferrite magnetic nanomaterials and hexaferrite (M-type), they can be used for the adsorption of various metals ions, cationic and anionic dyes from wastewater.19–23The design and synthesis of biocompatible magnetic nanoparticles are important subjects in green chemistry.24 Bio-polymers including alginate, cellulose, glucose, dextrin, chitosan, and starch are known as polysaccharides, which are present in the carbohydrates in plants, animals, microbes, and algae (Fig. 1).24 These bio-polymers have different properties such as biodegradable nature, biocompatibility, non-toxicity, availability, low cost, and heat resistantance.25 They are excellent agents for the functionalization of magnetic nanoparticles to yield nanomagnetic bio-polymers, promoting their longevity, hardness, and strength. These composites have many applications such as drug delivery,26–28 chemotherapy,29,30 magnetic resonance imaging (MRI) agents,31,32 solar cells,33 chemical sensors,34,35 catalysts,36 water treatment,37,38 and biomedical sensors.39
In comparison to conventional catalysts, heterogeneous magnetic bio-polymers have various advantages such as non-toxicity, easy separation, and eco-friendly nature. According to the functional groups on the magnetic nanoparticles, the catalysts can be grouped in various categories including, Lewis and Brønsted acids and bases.40,41 In continuation of our research,42–44 in this review, the importance of nanomagnetic bio-polymers is studied in multicomponent reactions. Multi-component reactions (MCRs) are essential tools in medicinal and organic chemistry, which have various advantages including simplicity, easy workup, availability, and reduced generation of waste. Hence, the design and application of MCRs for the synthesis of organic compounds are highly important.45–50
2. Synthesis of various magnetic bio polymers
2.1. Magnetic bio-polymers based on cellulose
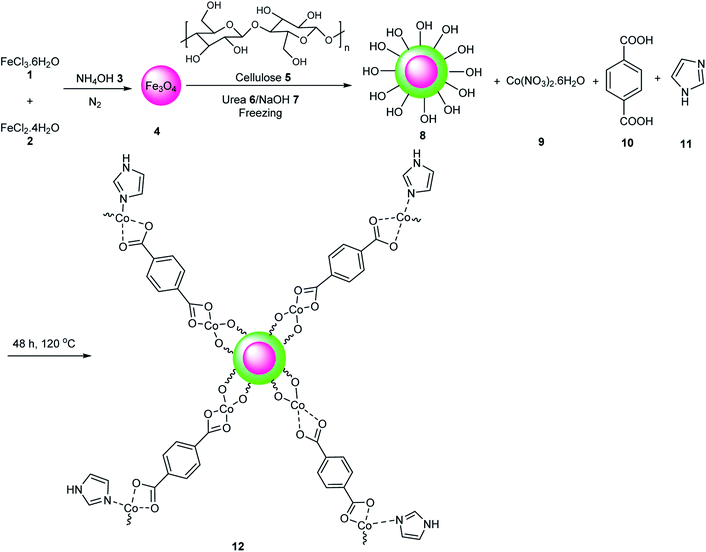
Fe3O4/cellulose/Co-MOF 12 has two sites including Lewis acidic sites (Co2+) and basic sites (IM), which were used in the Knoevenagel condensation reaction of aromatic aldehydes 13 with malononitrile 14 under solvent-free conditions (Scheme 2). This catalyst was reused five times without a decrease its catalytic activity.51
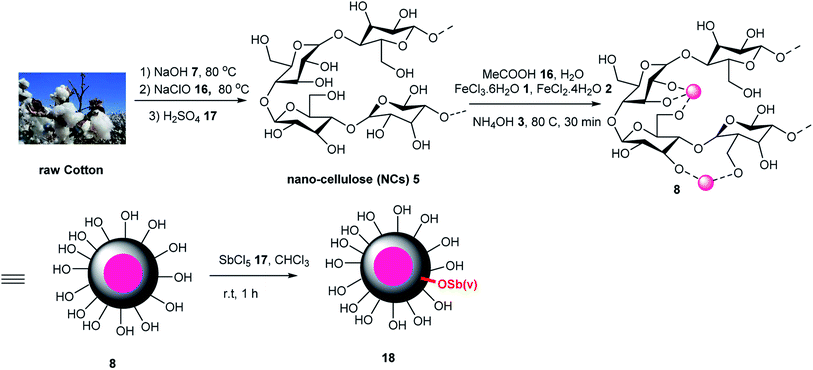
Fe3O4@NCs/Sb(V) was used as a Lewis acid for the activation of carbonyl groups, which was investigated in the synthesis of 4H-pyrimido[2,1-b]benzothiazoles 22 via the three-component reaction of aldehydes 19, 2-aminobenzothiazole 20, and ethylacetoacetate 21 under solvent-free conditions at 90 °C (Scheme 4). This catalyst was used five times without loss in its catalytic activity, which was compared with Fe3O4 with 39% yield in 3 h.52
Its catalytic activity was useful for the synthesis of 4H-pyrans via the multicomponent reaction of aldehydes, ethylacetoacetate, and malononitrile in H2O (Scheme 6).53
Also, the catalytic activity of 26 was tested for the synthesis of pyranopyrazole derivatives 29 via the four-component reaction of hydrazine hydrate 28, ethyl acetoacetate 21, benzaldehyde 19, and malononitrile 14 in H2O (Scheme 7). The metal ions on the catalyst surface act as Lewis acids, which were activated by the malononitrile and carbonyl groups. Also, the catalyst was used five times in the model reaction without a reduction in activity.53
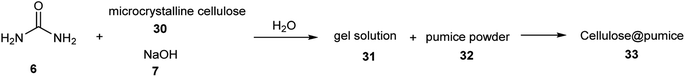
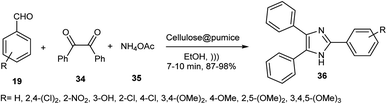
Cellulose@pumice as an acidic catalyst activated carbonyl groups in the synthesis of 2,4,5-triarylimidazoles 36 through the reaction of benzaldehyde 19 and ammonium acetate 35 in EtOH under ultrasonic irradiation (Scheme 9). In the reusability test, this catalyst was used ten times without a reduction in activity.54
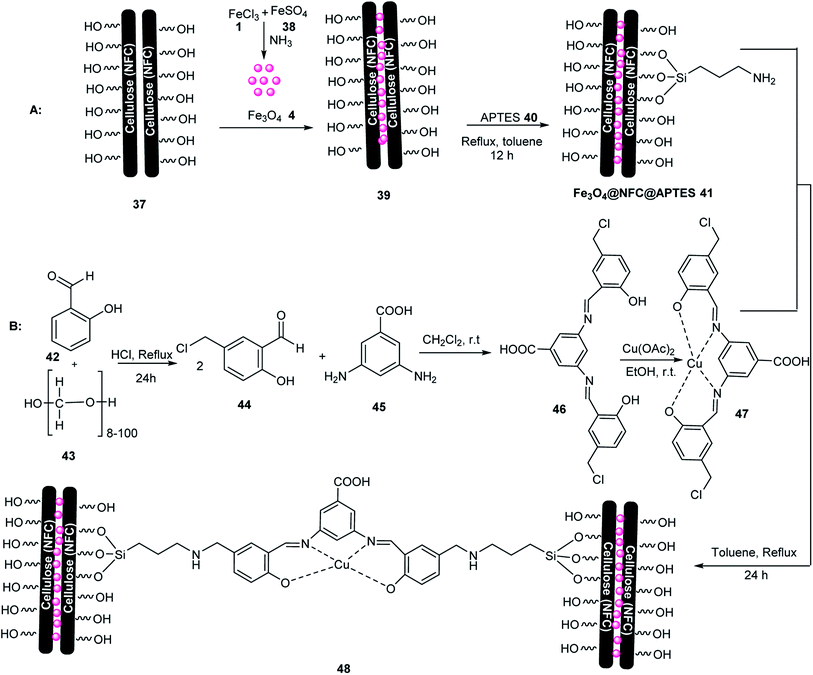
(Fe3O4@NFC@NSalophCu)CO2H 48 was tested in the synthesis of 5-substituted-1H-tetrazole 51 via the multicomponent reaction of various aldehydes 19, hydroxylamine 49, and sodium azide 50 (Scheme 11), and also in the synthesis of 1-substituted-1H-tetrazoles 54 via the multicomponent reaction of aniline 52, triethyl orthoformate 53, and sodium azide 50 (Scheme 12). This catalyst was used in the click reaction four times without a decrease in its activity. In this catalyst, copper metal plays a vital role in the click reactions.55
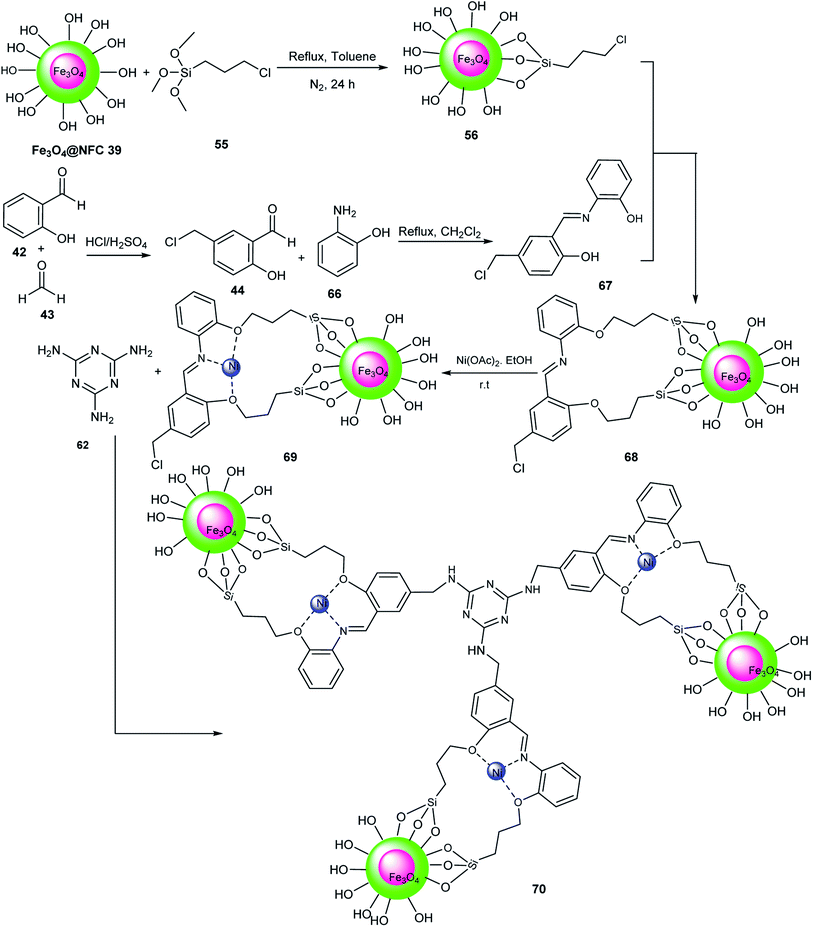
The activity of Fe3O4@NFC@NNSM-Mn(III) 63 was tested for the synthesis of xanthenes 65 via the pseudo-three-component reaction of aldehydes 19 and dimedone 64 in EtOH at 45 °C (Scheme 14). The manganese metal on the surface of Fe3O4@NFC@NNSM-Mn(III) acts as a Lewis acid to activate the carbonyl groups. This catalyst was used five times without a decrease in its activity.56
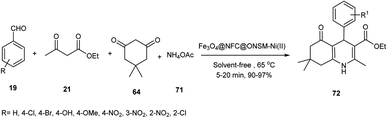
This catalyst was used in the synthesis of polyhydroquinolines 72 through the Hantzsch reaction among benzaldehydes 19, dimedone 64, ethyl acetoacetate 21, and ammonium acetate 71 (Scheme 16).57
Also, Bagherzade et al. applied this catalyst in the synthesis of 1,4-dihydropyridine 72 via the multicomponent reaction of aldehydes 19, ethyl acetoacetate 21, and ammonium acetate 71 (Scheme 17). The carbonyl groups in the multi-component reaction were activated in the presence of nickel metal on the surface of Fe3O4@NFC@ONSM-Ni(II) as a Lewis acid. Also, this catalyst was tested 6 times without loss in its activity.57
Cell-LA-TEA+/Fe3O4 was used as a catalyst for the regioselective synthesis of pyrazolo quinolones 82 via the three-component reaction of dimedone 64, 5-amino pyrazolone 81, and aromatic aldehydes 19 in EtOH/H2O under ultrasonic irradiation (Scheme 19). The catalyst was used with high stability in 7 cycles without loss in its activity.58
2,3-Dihydroquinazolin-4(1H)-ones 85 were prepared via the condensation reaction of 2-aminobenzamide 84 and aldehydes 19 in the presence of Fe3O4@NCS-PA 83 as a Brønsted acid in H2O: EtOH under reflux conditions (Scheme 21). The main advantages of this method are its excellent yields, simple workup, and eco-friendly catalyst. This reaction was accomplished in H2O: EtOH under reflux conditions in the presence of Fe3O4 with 60% yield; however, Fe3O4@NCS-PA performed better than Fe3O4 in this reaction.59
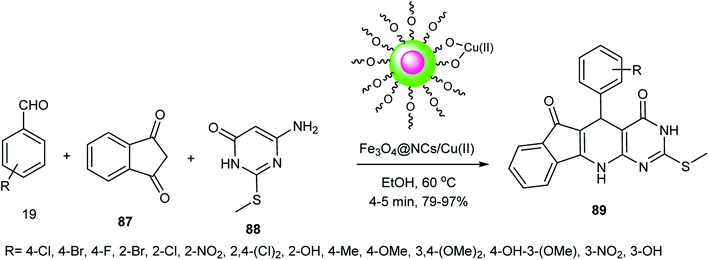
Fe3O4@NCs/Cu(II) as a Lewis acid activated the carbonyl groups of the starting materials by copper metal in the synthesis of indenopyrido[2,3-d]pyrimidines 89 through the three-component reaction of 6-amino-2-(methylthio)pyrimidin-4(3H)-one 88, 1,3-indanedione 87 and aldehydes 19 in EtOH (Scheme 23). The activity of the catalyst was preserved after four runs.60
Cu(II) in Fe3O4@nanocellulose/Cu(II), as a Lewis acid, activated the carbonyl groups in the three-component reaction of aromatic aldehydes 19, 2-aminobenzothiazole 20, and ethyl acetoacetate 21 for the synthesis of 4H-pyrimido[2,1-b]benzothiazoles 22 (Scheme 25). The catalyst activity of Fe3O4@nano-cellulose/Cu(II) was preserved after four runs. The yield of this reaction in the presence of Fe3O4 as a catalyst after 3 h was reported to be about 37%. Therefore, Fe3O4@nano-cellulose/Cu(II) is more active than Fe3O4.61
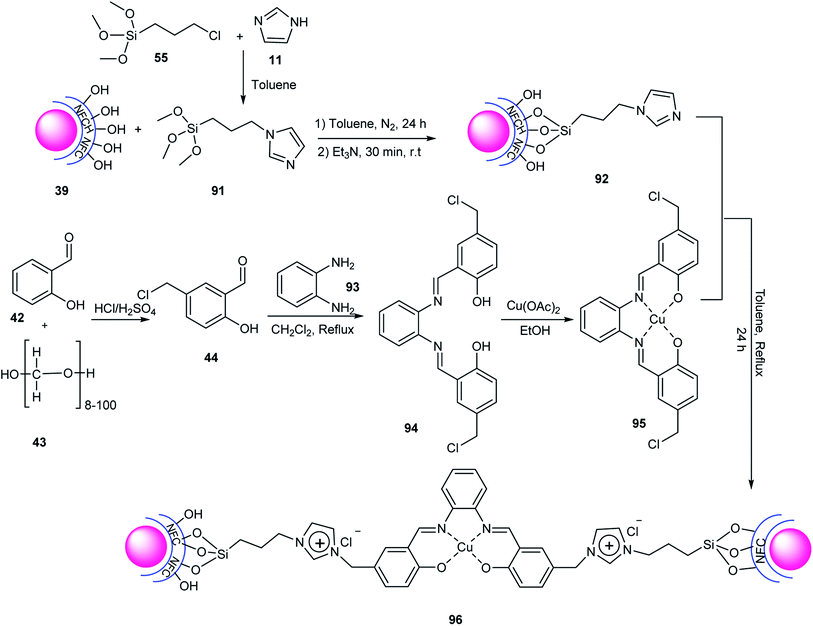 | ||
| Scheme 26 Synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives using Fe3O4@NFC-ImSalophCu(II) 96. | ||
The copper of Fe3O4@NFC-ImSalophCu 96 was applied in the click reaction of phenacyl bromides 97, sodium azide 90, and alkynes 98 in H2O to synthesize 1,2,3-triazoles 99 (Scheme 27). The reusability of this catalyst was tested in the click reaction four times without loss in its activity.62
Its catalytic activity was explored using 2-aryl/alkyl-2,3-dihydro-1H-naphtho[1,2-e][1,3]oxazines 102 or 104 or 106 via the pseudo-three-component reaction of β-naphthol 101 or α-naphthol 103 or phenol derivatives 105, formaldehyde 52, and various amines 43 (Scheme 29). The catalytic activity of nano-Fe3O4@walnut shell/Cu did not decrease after five-times use. The copper metal on the nano-Fe3O4@walnut shell/Cu(II) increased the reaction rate via interaction with the carbonyl group of the starting materials.63
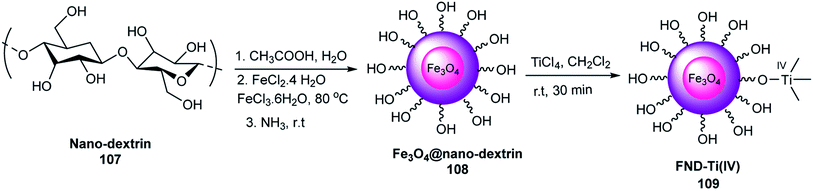
2.2. Magnetic bio-polymers based on dextrin
The magnetic dextrin was tested as a catalyst in the synthesis of polyhydroquinolines via four-component reactions of aromatic aldehydes 19, ethyl acetoacetate 21, dimedone 61, ammonium acetate 71 in EtOH under reflux conditions (Scheme 31). The yield of the products did not decrease after five runs. This reaction was performed in the presence of dextrin in ethanol in 28% yield, and thus magnetic dextrin is better than dextrin to catalyze this reaction.64
Maleki et al. studied the catalytic activity of magnetized dextrin 29 for the synthesis of dihydropyrano[2,3-c]pyrazoles 29 through the reaction of hydrazine hydrate 28, ethyl acetoacetate 21, aromatic aldehydes 19, and malononitrile 14 under reflux conditions in EtOH (Scheme 33). This catalyst was used for five runs without a decrease in its catalytic activity.65
Its catalytic activity as a Lewis acid was investigated in the synthesis of 2,3-dihydroquinazolin-4(1H)-ones 85 via the condensation reaction of 2-aminobenzamide 84 and aldehydes 19 under mild conditions (Scheme 35). This catalyst was used five times without loss in its catalytic activity.66
2.3. Magnetic bio-polymers based on starch
The catalytic activity of Ag/Fe3O4@starch as Lewis acid 115 was evaluated in the one-pot reaction of benzaldehydes 19, malononitrile 14, and dimedone 64 in EtOH for the synthesis of 4H-pyran 116 (Scheme 37). Also, the magnetic nanocatalyst was reused five times with no loss in its activities.67
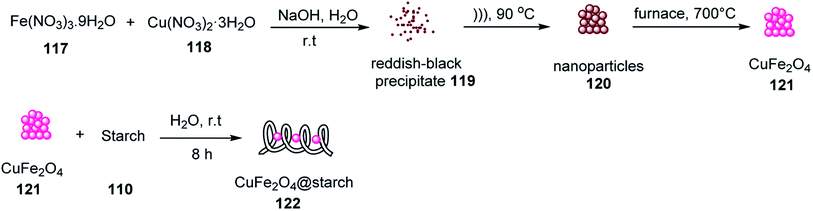
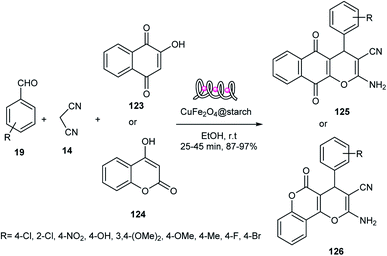
In another attempt, 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-benzo[b]pyrans 27 or 116 were synthesized via the reaction of aldehydes 19, malononitrile 14, and enolizable C–H-activated acidic compounds, including dimedone 64 and ethyl acetoacetate 21, in the presence of CuFe2O4@starch 122 as a Lewis acid in EtOH. The reaction rate for aldehydes with electron-withdrawing groups was faster than that with electron-donating groups (Scheme 39 and 40), respectively. This catalyst was used in six runs without a decrease in its activity.68
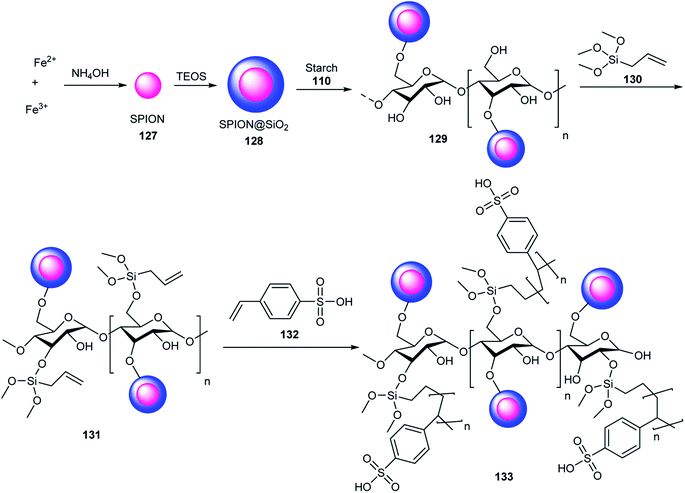
Starch/SPION@SO3H 133 as a Brønsted acid (SO3H) activated the carbonyl groups in the multicomponent reaction of 4-hydroxycoumarin 124, benzaldehydes 19, dimedone 64, and ammonium acetate 71 in the synthesis of chromeno[4,3-b]quinoline-6,8(9H)-dione derivatives 134 (Scheme 42). This catalyst was applied ten times without any loss in its activity.69
