 Open Access Article
Open Access ArticleComplete life of cobalt nanoparticles loaded into cross-linked organic polymers: a review
Muhammad Arif
 *
*
Department of Chemistry, School of Science, University of Management and Technology, Lahore 54770, Pakistan. E-mail: muhammadarif2861@yahoo.com; muhammadarif@umt.edu.pk
First published on 20th May 2022
Abstract
The unique combination of cobalt (Co) nanoparticles (NPs) and smart polymer microgels is of great interest and has received much attention over the past decade with respect to the production of hydrogen gas and its use in removing toxic dyes from water. The responsive behavior of microgels makes cobalt nanoparticle-loaded microgels most suitable for the production of hydrogen and for the reduction of pollutants in different environments. Different classes of Co NPs in microgels have been reported in the literature. Hybrid microgel formations play an important role in their use. Hence, a specific assembly of Co NPs in microgels has been designed for the synthesis and use of hydrogen to reduce toxic pollutants from water. All progress in the synthesis, classification, characterization, and applications of Co NPs in microgels has been reviewed in this report. Catalytic generation and the use of hydrogen for the reduction of pollutants in the presence of Co NPs loaded into microgels have been discussed in a tutorial manner.
1. Introduction
Green fuel consumption is an important task nowadays.1 All sources of consumption release heat along with toxic molecules into the environment, such as the burning of hydrocarbons that produces CO2 and CO.2 These produced gases are responsible for increasing the temperature of the environment and the deficiency of O2 in the human body that ultimately leads to death. Green fuels such as H2 gas are needed to avoid the creation of toxic environments.3 The burning of H2 gas produces energy along with water molecules. Water is essential for life. Thus, H2 gas is the best green fuel for consumption purposes.4 Hence, the production of hydrogen is very important due to its significant use. Generally, hydrogen gas is produced by catalysis.5 Different materials are used for catalysis, such as metal–organic frameworks,6 metal oxides,7 and metal nanoparticles.8 Nanoparticles are mostly used for the production of hydrogen, but the instability and cost of nanoparticles are the main issues facing their applicability. Stable metal nanoparticles are very costly but less stable nanoparticles are cheap. Co nanoparticles are of low cost and have catalytic activity comparable to that of noble metals for the catalytic production of hydrogen, but the instability of Co nanoparticles is their main drawback. This drawback can be diminished by using different stabilizers such as surfactants,9 dendrimers,10 micelles,11 and microgels.12 Microgels increase their stability as well as their catalytic performance to produce more hydrogen gas and are easily re-usable. Therefore, the best combination is that of cobalt nanoparticles with microgels. Microgels contain a cross-linked polymeric network in their structure. The microgels stabilize the metal nanoparticles due to the presence of electron donor sites in their structures.This unique combination of metal nanoparticles and organic cross-linked polymeric network microgels has received great interest during the last few years because of its many applications in different fields such as medicine,13 the purification of water,14 and catalysis.15 Hybrid microgels are mostly used for the catalytic generation of H2 gas from NaBH4 or NH3BH3 and then used to produce hydrogen for the reduction of toxic chemicals such as nitroarenes16 and organic dyes17 from water. Many review articles on different metal nanoparticle loaded microgels are reported in the literature18–20 but cobalt nanoparticles loaded into microgels are not. Hence, it is a requirement for the new researchers to provide review articles on other metal nanoparticle loaded microgels due to suitable applications and cobalt nanoparticles loaded microgels are the most suitable combination for the generation of hydrogen and its use for the reduction of toxic materials. During the last decade, many researchers have researched on Co nanoparticle loaded microgels for catalytic production of hydrogen, reduction of nitroarenes, and organic dyes. Therefore, it is necessary to provide a review to the researchers, which works on generation of hydrogen and its use through catalysis in the presence of cobalt nanoparticles in microgels.
2. Classifications of Co nanoparticle loaded microgels
Cobalt NPs loaded in microgels are divided into different classes based on the morphology of hybrid microgels.2.1 Microgel and metal nanoparticle-based hybrid microgels
Based on microgels and nanoparticles, hybrid microgels are divided into (i) monometallic (Co) nanoparticles loaded into homogeneous microgels21–23 (ii) bimetallic nanoparticles loaded into homogeneous microgels22 (iii) inorganic nanoparticles surrounded by cross-linked polymeric organic shell loaded with Co nanoparticles23 (iv) Co nanoparticles loaded into porous microgels.| Co NPs loaded into cross-linked polymer | Abbreviation of Co NPs loaded into polymer | Structure/morphology | Pictorial diagram | References |
|---|---|---|---|---|
| Monometallic (Co) nanoparticles loaded into homogenous microgel | Co NPs-AAMPSA | Monometallic homogenous structure |  |
20 |
| Bimetallic nanoparticles loaded into homogeneous microgels | Co–Fe NPs-P(MA-AN) | Bimetallic homogenous structure |  |
22 |
| Inorganic nanoparticles surrounded by cross-linked polymeric organic shell loaded with Co nanoparticles | Fe3O4–SiO2@Co NPs- P(4VP) | Core = Fe3O4–SiO2 | 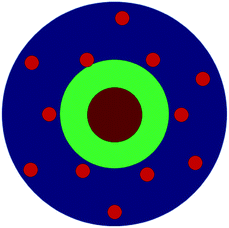 |
23 |
| Shell = Co NPs loaded P(4VP) | ||||
| Cobalt nanoparticles loaded into porous microgels | Co NPs-P(4VP) | Porous hybrid microgels |  |
26 |
3. Synthesis of Co nanoparticle loaded microgels
The synthesis of cobalt nanoparticles loaded in microgels can be synthesized by mixing microgels and cobalt nanoparticles or microgels are synthesized first, followed by loading cobalt nanoparticles into microgels. Cross-linked polymeric networks can also be synthesized by different ways based on temperature and be given different names. If microgels are synthesized at low temperatures, then the microgels are called cryogels, and at higher temperatures (generally at 70 °C) called as microgels. The synthesis of hybrid microgels or hybrid cryogels is given below.3.1 Synthesis of Co nanoparticles in microgel dispersion
In this process, microgel or cryogel is synthesized by adding the monomers along with a crosslinker and then adding the free radical initiator after maintaining the temperature at ≥70 °C under a nitrogen atmosphere. This is the free radical emulsion polymerization method (FREPM). Another method is the inverse suspension polymerization method (ISPM), in which both solvent and emulsifier are used during the synthesis of microgel.In this method, one phase is aqueous, and the other is oil. Cobalt ions are introduced after the synthesis of the microgel in both ways and then reduced to form nanoparticles. Farooqi et al.27 synthesized poly(N-isopropylacrylamide-co-methacrylic acid) (NipaM-co-MA) microgel by FREPM, as shown in Fig. 1. NipaM (monomer), MA (co-monomer), N,N-methylene bisacrylamide (bis) (crosslinker), and sodium dodecylsulphate (SDS) (stabilizer) were added into 95 mL de-ionized water in a three neck round bottom flask. Nitrogen purging was started along with the rising temperature with continuous stirring. 5 mL ammonium persulfate (APS) solution was added to the reaction mixture after maintaining the temperature at 70 °C. Reaction was continued for a further 4 h to obtain the microgel. Dialysis was done for 6 days to remove unreacted species from the mixture. Then, Co or Ni nanoparticles were introduced by reducing the respective salts.
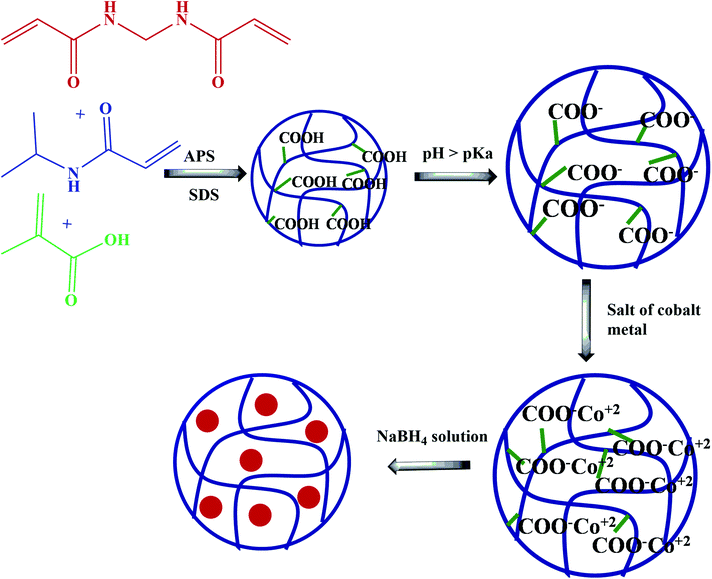 | ||
| Fig. 1 Synthesis of poly(N-isopropylacrylamide-co-methacrylic acid) (NipaM-co-MA) microgels by the free radical polymerization method and the introduction of cobalt nanoparticles by in situ reduction.27 | ||
Similarly, cryogels, which are basically microgels, have been synthesized at low temperatures. Sahiner and Yildiz28 have synthesized cryogels by FRPM. 4VP, poly(ethylene glycol) diacrylate P(EGDA), and TemeD were added to water and cooled (T = 0 °C) for 5 min. KPS solution was added to polymerize and then transferred into a freezer with the help of plastic straws at −18 °C for 24 h. The obtained cryogels were washed with ethanol and water and then dried at 50 °C and stored in a desiccator. Then, ions were introduced and reduced into nanoparticles with the help of NaBH4.
Rehman et al.17 have synthesized poly(3-acrylamidopropyl)-trimethylammonium chloride P(AAPTMAC) microgel with ISPM. They optimized the condition for high yield synthesis of microgels by using different amounts of emulsifier and solvent to enhance the cross-linking density of the microgel. They added AAPTMAC monomer and bis crosslinker in an aqueous solution and then transferred this solution into a mixture of span 80 and gasoline at 800 rpm. N,N,N′,N′-Tetramethylethylenediamine (TemeD) was added after 5 min. Then, APS solution was added after 15 min for polymerization. Polymerization was completed after 2 h. Synthesized microgel was poured into acetone solvent to remove impurities from the microgel. Then, cobalt, nickel, and copper solutions were prepared separately in ethanol. Then solution of metal salts is added into the microgel dispersion solution and reduced these salts into corresponding metal nanoparticles by in situ reduction with NaBH4.
3.2 Synthesis of hybrid microgels by mixing nanoparticles and microgels
In this method, microgels and cobalt nanoparticles are synthesized separately and then mixed to produce cobalt nanoparticles loaded into microgels. The loading of nanoparticles into microgels is due to the electrostatic interaction that occurs between cobalt nanoparticles and microgels. Generally, microgels have electron donor sites, which interact with the nanoparticles, causing the loading into microgels. In this way, Chen et al.29 have synthesized hybrid microgels, as shown in Fig. 2. They synthesized microgels by mixing NipaM, bis, and APS in deionized water in a vessel and sodium bisulphite (SBS) and a solution of NPs in another vessel. Both solutions were stirred for 30 min under a nitrogen atmosphere. Then, the first solution was poured into chlorobenzene (CB) and the other in n-hexane (NH). NH solution was added to the surface of CB. CB is denser than NH. So, the CB solution settles down, and NH is on the upper layer. The microgel and nanoparticles interact with each other due to electrostatic attraction at the interface region, forming hybrid microgels. Sidorov et al.30 also synthesized hybrid microgels using the same method.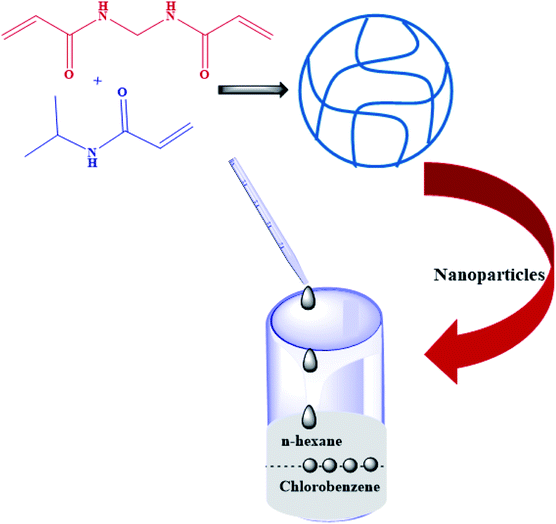 | ||
| Fig. 2 Synthesis of the poly(N-isopropylacrylamide) P(NipaM) microgel and the hybrid microgel produced by mixing metal nanoparticles with the P(NipaM) microgel.29 | ||
4. Characterization techniques used for microgels and hybrid microgels
Various techniques are used for the characterization of cobalt nanoparticles loaded in microgels. The reported techniques31 for the identification of various parameters of hybrid microgels are summarized in Table 2.| Co NPs loaded polymer | Used as catalyst | Characterization techniques | Identification by the techniques | Reference |
|---|---|---|---|---|
| Co NPs in P(VI) microgels (homogeneous hybrid microgel) | Generation of hydrogen by hydrolysis of NaBH4 | Zeta potential, FTIR, DLS, TGA, AAS, UV-Vis | Zeta-potential and DLS used to find the particle size, hydrodynamic radius, and surface charge. The size of P(VI)-por, P(VI), and P(VI)–Si microgels were 764 nm, 304 nm, and 530 nm, respectively, according to DLS technique. Hdr of P(VI)-por was greater than P(VI)–Si due to generation of pores after the removal of Si and more water intake. The zeta-potential value of P(VI)-por, P(VI), and P(VI)–Si microgels were −21.99 mV, 1.20 mV, and −19.86 mV, respectively. A more negative value of P(VI)-por microgel indicates that some amount of Si is present in the microgel in porous form. Different functionalities from microgel characterized with the help of FTIR. P(VI) microgels lost 90% weight, and weight-loss of P(VI)-por and P(VI)–Si were almost similar (31.3% wt and 34.4% wt, respectively), which was indicated by the TGA technique. The contents of Co nanoparticles in P(VI), P(VI)-por, and P(VI)–Si microgels were 7.71, 8.56, and 8.46, respectively, calculated with AAS | 26 |
| Co NPs loaded in P(4VP) cryogel (homogeneous hybrid microgel) | Hydrogen generation by hydrolysis of NaBH4 and NH3BH3 | FTIR, TGA, AAS | FTIR technique was used to identify the presence of different functional groups present in microgels and hybrid microgels. The content of Co nanoparticles in Co NPs loaded P(4VP) microgel was 11.5 wt%, which was found with the help of TGA in the first loading. This amount was increased by increasing the loading steps. This amount was 25.4 wt% in the third and 33 wt% in the fifth loading. The contents of Co NPs according to the AAS technique were 20.49, 49.97, 85.4, 94.5, and 97.3 mg g−1 in 1st, 2nd, 3rd, 4th, and 5th loading. In continuously loading the metal, the metal content increases and hence the active sites for catalytic performance | 28 |
| Metal NPs in P(NipaM)/P(HemA) (homogeneous hybrid microgel) | Can be used for catalysis | 1H NMR, TEM, FTIR, EDS, SEM, UV-Vis | The structure of the microgel has been characterized by 1H NMR. Functionalities of microgel and hybrid microgel identified with FTIR. Size of the microgel identified with SEM and metal nanoparticles with TEM techniques. The diameter of microgels was from 150 to 1000 μm and metal nanoparticles from 5 to 6 nm for Au, 20–25 for Ag and 30 for Co nanoparticles loading in microgels | 29 |
| Co NPs loaded into poly(ethylene imine) P(EI) (homogeneous hybrid microgel) | Generation of H2 by hydrolysis of NaBH4 and catalytic reduction of 4-nitrophenol | SEM, FTIR, TGA, DLS, zeta potential | SEM technique was used to determine the spherical morphology and size of the microgel in the swollen state. The particle size of microgels was in the range of 10 nm to 10 μm. Different functionalities of polymer particles and microgels were confirmed by FTIR. Property of thermal stability of polymer particle and microgels characterized with TGA technique. TGA indicated that the stability of the microgel is slightly more than the hybrid. The difference of wt% between polymer and microgels is 17.44%, which is the cross-linking density value. The size in diameter of microgel, obtained by zeta potential, without and with filtration were 1175 and 558 nm, respectively | 32 |
| Co NPs onto porous P(4VP) microgel (porous hybrid microgel) | Generation of H2 by hydrolysis of NaBH4 | DLS, zeta potential, FTIR, AAS, BET | FTIR technique has been used to identify different functionalities and incorporation of nanoparticles in the porous surface due to the shifting of stretching frequency. Particle sizes were 333, 398, and 478 nm for P(4VP), P(4VP)-silica, and P(4VP)-porous microgels, respectively, which were obtained by the DLS technique. Surface areas of these microgels obtained with the help of the BET technique were 43.78, 24.02, and 42.26 m2 g−1 for P(4VP)-silica, P(4VP), and P(4VP)-porous, respectively. TGA has been used to check the stability of microgels. P(4VP) lost 15.5% more amount as compared to P(4VP)-silica | 25 |
The techniques used are; Transmission Electron microscopy (TEM), Scanning electron microscopy (SEM), X-ray diffraction (XRD), High Resolution Transmission Electron microscopy (HRTEM), Fourier Transformed Infrared Spectroscopy (FTIR), dynamic light scattering (DLS), energy dispersive X-rays (EDX), Thermo-gravimetric analysis (TGA), Atomic absorption spectroscopy (AAS), and UV/visible spectroscopy (UV-Vis).
5. Applications of cobalt nanoparticles into microgels
5.1 Cobalt nanoparticle loaded microgels for hydrogen production
Different sources for the production of hydrogen gas have been reported in the literature33–36 but here, our main concern is the synthesis of hydrogen gas by cobalt nanoparticles loaded into microgels. According to my best effort of the literature survey, only ammonia borane and NaBH4 (substrates) are reported for hydrogen gas generation by Co NPs loaded into microgels. Ozay et al.20 synthesized cobalt, nickel, and copper NPs loaded microgels separately and reported that the catalytic activity of cobalt NPs loaded microgel was more than that of nickel or copper nanoparticle loaded microgels. 186 ml of hydrogen gas was produced in 25 min, 65 min, and 75 min by Co, Cu, and Ni NPs loaded microgel using ammonia borane. This catalytic performance may be due to a large amount of Co NPs than other NPs in the microgel because of more active sites. Seven and Sahiner21 reported the comparative study of NaBH4 and NH3BH3. They reported that NH3BH3 is a better source for hydrogen production than NaBH4 in a basic medium.5.1.1.1 Effect of temperature. The temperature has an important role in the generation rate of hydrogen gas by hybrid microgels and substrates. The kinetic energy of molecules is increased by increasing the temperature, and the kinetic energy of molecules is directly proportional to the number of collisions of hybrid microgels and the substrate. According to collision theory, when the number of collisions is increased, then the rate of product formation is also increased.
On the other hand, the diffusion rate of the substrate is also increased from the bulk region to the surface of nanoparticles. It means that when the temperature is increased, the rate of hydrogen gas production also increases, and the reaction occurs in a short time, as shown in Fig. 3. Sahiner and Yildiz28 have reported that cobalt NPs in P(4VP) produced hydrogen gas at a higher rate at high temperatures. Here, they did not study the generation of hydrogen at 0 °C. Turhan et al.35 studied the production of hydrogen gas at 0 °C. They indicated that the production of hydrogen gas may be applicable at low temperature as well as high temperature, but the rate of reaction increases continuously by increasing the temperature.
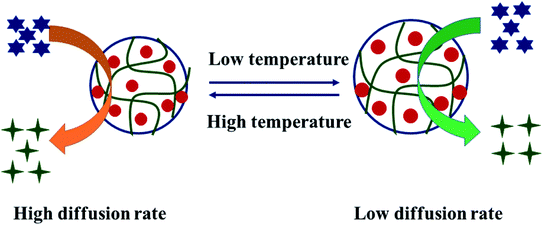 | ||
| Fig. 3 Production of hydrogen at low and high temperatures in the presence of Co nanoparticles in P(4VP) microgels.28 | ||
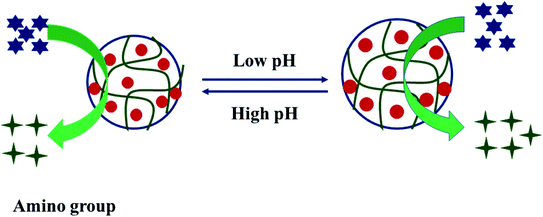
5.1.1.2 pH effect. The pH of a solution also affects the production rate of hydrogen gas by affecting the structure of hybrid microgels. The polar part of hybrid microgels have –COOH, –SO3H, and –NH– or –N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) groups in their structure. –COOH and –SO3H are acidic groups and can donate protons in water. In the deprotonated form, they have a negative charge, and due to the same charge, electrostatic repulsion occurs, and microgel swells. In the swollen state, a greater number of nanoparticles are loaded into microgels. So, such hybrid microgels show a higher rate at higher pH and a lower rate at a lower pH value of the medium, as shown in Fig. 4. On the other hand, in the swollen state, the substrate can easily reach the surface of nanoparticles, easily producing hydrogen gas. In the charged form, the hybrid microgel strongly interacts with water molecules, and water diffuses into the hybrid microgels. BH4− ions also easily diffuse and reach the surface of nanoparticles, and hence the rate of generation is also increased. On the other hand, the amino group is basic in nature, and it can accept protons. This group has a positive charge in the protonated form. Due to electrostatic repulsion, the Hdr of microgels increases, and a greater number of nanoparticles can be introduced into microgels. Their hydrogen gas production rate from substrate also increases due to the presence of more active sites of nanoparticles, as shown in Fig. 5. In this way, pH can affect the production rate of hydrogen gas by changing the protonated or deprotonated form of hybrid microgels.
groups in their structure. –COOH and –SO3H are acidic groups and can donate protons in water. In the deprotonated form, they have a negative charge, and due to the same charge, electrostatic repulsion occurs, and microgel swells. In the swollen state, a greater number of nanoparticles are loaded into microgels. So, such hybrid microgels show a higher rate at higher pH and a lower rate at a lower pH value of the medium, as shown in Fig. 4. On the other hand, in the swollen state, the substrate can easily reach the surface of nanoparticles, easily producing hydrogen gas. In the charged form, the hybrid microgel strongly interacts with water molecules, and water diffuses into the hybrid microgels. BH4− ions also easily diffuse and reach the surface of nanoparticles, and hence the rate of generation is also increased. On the other hand, the amino group is basic in nature, and it can accept protons. This group has a positive charge in the protonated form. Due to electrostatic repulsion, the Hdr of microgels increases, and a greater number of nanoparticles can be introduced into microgels. Their hydrogen gas production rate from substrate also increases due to the presence of more active sites of nanoparticles, as shown in Fig. 5. In this way, pH can affect the production rate of hydrogen gas by changing the protonated or deprotonated form of hybrid microgels.
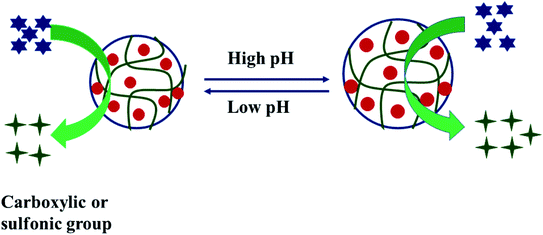 | ||
| Fig. 4 Effect of pH on the –COOH or –SO3H group-containing hybrid microgels (acidic group-containing hybrid microgels).24 | ||
 | ||
| Fig. 5 Effect of pH on amino group-containing hybrid microgels (basic group-containing hybrid microgels).36 | ||
Sahiner and Yasar36 have synthesized hybrid microgels using P(2VP) microgels and both P(2VP-co-4VP) microgels. More hydrogen production rate was observed at low pH due to protonation of amino groups. They also reported that the catalytic performance of hybrid microgels was increased by increasing the amount of 4VP as compared to 2VP. In 4VP, the nitrogen atom is away from the electron donating vinyl group, and hence it can easily interact with the ions without competition. So, more nanoparticles are loaded in microgels, and catalytic activity is also increased. Seven and Sahiner24 have synthesized nanoparticle loaded P(Am-Vsa) microgels. They also reported the same behavior by increasing the Vsa concentration, and the production rate was calculated at high pH due to swollen state.
5.1.1.3 Effect of cobalt NPs on microgels, macrogels, and cryogels. Basically, the rate of hydrogen gas generation is controlled by controlling the size of NPs and cross-linking area or the area of sieves and temperature. Cross-linking density is inversely proportional to the area of sieves. The size of nanoparticles can be controlled by controlling the area of sieves. If the area of sieves is small, then small sized nanoparticles are produced, which have more surface area. So, the rate of hydrogen production is increased. The area of sieves is greater in macrogels than in microgels. So, the catalytic activity for hydrogen production is more by hybrid microgels than hybrid macrogels. The catalytic performance of hybrid microgels is greater than hybrid cryogels because hybrid microgels uptake more water than hybrid cryogels. So, the Hdr of hybrid microgel is greater than hybrid cryogels.37 Hybrid cryogels are produced at low temperatures, usually 0 °C. So, the coagulation of nanoparticles can be controlled at 0 °C. Turhan et al.35 have synthesized 3-sulfopropyl methacrylate (3SPM) in micro and macrogel form with and without a stabilizer. Co and Ni NPs were introduced by in situ reduction. The rate of hydrogen production of hybrid microgels was greater than that of hybrid macrogels. Co NP-loaded P(3SPM)-micro and P(3SPM)-macrogels produced 248 ml hydrogen gas in 15 min and 26 min, respectively. Similarly, Sahiner and Demirci38 also reported the order of catalytic performance of cobalt NPs in micro-, macro-, and cryogels for hydrogen generation. Hybrid microgels showed better results than cryogels, followed by macrogels. 154 ml of hydrogen gas was produced by hybrid micro-, macro-, and cryogels in 55 min, 105 min, and 85 min, respectively.
5.1.1.4 Solvent effect. Solvents also play an important role in hydrogen gas generation. The solvent is the medium, which supports the substrate to reach the surface of nanoparticles. It also interacts with the substrate and hybrid microgels. If the interaction of the substrate or hybrid microgel is more with the solvent than between substrates and hybrid microgels, then the substrate reaches slowly on the surface of nanoparticles, and hence the rate of hydrogen generation becomes low. Sahiner et al.39 have used cobalt nanoparticle based P(4VP) microgels to generate hydrogen gas in aqueous and methanol solvents. 250 ml hydrogen gas is produced by methanolysis of NaBH4 in 5 min in the presence of cobalt nanoparticle composite with P(4VP) microgels, which is more than in water solvent. The oxygen atom is an inferior electron donor in methanol than in water. The solvent, which facilitates to donate the electrons, show a better result for catalytic hydrogen gas production. Another reason is the higher protonation of nitrogen atoms by methanol as compared to water, which is present in hybrid microgels. Due to this protonation, more electrostatic attraction is generated. Borohydride ions interact strongly with hybrid microgels and hence reach the surface of Co nanoparticles in microgels. Yildiz et al.40 have reported the hydrogen gas generation in deionized water, tap water, and seawater and reported that hydrogen gas is produced more rapidly in seawater than in tap water and then deionized water. More H2 gas is produced in seawater due to the effect of ions. Khan et al.41 also reported the studies in methanol solvent.
5.1.1.5 Effect of hydrophilic part of hybrid microgels. The hydrophilic part of hybrid microgels has an important role in hydrogen production by the substrate. The hydrophilic part contains partial positive and partial negative charges on hybrid microgels. These partial charges have the capacity to interact with ions or water or polar species. Due to stronger interaction with water, the hydrodynamic radius (Hdr) of microgels has been increased, and a large amount of metal ions can be loaded into the cross-linked polymeric structure of microgels. These ions are converted into NPs by reduction. In this way, more nanoparticles are introduced into the microgels. More nanoparticles mean more active sites and hence a more rapid hydrogen production rate. On the other hand, the hydrophilic part interacts with hydrides with more electrostatic force, and hence the substrate can easily reach the surface of nanoparticles. Seven and Sahiner24 have synthesized cobalt and nickel nanoparticles loaded poly(acrylamide-co-vinyl sulfonic acid) P(Am-Vsa) microgels separately. They reported that if the amount of Vsa is more in microgels, then a greater number of nanoparticles are introduced into it. They used 1.5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 ratios of Am and Vsa for the synthesis and used their hybrid microgels for hydrogen production by NaBH4 in an aqueous medium. The rate of hydrogen gas production was more in which the ratio of Am and Vsa was 1
1.5 ratios of Am and Vsa for the synthesis and used their hybrid microgels for hydrogen production by NaBH4 in an aqueous medium. The rate of hydrogen gas production was more in which the ratio of Am and Vsa was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. Similar behavior was reported by Sahiner and Yasar.36
1.5. Similar behavior was reported by Sahiner and Yasar.36
5.1.1.6 Salt effect. Salts also affect the H2 production rate. Salts prevent NaBO4 accumulation on the surface of nanoparticles. NaBO4 occupies the active sites of nanoparticles by accumulation. Therefore, it is essential to remove it from the surface of nanoparticles, and salts can do so, as shown in Fig. 6. So, salts increase the rate of hydrogen production. Ionic strength and size of ions can control the removal of NaBO4 from the nanoparticle surface. So, when the ionic strength is more, it interacts with more power to BO4− ions than others. Similarly, if the size of the ion is small, then it will interact with more power.
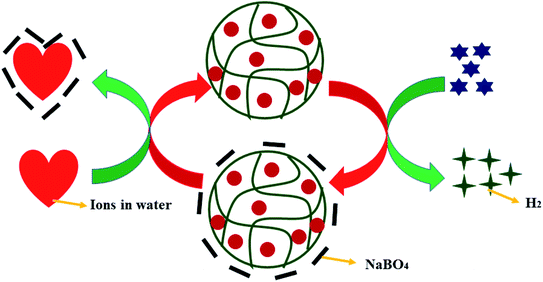 | ||
| Fig. 6 Effect of salts on the catalytic performance of cobalt nanoparticles loaded into P(3SPM) microgels.40 | ||
Yildiz et al.40 have synthesized metal NPs embedded P(3SPM) microgels. They studied the effect of ionic solutions on hydrogen generation. They used NaCl, KCl, and CaCl2 salts. The rate of hydrogen generation was NaCl > CaCl2 > KCl. Na+ ions have a smaller size as compared to K+ ions. So, Na+ ions interact more strongly with BO4− ions than K+ ions. Ca2+ ions have more ionic strength, but sulphonic groups interact more strongly than BO4− ions. So, Na+ ions interact more strongly with BO4− ions, and hence the production rate was high in the presence of NaCl.
5.2 Uses of produced hydrogen gas in the presence of cobalt nanoparticle loaded microgels
Hydrogen gas has various applications but its production by cobalt nanoparticle composites with microgels is rare. Mostly hydrogen, which is produced by Co NPs loaded into microgels and NaBH4 or NH3BH3 is used to remove toxic chemicals such as nitroarenes and organic dyes.16,42The temperature factor also controls the reduction rate of nitroarenes. Generally, the reduction rate of nitroarenes increases as the temperature increases because the diffusion rate of the substrate also increases from the bulk region to the surface of nanoparticles. As temperature increases, the collision number is also increased. So, the reduction rate increases along with the temperature. Sahiner and Demirci44 synthesized Co nanoparticle loaded (4VP) microgel and used it for the reduction of 4NP at various temperatures. The reduction rates of 4NP at 10, 20, 30, 40, and 50 °C were 0.0008, 0.0013, 0.0017, 0.0021, and 0.0023 s−1, respectively. This result showed that the reduction rate is increased with increasing temperature due to the high diffusion rate at high temperatures. Demirci and Sahiner also reported the same behavior for the reduction of 4NP and 2NP at different temperatures.
The pH of the medium also controls the rate of reduction of nitroarenes. The hybrid microgels, which have –COOH or –SO3H groups in their structure, rapidly increase their Hdr by increasing the pH value greater than the pKa value. This happens due to the electrostatic repulsion of anions (–COO−, –SO3−) of hybrid microgels, and under this condition, the substrate can easily diffuse from bulk to the surface of nanoparticles and then from the surface to bulk after conversion into the product. In this way, we can say that these hybrid microgels show better reduction performance in the basic medium. On the other hand, those hybrid microgels, which have an amino group in their structure, have high Hdr at low pH due to electrostatic repulsion between the same charges. Farooqi et al.27 studied the effect on Hdr of hybrid microgel and reported that at pH > pKa have a greater Hdr of hybrid microgels. They did not study the effect on catalytic behavior.
Catalytic reduction of nitroarenes consists of different steps as shown from a to d in Fig. 7.16,45 In the first step, a proton from water and hydride from BH4− attach with the nitro group of nitroarenes to form a. Then, rearrangement takes place to form b. In the next step, the water molecule detaches from the nitroso group to form c. Hydride and proton are attached to the nitroso group to form d. In the last, the d form is converted into an amino group, which detached from the surface of cobalt nanoparticles. The surface of cobalt nanoparticles provides a field to transport the electrons from borohydride to electron deficient nitroarenes and reduce this nitro group from nitroarenes.
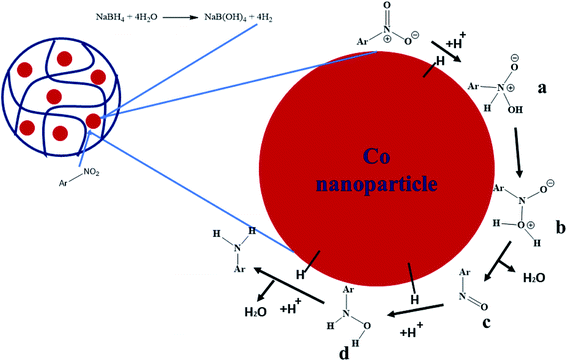 | ||
| Fig. 7 Proposed mechanism for the reduction of nitroarenes in the presence of Co NPs loaded into microgels.16,45 | ||
5.3 Adsorption of pollutants
Cobalt nanoparticle loaded microgels can be used as adsorbents to remove toxic chemicals such as metal ions, organic dyes, and herbicides from water. Hybrid microgels have polar parts in their structures. These polar parts interact with these toxic chemicals from water due to electrostatic interactions, as shown in Fig. 8. When toxic chemicals are attached to hybrid microgels, they can be removed by applying magnetic fields for ferronematic materials or centrifugation. Ajmal et al.22 have synthesized cobalt and iron nanoparticles in microgels and used them for the removal of Cd2+ ions, Cr3+ ions, paraquats (PQ), herbicides, Rh–B, and MB from water by adsorption process and separated simply by applying a magnetic field. They also reported that the adsorption property of hybrid microgels was increased by increasing the polarity (converting the nitrile group into an amidoxime group). The adsorption capacity increased from 56.3, 57.4, 75.3, 37.4, and 40.2 to 166.5, 334.5, 190.0, 89.9, and 88.1 for Cr3+, Cd2+, PQ, Rh–B, and MB respectively. Similarly, Bibi et al.48 have also used microgels and hybrid microgels to remove MB through the adsorption process.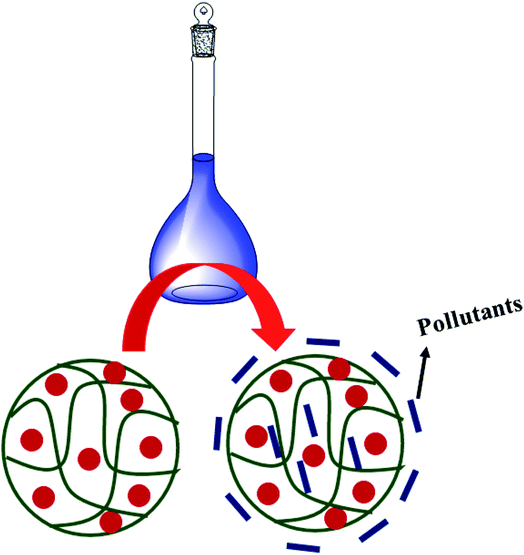 | ||
| Fig. 8 Adsorption process of Co–Fe nanoparticles loaded into P(MA-AN) microgels.22 | ||
6. Conclusions and future directions
The aim of this review article was to report the current research developments in the design, stabilization, and fabrication of cobalt nanoparticles loaded in microgels and the use of these cobalt nanoparticle loaded microgels for more hydrogen gas generation to adopt the most suitable condition. The suitable conditions for the generation of hydrogen gas were discussed in detail. This produced hydrogen gas was used to remove toxic chemicals such as nitroarenes and organic dyes from an aqueous medium. The catalytic reduction of nitroarenes and organic dyes can easily be monitored with a UV/visible spectrophotometer. Cobalt nanoparticle loaded microgels have a unique combination, which can remove toxic chemicals such as heavy metals ions, dyes, herbicides, paraquats, and nitroarenes by catalysis as well as adsorption process.15–24 Only ammonia borane and NaBH4 were used for hydrogen gas generation. Other substrates like water can also be studied in the future. Different morphologies of hybrid microgels for catalytic hydrogen generation and reduction of dyes are important topics for future studies. The monomers and co-monomers of microgels reported in the literature are non-biodegradable materials. They can be replaced by biodegradable materials such as 4-vinylcaprolactam in future studies. In the catalytic reduction of nitroarenes, mostly, 4NP is used as a model reaction. Catalytic reduction of a limited number of dyes by cobalt nanoparticle loaded microgels has been reported in the literature. Cobalt nanoparticle loaded microgels can be used for catalytic reduction of toxic metal ions such as Cr6+, Hg2+, Cd2+, etc., in the future.Conflicts of interest
There is no conflict of interest.List of abbreviations
| NPs | Nanoparticles |
| AAMPSA | 2-Acryl amido-2-methyl-1-propansulfonic acid |
| ABr | Ammonia borane |
| 4AP | 4-Aminophenol |
| NBH | Sodium borohydride |
| Hdr | Hydrodynamic radius |
| MA | Methacrylic |
| AN | Acrylonitrile |
| 4VP | 4-Vinyl pyridine |
| DLS | Dynamic light scattering |
| FREPM | Free radical emulsion polymerization method |
| ISPM | Inverse suspension polymerization method |
| EDX | Energy dispersive X-rays |
| FTIR | Fourier Transform Infrared Spectroscopy |
| NipaM | N-Isopropylacrylamide |
| HRTEM | High resolution transmission electron microscopy |
| LCST | Lower critical solution temperature |
| SDS | Sodium dodecylsulphate |
| bis | N,N-Methylene bisacrylamide |
| APS | Ammonium persulfate |
| P(EGDA) | Poly(ethylene glycol) diacrylate |
| P(AAPTMAC) | Poly(3-acrylamidopropyl)-trimethylammonium chloride |
| TemeD | N,N,N′,N′-Tetramethylethylenediamine |
| SBS | Sodium bisulphite |
| CB | Chlorobenzene |
| NH | n-Hexane |
| PnvcL | Poly(N-vinylcaprolactam) |
| SEM | Scanning electron microscopy |
| TGA | Thermo gravimetric analysis |
| AAS | Atomic absorption spectroscopy |
| 3SPM | 3-Sulfopropyl methacrylate |
| Vsa | Vinyl sulfonic acid |
| Am | Acrylamide |
| UV-Vis | UV/visible spectroscopy |
| VPTT | Volume phase transition temperature |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| MO | Methyl orange |
Acknowledgements
Muhammad Arif is thankful to the University of Management and Technology, Lahore for providing the facility to carry out a part of work.References
- K. Karagul, Y. Sahin, E. Aydemir and A. Oral, in Lean and green supply chain management, Springer, 2019, pp. 161–187 Search PubMed.
- P. Chiesa, S. Consonni, T. Kreutz and R. Williams, Int. J. Hydrogen Energy, 2005, 30, 747–767 CrossRef CAS.
- A. S. A. A. Hamaad, M. Tawfik, S. Khattab and A. Newir, Procedia Environ. Sci., 2017, 37, 564–571 CrossRef.
- A. Demirbas, Energy Resources, 2017, 24, 601–610 CrossRef.
- S. Demirci and N. Sahiner, Fuel Process. Technol., 2014, 127, 88–96 CrossRef CAS.
- X. Wang, J. He, B. Yu, B. Sun, D. Yang, X. Zhang, Q. Zhang, W. Zhang, L. Gu and Y. Chen, Appl. Catal., B, 2019, 258, 117996 CrossRef CAS.
- Y. Kojima, K. I. Suzuki, K. Fukumoto, M. Sasaki, T. Yamamoto, Y. Kawai and H. Hayashi, Int. J. Hydrogen Energy, 2002, 27, 1029–1034 CrossRef CAS.
- C. E. Bunker and M. J. Smith, J. Mater. Chem., 2011, 21, 12173–12180 RSC.
- Y. Bao, W. An, C. H. Turner and K. M. Krishnan, Langmuir, 2009, 26, 478–483 CrossRef PubMed.
- K. Aranishi, Q.-L. Zhu and Q. Xu, ChemCatChem, 2014, 6, 1375–1379 CAS.
- O. A. Platonova, L. M. Bronstein, S. P. Solodovnikov, I. M. Yanovskaya, E. S. Obolonkova, P. M. Valetsky, E. Wenz and M. Antonietti, Colloid Polym. Sci., 1997, 275, 426–431 CrossRef CAS.
- A. Biffis, N. Orlandi and B. Corain, Adv. Mater., 2003, 15, 1551–1555 CrossRef CAS.
- J. Ihsan, M. Farooq, M. A. Khan, M. Ghani, L. A. Shah, S. Saeed and M. Siddiq, Microsc. Res. Tech., 2021, 84, 1673–1684 CrossRef CAS PubMed.
- M. Ajmal, S. Demirci, M. Siddiq, N. Aktas and N. Sahiner, New J. Chem., 2016, 40, 1485–1496 RSC.
- M. Shahid, Z. H. Farooqi, R. Begum, M. Arif, A. Irfan and M. Azam, Chem. Phys. Lett., 2020, 754, 137645 CrossRef CAS.
- N. Jabeen, Z. H. Farooqi, A. Shah, A. Ali, M. Khurram, K. Mahmood, N. Sahiner and M. Ajmal, J. Porous Mater., 2021, 2021(1), 1–14 Search PubMed.
- S. Rehman, M. Siddiq, H. Al-Lohedan and N. Sahiner, Chem. Eng. J., 2015, 265, 201–209 CrossRef.
- M. Arif, Z. H. Farooqi, A. Irfan and R. Begum, J. Mol. Liq., 2021, 336, 116270 CrossRef CAS.
- R. Begum, K. Naseem and Z. H. Farooqi, J. Sol-Gel Sci. Technol., 2016, 77, 497–515 CrossRef CAS.
- O. Ozay, E. Inger, N. Aktas and N. Sahiner, Int. J. Hydrogen Energy, 2011, 36, 8209–8216 CrossRef CAS.
- F. Seven and N. Sahiner, Int. J. Hydrogen Energy, 2013, 38, 15275–15284 CrossRef CAS.
- M. Ajmal, M. Siddiq, N. Aktas and N. Sahiner, RSC Adv., 2015, 5, 43873–43884 RSC.
- N. Sahiner and A. O. Yasar, Fuel Process. Technol., 2016, 144, 124–131 CrossRef CAS.
- F. Seven and N. Sahiner, Int. J. Hydrogen Energy, 2013, 38, 777–784 CrossRef CAS.
- N. Sahiner and A. O. Yasar, Int. J. Hydrogen Energy, 2013, 38, 6736–6743 CrossRef CAS.
- N. Sahiner and A. O. Yasar, Fuel Process. Technol., 2013, 111, 14–21 CrossRef CAS.
- Z. H. Farooqi, S. Iqbal, S. R. Khan, F. Kanwal and R. Begum, e-Polymers, 2014, 14, 313–321 CAS.
- N. Sahiner and S. Yildiz, Fuel Process. Technol., 2014, 126, 324–331 CrossRef CAS.
- Z. Chen, L. Hu and M. J. Serpe, J. Mater. Chem., 2012, 22, 20998–21002 RSC.
- S. N. Sidorov, L. M. Bronstein, V. A. Davankov, M. P. Tsyurupa, S. P. Solodovnikov, P. M. Valetsky, E. A. W. and R. J. Spontak, Chem. Mater., 1999, 11, 3210–3215 CrossRef CAS.
- H. Park, L. Srisombat, A. Jamison, T. Liu, M. Marquez, H. Park, S. Lee, T.-C. Lee and T. Lee, Gels, 2018, 4, 28 CrossRef PubMed.
- N. Sahiner, Colloids Surf., A, 2013, 433, 212–218 CrossRef CAS.
- D. S. A. Simakov and M. Sheintuch, AIChE J., 2011, 57, 525–541 CrossRef CAS.
- L. Shi, Z. Chen, Z. Jian, F. Guo and C. Gao, Int. J. Hydrogen Energy, 2019, 44, 19868–19877 CrossRef CAS.
- T. Turhan, Y. A. Güvenilir and N. Sahiner, Energy, 2013, 55, 511–518 CrossRef CAS.
- N. Sahiner and A. O. Yasar, Int. J. Hydrogen Energy, 2014, 39, 10476–10484 CrossRef CAS.
- F. Seven and N. Sahiner, J. Power Sources, 2014, 272, 128–136 CrossRef CAS.
- N. Sahiner and S. Demirci, Eur. Polym. J., 2016, 76, 156–169 CrossRef CAS.
- N. Sahiner, A. O. Yasar and N. Aktas, Int. J. Hydrogen Energy, 2016, 41, 20562–20572 CrossRef CAS.
- S. Yildiz, N. Aktas and N. Sahiner, Int. J. Hydrogen Energy, 2014, 39, 14690–14700 CrossRef CAS.
- S. B. Khan, F. Ali and A. M. Asiri, Int. J. Hydrogen Energy, 2020, 45, 1532–1540 CrossRef CAS.
- H. Naeem, M. Ajmal, S. Z. Khan, M. N. Ashiq and M. Siddiq, Soft Mater., 2021, 19, 480–494 CrossRef CAS.
- S. Demirci, K. Sel and N. Sahiner, Sep. Purif. Technol., 2015, 155, 66–74 CrossRef CAS.
- N. Sahiner and S. Demirci, Asia-Pac. J. Chem. Eng., 2019, 14, e2305 CrossRef.
- M. Arif, M. Shahid, A. Irfan, J. Nisar, W. Wu, Z. H. Farooqi and R. Begum, RSC Adv., 2022, 12, 5105–5117 RSC.
- M. Farooq, J. Ihsan, S. Saeed, A. Haleem and M. Siddiq, J. Inorg. Organomet. Polym. Mater., 2021, 315(31), 2030–2042 CrossRef.
- M. Ajmal, M. Siddiq, H. Al-Lohedan and N. Sahiner, RSC Adv., 2014, 4, 59562–59570 RSC.
- F. Bibi, M. Ajmal, F. Naseer, Z. H. Farooqi and M. Siddiq, Int. J. Environ. Sci. Technol., 2017, 15, 863–874 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |

