 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Retracted Article: The structural and luminescence properties of plexcitonic structures based on Ag2S/L-Cys quantum dots and Au nanorods
Irina G. Grevtseva*a,
Oleg V. Ovchinnikov a,
Mikhail S. Smirnovab,
Aleksey S. Perepelitsa
a,
Mikhail S. Smirnovab,
Aleksey S. Perepelitsa *a,
Tamara A. Chevychelovaa,
Violetta N. Derepkoa,
Anna V. Osadchenkocd and
Alexandr S. Selyukovcde
*a,
Tamara A. Chevychelovaa,
Violetta N. Derepkoa,
Anna V. Osadchenkocd and
Alexandr S. Selyukovcde
aVoronezh State University, Department of Optics and Spectroscopy, Voronezh, Russia. E-mail: Grevtseva_IG@inbox.ru; A-Perepelitsa@yadex.ru
bVoronezh State University of Engineering Technologies, Voronezh, Russia
cBauman Moscow State Technical University, Moscow, Russia
dP.N. Lebedev Physical Institute of the Russian Academy of Sciences, Moscow, Russia
eMoscow Institute of Physics and Technology, Dolgoprudnyi, Moscow Oblast, Russia
First published on 24th February 2022
Abstract
A technique of obtaining plexitonic structures based on Ag2S quantum dots passivated with L-cysteine (Ag2S/L-Cys QDs) in the presence of Au nanorods passivated with cetyltrimethylammonium bromide molecules (Au/CTAB NRs) with controlled luminescence properties has been developed. The structural and luminescence properties of Ag2S/L-Cys QDs with Au/CTAB NRs are studied. The effect of plasmonic Au/CTAB NRs on IR trap state luminescence (750 nm) is considered. It has been found that the direct interaction between the components of the plexcitonic nanostructure leads to a significant luminescence quenching of Ag2S/L-Cys QDs, with the luminescence lifetime being constant. This is the evidence for photoinduced charge transfer. The spatial separation of the components of plexcitonic nanostructures due to the introduction of a polymer – poly(diallyldimethylammonium chloride) (polyDADMAC) provides a means to change their mutual arrangement and achieve an increase in the IR trap state luminescence intensity and a decrease in the luminescence lifetime from 7.2 ns to 4.5 ns. With weak plexcitonic coupling in the nanostructures [Ag2S QD/L-Cys]/[polyDADMAC]/[Au/CTAB NRs], the possibility of increasing the quantum yield of trap state luminescence for Ag2S QDs due to the Purcell effect has been demonstrated. In the case of formation [Ag2S QD/L-Cys]/[polyDADMAC]/[Au/CTAB NRs] a transformation of shallow trap state structure was established using the thermostimulated luminescence method.
1 Introduction
The formation of hybrid nanostructures based on semiconductor colloidal quantum dots (QDs) with unique photoluminescence properties, which are uncharacteristic of individual components, is relevant for photonic applications, such as optoelectronics, photovoltaics, bioimaging, and luminescent sensors.1–9 The luminescence properties of the hybrid nanostructures, such as high luminescence quantum yield and photostability, as well as the radiative lifetime, are key parameters that determine the area and potential for these applications.1–9In recent years, techniques for controlling the luminescence properties of QDs by plasmon–exciton interaction have been actively developed.9–27 It has been found that the “hybrid” luminescence properties of these nanostructures are unique due to their strong dependence on the features of the direct interaction between components of plasmon–exciton nanostructures, the distance between them, matching of spectral resonances, etc.9–27 The plasmon–exciton interaction provides a basis for changing the probabilities of radiative and nonradiative transitions in QDs due to the Purcell effect, transforming the shape of extinction and luminescence spectra of QDs as a result of the Rabi splitting, making Fano quantum interference possible.9–27
These effects are most clearly demonstrated in the framework of single-molecule spectroscopy.12–27 However, the use of plexcitonic effects in luminescence sensing requires an understanding of the conditions for their manifestation in the luminescence of quantum dot ensembles.9–11,28 The size dispersion of QDs strongly affects the spectral resonance due to the large luminescence bandwidth.9–11,28 In most situations described in the literature, QDs with exciton luminescence are considered.10–15,17–19,21–25 Under conditions of significant overlap of QD luminescence and extinction bands of plasmonic nanoparticles (NPs), processes of electronic excitation exchange, in particular, nonradiative energy transfer from QDs to plasmonic NPs, play a significant role in the plasmon–exciton interaction;29–31 charge phototransfer is also an important effect in these systems.32,33
Thus, in some situations, the changes in the parameters of QD luminescence do not have an obvious interpretation. The least studied are manifestations of the plexcitonic interaction in the case of trap state luminescence of QDs. Recently, several works have appeared that address this problem.34–36 Various samples of colloidal QDs, namely, CdS, CdTe, InP/ZnS, SiN, etc., and various plasmonic nanoparticles (nanorods, cones, as well as pyramidal and spherical nanoparticles made of silver and gold) have been considered. In most of the cited works, significant attention is given to quenching of trap state luminescence due to photoinduced electron transfer from plasmonic nanoparticles to QDs.32,35 It should be also noted that in the case of trap state luminescence, electron–phonon interaction and influence of nonradiative recombination centers are significant. Their contribution can largely determine the behavior of the luminescence intensity of QDs near plasmonic NPs.34 Thus, recognition of the effects responsible for the change in the spectral properties of hybrid plexcitonic nanostructures, which include QDs with trap state luminescence, is a relevant problem.
This paper presents experimental data demonstrating the possibility to control the luminescence properties of Ag2S QDs in the near field of plasmonic gold nanorods (Au NRs) by changing the plasmon–exciton coupling mode. For this purpose, a polymer was introduced to achieve the different mutual spatial arrangement of QDs and NRs. Note that for metal-conducting structures containing Ag2S QDs, plexcitonic effects have not yet been considered in detail with regard to the luminescence properties of such complex systems.
2 Sample preparation and experimental techniques
2.1 Sample preparation
Colloidal Ag2S QDs have been synthesized within the framework of a one-step synthesis, which involves the use of L-cysteine (L-Cys) amino acid molecules during crystallization. These molecules act as both a sulfur source and a passivator of QD interfaces (further referred to as Ag2S/L-Cys QDs, see Fig. 1). The procedure is as follows. Aqueous solutions of AgNO3 and L-Cys are mixed in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. After that, the pH is adjusted to 10 using a 1 M NaOH solution. Then, the reaction mixture is kept for 40 minutes at a temperature of 95 °C.
2. After that, the pH is adjusted to 10 using a 1 M NaOH solution. Then, the reaction mixture is kept for 40 minutes at a temperature of 95 °C.
 | ||
| Fig. 1 Schematic representation of the techniques for the formation of Ag2S/L-Cys QDs, Au NRs, and hybrid structures based on them. | ||
The colloidal Au NRs have been synthesized in the presence of a cetyltrimethylammonium bromide (CTAB) surfactant. Its aqueous solution forms cylindrical micelles, creating anisotropic conditions for the growth of the NRs. The gold NRs have been obtained by a multistage procedure. This includes sequential preparation and mixing of the seed and growth solutions (Fig. 1). The seed solution is the solution of spherical Au NRs (3 nm), obtained by chemical reduction of HAuCl4 (7 μL, 0.36 M) with a NaBH4 solution (1.0 mL, 5 mM) in the presence of CTAB (20 mL, 0.02 mM). The growth solution has been obtained by mixing HAuCl4 (28 μL, 0.36 M), CTAB (50 mL, 0.1 mM), AgNO3 (100 μL, 0.02 M), and C6H8O6 (5 mL, 0.05 μM). The aspect ratio (length![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diameter) of the Au NRs has been controlled by adding AgNO3 (50 μL, 0.02 M) to the growth solution. An increase in the aspect ratio of Au NRs provides a long-wavelength shift of the extinction peak of the longitudinal plasmon resonance, providing its spectral overlap with the luminescence spectrum of Ag2S/L-Cys QDs. The reaction byproducts were removed from the obtained solution of Au NRs by several cycles of centrifuging-redispersion in distilled water.
diameter) of the Au NRs has been controlled by adding AgNO3 (50 μL, 0.02 M) to the growth solution. An increase in the aspect ratio of Au NRs provides a long-wavelength shift of the extinction peak of the longitudinal plasmon resonance, providing its spectral overlap with the luminescence spectrum of Ag2S/L-Cys QDs. The reaction byproducts were removed from the obtained solution of Au NRs by several cycles of centrifuging-redispersion in distilled water.
The formation of hybrid structures was achieved by mixing colloidal solutions of Ag2S/L-Cys QDs and Au NRs in the molar ratio of [ν(NRs)]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ν(QD)] ∼ 10−4 (mole fraction, m.f.). This concentration ratio provides decoration of plasmonic Au NRs with colloidal Ag2S/L-Cys QDs (1
[ν(QD)] ∼ 10−4 (mole fraction, m.f.). This concentration ratio provides decoration of plasmonic Au NRs with colloidal Ag2S/L-Cys QDs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 104 pcs). The surface chemistry of the mixture components implies their direct contact due to the electrostatic interaction of the active carboxyl and amino groups of the L-Cys ligand, which passivates the Ag2S QDs interfaces, with the nitro groups of CTAB molecules, which coordinate the morphology of Au NRs (Fig. 1). Owing to the supplementary addition of 0.1 μL of a cationic polymer poly(diallyldimethylammonium chloride) (polyDADMAC) to the colloidal mixture, spatial separation of the mixture components is expected due to the deterioration of electrostatic interaction (Fig. 1).
104 pcs). The surface chemistry of the mixture components implies their direct contact due to the electrostatic interaction of the active carboxyl and amino groups of the L-Cys ligand, which passivates the Ag2S QDs interfaces, with the nitro groups of CTAB molecules, which coordinate the morphology of Au NRs (Fig. 1). Owing to the supplementary addition of 0.1 μL of a cationic polymer poly(diallyldimethylammonium chloride) (polyDADMAC) to the colloidal mixture, spatial separation of the mixture components is expected due to the deterioration of electrostatic interaction (Fig. 1).
2.2 Experimental techniques
The size and morphology of Ag2S QDs and Au NRs were determined using a Libra 120 transmission electron microscope (TEM) (Carl Zeiss, Germany) and a JEOL 2000FX high-resolution TEM (HR-TEM) (JEOL Ltd., Japan). The absorption properties were studied using a USB2000+ spectrometer (Ocean Optics, USA) with a USB-DT radiation source (Ocean Optics, USA). The luminescence spectra and luminescence decays of Ag2S QDs were studied using the USB2000+ device and a TimeHarp 260 Pico time-correlated single-photon counting board (PicoQuant, Germany) with a PMC-100-20 PMT module (Becker & Hickl, Germany) with a time resolution of 0.2 ns. To excite luminescence, we used an LD PLTB450 laser diode (Osram, Germany) operating at a wavelength of 445 nm (200 mW). The measurements were carried out at a temperature of 300 K.The luminescence quantum yield for Ag2S/L-Cys QDs was determined by the relative method using the expression
 | (1) |
Here QYR is the quantum yield of the reference standard, I and IR are the integrated luminescence intensities for the sample and reference standard, D and DR are the optical densities at the excitation wavelength for the sample and reference standard (in the experiments, the corresponding value was ∼0.1), n and nR are the refractive indices of the sample solution and the reference standard solution, respectively. Special attention was paid to the correction for the spectral sensitivity of the spectrometer, which was determined using the emission spectrum of a standard tungsten lamp with a known color temperature. A DMSO solution of Indocyanine green was chosen as a standard. Its quantum yield is 12% near 800 nm.37
For the study of spectral and luminescence properties of mixtures of Ag2S/L-Cys QDs and Au NRs, aqueous solutions containing Ag2S QDs or Au NRs with concentrations equivalent to those introduced during the formation of the mixtures were used as a reference. In the study of mixtures in the presence of the polyDADMAC polymer, the reference samples were polyDADMAC aqueous solutions containing Ag2S QDs or Au NRs with concentrations equivalent to those introduced during the formation of the mixtures.
Structure of trap states was studied by thermostimulated luminescence method (TSL), described in details in work.38,39 For obtaining of TSL dependences the sample was placed in the nitrogen cryostat and cooled from 330 K to 80 K. After that, the sample was kept at 80 K temperature for some time. Then the sample was heated to 330 K with average heating rate about β = 0.05 K s−1. Throughout the process of cooling and heating of the sample, photoexcitation and continuous registration of photoluminescence by USB2000+ spectrometer occurred. The experiment was repeated three times and the averaged temperature dependences were subsequently analyzed. In this approach, charge carriers, which were localized at shallow traps during the cooling stage under photoexcitation are released and participate in recombination IR luminescence at heating. This provides an increase in the intensity of luminescence at heating compared to cooling process. Thus, the curves of thermoluminescence were representing the difference of luminescence intensities for the heating and cooling process.38,39
3 Results and discussion
3.1 Structural data
Analysis of the TEM images of Ag2S/L-Cys QDs revealed the formation of individual nanocrystals with an average size of 2.1 ± 0.5 nm and size dispersion of ∼30%, which is due to the chosen approach of colloidal synthesis in an aqueous solution (Fig. 2a). According to the TEM data, the Au NRs have the average length and diameter of 30 and 9 nm with size dispersion ∼25% (Fig. 2b). According to HR-TEM and TEM data (Fig. 2c and c′), the approach used to obtain the mixtures of Ag2S/L-Cys QDs and Au NRs ensures compatibility of the components and formation of hybrid structures with good reproducibility. Analysis of the HR-TEM images showed the formation of particle agglomerates with interplanar distances of ∼0.196 nm, corresponding to the (422) crystallographic plane of the Ag2S monoclinic lattice. Particles with interplanar distances of 0.237 nm were obtained as well, corresponding to the (111) crystallographic plane of the cubic face-centered lattice of Au (Fig. 2c). Thus, plasmonic nanoparticles are adsorption centers for QDs. In turn, QDs decorate the interfaces of Au NRs.Analysis of the HR-TEM and TEM images (Fig. 2d and d′) of hybrid structures of Ag2S/L-Cys QDs and Au NRs in the presence of the polyDADMAC polymer showed agglomeration of the particles with interplanar distances of 0.251 nm, corresponding to the (022) crystallographic plane of the Ag2S monoclinic lattice, near the Au particles with interplanar distances of 0.237 nm, corresponding to the crystallographic plane (111) of the cubic facecentered lattice of gold (Fig. 2d). The agglomeration of nanoparticles is sparse. The presence of the polyDADMAC leads to separation of Au NRs and Ag2S QDs to a distances of about 4–10 nm. This is probably due to the presence of a polymer layer that leads to formation of contrast phase on TEM images around of Au NRs (Fig. 2d′) which was not observed at the absence of the polyDADMAC.
Thus, the chosen approach makes it possible to obtain mixtures of Au NRs with colloidal Ag2S QDs both with direct absorption of QDs onto the surface of NRs and with spatial separation of the mixture components when a cationic polyDADMAC polymer is introduced into the mixture.
3.2 Optical properties
In the UV-vis absorption spectrum of colloidal Ag2S/L-Cys QDs, a weak spectral shoulder is observed at 620 nm, associated with the exciton absorption, which is characteristic of the quantum confinement effect in nanocrystals (Fig. 3a, curve 1). The broadening of the distinct exciton peak in the UV-vis absorption spectrum is caused by the size dispersion of the Ag2S/L-Cys QDs in the ensemble. The spectrum corresponds to QDs with an average size of 2.1 nm.In the extinction spectrum of Au NRs, two peaks at 530 nm and 720 nm are observed. They correspond to the transverse and longitudinal dipole modes of localized plasmons40 (Fig. 3a, curve 2). Note that the presence of the polyDADMAC polymer in the colloidal solution does not affect the absorption and extinction spectra of the Ag2S QDs and Au NRs. Spectroscopic methods are not very informative for proving the adsorption of the polyDADMAC polymer on the Au NRs surface. In the UV-vis absorption spectra, the spectral bands of the polyDADMAC overlap with the absorption spectra of Ag2S QDS, which have a higher molar extinction. Luminescence spectroscopy makes it possible to establish only the presence of a polymer in solution. In turn, the FTIR spectroscopy data also turn out to be uninformative due to the superposition of the spectra of the polymer and stabilizer molecules (CTAB and L-Cys) presented in the solution. Thus, this is the subject of a separate spectroscopic study, which is not directly related to the results which are presented in this paper.
For colloidal Ag2S/L-Cys QDs, luminescence is peaked at 750 nm (Fig. 3b, curve 1). The Stokes shift is 0.35 eV. This is associated with the trap state luminescence of QDs. According to the data of ref. 41, luminescence results from radiative recombination of holes and trapped electrons, localized at the levels corresponding to structural impurity defects. Thus, the geometry and size of Au NRs provide a significant overlap of the peak of the longitudinal plasmon resonance with the absorption and luminescence spectra of the Ag2S QDs (Fig. 3a and b). Quantum yield of Ag2S/L-Cys QD luminescence was ∼0.2%.
For mixtures of Ag2S/L-Cys QDs with Au NRs, the resulting extinction spectrum is not a simple sum of the spectra of individual components (Fig. 3a, curve 3). The increase in the optical density over the entire extinction spectrum and the blue shift of the plasmon resonance peaks by 2–3 nm are not only due to the contribution from the Ag2S/L-Cys QDs but also due to the interaction between the mixture components (Fig. 3a, curves 3, 4).
The formation of mixtures of Ag2S/L-Cys QDs and Au NRs leads to a significant decrease (quenching) in the luminescence intensity for the Ag2S QDs (Fig. 3b, curve 2) (QY ∼ 0.05%). However, the average luminescence lifetime for Ag2S QDs does not change (Fig. 3c, curves 1, 2). The presented experimental data do not confirm nonradiative resonance energy transfer between the components of the hybrid mixtures. Significant luminescence quenching for Ag2S/L-Cys QDs in the presence of Au NRs, together with constant luminescence lifetime, is the evidence for photoinduced electron transfer (PET), similar to the results of works.32,33 The positions of the quantum confinement levels of Ag2S/L-Cys QDs and the Fermi level of gold imply that PET is possible in this hybrid system (Fig. 3d). For bulk silver sulfide, photoionization energy exceeds 5.24 eV.42 Due to the quantum confinement effect, this value is increased. For Ag2S/L-Cys QDs with an average size of 2.1 nm, the corresponding energy has been estimated to be 5.7 eV.41 The Fermi level of Au is at 5.1 eV.43 Therefore, the position of this Fermi level falls within the effective bandgap of the Ag2S/L-Cys QDs (Fig. 3d). In this case, efficient PET from the Ag2S/L-Cys QDs to Au NRs is possible, which leads to quenching of radiative recombination in the Ag2S/L-Cys QDs. PET can also be facilitated by the complex chemical interaction of the active carboxyl groups of the L-Cys ligand, which passivates the interfaces of the Ag2S QDs, with the nitro groups of the CTAB molecules, which coordinate the morphology of the Au NRs. This suggests direct contact between the Ag2S QDs and Au NRs (Fig. 1). The incomplete quenching of Ag2S/L-Cys luminescence in this situation is apparently due to the presence of interaction-free QDs in the mixture.
The addition of the polyDADMAC cationic polymer into the mixture of the Ag2S/L-Cys QDs and Au NRs stabilizes the complex chemical interaction between the mixture components. It also provides spatial separation of the components, which is confirmed by the TEM data (Fig. 2c). In this case, an increase in the intensity of IR luminescence (the peak at 750 nm) of Ag2S/L-Cys is observed evenly over the entire luminescence band of the QDs, with the intensity being increased by a factor of 1.5 (Fig. 3b, curve 3) (QY ∼ 0.3%). At the same time, acceleration of the luminescence decay was observed (Fig. 3c, curve 3). The average luminescence lifetime was determined from the experimental luminescence decays using the equation
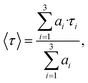 | (2) |
 | (3) |
The data indicate a decrease in the luminescence lifetime from 7.2 ns to 4.5 ns. Such behavior of the luminescence properties is the evidence of the Purcell effect.44 Apparently, the presence of the polyDADMAC polymer deteriorates the conditions for charge transfer between the mixture components. Here, Au NRs play to the recombination luminescence frequency of the Ag2S/L-Cys QDs. A slight increase in the luminescence intensity, in this case, is explained by the contribution from those Ag2S/L-Cys QDs that fall within the near field of Au NRs. The fraction of such QDs is much less than that of the QDs free from the plasmon–exciton interaction.
The formation of plexitonic structures is also manifested at the level of small localized states. Insert I in Fig. 3b shows the thermoluminescence curves of Ag2S/L-Cys QDs (Fig. 3b, insert I, curve 1), their mixtures with Au NRs in the presence of polyDADMAC (Fig. 3b, insert I, curve 2). The detected thermoluminescence curves are broad bands, the position of the maxima and the structure of which depends on the composition of the mixtures. Thus, in the case of Ag2S/L-Cys QDs, an asymmetric band is observed with a maximum in the region of 247 K with a continuing “tail” in the temperature range of 280–370 K. Addition of Au NRs in the presence of polyDADMAC leads to shifting of thermoluminescence peak maximum to high temperatures to 260 K and the appearance of a thermoluminescence peak with a maximum at 340 K. Such changes can be caused by both the polarizing effect of Au NRs and the introduction of polyDADMAC; therefore, the study of the nature of these changes requires a separate careful consideration.
4 Conclusions
Thus, the proposed approach to the formation of plexitonic structures based on Ag2S/L-Cys QDs in the presence of Au/CTAB NRs makes it possible to control the luminescent properties of Ag2S/L-Cys QDs. The relationship between the spatial separation of the components of the plexitonic structure and the change in the luminescent properties (quantum yield, luminescence lifetime) has been experimentally established. Significant quenching of the luminescence intensity ∼4 times has been observed for the Ag2S/L-Cys QDs upon their direct interaction with the Au NRs. Based on the absence of noticeable changes in the luminescence lifetime for Ag2S/L-Cys QDs, charge transfer between the mixture components has been proposed. It has been shown that the additional introduction of the polyDADMAC cationic polymer into the hybrid mixture leads to spatial separation of the mixture components (average distance between components about 4 nm) and blocks the charge transfer between them. At the same time, enhancement of the luminescence intensity of Ag2S/L-Cys QDs about 1.5 times, accompanied by a decrease in the luminescence lifetime has been found, which is the evidence for the Purcell effect. By TSL method the transformation of shallow trap states energy structure was found.The scientific results presented in this paper open up possibilities for controlling the quantum yield and luminescence lifetime of nonstoichiometric QDs, which is important in the development of highly efficient IR radiation sources for nanophotonics applications. The presence of luminescence in the considered plexitonic nanosystems based on low-toxicity and biocompatible Ag2S/L-Cys QDs in the region of the first therapeutic transparency window of biological tissues (NIR-I (750–900 nm)) makes it possible to use them for applications of luminescent biomarking and theranostics of severe human diseases.
Author contributions
Irina G. Grevtseva: methodology, validation, writing – original draft. Oleg V. Ovchinnikov: conceptualization, project administration, writing – review & editing. Mikhail S. Smirnov: methodology, validation, formal analysis. Aleksey S. Perepelitsa: methodology, investigation, writing – original draft. Tamara A. Chevychelova: investigation, writing – original draft. Violetta N. Derepko: investigation. Anna V. Osadchenko: investigation. Alexandr S. Selyukov: visualization, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by State Assignment to Higher Educational Institutions of Russian Federation (FZGU-2020-0035). Results of TEM investigations with the Libra 120 TEM were obtained on the equipment of the Center of Collective Usage of Scientific Equipment of Voronezh State University. High-resolution TEM images were obtained on the equipment of the Center of Collective Usage of Moscow Institute of Physics and Technology.Notes and references
- M. A. Cotta, ACS Appl. Nano Mater., 2020, 3, 4920–4924 CrossRef CAS.
- S. R. Patra and R. K. Bhuyan, J. Mater. Sci.: Mater. Electron., 2021, 32, 5538–5547 CrossRef CAS.
- S. Kargozar, S. J. Hoseini, P. B. Milan, S. Hooshmand, H.-W. Kim and M. Mozafari, Biotechnol. J., 2020, 15, 2000117 CrossRef CAS PubMed.
- Y. Jiang and E. A. Weiss, J. Am. Chem. Soc., 2020, 142, 15219–15229 CrossRef CAS.
- D. Dovzhenko, I. Martynov, P. Samokhvalov, E. Osipov, M. Lednev, A. Chistyakov, A. Karaulov and I. Nabiev, Opt. Express, 2020, 28, 22705 CrossRef CAS PubMed.
- V. Korshunov, S. Ambrozevich, I. Taydakov, A. Vashchenko, D. Goriachiy, A. Selyukov and A. Dmitrienko, Dyes Pigm., 2019, 163, 291–299 CrossRef CAS.
- B. del Rosal, E. Ximendes, U. Rocha and D. Jaque, Adv. Opt. Mater., 2016, 5, 1600508 CrossRef.
- M. Franke, S. Leubner, A. Dubavik, A. George, T. Savchenko, C. Pini, P. Frank, D. Melnikau, Y. Rakovich, N. Gaponik, A. Eychmüller and A. Richter, Nanoscale Res. Lett., 2017, 12, 314 CrossRef CAS PubMed.
- D. Ruiz, B. del Rosal, M. Acebrón, C. Palencia, C. Sun, J. Cabanillas-González, M. López-Haro, A. B. Hungría, D. Jaque and B. H. Juarez, Adv. Funct. Mater., 2016, 27, 1604629 CrossRef.
- R. Guo, S. Derom, A. I. Väkeväinen, R. J. A. van Dijk-Moes, P. Liljeroth, D. Vanmaekelbergh and P. Törmä, Opt. Express, 2015, 23, 28206 CrossRef CAS PubMed.
- J. Pan, J. Chen, D. Zhao, Q. Huang, Q. Khan, X. Liu, Z. Tao, Z. Zhang and W. Lei, Opt. Express, 2015, 24, A33 CrossRef.
- Y. Luo and J. Zhao, Nano Res., 2019, 12, 2164–2171 CrossRef CAS.
- W.-X. Yang, A.-X. Chen, Z. Huang and R.-K. Lee, Opt. Express, 2015, 23, 13032 CrossRef PubMed.
- H. Leng, B. Szychowski, M.-C. Daniel and M. Pelton, Nat. Commun., 2018, 9, 4012 CrossRef.
- S. N. Gupta, O. Bitton, T. Neuman, R. Esteban, L. Chuntonov, J. Aizpurua and G. Haran, Nat. Commun., 2021, 12, 1310 CrossRef CAS PubMed.
- N. S. Kurochkin, S. P. Eliseev, A. V. Gritsienko, V. V. Sychev and A. G. Vutukhnovsky, Nanotechnology, 2020, 31, 505206 CrossRef CAS PubMed.
- G.-Y. Chen, Y.-N. Chen and D.-S. Chuu, Opt. Lett., 2008, 33, 2212 CrossRef PubMed.
- A. V. Akimov, A. Mukherjee, C. L. Yu, D. E. Chang, A. S. Zibrov, P. R. Hemmer, H. Park and M. D. Lukin, Nature, 2007, 450, 402–406 CrossRef CAS PubMed.
- Y. He and K.-D. Zhu, Sensors, 2017, 17, 1445 CrossRef.
- A. V. Gritsienko, N. S. Kurochkin, A. G. Vitukhnovsky, A. S. Selyukov, I. V. Taydakov and S. P. Eliseev, J. Phys. D: Appl. Phys., 2019, 52, 325107 CrossRef CAS.
- H.-J. Chen, Laser Phys. Lett., 2020, 17, 025201 CrossRef CAS.
- E. Cao, W. Lin, M. Sun, W. Liang and Y. Song, Nanophotonics, 2018, 7, 145–167 Search PubMed.
- B. S. Nugroho, A. A. Iskandar, V. A. Malyshev and J. Knoester, Phys. Rev. B, 2019, 99, 075302 CrossRef CAS.
- D. E. Westmoreland, K. P. McClelland, K. A. Perez, J. C. Schwabacher, Z. Zhang and E. A. Weiss, J. Chem. Phys., 2019, 151, 210901 CrossRef.
- J. Zhang, Y. Fu, M. H. Chowdhury and J. R. Lakowicz, Nano Lett., 2007, 7, 2101–2107 CrossRef CAS PubMed.
- I. C. Serrano, C. Vazquez-Vazquez, A. M. Adams, G. Stoica, M. A. Correa-Duarte, E. Palomares and R. A. Alvarez-Puebla, RSC Adv., 2013, 3, 10691 RSC.
- S. Kühn, U. Håkanson, L. Rogobete and V. Sandoghdar, Phys. Rev. Lett., 2006, 97, 017402 CrossRef PubMed.
- S. Hu, Y. Ren, Y. Wang, J. Li, J. Qu, L. Liu, H. Ma and Y. Tang, Beilstein J. Nanotechnol., 2019, 10, 22–31 CrossRef CAS PubMed.
- E. Oh, A. L. Huston, A. Shabaev, A. Efros, M. Currie, K. Susumu, K. Bussmann, R. Goswami, F. K. Fatemi and I. L. Medintz, Sci. Rep., 2016, 6, 35538 CrossRef CAS PubMed.
- B. Nikoobakht, C. Burda, M. Braun, M. Hun and M. A. El-Sayed, Photochem. Photobiol., 2007, 75, 591–597 CrossRef.
- K. Hosoki, T. Tayagaki, S. Yamamoto, K. Matsuda and Y. Kanemitsu, Phys. Rev. Lett., 2008, 100, 207404 CrossRef PubMed.
- P. V. Kamat and B. Shanghavi, J. Phys. Chem. B, 1997, 101, 7675–7679 CrossRef CAS.
- N. Mondal and A. Samanta, J. Phys. Chem. C, 2015, 120, 650–658 CrossRef.
- C. Liao, L. Tang, X. Gao, R. Xu, H. Zhang, Y. Yu, C. Lu, Y. Cui and J. Zhang, Nanoscale, 2015, 7, 20607–20613 RSC.
- A. M. Flatae, F. Tantussi, G. C. Messina, F. D. Angelis and M. Agio, J. Phys. Chem. Lett., 2019, 10, 2874–2878 CrossRef CAS PubMed.
- J. Huang, O. S. Ojambati, R. Chikkaraddy, K. Sokołowski, Q. Wan, C. Durkan, O. A. Scherman and J. J. Baumberg, Phys. Rev. Lett., 2021, 126, 047402 CrossRef CAS PubMed.
- F. W. B. van Leeuwen, B. Cornelissen, F. Caobelli, L. Evangelista, L. Rbah-Vidal, S. D. Vecchio, C. Xavier, J. Barbet and M. de Jong, EJNMMI Radiopharmacy and Chemistry, 2017, 2, 15 CrossRef PubMed.
- A. Perepelitsa, M. Smirnov, O. Ovchinnikov, A. Latyshev and A. Kotko, J. Lumin., 2018, 198, 357–363 CrossRef CAS.
- O. Ovchinnikov, A. Perepelitsa, M. Smirnov and S. Aslanov, J. Lumin., 2022, 243, 118616 CrossRef CAS.
- S. Link, M. B. Mohamed and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 3073–3077 CrossRef CAS.
- M. Smirnov and O. Ovchinnikov, J. Lumin., 2020, 227, 117526 CrossRef CAS.
- S. Lin, Y. Feng, X. Wen, P. Zhang, S. Woo, S. Shrestha, G. Conibeer and S. Huang, J. Phys. Chem. C, 2014, 119, 867–872 CrossRef.
- J. R. Lombardi and R. L. Birke, J. Phys. Chem. C, 2008, 112, 5605–5617 CrossRef CAS.
- E. M. Purcell, Confined Electrons and Photons, Springer, US, 1995, pp. 839–839 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |


