Open Access Article
Open Access ArticleSingle-pot tandem oxidative/C–H modification amidation process using ultrasmall PdNP-encapsulated porous organosilica nanotubes†
Behnam Gholipour a,
Afsaneh Zonouzi*a,
Sadegh Rostamnia
a,
Afsaneh Zonouzi*a,
Sadegh Rostamnia *b and
Xiao Liu
*b and
Xiao Liu *c
*c
aSchool of Chemistry, College of Science, University of Tehran, P.O. Box 14155-6455, Tehran, Iran. E-mail: b.gholipour@ut.ac.ir; zonouziafsaneh@ut.ac.ir
bOrganic and Nano Group (ONG), Department of Chemistry, Iran University of Science and Technology (IUST), PO Box 16846-13114, Tehran, Iran. E-mail: rostamnia@iust.ac.ir; srostamnia@gmail.com
cKey Laboratory of Pesticide & Chemical Biology of the Ministry of Education, College of Chemistry, Central China Normal University, Wuhan 430079, P. R. China. E-mail: liuxiao71@tju.edu.cn
First published on 2nd February 2022
Abstract
Herein, we studied a single-pot method with a dual catalysis process towards the conversion of primary aromatic alcohols to amides using ultrasmall PdNPs of controlled uniform size (1.8 nm) inside hybrid mesoporous organosilica nanotubes (MO-NTs). The catalyst exhibited excellent performance in water under mild conditions and showed high stability. The catalytic activity towards the tandem oxidation of alcohols in the presence of amine salts and H2O2 to their corresponding amides without producing byproducts was evaluated, and high yields were obtained for all products. The structure of the organosilica nanotubes containing palladium nanoparticles was investigated using various characterization techniques such as XRD, TEM, BET, solid-state 29Si NMR and solid-state 13C CP MAS NMR. Catalyst recycling tests showed that the catalytic power of PdNPs@B-SNTs was preserved after 8 cycles and a slight decrease in catalyst activity was observed.
1. Introduction
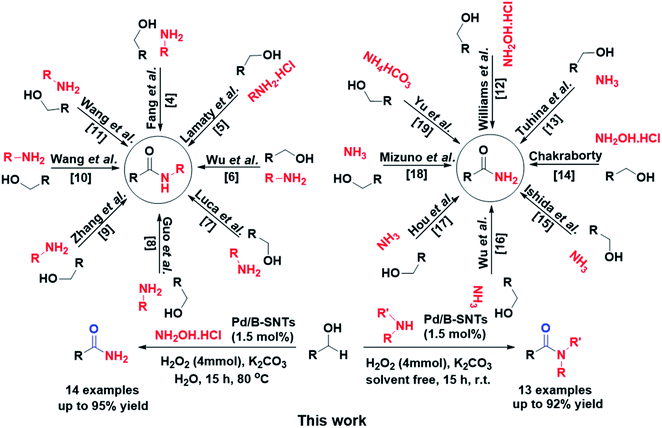
In a multitude of chemical structures, amide functional groups are important organic units. Moreover, due to the desirable properties of amides, such as high polarity, stability and conformational diversity, they have become one of the most beloved and reliable application groups in all branches of organic chemistry.1 For the formation of amide bonds, due to the applicability of this group in biological molecules, chemicals and pharmaceuticals, atom-efficient and green methods are a long-term consideration and a challenge in both research and industrial chemistry.2 Meanwhile, for diverse biologically active compounds and pharmaceuticals, primary and secondary amides are synthetically vital key intermediates as well as impressive progenitors.3 Among the methods proposed for the synthesis of amides, the use of alcohols has become one of the best alternatives. Indeed, alcohols can be converted to amides through a tandem oxidation process under oxidative conditions in situ via carboxylic acids or aldehydes4–19 (Scheme 1).In line with sustainable development and green chemistry, the utilization of heterogeneous catalysts versus conventional homogeneous catalysts has captured increasing attention.20 Among the heterogeneous catalysts, solid-based catalysts have many advantages such as lower corrosiveness, fewer disposal rates, and a higher selectivity to target products, as well as the simple separation of catalysts as an environmentally benign and economical route for the synthesis of chemicals.21 In this regard, the performance of heterogeneous catalysts can be amended by engineering the porosity of a solid catalyst to create a larger specific surface area, in addition to other parameters.22 In recent years, ordered mesoporous silica (OMS) has attracted research consideration in the field of catalysis because of its long-range order, high surface area, uniform silanol surface, ease of being functionalized, tunable hydrophobicity/hydrophilicity, periodically ordered pores (2–50 nm) and nanometer-thick walls.23 Accordingly, the immobilization of metal nanoparticles in solid supports such as PMO is an attractive strategy, which in addition to increasing lipophilicity can improve their recovery and diminish their tendency to agglomerate and leach.24 For this reason, many attempts have been reported that use PMOs for organic transformations, including Pd(nano)/MNP@IL-SiO2,25 Fe3O4@SiO2@PMO,26 Pd-MPMO,27 Sc(OTf)2-SO3Ph-PMO,28 Cu@PMO NCs,29 Porph@PMO and Pic@PMO.30 Despite the remarkable success of active and efficient heterogeneous catalysts, the design and development of sustainable and green catalysts continues to be an important and imperative challenge.
Our groups succeeded in engineering periodic mesoporous organosilicas (PMOs)31–37 and 1D organosilica nanotube linkages with organic ethane and phenylene groups.38 To further develop the application of organosilica nanotubes, we reported the enhanced durability of Ir-BPy-NT for water oxidation,39 PCN-SNT and PCN-BSNT as efficient photocatalysts for H2 generation40 and metal-free amino-incorporated organosilica nanotubes for the cycloaddition of CO2 to epoxides.41 In this work, in order to achieve an efficient and practical protocol, we developed the use of stabilized Pd nanoparticles in the inner channels of organosilica nanotubes assembled with 1,4-bis(triethoxysilyl)benzene and P123 precursors as active and reusable hybrid catalysts for the synthesis of primary and secondary amides in single-pot and mild conditions (Scheme 1).
2. Experimental
2.1. Chemicals and reagents
Triblock copolymer EO20PO70EO20 (Pluronic P123), 1,4-bis(triethoxysilyl)benzene (BTEB), tetraethoxysilane (99%, TEOS), and other reagents were purchased from Sigma-Aldrich (Shanghai Chemical Reagent., Inc. of Chinese Medicine Group). All materials were of analytical grade and used without any further purification.2.2. Characterization
Powder X-ray diffraction (PXRD) patterns were recorded on a Rigaku RINT D/Max-2500 powder diffraction system using Cu Kα radiation of 0.15406 nm wavelength over a 2θ range of 15–90° at a scan speed of 5° min−1 at room temperature. Nitrogen and water sorption experiments were performed at 77 K and 293 K, respectively, on a Micromeritics ASAP 2020 system. Prior to the measurement, the samples were out-gassed at 120 °C for at least 6 h. The Brunauer–Emmett–Teller (BET) specific surface areas were calculated using the adsorption data at a relative pressure range of P/P0 = 0.05–0.25. Pore size distributions were calculated from the adsorption branch using the Barrett–Joyner–Halenda (BJH) method. The total pore volumes were estimated from the amount adsorbed at a relative pressure (P/P0) of 0.99. Transmission electron microscopy (TEM) was performed using an FEI Tecnai G2 Spirit at an acceleration voltage of 120 kV. 13C (100.5 MHz) cross-polarization magic angle spinning (CP-MAS) and 29Si (79.4 MHz) MAS solid-state NMR experiments were conducted on a Varian Infinity-Plus 400 spectrometer equipped with a magic-angle spin probe in a 4 mm ZrO2 rotor. 13C and 29Si signals were referenced to tetramethylsilane. The experimental parameters were an 8 kHz spin rate, 3 s pulse delay, 4 min contact time, and 1500–3000 scans for the 13C CP-MAS NMR experiments, and a 4 kHz spin rate, 180 s pulse delay, 10 min contact time, and 116 scans for the 29Si MAS NMR experiments.2.3. Synthesis of benzene-silica nanotubes (B-SNTs)
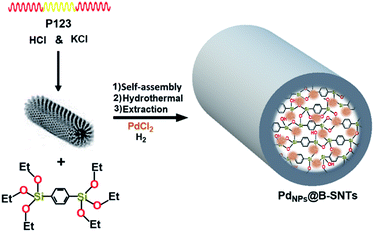
According to a previous report on the synthesis of benzene-silica nanotubes,38 0.55 g P123 and 3.49 g KCl were dissolved in 180 mL 2 M HCl at 38 °C. After complete copolymer dissolution, 3.50 mmol 1,4-bis(triethoxysilyl)benzene (BTEB) was added under vigorous shaking for 6 min, and the resulting mixture was kept for 24 h at the same temperature. Subsequently, the mixture was treated at 100 °C under static conditions for 24 h. The solid product was recovered by filtration and air-dried at ambient temperature overnight. Finally, for the extracted surfactant, 1.0 g of the as-synthesized solid in 200 mL of ethanol containing 1.5 g concentrated aqueous HCl solution was refluxed for 24 h.2.4. Encapsulation of Pd particles in the benzene-silica nanotubes (PdNPs@B-SNTs)
For the synthesis of PdNPs@B-SNTs, B-SNTs (0.3 g) were added to 10 mL ethanol solution containing PdCl2 (0.026 g). After 1 h of ultrasonic treatment, the solution was stirred at room temperature for 12 h. Finally, the obtained solids were reduced at 150 °C in H2 for 5 h.2.5. Synthesis of SBA-15 and the encapsulation of Pd particles in the pores
Synthesis of SBA-15 using an aqueous solution of the Pluronic copolymer P123 (E21P67E21), 2 M HCl and tetraethyl orthosilicate (TEOS) was executed step-by-step according to our previous method.42–54 Loading of PdNPs in SBA-15 pore channels was performed according to the abovementioned method.2.6. General procedure for the synthesis of primary and secondary amides
To synthesize primary and secondary amides, mixtures of preliminary substrates (amine salts and benzyl alcohol) in separate dry vials were subjected to intense stirring under obtained optimal conditions (Scheme 1). After the specified time, the reaction mixture was diluted in CH2Cl2/H2O solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After rinsing the organic phase with H2O several times (3 × 10 mL), the CH2Cl2 solvent was separated with a rotary. Finally, purification was performed via chromatography on silica gel (hexanes/EtOAc).
1). After rinsing the organic phase with H2O several times (3 × 10 mL), the CH2Cl2 solvent was separated with a rotary. Finally, purification was performed via chromatography on silica gel (hexanes/EtOAc).
3. Results and discussion
Fig. 1 summarizes the preparation of organosilica nanotubes to support palladium nanoparticles for the sustainable and green synthesis of primary and secondary amides. Benzene-bridged organosilica nanotubes (B-SNTs) were constructed with the incorporation of covalently bonded organic–inorganic 1,4-bis(triethoxysilyl)benzene (BTEB) ligands in the presence of P123 under acidic conditions, and the template was extracted using EtOH. Then, the as-synthesized B-SNTs were utilized for the encapsulation of palladium nanoparticles inside the pore channels.Solid-state 29Si NMR spectrum of the B-SNTs indicates the existence of Tn sites (Fig. 2a). The signals at −60.7, −70.2 and −80.3 ppm in the spectrum of the B-SNTs represent the silicone species T1 [SiC(OH)2(OSi)], T2 [SiC(OH)(OSi)2], and T3 [SiC(OSi)3], respectively, which confirm that the full framework joined the organosilica nanotubes. The lack of SiO4 species, such as Q3 [Si(OH)(OSi)3] and Q4 [Si(OSi)4], in the range of −90 to −120 ppm confirms that no carbon–silicon bond fracture occurred during the synthesis of the organosilica nanotubes.31 The chemical shift at 133.4 ppm is related to the signal of the benzene ring. The signals at 71.5, 55.6 and 18.8 ppm correspond to the remaining surfactants and ethoxy carbon groups formed during the surfactant extraction process (Fig. 2b).31 Wide-angle XRD analysis was used to confirm the structure and crystallinity of the as-synthesized PdNPs inside the B-SNTs and SBA-15. The scatter pattern for the Pd nanoparticles with five specific reflections at 40.06 (1 1 1), 47.02 (2 0 0), 68.03 (2 2 0), 81.68 (3 1 1) and 86.32 (2 2 2) shows that these characteristic reflections are related to the cubic (fcc) structure of the PdNPs (JCPDS: 87-0641, space group: Fm3m (225)) (Fig. 2c and d).38
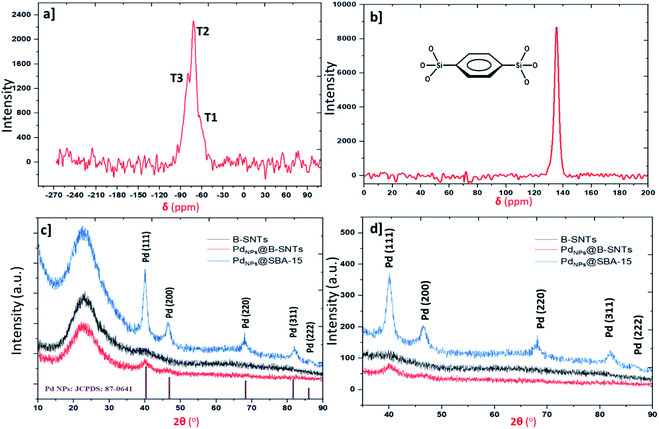 | ||
| Fig. 2 (a) 29Si NMR spectrum of the B-SNTs. (b) 13C CP MAS NMR spectra of the B-SNTs. (c) and (d) XRD patterns of PdNPs@B-SNTs and PdNPs@SBA-15. | ||
The surface oxidation and chemical modes of the PdNPs@B-SNT catalysts were analyzed by XPS spectroscopy. Fig. 3a exhibits the XPS spectra of PdNPs@B-SNTs. Several distinct peaks were observed in the XPS survey spectra at ∼105, ∼157, ∼290, ∼335–345, ∼537 and ∼560 eV, corresponding to Si 2p, Si 2s, C 1s, Pd 3d, O 1s and Pd 3p, respectively. The PdNPs@B-SNT spectrum revealed in Fig. 3b shows the presence of two chemical states of Pd. The binding energy range of 335–341 eV is correlated to Pd 3d5/2 and Pd 3d3/2 of metallic Pd, while the component at 338–344 eV is related to PdO owing to the partial oxidation of the PdNPs.55,56 The surface area, pore volume and diameter of the B-SNTs and PdNPs@B-SNTs are calculated based on the Brunauer–Emmett–Teller (BET) equation (Table 1).
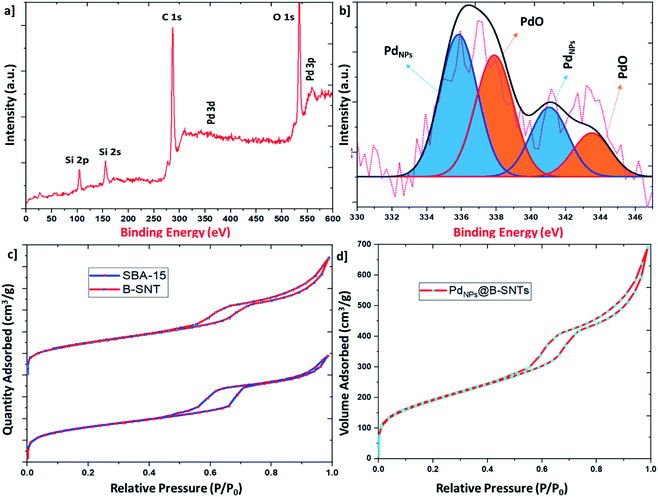 | ||
| Fig. 3 (a) and (b) XPS spectra of PdNPs@B-SNTs. (c) and (d) Nitrogen adsorption–desorption isotherms of the B-SNTs, SBA-15 and PdNPs/B-SNTs. | ||
| Sample | BET surface area (cm3 g−1) | Pore diameter (nm) | Total pore volume (cm3 g−1) |
|---|---|---|---|
| B-SNT | 701 | 5.5 | 0.79 |
| PdNPs-B-SNT | 690 | 6.1 | 1.07 |
| SBA-15 | 835.4 | 6.8 | 1.12 |
Nitrogen adsorption and desorption isotherms of the B-SNTs, SBA-15 and PdNPs@B-SNTs are shown in Fig. 3c and d. The hysteresis ring at a pressure of P/P0 = 0.5–0.7, which is consistent with the mesoporous materials, indicates the maintenance of the structure of the B-SNTs and PdNPs@B-SNTs after the loading of Pd nanoparticles. The decrease in the BET surface area of PdNPs@B-SNTs is attributed to the presence of Pd nanoparticles loaded inside the B-SNT channels, leading to the partial obstruction of the channels. A small difference in the surface areas of the B-SNTs and SBA-15 might be due to the presence of 1,4-bis(triethoxysilyl)benzene (BTEB) ligands in the B-SNTs.
Fig. 4a–d illustrates the TEM images of the B-SNTs before and after the encapsulation of palladium nanoparticles. The TEM images clearly demonstrate that the Pd nanoparticles are evenly distributed in the inner channel of the B-SNTs. Based on the TEM images, the size of the PdNPs in the B-SNTs is 1.8 nm. According to the results of TEM, the occurrence of much smaller Pd nanoparticles in the B-SNTs might be due to the stabilizing effect of the benzene ring in the B-SNT network.
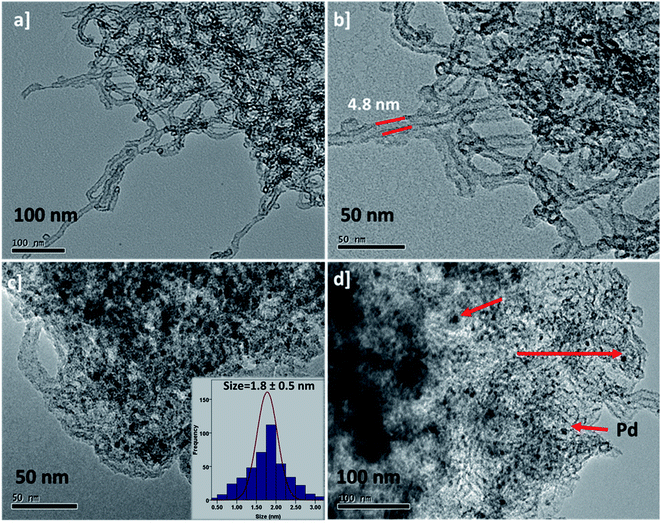 | ||
| Fig. 4 (a) and (b) TEM images of the B-SNTs and (c) and (d) PdNPs@B-SNTs, and the average diameter histogram of the PdNPs for PdNPs@B-SNTs. | ||
In line with the development of sustainable protocols and inspired by our previous work on the catalytic application of solid-based catalysts and porous materials (PMO, SBA-15, and MOF) in organic reaction transformations,31,34,52,53,55–65 especially the synthesis of amides,66–70 herein, we describe a one-pot tandem oxidative amidation reaction using PdNPs@B-SNTs as an active catalyst and H2O2 as a green oxidant for the synthesis of primary, secondary, and tertiary amides. The catalytic activity domain of PdNPs@B-SNTs for the synthesis of amides using various structural compounds (primary aromatic and aliphatic alcohols, amine salts, and NH2OH·HCl) through oxidative amidation was evaluated. The results are further elaborated and summarized in Table 2, and the reaction conditions such as different bases, oxidants, solvent and the amount of catalyst are explored. Assuming that the addition of water as a green solvent might increase product yields, it was used as the preliminary solvent. Initially, benzyl alcohol, hydroxylammonium chloride and K2CO3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 ratio) were selected for a model reaction in the presence of H2O2 (30%). First, the effects of H2O2 and PdNP amount using H2O as a solvent at 80 °C for the formation of amide were investigated. Comparison of the different values of H2O2 and PdNPs showed that the use of 4 mmol H2O2 and 1 mol% of PdNPs affords an excellent yield (Table 2, entries 1–10). On the other hand, the other oxidants did not show the desired yield compared to H2O2, except for TBHP (70% aqueous solution), which yielded 80% (Table 2, entries 11–13). In the different base study for the oxidative amidation of benzyl alcohol and hydroxyl ammonium chloride, the results of Cs2CO3 (95%) and K2CO3 (95%) were close, so K2CO3 was chosen as the appropriate base owing to its price and availability. No product was observed in the absence of a base. The results are summarized for other bases in Table 2 (entries 14–20). Furthermore, in the absence of a catalyst, no products were observed (Table 2, entry 21). A number of solvents including ethanol, dioxane, MeOH, toluene and DMSO were scrutinized in order to achieve further optimum conditions (Table 2). The catalyst performance was moderate in ethanol, dioxane, DMF, toluene and DMSO (yields of 20–75%), while amide yield was 95% in H2O (Table 2, entries 22–26). As a result, H2O was selected as the suitable solvent. The optimal conditions for PdNPs@B-SNTs were also evaluated for PdNPs@SBA-15 and a yield of 80% was obtained. The difference in the yield of the two studied catalysts could be related to the presence of aromatic phenyl groups in the body of the B-SNT channels, which increases the lipophilicity and thus leads to the effective mass transfer of organic lipophilic molecules to the inner surface of the channels to interact with the catalytic center.23,71,72
1.5 ratio) were selected for a model reaction in the presence of H2O2 (30%). First, the effects of H2O2 and PdNP amount using H2O as a solvent at 80 °C for the formation of amide were investigated. Comparison of the different values of H2O2 and PdNPs showed that the use of 4 mmol H2O2 and 1 mol% of PdNPs affords an excellent yield (Table 2, entries 1–10). On the other hand, the other oxidants did not show the desired yield compared to H2O2, except for TBHP (70% aqueous solution), which yielded 80% (Table 2, entries 11–13). In the different base study for the oxidative amidation of benzyl alcohol and hydroxyl ammonium chloride, the results of Cs2CO3 (95%) and K2CO3 (95%) were close, so K2CO3 was chosen as the appropriate base owing to its price and availability. No product was observed in the absence of a base. The results are summarized for other bases in Table 2 (entries 14–20). Furthermore, in the absence of a catalyst, no products were observed (Table 2, entry 21). A number of solvents including ethanol, dioxane, MeOH, toluene and DMSO were scrutinized in order to achieve further optimum conditions (Table 2). The catalyst performance was moderate in ethanol, dioxane, DMF, toluene and DMSO (yields of 20–75%), while amide yield was 95% in H2O (Table 2, entries 22–26). As a result, H2O was selected as the suitable solvent. The optimal conditions for PdNPs@B-SNTs were also evaluated for PdNPs@SBA-15 and a yield of 80% was obtained. The difference in the yield of the two studied catalysts could be related to the presence of aromatic phenyl groups in the body of the B-SNT channels, which increases the lipophilicity and thus leads to the effective mass transfer of organic lipophilic molecules to the inner surface of the channels to interact with the catalytic center.23,71,72
| Entry | Catalyst | Oxidant (mmol) | Solvent | Base | Pd (mol%) | T (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), NH2OH·HCl (1 mmol), base (1.5 mmol), H2O2 (4 mmol) in 3 mL of solvent.b Isolated yield. | ||||||||
| 1 | PdNPs@B-SNTs | H2O2 (1) | H2O | K2CO3 | 1 | 80 | 24 | 60 |
| 2 | PdNPs@B-SNTs | H2O2 (2) | H2O | K2CO3 | 1 | 80 | 24 | 75 |
| 3 | PdNPs@B-SNTs | H2O2 (3) | H2O | K2CO3 | 1 | 80 | 20 | 85 |
| 4 | PdNPs@B-SNTs | H2O2 (4) | H2O | K2CO3 | 1 | 80 | 20 | 95 |
| 5 | PdNPs@SBA-15 | H2O2 (4) | H2O | K2CO3 | 1 | 80 | 20 | 80 |
| 6 | PdNPs@B-SNTs | H2O2 (4) | H2O | K2CO3 | 1 | 100 | 20 | 95 |
| 7 | PdNPs@B-SNTs | H2O2 (4) | H2O | K2CO3 | 0.5 | 80 | 24 | 60 |
| 8 | PdNPs@B-SNTs | H2O2 (4) | H2O | K2CO3 | 1.5 | 80 | 20 | 95 |
| 9 | PdNPs@B-SNTs | H2O2 (4) | H2O | K2CO3 | 1 | 60 | 24 | 70 |
| 10 | PdNPs@B-SNTs | H2O2 (4) | H2O | K2CO3 | 1 | r.t. | 24 | 40 |
| 11 | PdNPs@B-SNTs | MnO2 (4) | H2O | K2CO3 | 1 | 80 | 20 | 25 |
| 12 | PdNPs@B-SNTs | TBHP (4) | H2O | K2CO3 | 1 | 80 | 20 | 80 |
| 13 | PdNPs@B-SNTs | SeO2 (4) | H2O | K2CO3 | 1 | 80 | 20 | 30 |
| 14 | PdNPs@B-SNTs | H2O2 (4) | H2O | NaOAc | 1 | 80 | 20 | 35 |
| 15 | PdNPs@B-SNTs | H2O2 (4) | H2O | NaHCO3 | 1 | 80 | 20 | 25 |
| 16 | PdNPs@B-SNTs | H2O2 (4) | H2O | Na2CO3 | 1 | 80 | 20 | 92 |
| 17 | PdNPs@B-SNTs | H2O2 (4) | H2O | Cs2O3 | 1 | 80 | 20 | 95 |
| 18 | PdNPs@B-SNTs | H2O2 (4) | H2O | NEt3 | 1 | 80 | 20 | 40 |
| 19 | PdNPs@B-SNTs | H2O2 (4) | H2O | Py | 1 | 80 | 20 | 30 |
| 20 | PdNPs@B-SNTs | H2O2 (4) | H2O | — | 1 | 80 | 20 | — |
| 21 | — | H2O2 (4) | H2O | K2CO3 | 1 | 80 | 20 | — |
| 22 | PdNPs@B-SNTs | H2O2 (4) | Ethanol | K2CO3 | 1 | 80 | 20 | 75 |
| 23 | PdNPs@B-SNTs | H2O2 (4) | Dioxane | K2CO3 | 1 | 80 | 20 | 50 |
| 24 | PdNPs@B-SNTs | H2O2 (4) | DMSO | K2CO3 | 1 | 80 | 20 | 55 |
| 25 | PdNPs@B-SNTs | H2O2 (4) | Toluene | K2CO3 | 1 | 80 | 20 | 20 |
| 26 | PdNPs@B-SNTs | H2O2 (4) | DMF | K2CO3 | 1 | 80 | 20 | 65 |
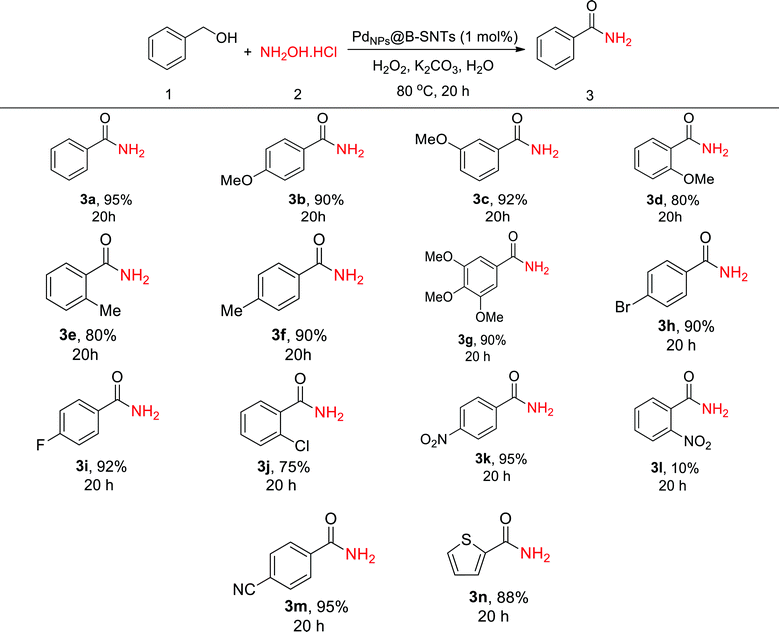
Taking into account the results obtained in Table 2, we examined the scope of the Pd-catalyzed tandem oxidative process for the synthesis of primary amides from primary aromatic alcohols and hydroxyl ammonium chloride under the optimized reaction conditions. Further screening of the electronic effect on the conversion of other alcohol derivatives into amides revealed that both electron-rich and electron-poor benzyl alcohols were successfully converted to their corresponding amides with high yield (Table 3). The results indicate that primary aromatic alcohols, particularly alcohols with electron withdrawing groups, are more active in the amidation reaction. A similar result was obtained with aromatic alcohols for heteroaromatic thiophene-2-carbaldehyde (Table 3).
| a Reaction condition: alcohol (1 mmol), NH2OH·HCl (1 mmol), K2CO3 (1.5 mmol), H2O2 (4 mmol) in 3 mL H2O at 80 °C. |
|---|
 |
In another method, the PdNPs@B-SNT catalyst was explored in one-pot tandem oxidative amidation reactions in the presence of H2O2 (30%) in solvent-free conditions. The catalytic activity of PdNPs@B-SNTs towards amide synthesis via the oxidative amidation of primary aromatic and aliphatic alcohols using aniline in the presence of H2O2 was evaluated as follows: PdNPs@B-SNTs (0.5 mol%), benzyl alcohol (1 mmol), aniline (1.2 mmol), H2O2 (2 mmol) and K2CO3 (1.5 mmol) were placed in a Teflon vessel with magnetic stirring at room temperature. Under these conditions, a conversion of 70% was obtained. By increasing the amount of PdNPs to 1.5 mol% and H2O2 to 4 mmol, the yield of conversion increased to 92% and the reaction time diminished to 12 h (Table 4, entries 1–5). Examination of various oxidants such as SeO2, MnO2, DDQ, NaClO and TBHP in comparison with H2O2 shows that H2O2 leads to the rapid conversion of benzyl alcohol to the desired amide in the presence of aniline. The results of the various oxidants are summarized in Table 4 (entries 6–10). As shown in Table 4, the study of different bases shows that the conversion percentage is higher when K2CO3 is used compared to other bases (Table 4, entries 11–17). No product was observed in the absence of base or catalyst (Table 4, entries 18–19). In the case of PdNPs@SBA-15 versus PdNPs@B-SNTs, 78% yield was achieved, which according to the above explanation on primary amide synthesis is due to the existence of aromatic phenyl groups (Table 4, entry 5).
| Entry | Catalyst | Pd (mol%) | Base | [O] | T (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: benzyl alcohol (1 mmol), aniline (1.2 mmol), base (1.5 mmol), oxidant (2–4 mmol) and PdNPs@B-SNTs (variant mol% of Pd) at room temperature in specific time.b Isolated yield. | |||||||
| 1 | PdNPs@B-SNTs | 0.5 | K2CO3 | H2O2 (2 mmol) | 25 | 20 | 70 |
| 2 | PdNPs@B-SNTs | 1 | K2CO3 | H2O2 (2 mmol) | 25 | 20 | 78 |
| 3 | PdNPs@B-SNTs | 1 | K2CO3 | H2O2 (4 mmol) | 25 | 20 | 80 |
| 4 | PdNPs@B-SNTs | 1.5 | K2CO3 | H2O2 (4 mmol) | 25 | 15 | 92 |
| 5 | PdNPs @SBA-15 | 1.5 | K2CO3 | H2O2 (4 mmol) | 25 | 15 | 78 |
| 6 | PdNPs@B-SNTs | 1.5 | K2CO3 | DDQ (4 mmol) | 25 | 15 | 25 |
| 7 | PdNPs@B-SNTs | 1.5 | K2CO3 | NaClO (4 mmol) | 25 | 15 | 20 |
| 8 | PdNPs@B-SNTs | 1.5 | K2CO3 | MnO2 (4 mmol) | 25 | 15 | 35 |
| 9 | PdNPs@B-SNTs | 1.5 | K2CO3 | SeO2 (4 mmol) | 25 | 15 | 30 |
| 10 | PdNPs@B-SNTs | 1.5 | K2CO3 | TBHP (4 mmol) | 25 | 15 | 70 |
| 11 | PdNPs@B-SNTs | 1.5 | Na2CO3 | H2O2 (4 mmol) | 25 | 15 | 85 |
| 12 | PdNPs@B-SNTs | 1.5 | NaOAc | H2O2 (4 mmol) | 25 | 15 | 40 |
| 13 | PdNPs@B-SNTs | 1.5 | NaHCO3 | H2O2 (4 mmol) | 25 | 15 | 35 |
| 14 | PdNPs@B-SNTs | 1.5 | Cs2CO3 | H2O2 (4 mmol) | 25 | 15 | 88 |
| 15 | PdNPs@B-SNTs | 1.5 | K3PO4 | H2O2 (4 mmol) | 25 | 15 | 25 |
| 16 | PdNPs@B-SNTs | 1.5 | NEt3 | H2O2 (4 mmol) | 25 | 15 | 30 |
| 17 | PdNPs@B-SNTs | 1.5 | Py | H2O2 (4 mmol) | 25 | 15 | 25 |
| 18 | PdNPs@B-SNTs | 1.5 | — | H2O2 (4 mmol) | 25 | 15 | — |
| 19 | — | 1.5 | K2CO3 | H2O2 (4 mmol) | 25 | 15 | — |
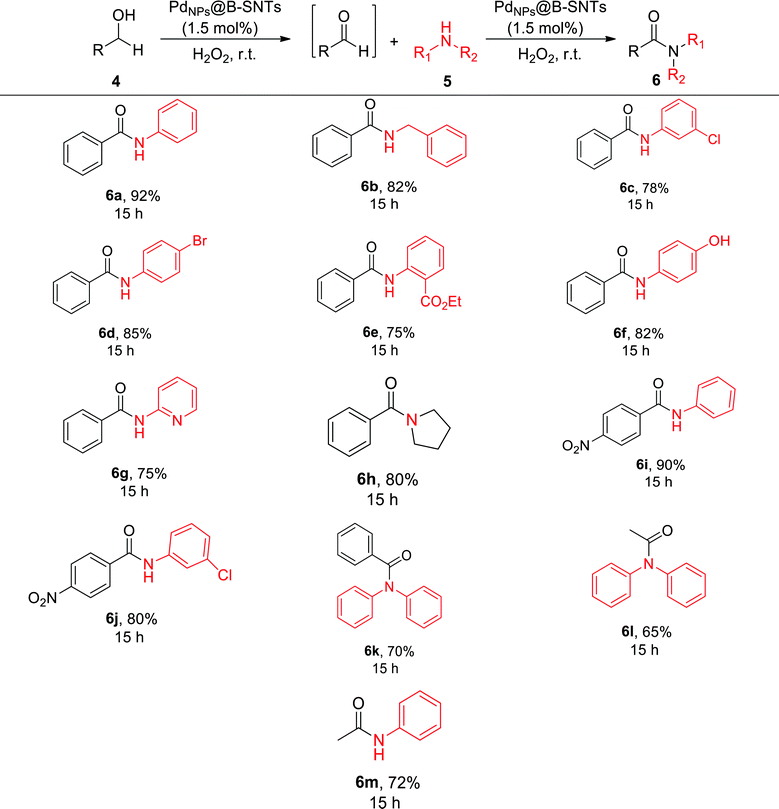
After achieving optimal conditions, further screening of the tandem oxidative reaction with different primary aromatic and aliphatic alcohols and various amines was performed to find the general mechanism and domain of the reaction. For all alcohol and amine derivatives with various substituents (Cl, Br, NO2, CO2Ee, OH and H) toward the synthesis of the desired amides, moderate to good reactivity was observed. Aromatic amines with electron-withdrawing groups on the aromatic ring provided higher yield in the synthesis of amides (Table 5). In the case of aliphatic alcohols, inferior yields were observed when compared to aromatic alcohols, which might be due to the side reactions such as oxidation to the corresponding carboxylic acids or aldol condensation. In addition to aniline, other amines such as pyrrolidine, diphenylamine, and phenyl methanamine were evaluated, and the results are summarized in Table 5.
| a Reaction conditions: benzyl alcohol (1 mmol), amine (1.2 mmol), base (1.5 mmol), H2O2 (4 mmol) and PdNPs@B-SNTs (1.5 mol% of Pd). |
|---|
 |
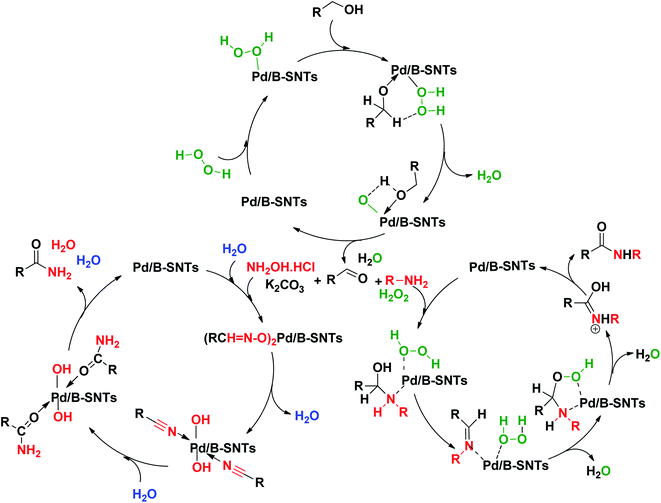
As explained in the literature,73,74 in the case of a plausible mechanism for the oxidation of alcohols, it can be said that at first the active site of the PdNPs interacts with H2O2 to form the PdNPs peroxo complex (I). The complex then reacts with benzyl alcohol, which leads to the formation of intermediate (II), which eventually regenerates the active sites of benzyl alcohol by two steps of dehydration and forms benzaldehyde. The primary amide formation mechanism proceeds through the reaction of an aldehyde with hydroxylammonium chloride in the presence of palladium with the formation of an aldoxime–PDNPs intermediate. Then, during the dehydration process, intermediate (I) forms. Finally, hydration of intermediate (I) leads to the formation of intermediate (II) and completes the catalytic cycle via dehydration processes.75 For secondary amides, the reaction mechanism involves oxidation and/or rearrangement via hydroperoxide intermediates formed from hemiaminal intermediates and/or imine intermediates.76 As shown in Scheme 2, the reaction mechanism involves the formation of a peroxide mediator in the oxidative process.
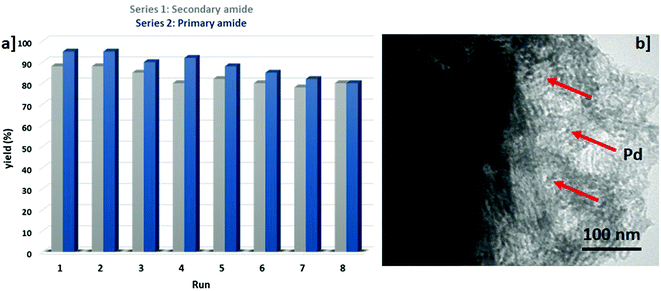
To further assess the performance of PdNPs@B-SNTs, the stability and reusability of PdNPs@B-SNTs toward the amidation reaction according to the model reaction were investigated. After the completion of the reaction, the catalyst and the product (PdNPs@B-SNTs + amide) were isolated from the reaction mixture using a biphasic system, and the catalyst was precipitated in the aqueous phase. After several washes, the catalyst was dried for 6 h at 70 °C and was reused for the next recycling run. We found that in addition to the high stability, the PdNPs@B-SNT catalyst can be reused for up to 8 consecutive cycles without noticeable loss of activity (Fig. 5a). The TEM image and the atomic absorption spectrometry (AAS) of PdNPs@B-SNTs after 8 successive uses also confirmed this fact and shows that the nanoparticles demonstrated reduced leaching (Fig. 5b).
A controlled leaching experiment was performed to attain an understanding of the heterogeneous nature of PdNPs@B-SNTs. The results of atomic absorption spectrometry (AAS) showed that after 8 runs, the leaching of Pd is below 1.0 ppm in each cycle. Thus, the AAS analysis confirmed that the PdNPs@B-SNT catalyst is heterogeneous in nature. The leaching of PdNPs@B-SNTs is quantified and presented in Table 6.
| Cycle | Scale (mol) | Leaching (ppm) | Yieldb (%) |
|---|---|---|---|
| a Benzyl alcohol (1 mmol), amine source (1.2 mmol), base (1.5 mmol), H2O2 (4 mmol) and PdNPs@B-SNTs (1.5 mol% of Pd); 15–20 h.b Isolated yields. | |||
| 1 | 1 | 0.1 | 95/92 |
| 2 | 0.8 | 0.15 | 95/92 |
| 3 | 0.78 | 0.25 | 90/88 |
| 4 | 0.74 | 0.38 | 88/85 |
| 5 | 0.7 | 0.4 | 88/88 |
| 6 | 0.65 | 0.48 | 85/85 |
| 7 | 0.62 | 0.53 | 83/83 |
| 8 | 0.58 | 0.58 | 83/83 |
The results of our work in comparison with catalysts previously reported in the literature are summarized in Table 7. As can be seen, this catalyst exhibited good catalytic activity with excellent yields (up to 95%) in mild conditions compared to most of the reported works.
| Entry | Catalyst | Oxidant | Solvent | T (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | MnO2 | O2 | Toluene | 100 | 24 | 57 | 11 |
| 2 | GO/PdNPs | H2O2 | H2O | 25 | 15–24 | 94 | 68 |
| 3 | MnO2/GO | O2 | H2O | 150 | 3–30 | 98 | 13 |
| 4 | PS-BHA-Cu | TBHP | DMF–H2O | 80 | 5 | 99 | 8 |
| 5 | AuPd/resin | H2O | O2 | 40 | 12 | 49–99 | 77 |
| 6 | CuSO4·5H2O | TBHP | Acetonitrile | 80 | 24 | 46–93 | 7 |
| 7 | FeCl3 | EDC | TEMPO | 90 | 8–32 | 75–92 | 10 |
| 8 | PdNPs@B-SNTs | H2O | H2O2 | 25–80 | 15–20 | 95 | This work |
4. Conclusion
In summary, we developed a sustainable and green catalytic protocol using encapsulated palladium nanoparticles within B-SNT organosilica cavities to synthesize a wide variety of amides using two different methods. All of the results indicate the high potential of PdNPs@B-SNTs as an exceptional hybrid catalyst for the amidation reaction under mild conditions with excellent performance. In this work, H2O2 was used as a mild and inexpensive oxidant, in accordance with the green chemistry landscape, as a promising oxidant and only produced water as a by-product. Due to the desirable results in this work, the high performance of this protocol can be studied for other cases, which will be reported in subsequent works.Conflicts of interest
The authors declare no competing financial interests.References
- V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471–479 CrossRef CAS PubMed.
- J. R. Dunetz, J. Magano and G. A. Weisenburger, Org. Process Res. Dev., 2016, 20, 140–177 CrossRef CAS.
- G. Li and M. Szostak, Nat. Commun., 2018, 9, 1–8 CrossRef PubMed.
- J. Gu, Z. Fang, C. Liu, Z. Yang, X. Li, P. Wei and K. Guo, RSC Adv., 2015, 5, 95014–95019 RSC.
- X.-F. Wu, M. Sharif, J.-B. Feng, H. Neumann, A. Pews-Davtyan and P. Langer, Green Chem., 2013, 15, 1956–1961 RSC.
- S. Gaspa, A. Porcheddu and L. De Luca, Org. Biomol. Chem., 2013, 11, 3803–3807 RSC.
- J. Gu, Z. Fang, Y. Yang, Z. Yang, L. Wan, X. Li, P. Wei and K. Guo, RSC Adv., 2016, 6, 89413–89416 RSC.
- R. A. Molla, K. Ghosh, K. Tuhina and S. Manirul Islam, New J. Chem., 2015, 39, 921–930 RSC.
- G. Wang, Q. Y. Yu, S. Y. Chen and X. Q. Yu, Org. Biomol. Chem., 2014, 12, 414–417 RSC.
- R. Das and D. Chakraborty, Catal. Commun., 2012, 26, 48–53 CrossRef CAS.
- T. Ishida, H. Watanabe, T. Takei, A. Hamasaki, M. Tokunaga and M. Haruta, Appl. Catal., A, 2012, 425–426, 85–90 CrossRef CAS.
- F. Paquin, J. Rivnay, A. Salleo, N. Stingelin and C. Silva, J. Mater. Chem. C, 2015, 3, 10715–10722 RSC.
- R. Nie, J. Shi, S. Xia, L. Shen, P. Chen, Z. Hou and F. S. Xiao, J. Mater. Chem., 2012, 22, 18115–18118 RSC.
- K. Yamaguchi, H. Kobayashi, T. Oishi and N. Mizuno, Angew. Chem., Int. Ed., 2012, 51, 544–547 CrossRef CAS PubMed.
- D. Q. Dong, S. H. Hao, H. Zhang and Z. L. Wang, Chin. Chem. Lett., 2017, 28, 1597–1599 CrossRef CAS.
- X. Guo, L. Tang, Y. Yang, Z. Zha and Z. Wang, Green Chem., 2014, 16, 2443–2447 RSC.
- N. A. Owston, A. J. Parker and J. M. J. Williams, Org. Lett., 2007, 9, 73–75 CrossRef CAS PubMed.
- X. Bantreil, C. Fleith, J. Martinez and F. Lamaty, ChemCatChem, 2012, 4, 1922–1925 CrossRef CAS.
- X. F. Wu, M. Sharif, A. Pews-Davtyan, P. Langer, K. Ayub and M. Beller, Eur. J. Org. Chem., 2013, 2783–2787 CrossRef CAS.
- A. Wang, J. Li and T. Zhang, Nat. Rev. Chem., 2018, 2, 65–81 CrossRef CAS.
- L. B. Sun, X. Q. Liu and H. C. Zhou, Chem. Soc. Rev., 2015, 44, 5092–5147 RSC.
- Y. Wang, H. Arandiyan, J. Scott, A. Bagheri, H. Dai and R. Amal, J. Mater. Chem. A, 2017, 5, 8825–8846 RSC.
- W. Wang, J. E. Lofgreen and G. A. Ozin, Small, 2010, 6, 2634–2642 CrossRef CAS PubMed.
- Y. Wu, Y. Zhang, J. Zhou and D. Gu, Emergent Mater., 2020, 3, 247–266 CrossRef CAS.
- S. Omar and R. Abu-Reziq, J. Phys. Chem. C, 2014, 118, 30045–30056 CrossRef CAS.
- J. Dai, H. Zou, R. Wang, Y. Wang, Z. Shi and S. Qiu, Green Chem., 2017, 19, 1336–1344 RSC.
- S. Elavarasan, K. Kala, I. Muhammad, A. Bhaumik and M. Sasidharan, Mol. Catal., 2019, 476, 110521 CrossRef.
- M. Chen, C. Liang, F. Zhang and H. Li, ACS Sustainable Chem. Eng., 2014, 2, 486–492 CrossRef CAS.
- H. Naeimi, V. Nejadshafiee and S. Masoum, RSC Adv., 2015, 5, 15006–15016 RSC.
- L. Bourda, H. S. Jena, R. Van Deun, A. M. Kaczmarek and P. Van Der Voort, J. Mater. Chem. A, 2019, 7, 14060–14069 RSC.
- E. Doustkhah, S. Rostamnia, M. Imura, Y. Ide, S. Mohammadi, C. J. T. Hyland, J. You, N. Tsunoji, B. Zeynizadeh and Y. Yamauchi, RSC Adv., 2017, 7, 56306–56310 RSC.
- A. Eftekhari, M. Dalili, Z. Karimi, S. Rouhani, A. Hasanzadeh, S. Rostamnia, S. Khaksar, A. O. Idris, H. Karimi-Maleh and M. L. Yola, Food Chem., 2021, 358, 129763 CrossRef CAS PubMed.
- E. Doustkhah, H. Mohtasham, M. Farajzadeh, S. Rostamnia, Y. Wang, H. Arandiyan and M. H. N. Assadi, Microporous Mesoporous Mater., 2020, 293, 109832 CrossRef CAS.
- E. Doustkhah, H. Mohtasham, M. Hasani, Y. Ide, S. Rostamnia, N. Tsunoji and M. H. N. Assadi, Mol. Catal., 2020, 482, 110676 CrossRef CAS.
- A. Ahadi, H. Alamgholiloo, S. Rostamnia, X. Liu, M. Shokouhimehr, D. A. Alonso and R. Luque, ChemCatChem, 2019, 11, 4803–4809 CrossRef CAS.
- A. G. Moaser, A. Ahadi, S. Rouhani, B. B. Mamba, T. A. M. Msagati, S. Rostamnia, T. Kavetskyy, S. Dugheri, S. Khaksar, A. Hasanzadeh and M. Shokouhimehr, J. Mol. Liq., 2020, 312, 113388 CrossRef CAS.
- A. Hasanzadeh, B. Gholipour, S. Rostamnia, A. Eftekhari, A. Tanomand, S. Khaksar and R. Khalilov, J. Colloid Interface Sci., 2021, 585, 676–683 CrossRef CAS PubMed.
- X. Liu, X. Li, Z. Guan, J. Liu, J. Zhao, Y. Yang and Q. Yang, Chem. Commun., 2011, 47, 8073–8075 RSC.
- S. Zhang, H. Wang, M. Li, J. Han, S. Inagaki and X. Liu, Dalton Trans., 2017, 46, 9369–9374 RSC.
- M. Li, H. Wang, X. Li, S. Zhang, J. Han, A. F. Masters, T. Maschmeyer and X. Liu, ChemCatChem, 2018, 10, 581–589 CrossRef CAS.
- S. Zhang, X. Liu, M. Li, Y. Wei, G. Zhang, J. Han, X. Zhu, Q. Ge and H. Wang, Catal. Today, 2019, 324, 59–65 CrossRef CAS.
- S. Rostamnia, X. Liu and D. Zheng, J. Colloid Interface Sci., 2014, 432, 86–91 CrossRef CAS PubMed.
- E. Doustkhah, S. Rostamnia, H. G. Hossieni and R. Luque, ChemistrySelect, 2017, 2, 329–334 CrossRef CAS.
- H. Alamgholiloo, S. Rostamnia and N. Noroozi Pesyan, Appl. Organomet. Chem., 2020, 34, e5452 CrossRef CAS.
- S. Rostamnia, E. Doustkhah and A. Nuri, J. Fluorine Chem., 2013, 153, 1–6 CrossRef CAS.
- S. Rostamnia and E. Doustkhah, Tetrahedron Lett., 2014, 55, 2508–2512 CrossRef CAS.
- S. Rostamnia and T. Rahmani, Appl. Organomet. Chem., 2015, 29, 471–474 CrossRef CAS.
- S. Rostamnia and E. Doustkhah, J. Mol. Catal. A: Chem., 2016, 411, 317–324 CrossRef CAS.
- S. Rostamnia, H. G. Hossieni and E. Doustkhah, J. Organomet. Chem., 2015, 791, 18–23 CrossRef CAS.
- S. Rostamnia and E. Doustkhah, Synlett, 2015, 26, 1345–1347 CrossRef CAS.
- S. Rostamnia, K. Lamei and F. Pourhassan, RSC Adv., 2014, 4, 59626–59631 RSC.
- S. Rostamnia, A. Hassankhani, H. G. Hossieni, B. Gholipour and H. Xin, J. Mol. Catal. A: Chem., 2014, 395, 463–469 CrossRef CAS.
- S. Rostamnia, B. Gholipour and H. G. Hosseini, Process Saf. Environ. Prot., 2016, 100, 74–79 CrossRef CAS.
- M. Farajzadeh, H. Alamgholiloo, F. Nasibipour, R. Banaei and S. Rostamnia, Sci. Rep., 2020, 10, 1–9 CrossRef PubMed.
- S. Rostamnia, K. Lamei and F. Pourhassan, RSC Adv., 2015, 5, 1033 RSC.
- N. Nouruzi, M. Dinari, N. Mokhtari, B. Gholipour, S. Rostamnia, S. Khaksar and R. Boluki, Appl. Organomet. Chem., 2020, 34, 1–10 CrossRef.
- S. Rostamnia and A. Morsali, RSC Adv., 2014, 4, 10514–10518 RSC.
- H. G. Hosseini, E. Doustkhah, M. V. Kirillova, S. Rostamnia, G. Mahmoudi and A. M. Kirillov, Appl. Catal., A, 2017, 548, 96–102 CrossRef CAS.
- H. Golchin Hosseini and S. Rostamnia, New J. Chem., 2018, 42, 619–627 RSC.
- A. Hassankhani, B. Gholipour and S. Rostamnia, Polyhedron, 2020, 175, 114217 CrossRef.
- H. Mohtasham, B. Gholipour, S. Rostamnia, A. Ghiasi-Moaser and M. Shokouhimehr, Colloids Surf., A, 2021, 614, 126187 CrossRef CAS.
- S. Rostamnia and S. Kholdi, Adv. Powder Technol., 2018, 29, 1167–1174 CrossRef CAS.
- S. Rostamnia, B. Gholipour, X. Liu, Y. Wang and H. Arandiyan, J. Colloid Interface Sci., 2018, 511, 447–455 CrossRef CAS PubMed.
- S. Rostamnia and M. Jafari, Appl. Organomet. Chem., 2017, 31, 1–6 CrossRef.
- S. Rostamnia, E. Doustkhah and B. Zeynizadeh, Microporous Mesoporous Mater., 2016, 222, 87–93 CrossRef CAS.
- S. Rostamnia, E. Doustkhah and B. Zeynizadeh, J. Mol. Catal. A: Chem., 2016, 416, 88–95 CrossRef CAS.
- S. Rostamnia, N. Nouruzi, H. Xin and R. Luque, Catal. Sci. Technol., 2015, 5, 199–205 RSC.
- S. Rostamnia, E. Doustkhah, H. Golchin-Hosseini, B. Zeynizadeh, H. Xin and R. Luque, Catal. Sci. Technol., 2016, 6, 4124–4133 RSC.
- A. Alizadeh, N. Zohreh and S. Rostamnia, Tetrahedron, 2007, 63, 8083–8087 CrossRef CAS.
- K. Zhu, Y. Du, J. Liu, X. Fan, Z. Duan, G. Song, A. Meng, Z. Li and Q. Li, J. Nanosci. Nanotechnol., 2017, 17, 2515–2519 CrossRef CAS PubMed.
- P. Van Der Voort, D. Esquivel, E. De Canck, F. Goethals, I. Van Driessche and S. S. Francisco Romero, Chem. Soc. Rev., 2013, 42, 3913–3955 RSC.
- E. De Canck, Versatile Hybrid Nanomaterials: Periodic Mesoporous Organosilicas as Adsorbent and Catalyst, 2013 Search PubMed.
- Y. Yu, B. Lu, X. Wang, J. Zhao, X. Wang and Q. Cai, Chem. Eng. J., 2010, 162, 738–742 CrossRef CAS.
- X. Xia, X. Gao, J. Xu, C. Hu and X. Peng, J. Saudi Chem. Soc., 2017, 21, 334–340 CrossRef CAS.
- M. A. Ali and T. Punniyamurthy, Adv. Synth. Catal., 2010, 352, 288–292 CrossRef CAS.
- Y. Suto, N. Yamagiwa and Y. Torisawa, Tetrahedron Lett., 2008, 49, 5732–5735 CrossRef CAS.
- L. Zhang, W. Wang, A. Wang, Y. Cui, X. Yang, Y. Huang, X. Liu, W. Liu, J. Y. Son, H. Oji and T. Zhang, Green Chem., 2013, 15, 2680–2684 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08682k |
| This journal is © The Royal Society of Chemistry 2022 |