Open Access Article
Open Access ArticleLight-activated hydrolysis properties of Mg-based materials
Daifeng Wu *,
Rui Li,
Qing Zhou,
Renheng Tang and
Fangming Xiao
*,
Rui Li,
Qing Zhou,
Renheng Tang and
Fangming Xiao
Guangdong Province Key Laboratory of Rare Earth Development and Application, Institute of Resources Utilization and Rare Earth Development, Guangdong Academic of Science, Guangzhou, 510650, People's Republic of China. E-mail: wudaifeng@irmgdas.gd.com
First published on 24th February 2022
Abstract
Hydrolysis of Mg-based materials is a promising technology for the development of portable hydrogen fuel cells. However, the Mg(OH)2 layer impedes the diffusion of water molecules into inner particles, resulting in sluggish hydrolysis performance. The hydrolysis performances of Mg-based materials (Mg, MgH2, MgH2-BM and MgH2-RBM) with water are effectively improved under light-activation. The hydrolysis performance could be tailored by the light energy (frequency and intensity). The combination of ball-milling and light-activation could further enhance the hydrolysis performance of MgH2. In particular, the hydrolysis yield of MgH2-RBM reached 95.7% of the theoretical yield under 90 W green light-activation. Thus, rasing the light energy (by using purple light and UV, or higher power lights) and the combination of ball-milling could lead to better hydrolysis performance of Mg-based materials. The Mg(OH)2 layer was considered as a barrier to MgH2 hydrolysis of MgH2. Interestingly, under light-activation, the Mg(OH)2 layer can act as a catalyst to enhance the decomposition of MgH2, and improve the hydrolysis yield and kinetics of Mg-based materials.
1. Introduction
Hydrolysis is an efficient and convenient hydrogen generation method with high purity, great yield and low operation temperature. Generally, studies on hydrolysis have focused on the materials of metals, metal hydrides, borohydrides and ammonia borane.1 Among them, Mg-based materials have received immense attention due to their higher hydrogen generation capacity (1703 mL g−1 for MgH2 and 921 mL g−1 for Mg), abundance of raw materials, desirable hydrogen generation conditions and eco-friendly byproduct.2,3 Nevertheless, the insoluble and dense hydroxide layer deposited on the MgH2 surface hinders the diffusion of water molecules into inner particles, leading to undesirable and uncontrollable hydrolysis performance.4Various strategies, such as the acidification of the reaction environment,5,6 the use of salt solutions,5,7 the modification of the morphology to obtain nanostructured hydrolysable materials,8,9 and the incorporation of additives with different functions,10,11 have been adopted and verified effective for activating the reaction of MgH2 with water. However, these strategies still face many challenges, such as complex operation, high-cost or the pollution of additives. To develop alternative solutions, light-activated may be a good choice. Sunlight is a “free” and widely available source of energy. Recently, Sun et al. studied the light-activated hydrogen storage in Mg, LiH and NaAlH4.12 They found that light-activated hydrogen storage could lead to an effective approach for hydrogen uptake and release from hydrides at low temperature.
Sunlight may also be an effective means of driving hydrolysis reactions. In this work, the influence of light-activated the hydrolysis properties of Mg-based materials were investigated at RT (25 °C). Since the potential for damage to the skin and eye form UV, we limit the experiment scope in visible spectrum. According to standard electrode potentials of Mg2+/Mg (−2.36 eV) and H+/H (0 eV),13 we assume the band gap energy of Mg is 2.36 eV. According to the Scherer equation λ = 1240/Eg (λ was the limiting wavelength, Eg was the band gap energy).14 We found the corresponding wavelength is 525 nm, which is in the green light spectrum (530 to 520 nm). So we chose green light and the adjacent light (blue and yellow light) as experiment light source. An unexpectedly light-activated of the hydrolysis of Mg-based materials was found. Interestingly, the hydrolysis performance can be tailored by changing the color and power of the spotlight. Our findings provide a green, simple and effective solution in enhance the hydrolysis performance of metal-based materials, and this solution is expected to be used for other hydrogen generation materials, such as borohydrides and ammonia borane.
2. Results and discussion
2.1 Hydrolysis kinetics of Mg and MgH2 under light-activated
Firstly, the hydrolysis kinetics of Mg and MgH2 under light-activated were investigated. Fig. 1 shows the hydrogen evolution curves of (a) Mg and (b) MgH2 under different colors (yellow, green and blue) light-activated at RT. As shown in Fig. 1(a), the hydrolysis performance of the single Mg without light-activated is rather sluggish, delivering only 2.3 mL g−1 H2 in 60 min. Under light-activated, the hydrolysis kinetics of Mg was significantly improved. Under yellow, green and blue light-activated, the hydrogen yields of Mg increased to 14.3, 16.4 and 20.1 mL g−1 in 60 min, respectively. Furthermore, the hydrolysis yields and kinetics of Mg increase with the color frequency of light. | ||
| Fig. 1 Hydrogen evolution curves of (a) Mg and (b) MgH2 under different color (yellow, green and blue) light-activated at RT. | ||
Fig. 1(b) shows the hydrogen evolution curves of MgH2 under different color (yellow, green and blue) light-activated. Similar to Mg, the hydrolysis kinetics of MgH2 was significantly improved by light-activated. The hydrogen yields of MgH2 under yellow, green and blue light-activated are 29.1, 45.1 and 52.9 mL g−1 H2 in 60 min, while the hydrogen yields of MgH2 without light-activated is only 25.6 mL g−1 H2 in 60 min. The hydrolysis yields and kinetics of MgH2 also increase with the color frequency of light.
Thus, the hydrolysis performance of Mg and MgH2 are improved by light-activated and can be tuned by the light color (frequency).
2.2 Hydrolysis kinetics of MgH2-BM and MgH2-RBM with light-activated
Ball-milling is an effective method to improve the hydrolysis performance of MgH2 since it can reduce the particle size of MgH2.9 MgH2-BM and MgH2-RBM were prepared by planetary ball mill under argon and hydrogen atmosphere, and their hydrogen evolution curves under light-activated were tested (Fig. 2). As shown in Fig. 2(a), MgH2-BM could generate 864.6 mL g−1 H2 in 120 min without light-activated. While the hydrogen yields of MgH2-BM under yellow, green and blue light-activated increased to 972.5, 1066.7 and 1137.7 mL g−1 in 120 min, respectively. | ||
| Fig. 2 Hydrogen evolution curves of the (a) MgH2-BM and (b) MgH2-RBM under different color (yellow, green and blue) light-activated at RT. | ||
The hydrogen evolution curves of MgH2-RBM under light-activated were shown in Fig. 2(b). MgH2-RBM can release 1039.1 mL g−1 H2 in 120 min without light-activated. The hydrogen yields of MgH2-RBM under yellow, green and blue light-activated were enhanced to 1287.7, 1334.2 and 1385.8 mL g−1 in 120 min, respectively. The hydrolysis yield of MgH2-RBM under blue light-activated is up to 78.3% of the theoretical yield.
Thus, light-activated is also work on the hydrolysis performance of MgH2-BM and MgH2-RBM, and the strategy that combined ball-milling and light-activated is an effective method to enhance the hydrolysis performance of MgH2.
2.3 Effect of stirring combined with light-activated
To further improve the hydrolysis performance, the effect of stirring combined with light-activated was investigated. Fig. 3 is the hydrogen evolution curves of MgH2-BM with (a) stirring and (b) stirring combined green light-activated at RT. Unexpectedly, the hydrolysis performance of MgH2-BM with stirring was inferior to that without stirring. Under green light, the hydrolysis yield of MgH2-BM with and without stirring were 1019.7 and 1066.7 mL g−1 in 120 min. Thus, the hydrolysis performance of MgH2-BM was hinder by stirring. | ||
| Fig. 3 Hydrogen evolution curves of MgH2-BM with and without stirring under green light-activated at RT. | ||
2.4 Effect of heating combined with light-activated
To further study the hydrolysis properties of MgH2 under light-activated, the hydrogen evolution curves for MgH2 at different temperatures were investigated. The hydrogen evolution curves for MgH2 under different color ((a) void, (b) yellow, (c) green, and (d) blue) light-activated at various temperature (35, 45 and 55 °C) were shown in Fig. 4, and their hydrolysis yield were shown in Table 1. The hydrogen evolution curves of MgH2 under light-activated is similar to those without light-activated. The hydrolysis yields of MgH2 under different color light-activated were irregular due to the temperature fluctuation of the water bath. Additionally, the hydrolysis activation energies were failed to calculate, since the hydrogen evolution curves are so straight. | ||
| Fig. 4 Hydrogen evolution curves of MgH2 under different color ((a) void, (b) yellow, (c) green, and (d) blue) light-activated at various temperature (35, 45 and 55 °C). | ||
| Light-activated | Hydrolysis yield at 35 °C | Hydrolysis yield at 45 °C | Hydrolysis yield at 55 °C |
|---|---|---|---|
| Void | 61.5 | 91.8 | 194.0 |
| Yellow | 61.9 | 105.5 | 201.1 |
| Green | 67.9 | 105.1 | 195.6 |
| Blue | 69.8 | 97.2 | 205.8 |
2.5 Effect of higher power (90 W) light-activated
To study the effect of light power, we doubled the power of light-activated by adding spot another light source. Fig. 5 is the hydrogen evolution curves of (a) Mg, (b) MgH2, (c) MgH2-BM and (d) MgH2-RBM under different power (0, 45 and 90 W) green light-activated at RT, and their hydrolysis yields of Mg-based materials (Mg, MgH2, MgH2-BM and MgH2-RBM) are summary in Table 2. As the Fig. 5 shows, the light power can significantly enhance the hydrolysis performance of Mg-based materials. Compared with that at 45 W, the hydrolysis yield of Mg and MgH2 are raised 60.4% and 166.3% under 90 W light-activated. Meantime, the hydrolysis yield of MgH2-BM and MgH2-RBM are raised 15.3% and 22.2% at double power light-activated. Thus, the hydrolysis performance of Mg-based materials can be tuned by the light power. | ||
| Fig. 5 Hydrogen evolution curves for (a) Mg, (b) MgH2, (c) MgH2-BM and (d) MgH2-RBM at different power (0, 45 and 90 W) green light-activated at RT. | ||
| Mg-based materials | 0 W | 45 W | 90 W | Increasing rate (90/45 W) |
|---|---|---|---|---|
| Mg | 2.3 | 16.4 | 26.3 | 60.4% |
| MgH2 | 25.6 | 45.1 | 106.8 | 136.8% |
| MgH2-BM | 864.6 | 1066.7 | 1230 | 15.3% |
| MgH2-RBM | 1039.1 | 1334.2 | 1630.6 | 22.2% |
Furthermore, the hydrolysis yield of MgH2-BM and MgH2-RBM under 90 W green light-activated is up to 72.2% and 95.7% of the theoretical yield, respectively. Table 3 Hydrolysis properties at ambient temperature of MgH2 reported in the literature. As Table 3 shows, the hydrolysis properties of MgH2-BM and MgH2-RBM under 90 W green light-activated are superior to that without additive. The hydrolysis properties of MgH2-RBM under 90 W green light-activated is comparable to that with NH4Cl and CaH2 additive. Mentionable, the method of light-activated has more advantage: (1) the hydrolysis kinetics are controlled during the hydrolysis process since the power of light-activated can be tuned; (2) there is not impurity in the byproduct after hydrolysis process.
| Material | Hydrolysis yield (mL g−1) | Hydrolysis time (min) | Ref. |
|---|---|---|---|
| MgH2 milled 20 h in Ar (Spex 8000) | 1370 | 1200 | 15 |
| MgH2 milled 1 h in Ar (P6) | 470 | 50 | 16 |
| MgH2 milled 10 h in Ar (Spex 8000) | 300 | 20 | 17 |
| MgH2-BM under 90 W green light-activated | 912/1230 | 60/120 | This work |
| MgH2 milled 3 h in H2 (Retsch PM100) | 580 | 23 | 18 |
| MgH2-RBM under 90 W green light-activated | 1331/1630.6 | 60/120 | This work |
| MgH2/3 M MgCl2 milled for 30 min | 946 | 60 | 19 |
| MgH2/5% MgCl2 milled for 5 h | 1094 | 60 | 7 |
| MgH2/10% NH4Cl milled for 5 h | 1331 | 60 | 7 |
| MgH2/10 mole% CaH2 milled for 1 h | 1389 | 60 | 20 |
2.6 Hydrolysis mechanism of MgH2 under light-activated
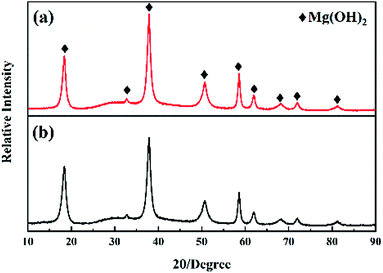
To study the catalytic mechanism of MgH2 under light-activated, the hydrolysis product after freeze-drying was characterized by XRD. Fig. 6 shows the XRD patterns of MgH2 hydrolysis product with and without green light-activated. As Fig. 6 shows, the MgH2 hydrolysis product under light-activated is the same as that without light-activated, both of them are merely composed of Mg(OH)2.Considering that Mg(OH)2 is the only hydrolysis product after the hydrolysis reaction of MgH2, the photocatalytic activity of Mg(OH)2 may effect on the hydrolysis mechanism. In fact, Mg(OH)2 has been widely applied in photovoltaic devices and solid-state electronics owing to its rapid electron transport, low exciton recombination rate and efficient photon-harvesting capacity.21–23
In Section 3.1, 3.2 and 3.5, we found that the hydrolysis yield and kinetics of MgH2 can be tuned by the energy of light-activated (frequency and power). However, in Section 3.3 and 3.4, we found that other driving force (stirring and heating) could not improve the hydrolysis performance. Therefore, the light energy and the photocatalytic activity of Mg(OH)2 play the key role on the hydrolysis mechanism of MgH2. Usually, Mg(OH)2 layer is deposited on the MgH2 surface impedes the diffusion of water, resulting in the sluggish hydrolysis kinetics of MgH2. However, Mg(OH)2 layer may provide the rapid transport for H− under light-activated, improving the hydrolysis performance of MgH2.
Consequently, the hydrolysis mechanism of MgH2 in the presence of light-activated may be as shown in Fig. 7. Under light-activated, the Mg(OH)2 layer on the surface of MgH2 is excited and functions as a catalyst, accelerating the decomposition of MgH2, similar to the photocatalytic water splitting. And then, Mg2+ combine with OH− to form new Mg(OH)2 layer, while H− transfer through the Mg(OH)2 layer and react with H2O to generate H2. Finally, the Mg(OH)2 layer becomes thicker during the reaction progress, reducing the lighting energy reach the interface of MgH2/Mg(OH)2, so the hydrolysis yield of MgH2 comes to a platform.
3. Experimental
3.1 Preparation
The starting materials, Mg (99.8%, 300 mesh, Tangshan Weihao Magnesium Powder) and MgH2 (99.5%, 400 mesh, MG Power) were used as raw materials without other treatment.MgH2-BM and MgH2-RBM were prepared by ball mill under argon and 2 MPa hydrogen atmosphere, respectively. Around 2 g sample and 80 g steel ball was loaded in the pressure ball-milled jar, and milled for 4 h at 400 rpm at room temperature by planetary ball mill (P5, FRITSCH), as Fig. 8 shown.
The 45 W spot lights with different color (blue: 470–465 nm; green: 530–520 nm; yellow: 595–585 nm) were purchased from Jiangsu Xincai Illumination.
3.2 Characterization
The phase structure of samples was characterized by X-ray diffraction (Bruker, D8 advance X-ray Diffraction) with Cu-Kα radiation, performed with a step size of 0.02° in the range of 10–90°.Hydrogen-generation measurements of samples were performed using in house-developed equipment, as Fig. 9 shown. The test steps are as follow:
(1) 0.1 g sample was loaded in a 100 mL flask
(2) The spot lights (yellow, green and blue) was turned on
(3) 20 mL deionized water was injected into the flask. The hydrogen was generated, pushing the water from the Monteggia washing bottle into a beaker placed on an electronic scale, which was recorded by the computer
(4) After converter, the hydrogen evolution curves (hydrogen volume against time) were drawn, and the hydrogen generation rate and yield were determined.
4. Conclusions
The hydrolysis reaction of Mg-based materials (Mg, MgH2, MgH2-BM and MgH2-RBM) with water has been effectively improved by light-activated. In addition, the hydrolysis performance of Mg-based materials could be tuned by the light energy (frequency and intensity). The combination of ball-milling and light-activated could further enhance the hydrolysis performance of MgH2. The degree of conversion of MgH2-RBM with 90 W green light-activated is up to 95.7%. Thus, rasing the light energy (by using purple light and UV, or higher power lights) and combination of ball-milling could lead to better hydrolysis performance of Mg-based materials.Due to the photocatalytic activity of Mg(OH)2, Mg(OH)2 layer under light-activated can act as a catalyst and enhances decomposition of MgH2, improving the hydrolysis yield and kinetics of Mg-based materials.
This work provide a green, simple and effective solution in enhance the hydrolysis performance of metal-based materials, and this solution is hopefully to work on other hydrogen generation materials, such as borohydrides and ammonia borane.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by MOST Scientific Project (2018YFE0100700), Guangdong Scientific Project (No. 2017B030314081), GDAS Project of Science and Technology Development (No. 2020GDASYL-20200103105), Regional Joint Fund project of Basic and Applied Basic Research Fund of Guangdong Province (No. 2020B1515120006) and Baotou Natural Science Foundation (Grant No. XM2020BT04).Notes and references
- D. Wu, et al., Hydrogen generation properties and the hydrolysis mechanism of Zr(BH4)4·8NH3, J. Mater. Chem. A, 2017, 5(32), 16630–16635 RSC.
- L. Ouyang, et al., Magnesium-based hydrogen storage compounds: A review, J. Alloys Compd., 2020, 832 Search PubMed.
- Z. Liu, et al., Hydrolysis of Mg-based alloys and their hydrides for efficient hydrogen generation, Int. J. Hydrogen Energy, 2021, 46(36), 18988–19000 CrossRef CAS.
- C. Zhou, et al., Controllable hydrogen generation behavior by hydrolysis of MgH2-based materials, J. Power Sources, 2021, 494 Search PubMed.
- Y. Liu, et al., Hydrogen generation from the hydrolysis of Mg powder ball-milled with AlCl3, Energy, 2013, 53, 147–152 CrossRef CAS.
- S. D. Kushch, et al., Hydrogen-generating compositions based on magnesium, Int. J. Hydrogen Energy, 2011, 36(1), 1321–1325 CrossRef CAS.
- S. Li, et al., Influence of chloride salts on hydrogen generation via hydrolysis of MgH 2 prepared by hydriding combustion synthesis and mechanical milling, Trans. Nonferrous Met. Soc. China, 2017, 27(3), 562–568 CrossRef CAS.
- S. A. Pighin, et al., Nanostructured Mg for hydrogen production by hydrolysis obtained by MgH2 milling and dehydriding, J. Alloys Compd., 2020, 827 Search PubMed.
- M. Grosjean, et al., Hydrogen production via hydrolysis reaction from ball-milled Mg-based materials, Int. J. Hydrogen Energy, 2006, 31(1), 109–119 CrossRef CAS.
- L. Xie, et al., Improved Hydrogen Generation Performance via Hydrolysis of MgH2 with Nb2O5 and CeO2 Doping, Mater. Trans., 2021, 62(6), 880–886 CrossRef CAS.
- K. Yang, et al., The Effect of Graphite and Fe2O3 Addition on Hydrolysis Kinetics of Mg-Based Hydrogen Storage Materials, Int. J. Photoenergy, 2021, 2021, 1–8 Search PubMed.
- Y. Sun and K. F. Aguey-Zinsou, Light-Activated Hydrogen Storage in Mg, LiH and NaAlH4, Chempluschem, 2018, 83(10), 904–908 CrossRef CAS PubMed.
- S. G. Bratsch, Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K, J. Phys. Chem. Ref. Data, 1989, 18(1), 1–21 CrossRef CAS.
- X. Li, et al., Synthesis and Photocatalytic Activity of TiO2/BiVO4 Layered Films under Visible Light Irradiation, J. Korean Ceram. Soc., 2016, 53(6), 665–669 CrossRef CAS.
- J. Huot, G. Liang and R. Schulz, Magnesium-based nanocomposites chemical hydrides, J. Alloys Compd., 2003, 353(1–2), L12–L15 CrossRef CAS.
- M. Tegel, et al., An efficient hydrolysis of MgH2-based materials, Int. J. Hydrogen Energy, 2017, 42(4), 2167–2176 CrossRef CAS.
- J. P. Tessier, et al., Hydrogen production and crystal structure of ball-milled MgH2–Ca and MgH2–CaH2 mixtures, J. Alloys Compd., 2004, 376(1–2), 180–185 CrossRef CAS.
- T. Tayeh, et al., Production of hydrogen from magnesium hydrides hydrolysis, Int. J. Hydrogen Energy, 2014, 39(7), 3109–3117 CrossRef CAS.
- M.-H. Grosjean and L. Roué, Hydrolysis of Mg–salt and MgH2–salt mixtures prepared by ball milling for hydrogen production, J. Alloys Compd., 2006, 416(1–2), 296–302 CrossRef CAS.
- Y. Xiao, et al., Hydrogen generation by CaH2-induced hydrolysis of Mg17Al12 hydride, Int. J. Hydrogen Energy, 2011, 36(24), 15698–15703 CrossRef CAS.
- B.-J. Wang, et al., Blue Phosphorus/Mg(OH)2 van der Waals Heterostructures as Promising Visible-Light Photocatalysts for Water Splitting, J. Phys. Chem. C, 2018, 122(13), 7075–7080 CrossRef CAS.
- Y. Luo, et al., Transition-metal dichalcogenides/Mg(OH)2 van der Waals heterostructures as promising water-splitting photocatalysts: a first-principles study, Phys. Chem. Chem. Phys., 2019, 21(4), 1791–1796 RSC.
- X. Zheng, et al., CuInS2/Mg(OH)2 Nanosheets for the Enhanced Visible-Light Photocatalytic Degradation of Tetracycline, Nanomaterials, 2019, 9(11), 1–12 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |