Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Insight into the exemplary structural, elastic, electronic and optical nature of GaBeCl3 and InBeCl3: a DFT study
Saima Ahmad Shahab,
Mudasser Husainc,
Nasir Rahman *b,
Mohammad Sohailc,
Rajwali Khanc,
Abid Ali Khand,
Asad Ullahe,
Shaimaa A. M. Abdelmohsenf,
Ashraf M. M. Abdelbackig,
Ahmed M. El-Sabrout
*b,
Mohammad Sohailc,
Rajwali Khanc,
Abid Ali Khand,
Asad Ullahe,
Shaimaa A. M. Abdelmohsenf,
Ashraf M. M. Abdelbackig,
Ahmed M. El-Sabrout h,
Hosam O. Elansaryi and
Aurangzeb Khan*bj
h,
Hosam O. Elansaryi and
Aurangzeb Khan*bj
aDepartment of Physics, Shaheed Benazir Bhutto Women University, Peshawar, Pakistan
bDepartment of Physics, Abdul Wali Khan University, Mardan, Pakistan. E-mail: nasir@ulm.edu.pk; akhan@awkum.edu.pk
cDepartment of Physics, University of Lakki Marwat, 28420, Lakki Marwat, Khyber Pukhtunkhwa, Pakistan
dDepartment of Chemical Sciences, University of Lakki Marwat, 28420, Lakki Marwat, Khyber Pukhtunkhwa, Pakistan
eDepartment of Physics, College of Science, Princess Nourah Bint Abdulrahman University, P. O. Box 84428, Riyadh 11681, Saudi Arabia
fDeanship of Skills Development, King Saud University, Riyadh 11451, Saudi Arabia
gApplied Studies and Community Service College, King Saud University, Riyadh, 11451, Saudi Arabia
hDepartment of Applied Entomology and Zoology, Faculty of Agriculture (EL-Shatby), Alexandria University, Alexandria, 21545, Egypt
iPlant Production Department, College of Food and Agriculture Sciences, King Saud University, Riyadh, 11451, Saudi Arabia
jUniversity of Lakki Marwat, 28420, Lakki Marwat, Khyber Pukhtunkhwa, Pakistan
First published on 15th March 2022
Abstract
In the scheme of density functional theory (DFT), Structural, elastic, electronic, and optical properties calculations of GaBeCl3 and InBeCl3 are carried out using Tran–Blaha modified Becke–Johnson exchange potential approximation (TB-mBJ) installed in Wein2k software. Structurally the compounds of interest are found to be stable. Both compounds possess elastic stability, anisotropy, and ductility determined by the elastic studies. The electronic-band structure analysis shows the semiconductor nature of GaBeCl3 and InBeCl3 compounds with indirect band gaps of ∼3.08 eV for GaBeCl3 and ∼2.04 eV for InBeCl3 along with the symmetrical points from (X–Γ). The calculated total density of states (TDOS) and partial density of states (PDOS) of these compounds reveal that for the GaBeCl3 compound, the contribution of Ga (4p) and Cl (3p) orbital states in the valence, as well as the conduction band, is dominant. While for InBeCl3, the contribution of Cl (3p) states as well as In (5s) is large in the valence band and in that of Cl (3p-states) states in the conduction band. The type of chemical bonding is found to be ionic in both compounds. The optical properties i.e., the real (ε1(ω)) and imaginary (ε2(ω)) parts of dielectric function, refractive index n(ω), optical reflectivity R(ω), optical conductivity σ(ω), absorption coefficient α(ω), energy loss L(ω) and electron extinction coefficient k(ω) are also discussed in terms of optical spectra. It is reported that n(ω) and k(ω) exhibit the same characteristics as ε1(ω) and ε2(ω) respectively. Efficient application of these materials can be seen in semiconducting industries and many modern electronic devices.
Introduction
It is well known that perovskite compounds show significant diversity in mechanical,1,2 electrical,3,4 magnetic,5 optical,6,7 and transport properties8–12 i.e., magnetism, ferroelectricity, multiferroicity, superconductivity, piezoelectricity, colossal magnetoresistance, etc.8–16 And due to these properties, perovskites are the key materials for several technological applications including optoelectronic, photonic, and spintronic appliances i.e., a promising candidate for radiation detection, photovoltaic absorbance and a laser medium.17–21 Literally, the flexible crystal structure and elements of which a perovskite is composed of are responsible for some of the characteristics of these perovskites.22–24 An ideal perovskite having a cubic crystal structure has the general formula ABX3, in which, “A” (occupying the corners) and “B” (occupying the center) are metallic cations while X (occupying the face centers) is an oxide or halide i.e., Cl, Br, I and F. The cubic perovskites are considered as the most stable. It is observed experimentally and theoretically by many scientists that such type of perovskites exhibit the cubic structure possessing the space group p![[m with combining macron]](https://www.rsc.org/images/entities/i_char_006d_0304.gif) 3m (#221).25,26
3m (#221).25,26
Metal halide perovskites have several similar phenomena, which exist in the oxide perovskites. Although many perovskites have been found experimentally, the huge number of possible combinations of three chemical elements, the perovskites family may be vast, and possibly many systems are still waiting for researcher discovery.
This work is based on the study of the structural, elastic, electronic, and optical properties of GaBeCl3 and InBeCl3 perovskites. So far, there has been no experimental or theoretical study on these perovskite materials. Therefore, the work of this paper can be used for reference in the future.
Computational methodology
The following investigation is performed using TB-mBJ in the framework of DFT, implemented in the WIE2K code.27 The TB-mBJ potential is used to investigate the elastic, electronic, and optical properties. Structural properties are computed by fitting the Birch–Murnaghan equation of state (EOS), in which the unit cell volume versus unit cell energy is fitted to attain the ground state parameters. The value of RMT is chosen such that no charge leaks out of the sphere. The energy difference of −6 Ry is taken between the core and valence band. The RMT × Kmax cutoff is chosen to be 7. The IRelast package28 is used for the computation of elastic properties. For precise, reliable, and better results, 2000 k-points are taken for computation of all the properties.Results and discussions
Structural characteristics
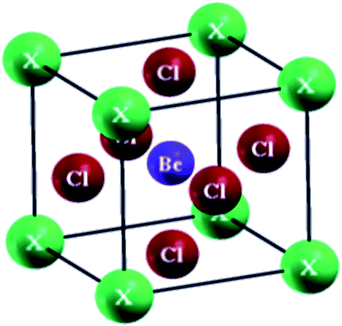
The perovskites compounds XBeCl3 have an ideal cubic structure exhibiting the space group p![[m with combining macron]](https://www.rsc.org/images/entities/i_char_006d_0304.gif) 3m (#221). The occupied Wyckoff positions in the elementary cell are (0, 0, 0), (0.5, 0.5, 0.5) and (0, 0.5, 0.5) as depicted in Fig. 1. The volume vs. energy is fitted by the Birch–Murnaghan equation of state (EOS) as shown in Fig. 2.29 From this fit, the determined ground state characteristics i–e the lattice constant (ao), bulk modulus (B), the minimum energy (Eo), and its pressure derivative (B′) are given in the table. No previous experimental or theoretical results are available for the GaBeCl3 and InBeCl3 compounds for comparison with the current calculations (Table 1).
3m (#221). The occupied Wyckoff positions in the elementary cell are (0, 0, 0), (0.5, 0.5, 0.5) and (0, 0.5, 0.5) as depicted in Fig. 1. The volume vs. energy is fitted by the Birch–Murnaghan equation of state (EOS) as shown in Fig. 2.29 From this fit, the determined ground state characteristics i–e the lattice constant (ao), bulk modulus (B), the minimum energy (Eo), and its pressure derivative (B′) are given in the table. No previous experimental or theoretical results are available for the GaBeCl3 and InBeCl3 compounds for comparison with the current calculations (Table 1).
| Compounds | ao | B | B′ | Eo |
|---|---|---|---|---|
| GaBeCl3 | 4.621 | 65.578 | 5.371 | −6687.0977 |
| InBeCl3 | 4.586 | 193.255 | 42.380 | −14565.4784 |
Elastic characteristics
The elastic constants are basic and necessary parameters used to describe the mechanical properties of materials.30 For cubic systems, there are three completely independent elastic constants i–e C11, C12, and C44.31 Using these elastic constants, the other parameters e.g. G, ν, A, E, and B/G can be calculated using Voigt–Reuss–Hill eqn (1)–(6).32–34
 | (1) |
 | (2) |
 | (3) |
 | (4) |
 | (5) |
 | (6) |
The “B” and “G” determine the hardness of the materials.35 Similarly, the material will be brittle, if υ <0.26 and B/G <1.75 otherwise it will exhibit a ductile nature.36 Here both the compounds show the ductile nature. Furthermore, as for isotropic nature, the value of “A” should be equal to 1, if not then the material will have anisotropic nature. Therefore, GaBeCl3 and InBeCl3 compounds show anisotropic properties. All the obtained results are summarized in Table 2.
| Compounds | C11 | C12 | C44 | A | G | E | v | B/G |
|---|---|---|---|---|---|---|---|---|
| GaBeCl3 | 94.9636 | 48.1999 | 52.4496 | 2.2432 | 38.6754 | 96.9643 | 0.3515 | 1.6956 |
| InBeCl3 | 332.7265 | 107.3298 | 161.7959 | 1.4356 | 139.9710 | 338.2573 | 0.3582 | 1.3807 |
Electronic characteristics
To study the electronic properties of the compounds, their energy band structures along the highly symmetrical points in the first Brillouin zone are calculated. Fig. 3(a) and (b) illustrates the band-structures of GaBeCl3 and InBeCl3 respectively. It can be observed that in both the compounds, the maximum of the valence band and minimum of the conduction band is located at X and Γ and the two bands do not overlap each other. Therefore, the material GaBeCl3 can be regarded as semiconducting which possessing an indirect band gap of ∼3.08 eV, while InBeCl3 possesses a band gap of 2.04 eV and thus a semiconductor.To better understand the electronic properties, the TDOS and PDOS are calculated for both compounds. Fig. 4(a–d) illustrates the TDOS and PDOS for the GaBeCl3 compound. TDOS is divided into three regions. In the first region from −7 to −1, Cl (p-states) contributes the maximum. In the second region from −1 to 0, the contribution of Ga (4p-states), as well as Cl (3p-states) atoms, is large. These are the regions below the Fermi energy (EF). Above EF, again the contribution of Ga (4p-states) and Cl (3p-states) atoms is large. Consequently, in both the valence band as well as the conduction band Ga and Cl, p orbital states are maximum. Similarly, Fig. 4(e–h) shows the TDOS and PDOS for the InBeCl3 compound. Here the contribution of Cl (3p-states) is large in the energy range of −12 to −3 eV. However, in the region from −3 to 0, In (5s-states) atoms contribution is large. In addition, a Cl (3p-states) atom also makes a certain contribution. Above EF, in the energy range from 3–8 eV, Cl (3p-states) atoms participation is maximum. Hence the contribution of states in the valence band of InBeCl3 is due to Cl (3p-states) and In (5s-states) and in that of the conduction band is due to the Cl (3p-states) only. The result of TDOS and PDOS confirms the band structure reports.
The electronic properties or the band gap varies with the change in A-site, B-site and X-site (halide) ion. As well as when the calculations method is changed. Several examples are listed in Table 3.
Optical properties
The response of the materials to the incident photons are calculated in the energy range 0–40 eV. The complex dielectric function (eqn (7)) can be separated into two parts i–e real (ε1(ω)) and imaginary (ε2(ω)) part.39| ε(ω) = ε1(ω) + iε2(ω) | (7) |
Here ε1(ω) and ε2(ω) are related to the absorption and the band structure respectively.40,41 Fig. 5(a and c) illustrates ε1(ω) and ε2(ω) spectrum respectively. From Fig. 5(a) it can be seen that the threshold energy or absorption edge20 for GaBeCl3 and InBeCl3 is located at ∼7.178 eV and ∼3.27 eV respectively. Similarly, the static dielectric constant ε1(0) (5(c)) for GaBeCl3 and InBeCl3 is situated at 5.1 and 8.08, while the maximum peaks are found at 4.37 and 3.10 after this a decrease can be seen in both the plots, following another maximum.
 | ||
| Fig. 5 Calculated (a) ε1(ω), (b) n(ω), (c) ε2(ω) (d) k(ω) (e) R(ω) (f) σ(ω) (g) L(ω) and (h) α(ω) of XBeCl3 compounds. | ||
Fig. 5(b) shows n(ω) for both the compounds the static refractive index n(0) is found to be 2.32 and 2.88 for GaBeCl3 and InBeCl3 respectively. The value of n(ω) increases and reaches a maximum value of 3.27 at 4.40 and 4.26 at 3.1 eV. This lies in the UV region of the light spectrum. Furthermore, it is observed that behavior of n(ω) and k(ω) is similar to ε1(ω) and ε2(ω) for both the compounds.
Fig. 5(e) displays the R(ω) spectra for both the compounds. The results depict the maximum reflectivity of 38% and 48% at 19.24 eV and 3.3 eV for GaBeCl3 and InBeCl3 respectively. Similarly, Fig. 5(f–h) depicts (σ(ω), L(ω)) and α(ω) for GaBeCl3 and InBeCl3 compounds. The highest conductivity i–e 6.71 Ω−1 cm−1 is obtained at 7.04 eV and 9.98 Ω−1 cm−1 at 10.50 eV for GaBeCl3 and InBeCl3 respectively. The resonant energy losses are observed at 23.25 eV and 25.97 eV for both the compounds respectively. In addition, the point at which the material starts absorption is found to be at 2.23 eV and 3.23 eV for GaBeCl3 and InBeCl3 compounds respectively.
Conclusion
In summary, the TB-mBJ approximation based on DFT is used to investigate the structural, elastic, electronic, and optical properties of ABeCl3 (A = Ga, In). It is reported that with the change in cation from Ga to In, the lattice constant and ground state energy decreases while bulk modulus and pressure derivative of bulk modulus increase. The elastic properties confirmed the anisotropic and ductile nature of both the compounds. Furthermore, the studied compounds have mechanical stability which is the key requirement for applications in high-performance electronic devices. The ionic type of chemical bonding is present in the compounds. In addition, the investigated electronic-band structure reveals the narrow band-gap semiconducting nature of the compounds with the indirect gap of ∼3.1 eV along (X–Γ) for GaBeCl3 and InBeCl3compounds. The results of TDOS and PDOS for GaBeCl3 and InBeCl3 compound shows that in both the valence band as well as the conduction band Ga (4p-states) and Cl (3p-states) atoms contribution are maximum for GaBeCl3. However, in InBeCl3 the contribution of Cl (3p-states) and In (5s-states) in the conduction band and that of Cl (3p-states) in the valence band is large. In addition, the optical properties of GaBeCl3 and InBeCl3 are calculated in the photon energy range of 0–40 eV. It is reported that n(ω) and k(ω) exhibit the same characteristics as ε1(ω) and ε2(ω) respectively. The computed properties of this work must be considered to understand and utilize the possible technical benefits in device manufacturing in semiconducting industries. Optical properties are of great significance in radiation detection and laser technology.Conflicts of interest
There are no conflicts of interest.Acknowledgements
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R61) Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.References
- M. S. Ahmad, A. Habib, A. Rauf, M. U. Haq, J. Saddique, M. Nisar, M. U. Rehman and M. Imran, Theoretical investigation, 2020, 1, 1 Search PubMed.
- N. Rahman, M. Husain, J. Yang, G. Murtaza, M. Sajjad, A. Habib, A. Karim, M. U. Haq, A. Rauf and M. Nisar, J. Supercond. Novel Magn., 2020, 33, 3915 CrossRef CAS.
- N. Rahman, M. Husain, J. Yang, M. Sajjad, G. Murtaza, M. U. Haq, A. Habib, A. Rauf, A. Karim and M. Nisar, Eur. Phys. J. Plus, 2021, 136, 1 CrossRef.
- S. U. Zaman, N. Rahman, M. Arif, M. Saqib, M. Husain, E. Bonyah, Z. Shah, S. Zulfiqar and A. Khan, AIP Adv., 2021, 11, 015204 CrossRef CAS.
- M. Husain, M. S. Ahmad, N. Rahman, M. Sajjad, A. Rauf, A. Habib, M. U. Haq, M. Nisar, A. Hussain and M. Imran, Fluoride, 2020, 53, 657 Search PubMed.
- M. Husain, N. Rahman, A. H. Reshak, A. Habib, S. Ali, A. Laref, A. M. Al Bakri and J. Bila, Eur. Phys. J. Plus, 2021, 136, 1 CrossRef.
- M. Husain, N. Rahman, R. Khan, S. Zulfiqar, S. A. Khattak, S. N. Khan, M. Sohail, A. Iqbal, A. H. Reshak and A. Khan, Int. J. Energy Res., 2022, 46, 2446–2453 CrossRef CAS.
- T. Y. Ansell and D. P. Cann, Mater. Lett., 2012, 80, 87 CrossRef CAS.
- M. E. Lines and A. M. Glass, Principles and applications of ferroelectrics and related materials, Oxford university press, 2001 Search PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050 CrossRef CAS PubMed.
- J. Wang, J. Neaton, H. Zheng, V. Nagarajan, S. Ogale, B. Liu, D. Viehland, V. Vaithyanathan, D. Schlom and U. Waghmare, Science, 2003, 299, 1719 CrossRef CAS PubMed.
- S. Aharon, A. Dymshits, A. Rotem and L. Etgar, J. Mater. Chem. A, 2015, 3, 9171 RSC.
- M. Eibschütz and H. Guggenheim, Solid State Commun., 1968, 6, 737 CrossRef.
- N. A. Spaldin and R. Ramesh, Nat. Mater., 2019, 18, 203 CrossRef CAS PubMed.
- R. Dubrovin, A. Garcia-Castro, N. Siverin, N. Novikova, K. Boldyrev, A. H. Romero and R. Pisarev, Phys. Rev. B, 2021, 104, 144304 CrossRef CAS.
- F. Pascale, P. D’arco, V. Lacivita and R. Dovesi, J. Phys.: Condens. Matter, 2021, 34, 074002 CrossRef PubMed.
- D. Sardar, W. Sibley and R. Alcala, J. Lumin., 1982, 27, 401 CrossRef.
- A. Gektin, I. Krasovitskaya and N. Shiran, J. Lumin., 1997, 72, 664 CrossRef.
- M. Mortier, J. Gesland and M. Rousseau, Solid State Commun., 1994, 89, 369 CrossRef CAS.
- A. Mubarak and A. Mousa, Comput. Mater. Sci., 2012, 59, 6 CrossRef CAS.
- Y. Fu, H. Zhu, J. Chen, M. P. Hautzinger, X.-Y. Zhu and S. Jin, Nat. Rev. Mater., 2019, 4, 169 CrossRef CAS.
- A. G. Ricciardulli, S. Yang, J. H. Smet and M. Saliba, Nat. Mater., 2021, 1 Search PubMed.
- T. Neumann, S. Feldmann, P. Moser, A. Delhomme, J. Zerhoch, T. van de Goor, S. Wang, M. Dyksik, T. Winkler and J. J. Finley, Nat. Commun., 2021, 12, 1 CrossRef PubMed.
- K. Koteras, J. Gawraczyński, M. Derzsi, Z. Mazej and W. Grochala, Chemistry, 2021, 3, 94 CrossRef CAS.
- G. Vaitheeswaran, V. Kanchana, R. S. Kumar, A. Cornelius, M. Nicol, A. Svane, A. Delin and B. Johansson, Phys. Rev. B, 2007, 76, 014107 CrossRef.
- G. Vaitheeswaran, V. Kanchana, R. S. Kumar, A. Cornelius, M. Nicol, A. Svane, N. Christensen and O. Eriksson, Phys. Rev. B, 2010, 81, 075105 CrossRef.
- R. M. Almeida, A. L. da Rosa, T. Frauenheim and J. S. de Almeida, Phys. Status Solidi B, 2019, 256, 1800380 CrossRef.
- M. Jamal, M. Bilal, I. Ahmad and S. Jalali-Asadabadi, J. Alloys Compd., 2018, 735, 569 CrossRef CAS.
- F. Murnaghan, Proc. Natl. Acad. Sci. U. S. A., 1944, 30, 244 CrossRef CAS PubMed.
- K. E. Babu, N. Murali, K. V. Babu, P. T. Shibeshi and V. Veeraiah, Acta Phys. Pol., A, 2014, 125, 1179 CrossRef.
- M. Harmel, H. Khachai, A. Haddou, R. Khenata, G. Murtaza, B. Abbar, S. B. Omran and M. Khalfa, Acta Phys. Pol., 2015, 128, 34 CrossRef CAS.
- C.-S. Man and M. Huang, J. Elasticity, 2011, 105, 29 CrossRef.
- A. Ballato, Voigt-Reuss-Hill moduli for ferroelectric aggregates, 2006 15th ieee international symposium on the applications of ferroelectrics, IEEE, 2006, p. 208 Search PubMed.
- C.-S. Man and M. Huang, J. Elasticity, 2012, 106, 105 CrossRef.
- M. H. Sadd, Elasticity: Theory, Applications and Numerics, Elsevier Academic Press, Burlington, MA, 2005, pp. 123–259 Search PubMed.
- D. Pettifor, Mater. Sci. Technol., 1992, 8, 345 CrossRef CAS.
- G. Vaitheeswaran, V. Kanchana, X. Zhang, Y. Ma, A. Svane and N. Christensen, J. Phys.: Condens. Matter, 2016, 28, 315403 CrossRef CAS PubMed.
- R. B. Behram, M. Iqbal, S. Alay-e-Abbas, M. Sajjad, M. Yaseen, M. I. Arshad and G. Murtaza, Mater. Sci. Semicond. Process., 2016, 41, 297 CrossRef CAS.
- M. Fox, Optical Properties of Solids, Oxford University Press Inc., New York, 2001, p. 25 Search PubMed.
- N. V. Smith, Phys. Rev. B, 1971, 3, 1862 CrossRef.
- Z. Feng, H. Hu, S. Cui, W. Wang and C. Lu, Open Phys., 2009, 7, 786 CAS.
| This journal is © The Royal Society of Chemistry 2022 |