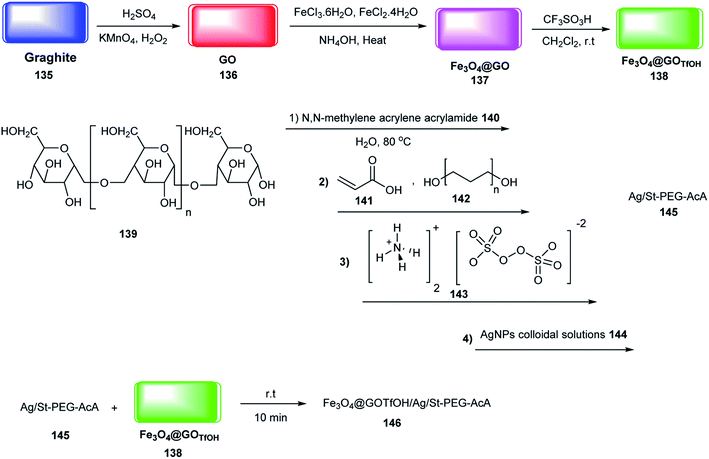
The catalytic activity of Fe3O4@GOTfOH/Ag/St-PEG-AcA 146 was examined in the synthesis of 2,4,6-triarylpyridines 137 through the pseudo-three-component reaction of aryl aldehydes 19, acetophenone 147 and ammonium acetate 71 (Scheme 44). According to the catalyst structure, the carbonyl groups were activated via interaction with the Brønsted acid site and Lewis acid of Fe3+. Also, the catalytic activity of 146 did not decrease after 10 runs.70
γ-Fe2O3@starch-n-butylSO3H 152 as Brønsted acid activated the carbonyl groups in the multicomponent reactions of aldehydes 19, dimedone 64, and 2-aminobenzimidazole 153 or phthalhydrazide 154 to synthesize tetrahydrobenzimidazo[2,1-b]quinazolin-1(2H)-ones 155 or 2H-indazolo[2,1-b]phthalazine-trione 156 (Scheme 46). This catalyst was used seven times without loss in any of its activities.71
2.4. Magnetic bio-polymers based on alginate
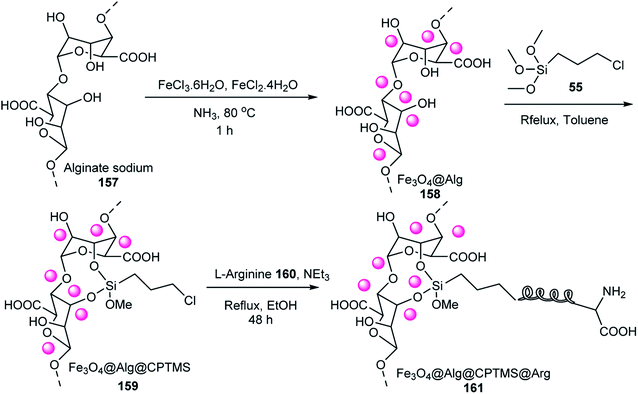
Subsequently, Fe3O4@Alg@CPTMS@Arg, which has two acidic and basic functional sites, activated the carbonyl groups in the synthesis of 2,4,5-triarylimidazoles through the reaction of ammonium acetate, aldehydes, and benzil in EtOH under reflux conditions (Scheme 48). The recyclability of the catalyst was tested 7 times using the model reaction without loss in any of its activities. When Fe3O4 was used as the catalyst, the yield of this reaction was about 65%.72
The activity of Fe3O4@Alg@CPTMS@Arg was tested as a catalyst in the synthesis of 2,4,5-triarylimidazoles 36 via the reaction of ammonium acetate 71, aldehyde derivatives 19, and benzil 34 in EtOH (Scheme 50). Its catalytic activity did not decrease after seven uses. It has two functional groups including a Lewis base (NH2) and Brønsted acidic (COOH), which catalyzed the synthesis of 2,4,5-triarylimidazoles.73
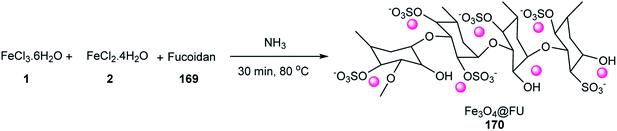
The activity of Fe3O4@FU was evaluated as a catalyst in the synthesis of tri- and tetra-substituted imidazoles 36 or 171 via three- and four-component reactions of benzil 34, aldehydes 19, NH4OAc 71, and amine 52 under reflux conditions in EtOH (Scheme 52), respectively. The catalyst was used six times in the model reaction without loss in any of its activities. This reaction was accomplished in ethanol under reflux conditions in the presence of Fe3O4 as a catalyst after 40 min with 55% yield. The carbonyl groups were activated via hydrogen bonding with Fe3O4@FU as a catalyst.74
2.5. Magnetic bio-polymers based on glucose
Subsequently, the catalytic activity of Fe3O4@C@ONa was tested for the synthesis of 4H-chromene derivatives 174 via the reaction of salicylaldehyde 42, dimedone 64, and β-naphthol 101 in water at 60 °C (Scheme 54). Also, the catalyst was used five times in the model reaction without loss in any of its activities. This catalyst has two functional groups, including Fe3+ as a Lewis acid and oxygen group as a Lewis base, which increased the reaction rate.75
2.6. Magnetic bio-polymers based on chitosan
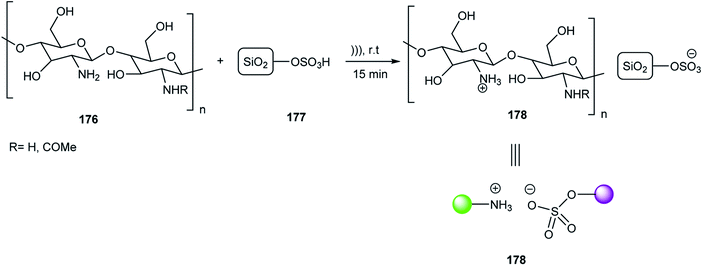
A green method for the synthesis of 3,4-dihydropyrimidine-2(1H)-one/thione derivatives 179 was described by Behrouz and co-workers via the Biginelli reaction of (thio)urea 23 or urea 6, methyl acetoacetate 21, and aldehydes 19 using CSSNH@Arg 178 under solvent-free conditions (Scheme 56). According to the reusability test, this catalyst was used five times without a decrease in any of its activities. The various functional groups including hydroxyl and amine on the CSSNH@Arg can activate the carbonyl groups via hydrogen bonding.76
CSSNH was applied as a Brønsted acid catalyst in the synthesis of 2-amino-3-cyano-4H-pyrans 126 and 2-amino-4H-chromenes 116 or 182 via the three-component reactions of malononitrile 14, benzaldehyde 19, and β-naphthol 101 or dimedone 64 or 4-hydroxycoumarin 124, respectively (Scheme 58). This catalyst was used four times without any loss in its activity.77
Its catalytic activity was tested in the synthesis of polyhydroquinolines 72 via the reaction of aldehydes 19, dimedone 67, ammonium acetate 71, and ethyl acetoacetate or methyl acetoacetate 21 in EtOH (Scheme 60). In another attempt, it was used in the synthesis of 1,4-dihydropyridines 73 via the pseudo-three-component reaction of aldehydes 19, methyl acetoacetate 21, and ammonium acetate 71 (Scheme 61). The acidic site of the catalyst activated the carbonyl groups of the primary compounds. The activity of this catalyst did not decrease after use eight times.78
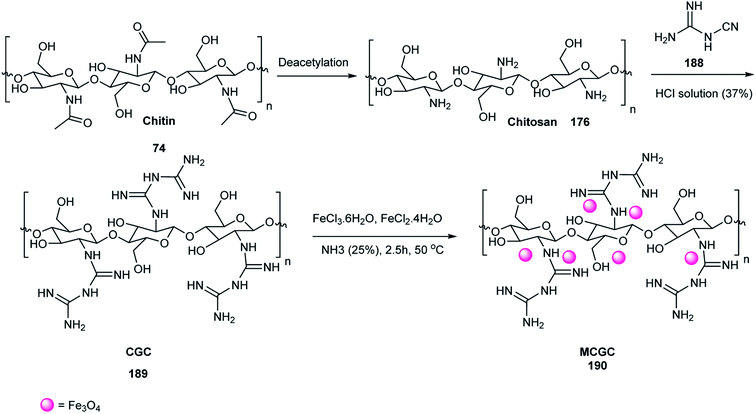
1,4-Dihydropyridines 73 were synthesized via the Hantzsch reaction of ethyl acetoacetate 35, aromatic aldehydes 19, and ammonium acetate 71 under ultrasonic irradiation in the presence of MCGC 190 as a catalyst, which activated the carbonyl groups via hydrogen bonding. Also, the yield of the reaction did not decrease after eight times usage of the catalyst (Scheme 64).79
The catalyst activity of MnFe2O4-CS-Bu-SO3H was investigated in the synthesis of spiro[acenaphthylene-1,9′-acridine]triones 194 via the multicomponent reaction of dimedone 64, aldehydes 19, and acenaphthoquinone 193 under ultrasonic irradiation in H2O (Scheme 66). Also, MnFe2O4-CS-Bu-SO3H as a Brønsted acid increased the reaction rate via hydrogen bonding with the carbonyl group. According to the reusability test, this catalyst was used five times without any loss in its activity.80
The catalytic activity of Cu-MCS was verified in the synthesis of various tetrazoles 198 or 199 via the reaction of cyanamides 197 and NaN3 50 in H2O under reflux conditions (Scheme 68). This catalyst could be used five times without loss in its activity.81
The condensation reaction of benzaldehyde 19, ethyl acetoacetate 35 or dimedone 64, and ammonium acetate 71 gave 1,4-dihydropyridine derivatives 73 or 202 in the presence of Ch-rhomboclase NCs 201 under solvent-free conditions at 80 °C (Scheme 70). In the Hantzsch reaction, this acidic catalyst activated carbonyl groups via hydrogen bonding. Also, according to the reusability test, the catalyst was used seven times without any decrease in its activity.82
The catalytic activity of Fe3O4/CS/COF/Cu 207 was tested in the synthesis of polyhydroquinolines 72 via the Hantzsch reaction of aldehydes 19, dimedone 64, ammonium acetate 71, and ethyl acetoacetate 35 (Scheme 72). Fe3O4/CS/COF/Cu with two sites including a Lewis acid (Cu2+) and Lewis base (imine) catalyzed the Hantzsch reaction, which was used five times without a decrease in its activity.83
2.7. Synthesis and application of magnetic ZnS/CuFe2O4/agar
FeCl3·6H2O 1, CuCl2·2H2O 208, Zn(OAc)2.2H2O 209, and thioacetamide were dissolved in distilled H2O. Then, agar 211 and ammonia solution were added to the reaction mixture to obtain ZnS/CuFe2O4/agar 212 (Scheme 73).84The catalytic activity of ZnS/CuFe2O4/agar 212 was examined in the reaction of dimedone 64, malononitrile 14, and aldehydes 19 to synthesize 2-amino-tetrahydro-4H-chromene-3-carbonitriles 116 (Scheme 74). The carbonyl groups were activated in the presence of ZnS/CuFe2O4 via interaction with the hydroxyl groups of agar and Zn metal as a Lewis acid. The catalyst was reused five times with no reduction in its activity.84
2.8. Synthesis and application of magnetic Fe3O4@xanthan gum
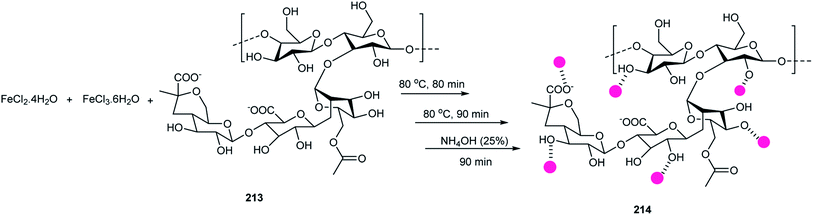
NH4OH solution was added to a mixture of the FeCl2·4H2O, FeCl3·6H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and an aqueous suspension of xanthan gum 213 to give Fe3O4@xanthan gum 214 (Scheme 75).85
2), and an aqueous suspension of xanthan gum 213 to give Fe3O4@xanthan gum 214 (Scheme 75).85
Fe3O4@xanthan gum was applied in the synthesis of 2-amino-3-cyano-4H-pyran derivatives 116 via the reaction of aldehydes 19, dimedone 64, and malononitrile 14 in EtOH (Scheme 76). The model reaction was performed about nine times and the yields did not decrease. This reaction was accomplished in the presence of Fe3O4 after 45 min in 30% yield. It was shown that Fe3O4@xanthan gum activated the carbonyl groups via hydrogen bonding, which was more effective than Fe3O4.85
3. Conclusion
Many bio-polymers can be obtained from natural sources. According to the importance of green chemistry in organic reactions, in this review, the application of bio-polymers as a catalyst in multicomponent reactions was grouped and summarized. Herein, we highlighted the immobilization of magnetic nanoparticles with bio-polymers. Due to the excellent properties of magnetic nano-catalysts, including their non-toxic nature, high surface area, simple preparation, easy surface modification, and simple separation, these systems have been applied as catalysts in multicomponent reactions. Various organic compounds such as bio-polymers were used for the modification of magnetic nanoparticles. Bio-polymers have various advantages such as biodegradable, biocompatible, and heat-resistant nature. Therefore, herein, their synthesis and catalytic activities in multicomponent reactions were studied. We believe that this article will guide researchers in the design and synthesis of various compounds according to green chemistry. Magnetic nanocomposites have many applications in various fields including, drug delivery, solar cells, chemical sensors, water treatment, biomedical sensors, and catalysts. The importance of these topics could be discussed as a review article in the future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the Research Council's support of Alzahra University.References
- A. Ali, H. Zafar, M. Zia, U. HAQ, A. Phull, J. Ali and A. Hussain, Nanotechnol., Sci. Appl., 2016, 9, 49–67 CrossRef CAS PubMed.
- F. Erogbogbo, K.-T. Yong, R. Hu, W.-C. Law, H. Ding, C.-W. Chang, P. N. Prasad and M. T. Swihart, ACS Nano, 2010, 4, 5131–5138 CrossRef CAS PubMed.
- M. Esmaeilpour, J. Javidi, F. N. Dodeji and H. Hassannezhad, J. Iran. Chem. Soc., 2014, 11, 1703–1715 CrossRef CAS.
- L. Lai, Q. Xie, L. Chi, W. Gu and D. Wu, J. Colloid Interface Sci., 2016, 465, 76–82 CrossRef CAS PubMed.
- A. Abou-Hassan, O. Sandre, S. Neveu and V. Cabuil, Angew. Chem., 2009, 121, 2378–2381 CrossRef.
- N. O. Mahmoodi, F. Ghanbari Pirbasti and Z. Jalalifard, J. Chin. Chem. Soc., 2018, 65, 383–394 CrossRef CAS.
- M. Esmaeilpour, J. Javidi and M. Zandi, New J. Chem., 2015, 39, 3388–3398 RSC.
- X. Chen, Y. Zhou, H. Han, X. Wang, L. Zhou, Z. Yi and L. Zeng, Mater. Today Chem., 2021, 22, 100556 CrossRef CAS.
- G. U. I. Sheng, S. H. E. N. Xiaodong and L. I. N. Benlan, Rare Met., 2006, 25, 426–430 CrossRef.
- S. S. Khasraghi, M. Shojaei, M. Janmaleki and U. Sundararaj, Eur. Polym. J., 2021, 159, 110735 CrossRef.
- X. Liu, J. Tian, Y. Li, N. Sun, S. Mi, Y. Xie and Z. Chen, J. Hazard. Mater., 2019, 373, 397–407 CrossRef CAS PubMed.
- M. Muthiah, I. K. Park and C. S. Cho, Biotechnol. Adv., 2013, 3, 1224–1236 CrossRef PubMed.
- R. Mohammadi, A. Saboury, S. Javanbakht, R. Foroutan and A. Shaabani, Eur. Polym. J., 2021, 153, 110500 CrossRef CAS.
- M. A. Almessiere, Y. Slimani, A. Demir Korkmaz, A. Baykal, H. Albetran, T. A. Saleh, M. Sertkol and I. Ercan, Ultrason. Sonochem., 2020, 62, 104847 CrossRef CAS PubMed.
- H. Javadian, M. Ruiz, T. A. Saleh and A. M. Sastre, J. Mol. Liq., 2020, 306, 112760 CrossRef CAS.
- A. A. Basaleh, M. H. Al-Malack and T. A. Saleh, J. Environ. Chem. Eng., 2021, 9, 105126 CrossRef CAS.
- A. A. Basaleh, M. H. Al-Malack and T. A. Saleh, Sustainable Chem. Pharm., 2021, 23, 100503 CrossRef.
- N. Dhenadhayalan, K. C. Lin and T. A. Saleh, Small, 2020, 6, 1905767 CrossRef PubMed.
- A. M. Alansi, M. Al-Qunaibit, I. O. Alade, T. F. Qahtan and T. A. Saleh, J. Mol. Liq., 2018, 253, 297–304 CrossRef CAS.
- A. M. Alansi, T. F. Qahtan and T. A. Saleh, Adv. Mater. Interfaces, 2021, 8, 2001463 CrossRef CAS.
- A. M. Alansi, T. F. Qahtan, N. Al Abass, J. M. AlGhamdi, M. Al-Qunaibit and T. A. Saleh, Adv. Sustainable Syst., 2021, 2100267 Search PubMed.
- T. A. Saleh, Environ. Technol. Innovation, 2021, 24, 101821 CrossRef CAS.
- J. Wang, Z. Shi, J. Fan, Y. Ge, J. Yin and G. Hu, J. Mater. Chem., 2012, 22, 22459 RSC.
- T. A. Debele, S. L. Mekuria and H. C. Tsai, Mater. Sci. Eng., C, 2016, 68, 964–981 CrossRef CAS PubMed.
- A. Maleki and M. Kamalzare, Catal. Commun., 2014, 53, 67–71 CrossRef CAS.
- V. Omid, F. M. Kievit, C. Fang, N. Mu, S. Jana, M. C. Leung, H. Mok, R. G. Ellenbogen, J. O. Park and M. Zhang, Biomaterials, 2010, 31, 8032–8042 CrossRef PubMed.
- Z. M. Avval, L. Malekpour, F. Raeisi, A. Babapoor, S. M. Mousavi, S. A. Hashemi and M. Salari, Drug Metab. Rev., 2020, 52, 157–184 CrossRef PubMed.
- S. Mirza, M. S. Ahmad, M. I. A. Shah and M. Ateeq, In Metal nanoparticles for drug delivery and diagnostic applications, Elsevier, 2020, pp. 189–213 Search PubMed.
- A. Gholami, S. M. Mousavi, S. A. Hashemi, Y. Ghasemi, W.-H. Chiang and N. Parvin, Drug Metab. Rev., 2020, 52, 205–224 CAS.
- X. Sun, G. Zhang, R. Du, R. Xu, D. Zhu, J. Qian, G. Bai, C. Yang, Z. Zhang, X. Zhang and D. Zou, Biomaterials, 2019, 194, 151–160 CrossRef CAS PubMed.
- Z. Zhou, L. Yang, J. Gao and X. Chen, Adv. Mater., 2019, 31, 1804567 CrossRef PubMed.
- D. D. Stueber, J. Villanova, I. Aponte, Z. Xiao and V. L. Colvin, Pharmaceutics, 2021, 13, 943 CrossRef CAS PubMed.
- N. M. El-Shafai, M. M. Abdelfatah, M. E. El-Khouly, I. M. El-Mehasseb, A. El-Shaer, M. S. Ramadan, M. S. Masoud and M. A. El-Kemary, Appl. Surf. Sci., 2020, 506, 144896 CrossRef CAS.
- L. Gloag, M. Mehdipour, D. Chen, R. D. Tilley and J. J. Gooding, Adv. Mater., 2019, 31, 1904385 CrossRef CAS PubMed.
- S. Khizar, H. Ben Halima, N. M. Ahmad, N. Zine, A. Errachid and A. Elaissari, Electrophoresis, 2020, 41, 1206–1224 CrossRef CAS PubMed.
- M. P. Conte, J. K. Sahoo, Y. M. Abul-Haija, K. A. Lau and R. V. Ulijn, ACS Appl. Mater. Interfaces, 2018, 10, 3069–3075 CrossRef CAS PubMed.
- S. Mallakpour and M. Hatami, Appl. Clay Sci., 2019, 174, 127–137 CrossRef CAS.
- N. Shabani, A. Javadi, H. Jafarizadeh-Malmiri, H. Mirzaei and J. Sadeghi, Food Hyg., 2021, 10, 61–71 Search PubMed.
- R. Banerjee, Y. Katsenovich, L. Lagos, M. McIintosh, X. Zhang and C.-Z. Li, Curr. Med. Chem., 2010, 17, 3120–3141 CrossRef CAS PubMed.
- S. Mallakpour, M. Tukhani and C. M. Hussain, Int. J. Biol. Macromol., 2021, 179, 429 CrossRef CAS PubMed.
- S. Kamel and T. A. Khattab, Cellulose, 2021, 28, 4545 CrossRef CAS.
- G. Mohammadi Ziarani, Z. Kheilkordi, F. Mohajer, A. Badiei and R. Luque, RSC Adv., 2021, 11, 17456–17477 RSC.
- G. Mohammadi Ziarani, S. Rohani, A. Ziarati and A. Badiei, RSC Adv., 2018, 8, 41048–41100 RSC.
- F. Mohajer, G. Mohammadi Ziarani and A. Badiei, RSC Adv., 2021, 11, 6517–6525 RSC.
- Z. Kheilkordi, G. Mohammadi Ziarani and F. Mohajer, Curr. Org. Chem., 2022, 26, 287–298 CrossRef.
- G. Mohammadi Ziarani, R. Moradi, T. Ahmadi and N. Lashgari, RSC Adv., 2018, 8, 12069–12103 RSC.
- G. Mohammadi Ziarani, F. Aleali and N. Lashgari, RSC Adv., 2016, 6, 50895–50922 RSC.
- G. Mohammadi Ziarani, N. H. Nasab and N. Lashgari, RSC Adv., 2016, 6, 38827–38848 RSC.
- L. A. Thompson, Curr. Opin. Chem. Biol., 2000, 4, 324–337 CrossRef CAS PubMed.
- A. Dömling, Curr. Opin. Chem. Biol., 2002, 6, 306–313 CrossRef.
- E. Zare and Z. Rafiee, Appl. Organomet. Chem., 2020, 34, e5516 CrossRef CAS.
- S. S. Hosseinikhah and B. B. F. Mirjalili, RSC Adv., 2020, 10, 40508–40513 RSC.
- P. G. Kargar, G. Bagherzade and H. Eshghi, RSC Adv., 2020, 10, 37086–37097 RSC.
- A. Maleki, S. Gharibi, K. Valadi and R. Taheri-Ledari, J. Phys. Chem. Solids, 2020, 142, 109443 CrossRef CAS.
- G. Bagherzade, RSC Adv., 2021, 11, 19203–19220 RSC.
- G. Bagherzade and H. Eshghi, RSC Adv., 2021, 11, 4339–4355 RSC.
- P. G. Kargar, M. Noorian, E. Chamani, G. Bagherzade and Z. Kiani, RSC Adv., 2021, 11, 17413–17430 RSC.
- M. Zahedifar, B. Pouramiri and R. Razavi, Res. Chem. Intermed., 2020, 46, 2749–2765 CrossRef CAS.
- B. B. F. Mirjalili, Z. Zaghaghi and A. Monfared, J. Chin. Chem. Soc., 2020, 67, 197–201 CrossRef CAS.
- N. Safajoo, B. B. F. Mirjalili and A. Bamoniri, Polycyclic Aromat. Compd., 2021, 41, 1241–1248 CrossRef CAS.
- N. Safajoo, B. B. F. Mirjalili and A. Bamoniri, RSC Adv., 2019, 9, 1278–1283 RSC.
- G. Bagherzade and H. Eshghi, RSC Adv., 2020, 10, 32927–32937 RSC.
- A. D. Tafti and B. B. F. Mirjalili, RSC Adv., 2020, 10, 31874–31880 RSC.
- A. Maleki, F. Hassanzadeh-Afruzi, Z. Varzi and M. S. Esmaeili, Mater. Sci. Eng., C, 2020, 109, 110502 CrossRef CAS PubMed.
- Z. Amiri-Khamakani, F. Hassanzadeh-Afruzi and A. Maleki, Chem. Process., 2020, 3, 101 Search PubMed.
- E. Babaei and B. B. F. Mirjalili, Iran. J. Catal., 2020, 10, 219–226 CAS.
- A. Mohammadzadeh, A. P. Marjani and A. Zamani, S. Afr. J. Chem., 2020, 73, 55–63 CrossRef CAS.
- M. Kamalzare, M. Bayat and A. Maleki, R. Soc. Open Sci., 2020, 7, 200385 CrossRef CAS PubMed.
- M. H. Sayahi, F. Shamkhani, M. Mahdavi and S. Bahadorikhalili, Starch-Stärke, 2021, 73, 2000257 CrossRef CAS.
- S. Forouzandehdel, M. Meskini and M. R. Rami, J. Mol. Struct., 2020, 1214, 128142 CrossRef CAS.
- M. Shamsi-Sani, F. Shirini and M. Mohammadi-Zeydi, J. Nanosci. Nanotechnol., 2019, 19, 4503–4511 CrossRef CAS PubMed.
- S. Amirnejat, A. Nosrati and S. Javanshir, Appl. Organomet. Chem., 2020, 34, e5888 CAS.
- S. Amirnejat, A. Nosrati, S. Javanshir and M. R. Naimi-Jamal, Int. J. Biol. Macromol., 2020, 152, 834–845 CrossRef CAS PubMed.
- M. Banazadeh, S. Amirnejat and S. Javanshir, Front. Chem., 2020, 8, 1023 Search PubMed.
- H. Ghavidel, B. Mirza and S. Soleimani-Amiri, Polycycl. Aromat. Compd., 2021, 41, 604–625 CrossRef CAS.
- S. Behrouz, M. N. Abi and M. A. Piltan, Acta Chim. Slov., 2021, 68, 374–386 CrossRef CAS.
- S. Behrouz, M. N. Abi and M. A. Piltan, J. Appl. Chem., 2019, 14, 109–124 Search PubMed.
- S. Asgharnasl, R. Eivazzadeh-Keihan, F. Radinekiyan and A. Maleki, Int. J. Biol. Macromol., 2020, 144, 29–46 CrossRef CAS PubMed.
- K. Javanmiri and R. Karimian, Monatsh. Chem., 2020, 151, 199–212 CrossRef CAS.
- H. Naeimi and S. Lahouti, J. Org. Chem. Res., 2020, 6, 54–68 Search PubMed.
- N. Motahharifar, M. Nasrollahzadeh, A. Taheri-Kafrani, R. S. Varma and M. Shokouhimehr, Carbohydr. Polym., 2020, 232, 115819 CrossRef CAS PubMed.
- P. Kamalzare, B. Mirza and S. Soleimani-Amiri, J. Nanostructure Chem., 2021, 11, 229–243 CrossRef CAS.
- E. Zare and Z. Rafiee, J. Taiwan Inst. Chem. Eng., 2020, 116, 205–214 CrossRef CAS.
- S. Bahrami, F. Hassanzadeh-Afruzi and A. Maleki, Appl. Organomet. Chem., 2020, 34, e5949 CrossRef CAS.
- M. S. Esmaeili, M. R. Khodabakhshi, A. Maleki and Z. Varzi, Polycyclic Aromat. Compd., 2021, 41, 1953–1971 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |