Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Free-standing conductive hydrogel electrode for potentiometric glucose sensing†
Shogo Himori and
Toshiya Sakata *
*
Department of Materials Engineering, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656, Japan. E-mail: sakata@biofet.t.u-tokyo.ac.jp
First published on 14th February 2022
Abstract
Flexible conductive polymer hydrogels are attracting attention as an electrode material. Electrochemical biosensors with conductive polymer hydrogels have been developed because they have some advantages such as biocompatibility, high conductivity, 3D nanostructure, solvated surface, and enlarged interface. Conductive polymer hydrogels bearing receptor molecules such as enzymes in its 3D nanostructure enable the detection of target analytes with high sensitivity. However, because such hydrogels are fragile, they cannot stand on their own and a supporting substrate is required to fabricate them. This means that the loss of mechanical toughness is detrimental for their application to flexible biosensors. In this study, we have proposed a free-standing conductive hydrogel electrode with no coating on a substrate, which is composed of polyaniline with phenyl boronic acid including polyvinyl alcohol, for potentiometric glucose sensing. In addition, its electrical responsivity to glucose has been confirmed by investigating its mechanical properties at various glucose concentrations, considering the hydrogel compositions.
1. Introduction
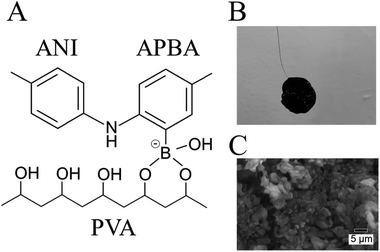
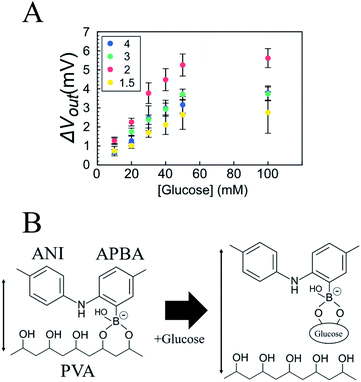
Electrochemical biosensors are attractive tools for measuring biomarkers in humans for medical diagnosis. Electrochemical methods are conceptually based on a potentiometric or amperometric measurement principle. Potentiometric biosensors such as field-effect transistors (FETs) enable the direct detection of biomolecular charges based on biomolecular recognition events without redox reactions, regardless of their molecular sizes; therefore, such potentiometric biosensors are suitable for the direct detection of small biomolecules.1,2 As electrode materials, metal, carbon, oxide films, and so forth are often used.3–6 However, these electrode materials have a problematic issue of flexibility in the human body owing to their stiffness. Therefore, flexible conductive polymer hydrogels are attracting attention as an alternative electrode material.7,8 Moreover, conductive hydrogels have recently been developed for energy9,10 and biomedical applications.11,12 Electrochemical biosensors with conductive polymer hydrogels have been developed because they have some advantages such as biocompatibility, high conductivity, 3D nanostructure, solvated surface, and enlarged interface.13 Conductive polymer hydrogels bearing enzymes in its 3D nanostructure enable the detection of target analytes with high sensitivity.14,15 However, because such hydrogels are fragile, they cannot stand on their own and a supporting substrate is required to fabricate them. This means that the loss of mechanical toughness is detrimental for their application to flexible biosensors. In addition, the supporting substrate interferes with the utilization of hydrogels with their flexibility maintained and is a hurdle for the miniaturization of devices. Moreover, the contact between the hydrogel and the supporting electrode complicates the fabrication process. This is why the interface may be easily broken by some mechanical stimulations. Thus, mechanical toughness is necessary for the long-term stability toward a practical usage of flexible conductive polymer hydrogels. In this context, a free-standing conductive polymer hydrogel (FSC hydrogel) with high mechanical toughness can be applied to electrochemical biosensors.Recently, FSC hydrogels have been developed for supercapacitors.16–19 One of the synthetic routes for the FSC hydrogels is based on in situ polymerization of monomers in an insulative matrix polymer.17 In particular, polyaniline (PANI), which is used as a conductive polymer, shows the expected electrochemical properties in polyvinyl alcohol (PVA) as an insulative matrix.19 In this synthetic route, amino phenylboronic acid (APBA) is copolymerized with ANI [P(ANI-APBA)] and used as a cross-linker with the PVA matrix on the basis of the specific binding between PBA and diol molecules such as PVA (Fig. 1A). The mechanical properties of the P(ANI-APBA)-PVA-based FSC hydrogels can be controlled by adjusting the composition ratio of PVA to copolymerized (ANI + APBA) because they cannot stand on their own. In particular, we pay attention to PBA as a recognition site of small biomolecules such as glucose and dopamine, which have diol groups in their chemical structures.20,21 It means the PBA–biomolecule binding, which induces molecular charges,21 contributes to a specific biomolecular recognition for next-generation electrochemical biosensors in an enzyme-free manner.1 On the other hand, the hydrogel-based biosensors with PBA have been recently developed for glucose detection in optical sensors.22–27 In these systems, the specific binding of glucose to PBA increases the density of negative charges and then induces repulsive force in the hydrogels; as a result, the hydrogels swell with increasing glucose concentration, resulting in changes in the optical properties of hydrogels. Thus, P(ANI-APBA)-PVA-based FSC hydrogels may enable the electrochemical detection of glucose as well.
In this study, an FSC hydrogel electrode was fabricated from ANI, APBA, and PVA in a one-step facile process, and its electrochemical responsivity toward glucose was investigated using a FET potential measurement system. In addition, its mechanical properties were analyzed by atomic force microscopy (AFM) to investigate their correlation with its electrochemical properties for glucose detection. This study can contribute to the utilization of conductive hydrogel biosensors with high mechanical toughness.
2. Experimental
2.1 Chemicals
Ammonium persulfate (APS), hydrochloric acid (HCl), aminophenyl boronic acid (APBA), aniline (ANI), polyvinyl alcohol (PVA), and glucose were purchased from Wako Pure Chemical Industries, Ltd. Phosphate-buffered saline (1× PBS, pH 7.4) was purchased from Thermo Fisher Scientific Inc.2.2 Hydrogel electrode fabrication
For the fabrication of free-standing conductive hydrogel (FSC hydrogel), two types of solution (A and B) were prepared.19 Solution A was prepared by mixing 0.2 mmol of APS and 100 μl of HCl. Solution B was prepared by mixing 10.5 μmol of APBA, 0.15 mmol of ANI, PVA, and 400 μl of HCl. To investigate the effect of PVA amount on the free-standing property, solution B with 0.24, 0.32, 0.48, or 0.64 mmol of PVA was prepared. 18.8 μl of solution A and 100 μl of solution B were mixed and allowed to react overnight at 0 °C. The molar ratio of APBA + ANI to APS was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and that of APBA + ANI to PVA was 1
1 and that of APBA + ANI to PVA was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x (x = 1.5, 2, 3, 4). Unless otherwise noted, the ratio of APBA + ANI to PVA was fixed at 1
x (x = 1.5, 2, 3, 4). Unless otherwise noted, the ratio of APBA + ANI to PVA was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The reaction solution was sandwiched at the top and the bottom by 12 mm-diameter round cover glasses (Matsunami Glass Ind., Ltd.) to keep the gel shape uniform, and part of the 0.10 mm-diameter platinum wire (The Nilaco Corporation) was immersed in the reaction solution to connect the gel to the measurement device. After gelation, the gel was kept in PBS for further experiments.
2. The reaction solution was sandwiched at the top and the bottom by 12 mm-diameter round cover glasses (Matsunami Glass Ind., Ltd.) to keep the gel shape uniform, and part of the 0.10 mm-diameter platinum wire (The Nilaco Corporation) was immersed in the reaction solution to connect the gel to the measurement device. After gelation, the gel was kept in PBS for further experiments.
2.3 Electrochemical measurement
An electrochemical analyzer (618E, CH Instruments) was used to investigate the electrochemical properties of gel electrodes. The Ag/AgCl electrode was immersed in saturated KCl solution as a reference electrode. Pt was used as a counter electrode. The gel electrode was connected to the analyzer through a Pt wire.Specific capacitance was calculated using the following equation using the result of cyclic voltammetry.28
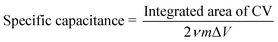 | (1) |
For the FET potential measurement, the FSC hydrogel electrode was connected to the gate electrode of a silicon-based n-channel junction-type FET (K246, Toshiba), which is called an extended-gate FET, and a gate voltage was applied through the Ag/AgCl reference electrode (Fig. S1†). The surface potential at the FSC hydrogel gate electrode (Vout) was measured in real time using a FET real-time monitoring system (PROVIGATE Inc.). In this study, the gate voltage (VG), drain voltage (VD), and drain–source current (IDS) were set to constant values, and the change in Vout (ΔVout) at the gate electrode was measured using a source follower circuit. In the electrical measurement, the reference electrode and the FSC hydrogel were put in 10 ml of PBS (Fig. S1A†). ΔVout was monitored under the constant conditions of IDS = 700 μA and VG = 0 V. After the surface potential stabilized, small biomolecules were gradually titrated from a low concentration. To suppress the spike signals induced upon the addition of the sample, the introduced volume was 1/10 of the total volume of the measurement solution.
2.4 Force curve measurement by AFM
Force curve analysis was performed by atomic force microscopy (AFM; 5500 AFM, Keysight). The spring constant of the cantilever was 0.06 N m−1 and the radius of curvature was 20 nm with NP-O10 (Veeco) made of Si3N4. The FSC hydrogel was analyzed in the liquid cell with PBS. The force curve was taken from 4 × 4 points in 1 μm2 of each FSC hydrogel sample and used for calculation after omitting inconsistent curves. Each hydrogel sample was measured after immersing into each glucose solution for over 30 min for glucose to be absorbed into the hydrogel sufficiently.3. Results and discussion
The P(ANI-APBA)-PVA-based FSC hydrogel was formed with a 12 mm-diameter cylinder shape (Fig. 1B). The gel surface appeared flat, but the porous structure was observed by scanning electron microscopy (SEM) (Fig. 1C). Considering the adhesiveness of PVA in the P(ANI-APBA)-PVA-based FSC hydrogel, it was connected to the platinum wire as a gate electrode by physical bonding. The chemical composition and structure of the hydrogel were analyzed by attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR). From the FTIR spectra (Fig. S2†), the characteristic peaks of PANI appeared at 1540 and 1500 cm−1, as derived from the vibrations of the quinoid and benzenoid rings, respectively.29 In addition, the peaks at 2920 and 1020 cm−1 were derived from the vibrations of C–H and C–O of PVA, respectively,30 and the peak of 690 cm−1 was attributed to the O–B–O bending of APBA.31 To investigate its free-standing property, force curve measurement was conducted by AFM. In the force curve analysis, the force is recorded when a cantilever is contacted with or retracted from the hydrogel.32 In the approaching curve, the cantilever pushes the hydrogel, resulting in the depression of the hydrogel as the force increases. On the other hand, in the retraction curve, the adhesion of the cantilever to the hydrogel caused the hydrogel to rise after the depression returned, and a negative force was observed (Fig. S3†). The maximum adhesion force (Fad) in the retraction force curve was calculated using the following eqn (2) based on the Johnson–Kendall–Roberts model:32
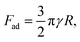 | (2) |
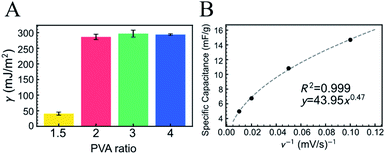 | ||
| Fig. 2 (A) Surface free energy (γ) of FSC hydrogels with varying PVA ratios. The molar ratio of PVA to copolymerized (ANI + APBA) was 1.5, 2, 3, or 4. γ was calculated using eqn (2) for the retraction force curve. The error bar shows the standard error of the mean and the measurement points were over 9. (B) Specific capacitance of FSC hydrogel for scan rate (ν) of cyclic voltammetry in PBS. The hydrogel with the PVA ratio of 2 was used. | ||
The electrochemical property of the P(ANI-APBA)-PVA-based FSC hydrogel was examined by cyclic voltammetry (CV) in PBS (Fig. 2B and S4†). The specific capacitance of the P(ANI-APBA)-PVA-based FSC hydrogel with the PVA ratio of 2 was calculated using eqn (1) from the CV diagram (Fig. S4†) and analyzed for the sweep rate, as shown in Fig. 2B.28 The specific capacitance changed, depending on the reciprocal of the scan rate (ν−1), when other factors such as the mass and resistance of the electrode were constant. However, the specific capacitance was significantly smaller at a higher scan rate than at a lower one. This is a specific characteristic of porous materials owing to the change in the reactive area.36,37 At a higher scan rate, few ions in the measurement solution diffused into the electrode matrix; therefore, only the outer surface of P(ANI-APBA)-PVA-based FSC hydrogel electrochemically reacted with ions, resulting in the smaller specific capacitance. On the other hand, the lower scan rate contributed to the generation of greater electrical doping inside the porus hydrogel; therefore, the conductivity of the hydrogel increased, that is, the specific capacitance increased. Thus, the scan rate dependence of specific capacitance was useful for evaluating the porosity of FSC hydrogels.
The electrical responsivity of the P(ANI-APBA)-PVA-based FSC hydrogel electrode to glucose was investigated using the FET potential measurement system. The P(ANI-APBA)-PVA-based hydrogel was used as a gate electrode for an extended-gate FET (Fig. S1†). The change in the potential (ΔVout) of the P(ANI-APBA)-PVA-based FSC hydrogel electrode with the change in glucose concentration is shown in Fig. 3A. From the result, the potential increased with increasing glucose concentration, regardless of the PVA ratio. This may be because the capacitance of the FSC hydrogels increased upon adding glucose to them. ΔVout was output with a source-follower circuit in this FET measurement system (see ESI†), on the basis of eqn (3) and (4):38
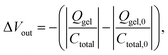 | (3) |
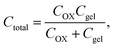 | (4) |
Furthermore, we also examined the reactivity of P(ANI-APBA)-PVA-based FSC hydrogel with glucose on the basis of the mechanical measurement by AFM. The change in the Young's modulus (E) of the FSC hydrogel with the change in the glucose concentration was evaluated from the AFM force curve. On the basis of the Hertz model, E is obtained, according to eqn (5) from the approaching curve of AFM (Fig. S6†):40
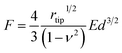 | (5) |
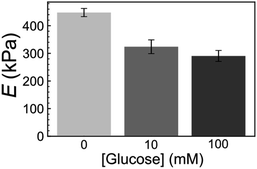 | ||
| Fig. 4 Young's modulus (E) of FSC hydrogel with PVA ratio of 2 at various glucose concentrations. E was calculated from eqn (5) using the approach force curve. The error bar shows the standard error of the mean and the measurement points were over 9. | ||
4. Conclusions
In conclusion, we developed the P(ANI-APBA)-PVA-based FSC hydrogel electrode, which included the cross-links between PBA and PVA, and investigated its reactivity to glucose as a small biomolecule, focusing on both electrical and mechanical properties. In the electrical measurement using the extended-gate FET, the gate potential at the P(ANI-APBA)-PVA-based FSC hydrogel gate electrode increased with increasing glucose concentration, which was induced by increase in the capacitance of the FSC hydrogel owing to its swelling. In addition, the reactivity of the P(ANI-APBA)-PVA-based FSC hydrogel electrode to glucose was also clarified from its mechanical properties on the basis of the AFM force curve. The P(ANI-APBA)-PVA-based FSC hydrogel is suitable as a base electrode of electrochemical biosensors because it enables further chemical modifications of biorecognition units in/on its structure in the solution.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by JSPS KAKENHI Grant Number JP20J21302.Notes and references
- T. Sakata, ACS Omega, 2019, 4, 11852–11862 CrossRef CAS PubMed.
- S. Himori, S. Nishitani and T. Sakata, Electrochim. Acta, 2021, 368, 137631 CrossRef CAS.
- T. Sakata, K. Nishimura, Y. Miyazawa, A. Saito, H. Abe and T. Kajisa, Anal. Chem., 2017, 89, 3901–3908 CrossRef CAS PubMed.
- S. Himori, S. Nishitani and T. Sakata, Langmuir, 2019, 35, 3701–3709 CrossRef CAS PubMed.
- N. T. Tung, P. T. Tue, T. Thi Ngoc Lien, Y. Ohno, K. Maehashi, K. Matsumoto, K. Nishigaki, M. Biyani and Y. Takamura, Sci. Rep., 2017, 7, 17881 CrossRef PubMed.
- C. Reiner-Rozman, M. Larisika, C. Nowak and W. Knoll, Biosens. Bioelectron., 2015, 70, 21–27 CrossRef CAS PubMed.
- A. Koklu, D. Ohayon, S. Wustoni, V. Druet, A. Saleh and S. Inal, Chem. Rev., 2021 DOI:10.1021/acsacs.chemrev.1c00395.
- T. Nezakati, A. Seifalian, A. Tan and A. M. Seifalian, Chem. Rev., 2018, 118, 6766–6843 CrossRef CAS PubMed.
- H. H. Hsu, X. Zhang, K. Xu, Y. Wang, Q. Wang, G. Luo, M. Xing and W. Zhong, Chem. Eng. J., 2021, 422, 129499 CrossRef CAS.
- J. Liu, Y. Jia, Q. Jiang, F. Jiang, C. Li, X. Wang, P. Liu, P. Liu, F. Hu, Y. Du and J. Xu, ACS Appl. Mater. Interfaces, 2018, 10, 44033–44040 CrossRef CAS PubMed.
- Y. Liu, J. Liu, S. Chen, T. Lei, Y. Kim, S. Niu, H. Wang, X. Wang, A. M. Foudeh, J. B. H. Tok and Z. Bao, Nat. Biomed. Eng., 2019, 3, 58–68 CrossRef CAS PubMed.
- C. Rinoldi, M. Lanzi, R. Fiorelli, P. Nakielski, K. Zembrzycki, T. Kowalewski, O. Urbanek, V. Grippo, K. Jezierska-Woźniak, W. Maksymowicz, A. Camposeo, R. Bilewicz, D. Pisignano, N. Sanai and F. Pierini, Biomacromolecules, 2021, 22, 3084–3098 CrossRef CAS PubMed.
- L. Li, Y. Shi, L. Pan, Y. Shi and G. Yu, J. Mater. Chem. B, 2015, 3, 2920–2930 RSC.
- L. Pan, G. Yu, D. Zhai, H. R. Lee, W. Zhao, N. Liu, H. Wang, B. C.-K. Tee, Y. Shi, Y. Cui and Z. Bao, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 9287–9292 CrossRef CAS PubMed.
- D. Zhai, B. Liu, Y. Shi, L. Pan, Y. Wang, W. Li, R. Zhang and G. Yu, ACS Nano, 2013, 7, 3540–3546 CrossRef CAS PubMed.
- D. Ni, Y. Chen, H. Song, C. Liu, X. Yang and K. Cai, J. Mater. Chem. A, 2019, 7, 1323–1333 RSC.
- Z. Yang, D. Shi, W. Dong and M. Chen, Chem. –A Eur. J., 2020, 26, 1846–1855 CrossRef CAS PubMed.
- Z. Yang, J. Ma, B. Bai, A. Qiu, D. Losic, D. Shi and M. Chen, Electrochim. Acta, 2019, 322, 134769 CrossRef CAS.
- W. Li, F. Gao, X. Wang, N. Zhang and M. Ma, Angew. Chem., Int. Ed., 2016, 55, 9196–9201 CrossRef CAS PubMed.
- T. Kajisa and T. Sakata, ACS Appl. Mater. Interfaces, 2018, 10, 34983–34990 CrossRef CAS PubMed.
- T. Kajisa, W. Li, T. Michinobu and T. Sakata, Biosens. Bioelectron., 2018, 117, 810–817 CrossRef CAS PubMed.
- M. Elsherif, M. U. Hassan, A. K. Yetisen and H. Butt, ACS Nano, 2018, 12, 2283–2291 CrossRef CAS PubMed.
- M. Elsherif, M. U. Hassan, A. K. Yetisen and H. Butt, ACS Nano, 2018, 12, 5452–5462 CrossRef CAS PubMed.
- Y. Guan and Y. Zhang, Chem. Soc. Rev., 2013, 42, 8106–8121 RSC.
- C. Zhang, M. D. Losego and P. V. Braun, Chem. Mater., 2013, 25, 3239–3250 CrossRef CAS.
- X. Zhang, Y. Guan and Y. Zhang, Biomacromolecules, 2012, 13, 92–97 CrossRef CAS PubMed.
- V. L. Alexeev, A. C. Sharma, A. V. Goponenko, S. Das, I. K. Lednev, C. S. Wilcox, D. N. Finegold and S. A. Asher, Anal. Chem., 2003, 75, 2316–2323 CrossRef CAS PubMed.
- V. D. Nithya, R. K. Selvan, D. Kalpan, L. Vasylechko and C. Sanjeeviraj, Electrochim. Acta, 2013, 109, 720–731 CrossRef CAS.
- J. Xu, K. Wang, S. Z. Zu, B. H. Han and Z. Wei, ACS Nano, 2010, 4, 5019–5026 CrossRef CAS PubMed.
- S. Majumdar and B. Adhikari, Sens. Actuators, B, 2006, 114, 747–755 CrossRef CAS.
- Y. Huang, M. Zhang and W. Ruan, J. Mater. Chem. A, 2014, 2, 10508–10515 RSC.
- Y. M. Efremov, D. V. Bagrov, M. P. Kirpichnikov and K. V. Shaitan, Colloids Surf., B, 2015, 134, 131–139 CrossRef CAS PubMed.
- M. Levine, G. Ilkka and P. Weiss, J. Polym. Sci., Part B: Polym. Lett., 1964, 2, 915–919 CrossRef CAS.
- K. Grennan, A. J. Killard and M. R. Smyth, Electroanalysis, 2005, 17, 1360–1369 CrossRef CAS.
- O. Ngamna, A. Morrin, A. J. Killard, S. E. Moulton, M. R. Smyth and G. G. Wallace, Langmuir, 2007, 23, 8569–8574 CrossRef CAS PubMed.
- J. Shabani Shayeh, P. Norouzi and M. R. Ganjali, RSC Adv., 2015, 5, 20446–20452 RSC.
- R. K. Sharma and L. Zhai, Electrochim. Acta, 2009, 54, 7148–7155 CrossRef CAS.
- T. Masuda, T. Kajisa, A. M. Akimoto, A. Fujita, K. Nagase, T. Okano, T. Sakata and R. Yoshida, RSC Adv., 2017, 7, 34517–34521 RSC.
- A. Matsumoto, T. Ishii, J. Nishida, H. Matsumoto, K. Kataoka and Y. Miyahara, Angew. Chem., Int. Ed., 2012, 51, 2124–2128 CrossRef CAS PubMed.
- S. Schmidt, M. Zeiser, T. Hellweg, C. Duschl, A. Fery and H. Möhwald, Adv. Funct. Mater., 2010, 20, 3235–3243 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08956k |
| This journal is © The Royal Society of Chemistry 2022 |