Open Access Article
Open Access ArticleMolecular evidence for sulfurization of molybdenum dithiocarbamates (MoDTC) by zinc dithiophosphates: a key process in their synergetic interactions and the enhanced preservation of MoDTC in formulated lubricants?†
Yu Min Kiw ab,
Pierre Adama,
Philippe Schaeffer*a,
Benoît Thiébautb and
Chantal Boyerb
ab,
Pierre Adama,
Philippe Schaeffer*a,
Benoît Thiébautb and
Chantal Boyerb
aUniversity of Strasbourg, CNRS, Institut de Chimie de Strasbourg UMR 7177, F-67000 Strasbourg, France. E-mail: p.schaef@unistra.fr
bTotalEnergies Solaize Research Center, BP22-69360 Cedex, France
First published on 26th January 2022
Abstract
Molybdenum dithiocarbamates (MoDTC) are widely used in automotive industries as lubricant additives to reduce friction and to enhance fuel economy. Sulfur-containing additives such as zinc dithiophosphates (ZnDTP) are proposed to play a key role in the improvement of friction reducing properties of MoDTC in formulated lubricants by facilitating the formation of MoS2 tribofilm at the rubbing contacts. This study focuses on the interactions between MoDTC and ZnDTP under conditions comparable with those prevailing in operating engines. The capacity of ZnDTP to sulfurize MoDTC in solution in a hydrocarbon base oil could be demonstrated. Sulfurized Mo complexes bearing one or two additional sulfur atoms (1S-MoDTC and 2S-MoDTC, respectively) which have replaced the genuine oxygen atom(s) from the MoDTC core were detected and quantified using a specifically developed HPLC-MS analytical method. A possible sulfurization mechanism relying on the higher affinity of phosphorus from ZnDTP for oxygen could be proposed. In parallel, the evolution and molecular transformation of the prepared 2S-MoDTC in hydrocarbon base oil under thermal and thermo-oxidative conditions were followed using HPLC-MS and compared with the evolution of their friction coefficients. 2S-MoDTC complexes were shown to exhibit a better retention of friction reducing capability under oxidative conditions than the “classical” MoDTC, although they did not seem to significantly reduce the friction coefficients of lubricants as compared to the “classical” MoDTC. Therefore, sulfurization of MoDTC by ZnDTP might contribute to delaying the progressive consumption of MoDTC and the loss of their friction-reducing efficiency in lubricants under thermo-oxidative conditions.
1. Introduction
Molybdenum-based compounds have a wide range of applications in chemistry, and are used for instance as catalysts to speed-up chemical reactions1–3 or occur as lubricant additives in automotive applications.4,5 Among the different molybdenum-based additives, oil-soluble molybdenum dithiocarbamates (MoDTC) have been used as highly effective friction modifiers since the early 1970s6–8 in engine lubricants for energy saving. Indeed, MoDTC are able to generate molybdenum disulfide (MoS2) at the tribo-contact, thereby providing ultralow friction coefficients which improve fuel efficiency.9–15 Different analytical techniques such as X-ray spectroscopy,16,17 electron diffraction,18 Raman spectroscopy19,20 and high-resolution transmission electron microscopy8 have been employed to detect and to study MoS2 tribofilms. Although numerous investigations have been conducted to explain the mechanisms of MoS2 formation from Mo-based additives,9,13,21–25 the molecular transformations involved in the processes have not yet been completely clarified. The main limitation of employing MoDTC as friction modifiers is related to their deterioration over time upon oil oxidation, leading to the loss of their friction-reducing effectiveness during engine operation.11,26–30 Therefore, understanding the mechanisms of MoDTC decomposition and their interactions with other additives from formulated lubricants at the molecular level is crucial in order to (i) predict their friction reduction efficiency (ii) provide guidance for the optimization of MoDTC tribological performance in lubricant formulation (iii) extend the functional lifespan of MoDTC during engine oil ageing and (iv) provide information for future development of new eco-friendly additives.Modern lubricant formulations also require anti-wear action and thus additional multifunctional additives are used to achieve optimal engine efficiency. The presence of these additives in formulated lubricants could exhibit synergistic or antagonistic interactions with MoDTC, resulting in either enhancement or decrease in friction reduction efficiency of MoDTC. Zinc dithiophosphates (ZnDTP) show antiwear, anticorrosive and antioxidant properties,31 thus representing one of the most widely used multifunctional additives in engine lubricants. Tribo-chemical interactions between ZnDTP and MoDTC have been shown to exhibit positive effects on both friction and wear reduction in boundary lubrication regime.32–35
In this connection, it has been proposed that the presence of both molybdenum and sulfur-containing species such as ZnDTP in lubricants is required for the generation of a MoS2 tribofilm.36 Similarly, Sogawa et al. (1999)37 showed that ca. 40% of the sulfur atoms present in the MoS2 tribofilm derive from ZnDTP when used in combination with MoDTC, suggesting that the presence of a sulfur source together with zinc atoms are important to promote the generation of MoS2 at the tribo-contacts. Umehara et al. (2013)38 also asserted that ZnDTP could provide sulfur atoms to MoDTC and affirmed the importance of sulfur suppliers to form MoS2 layers at the sliding surfaces. In addition, some sulfur-free organic molybdenum-based friction modifiers were also proved to be effective when used in conjunction with sulfur-containing additives such as ZnDTP.39,40
Therefore, given the numerous studies emphasizing on the importance of sulfur suppliers such as ZnDTP to improve the tribological performance of MoDTC in lubricants, this study focuses on the investigation at the molecular level of the interactions between MoDTC and ZnDTP using laboratory oil ageing experiments. We report here the transformation of MoDTC into mono- and di-sulfurized analogues (hereinafter noted as 1S- and 2S-MoDTC) induced by ZnDTP in lubricants under thermal non-oxidizing conditions (Fig. 1a). The formation of the sulfurized Mo complexes was investigated using a specifically developed HPLC-MS analytical method allowing their unambiguous detection. Besides, 2S-MoDTC reference compounds were synthesized using Lawesson's reagent (Fig. 1b) for the investigation of their tribological performances, evolution and chemical transformation in hydrocarbon base oils under thermal and thermo-oxidative conditions. The possible implications of the sulfurization processes of MoDTC on the tribological performances of lubricants and on the persistence of MoDTC in lubricants under thermo-oxidative conditions have been investigated using the synthesized 2S-MoDTC.
2. Experimental section
2.1 Materials (base oil and additives)
The mineral base oil used in this work was provided by TotalEnergies Marketing Services. The chemical structures of the Mo and Zn complexes discussed in this study are shown in Fig. 2. MoDTC 1a–1c were purchased from Adeka as a mixture in base oil (MoDTC 1a–1c: 10 wt% Mo; trade name: Sakuralube 525), whereas MoDTC 1d (trade name Sakuralube 600) occurs as a pure solid material. ZnDTP 2a–2c (in solution in a hydrocarbon base oil) were purchased from Infineum. ZnDTP 2a (7.3 wt% P, trade name: OLOA 269R) corresponds to a primary dithiophosphate complex with C8 (2-ethylhexyl) alkyl chains. Secondary ZnDTP (trade name: Infineum C9421) additives were bought as a mixture of compounds 2b (8.0 wt% P) with C3 and C6 alkyl chains and ZnDTP 2c (7.0 wt% P, trade name: Infineum C9426) is constituted of a mixture of ZnDTP with a complex mixture of isomeric C8 (isooctyl) alkyl chains (see ESI Fig. 7S†). Lawesson's reagent 3 was purchased from Sigma-Aldrich. All the purchased compounds were used without any prior purification.2.2 Laboratory ageing experiments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, wt./wt.) were vacuum-sealed in NMR glass tubes without dilution in hydrocarbon base oil or solvents. The glass tubes were heated at 150 °C for 3 h or 6 h.
1, wt./wt.) were vacuum-sealed in NMR glass tubes without dilution in hydrocarbon base oil or solvents. The glass tubes were heated at 150 °C for 3 h or 6 h.2.3 Ball-on-flat tribotests
Tribological tests were carried out by using an Anton Paar MCR302 rheometer with a T-PTD-200 tribology assembly (ball on three flats). Test temperature was 110 °C, normal load 5 N and sliding speed was kept constant throughout 15 min test at 0.1 m s−1. Upper ball was made of AISI 52100 hardened steel (roughness) and flats was made of 100Cr6 hardened steel (60–66 HRC). Roughness of the balls was 0.032 μm (Ra) and 0.06 μm (Ra) for the flats. Test conditions are representative of a “boundary lubrication regime” where important asperity to asperity contacts occur favouring the reaction of additives and tribofilm formation. Only final friction coefficients are reported in this paper.2.4 High pressure liquid chromatography-mass spectrometry (HPLC-MS) analyses
MoDTC and their sulfurized analogues were analyzed by HPLC-MS using an Agilent HP 1100 series instrument equipped with an auto-injector, and connected to a Bruker Esquire 3000Plus ion trap mass spectrometer as described in Kiw et al. (2020).41 Sample preparation and quantification of MoDTC derivatives were carried out as described in Kiw et al. (2020)41 using 2,3-bis(n-octadecyloxy)-propan-1-ol as internal standard which was added prior HPLC-MS analysis. ZnDTP 2a–2c could not be detected using the developed HPLC-MS analytical method. In the case of the experiments involving concentrated solutions, samples were prepared following a similar procedure, but MoDTC derivatives were not quantified (no internal standard added).2.5 1H and 13C NMR (analysis of 2S-1d)
NMR spectra were recorded on a Bruker Avance I 500 MHz spectrometer (500 MHz for 1H; 125 MHz for 13C) for NMR measurements. The 1H- and 13C-chemical shifts are reported in ppm relative to tetramethylsilane with the residual protons and carbon atoms of the solvent used as internal standards (CDCl3: δ 1H 7.26 ppm; δ 13C 77.2 ppm). 5 mg of the synthesized 2S-1d was diluted in ca. 0.8 mL of CDCl3 for NMR analysis.2.6 GC-MS (analysis of ligands from ZnDTP 2c)
GC-MS analysis was carried out using a Thermo Trace gas chromatograph (Thermo Scientific) coupled to a Thermo Scientific TSQ Quantum mass spectrometer equipped with a programmed temperature vaporizing (PTV) injector. The temperature of the source was set at 220 °C. The mass spectrometer was operating in the electron ionization (EI) mode at 70 eV and scanning m/z 50 to 700. Gas chromatographic separation was performed on a HP5-MS column (30 m × 0.25 mm; 0.1 μm film thickness) using He as carrier gas at a constant flow rate of 1.2 mL min−1. The oven temperature was programmed as followed: 40 °C (1 min), 40–200 °C (10 °C min−1), 200–300 °C (4 °C min−1), isothermal at 300 °C for 30 min.2.7 Synthesis of 2S-MoDTC
MoDTC 1d (5 g, 7.1 mmol) and Lawesson's reagent 3 (2.9 g, 7.1 mmol) were stirred under reflux in toluene for 1.5 h. The reaction mixture was then cooled down to room temperature and washed with 200 mL of water. The organic phase was dried over magnesium sulfate, filtrated and concentrated in vacuo to afford 2S-1d as a dark red solid. 1H- and 13C- NMR data as well as HPLC-MS analysis showed that the starting product 1d was completely converted into its corresponding disulfurized MoDTC complex 2S-1d, indicating that the sulfurization reaction was quantitative. It is worth noting that 2S-1d is barely soluble in a hydrocarbon medium, and it was thus not further used for laboratory experiments. It was synthesized essentially to obtain a pure reference compound of 2S-MoDTC for 1H- and 13C-NMR characterization and allowed the yield of the sulfurization reaction to be evaluated.Following this, the same procedure as above was carried out using a mixture containing MoDTC 1a–1c (8.2 g, 4.7 mmol) and Lawesson's reagent 3 (2.0 g, 4.7 mmol), leading to the complete conversion of 1a–1c into the corresponding mixture of 2S-1a–1c as a dark red liquid, as determined by HPLC-MS (APPI, positive mode). 2S-1a–1c existed in the form of a mixture of three 2S-MoDTC complexes, therefore their formation was hard to be confirmed by 1H-NMR. However, the reaction was believed to give an almost quantitative yield of 2S-1a–1c, based on the 1H- and 13C-NMR results obtained from the pure disulfurized MoDTC complexes 2S-1d, which was synthesized using the same procedure (see above).
This sulfurization reaction involving either MoDTC 1a–1c or MoDTC 1d was repeated twice and led to the same results as those reported above (i.e., complete sulfurization of the starting MoDTC products). Direct evaluation of the sulfurization yield using NMR would be difficult in the case of the reaction involving the mixture of MoDTC 1a–1c since the chemical shifts of the protons from the methylenes adjacent to N of the various MoDTC involved (1a–1c) and of their related sulfurized analogues 2S-1a–1c must have similar though not identical chemical shifts, making the overall complex signal hard to interpret.
2S-1d: 1H-NMR (500 MHz; CDCl3): 4.00–3.93 (m, 4H), 3.92–3.85 (m, 4H), 1.88–1.7 (m, 8H), 1.49–1.36 (m, 8H), 1.00 (t, J = 7.5 Hz, 12H). 13C-NMR (125 MHz; CDCl3): 207.4, 51.6, 29.7, 20.2, 13.9.
2S-1d: HPLC-MS (APPI, positive mode), [M + H]+: m/z (relative intensity) 772 (11%), 723 (27), 724 (42), 725 (44), 726 (78),727 (65), 728 (98), 729 (91), 730 (100), 731 (67), 732 (72), 733 (36), 734 (42), 735 (12), 736 (17) (see also ESI Fig. S1† for the mass spectrum of 2S-1d).
2S-1a: HPLC-MS (APPI, positive mode), [M + H]+: m/z (relative intensity) 948 (13%), 949 (31), 950 (43), 951 (61), 952 (54), 953 (83), 954 (87), 955 (100), 956 (73), 957 (62), 958 (55), 959 (36), 960 (15).
2S-1b: HPLC-MS (APPI, positive mode), [M + H]+: m/z (relative intensity) 1087 (17%), 1088 (24), 1089 (52), 1090 (48), 1091 (72), 1092 (67), 1093 (92), 1094 (100), 1095 (96), 1096 (82), 1097 (91), 1098 (57), 1099 (52), 1100 (25), 1101 (24).
2S-1c: HPLC-MS (APPI, positive mode), [M + H]+: m/z (relative intensity) 1226 (10%), 1227 (17), 1228 (31),1229 (34), 1230 (36), 1231 (74), 1232 (56), 1233 (83), 1234 (100), 1235 (86), 1236 (93), 1237 (75), 1238 (52), 1239 (45), 1240 (26), 1241 (19), 1242 (10) (see also ESI Fig. S2† for the mass spectra of 2S-1a–1c).
2.8 GC-MS ligand analysis of ZnDTP 2c
5 mg of ZnDTP 2c were dissolved in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v mixture of methanol and dichloromethane (1 mL). Methyl iodide (500 μL) was then added and the mixture was left to react at room temperature for 1 h. After removal of methyl iodide and of the solvents under a stream of argon, the crude mixture was separated by column chromatography on silica gel (H: 4 cm, Ø: 1.5 cm). A first elution using cyclohexane (20 mL; 2 dead volumes) yielded a fraction containing the hydrocarbon base oil present with the additive. A second fraction was obtained by elution with dichloromethane (30 mL; 3 dead volumes) and contained the dithiophosphate ligands with isomeric C8 (isooctyl) alkyl chains which were analyzed as methylated derivatives using GC-MS.
1 v/v mixture of methanol and dichloromethane (1 mL). Methyl iodide (500 μL) was then added and the mixture was left to react at room temperature for 1 h. After removal of methyl iodide and of the solvents under a stream of argon, the crude mixture was separated by column chromatography on silica gel (H: 4 cm, Ø: 1.5 cm). A first elution using cyclohexane (20 mL; 2 dead volumes) yielded a fraction containing the hydrocarbon base oil present with the additive. A second fraction was obtained by elution with dichloromethane (30 mL; 3 dead volumes) and contained the dithiophosphate ligands with isomeric C8 (isooctyl) alkyl chains which were analyzed as methylated derivatives using GC-MS.
3. Results
3.1 Sulfurization of MoDTC by ZnDTP in a concentrated medium
The interactions between MoDTC and ZnDTP have first been investigated under concentrated conditions by heating a mixture of commercial MoDTC 1a–1c and primary ZnDTP 2a (secondary ZnDTP 2b or 2c, respectively) in vacuum-sealed glass tubes at 150 °C for 3 h and 6 h. HPLC-MS analysis of the resulting crude mixtures showed the formation of two new series of compounds. Based on the interpretation of their mass spectra, it could be established that the newly-formed compounds corresponded to Mo derivatives, given the typical complex isotopic pattern observed for the pseudo-molecular ions (Fig. 3).41 Since these pseudo-molecular ions were shown to be shifted upwards by 16 mass units (first series) or 32 mass units (second series) relative to the MoDTC substrates, it could be deduced that the new compound series corresponded to MoDTC derivatives bearing one or two additional sulfur atoms (1S-MoDTC and 2S-MoDTC, respectively; Fig. 3) which have replaced the genuine oxygen atom(s) from the MoDTC core. It is worth mentioning that the formation of MoDTC derivatives showing an upward molecular shift of 16 and 32 mass units has been previously observed by electrospray mass spectrometry (direct inlet) by De Feo (2015)26 in experiments involving MoDTC and ZnDTP in solution in a hydrocarbon base oil, these authors suggesting the possible sulfur–oxygen exchange on the genuine MoDTC, without providing further explanation on the sulfurization mechanisms of MoDTC by ZnDTP and on the possible implications of sulfurized MoDTC on tribological performances.HPLC-MS analysis of MoDTC species showed that at T = 0 h, in the presence of ZnDTP 2b or 2c, MoDTC 1a–1c could be mainly detected. The small amounts of 1S-MoDTC detected at T = 0 h were likely already present in the commercial MoDTC additive. After 3 h at 150 °C, the relative proportions of 1S-MoDTC (1S-1a–1c) increased and 2S-MoDTC species (2S-1a–1c) progressively appeared, the kinetics of formation of the sulfurized species differing in function of the nature of the ZnDTP substrates used (i.e., mixture of ZnDTP 2b or 2c, respectively) (Fig. 3a and b). Comparison of the relative abundance of 2S-MoDTC after 3 h of reaction (Fig. 3a and b) showed that sulfurization of MoDTC occurred faster in the presence of di-isooctyl ZnDTP 2c as compared to the secondary ZnDTP 2b. After 6 h at 150 °C, the complete disappearance of the MoDTC substrates 1a–1c and of the related 1S-MoDTC was observed as a result of their extensive transformation into 2S-MoDTC species (Fig. 3a and b). By contrast, the reaction with primary ZnDTP 2a resulted in a very low degree of sulfurization of MoDTC under the same experimental conditions, leading to moderate formation of 1S-MoDTC even after 6 h at 150 °C (Fig. 3c). These results thus clearly demonstrate the capacity of di-isooctyl ZnDTP 2c and secondary ZnDTP 2b to efficiently sulfurize MoDTC in a concentrated medium without hydrocarbon base oil, whereas primary ZnDTP 2a is apparently much less efficient.
3.2 Sulfurization of MoDTC by ZnDTP in diluted medium
Heating experiments involving MoDTC 1a–1c and ZnDTP (2a, mixture of 2b, or 2c) have also been performed in a diluted medium at concentrations compatible with those used in commercial formulated oils (i.e., 1 wt%) using a hydrocarbon base oil at 135 °C under argon atmosphere for a period of 15 h. The evolution of the sulfurization reaction was monitored by HPLC-MS analysis of the samples collected at every 3 h intervals.Fig. 4a (respectively Fig. 4d) shows the evolution of the relative concentrations of MoDTC substrates 1a–1c upon heating with ZnDTP 2b (respectively 2c). It could be determined that the concentrations of the related sulfurized MoDTC species progressively increased over time (Fig. 4b and e), whereas those of the MoDTC substrates decreased gradually, notably due to oxygen–sulfur substitution on MoDTC derivatives. Detection of 1S-MoDTC and of traces of 2S-MoDTC at the starting point of the reaction (T = 0 h) could be partly explained, as mentioned above, by the presence of minor amounts of 1S-MoDTC (but not 2S-MoDTC) in the commercially purchased MoDTC substrates. Furthermore, since the initial sample (T = 0 h) was collected only when the reaction mixture has reached the temperature of 135 °C, it is likely that sulfurization of MoDTC by ZnDTP already started during the preliminary heating step, thus explaining the formation of 2S-MoDTC at this early stage. Nonetheless, the gradual increase of the different sulfurized MoDTC species over time clearly showed the capacity of the secondary ZnDTP 2b and of the di-isooctyl ZnDTP 2c to efficiently sulfurize MoDTC in solution at concentrations similar to those used in formulated lubricants. Fig. 4c and f show the global evolution of the proportion of sulfurized MoDTC (sum of 1S- and 2S-MoDTC) relative to the sum of the total MoDTC pool (i.e., MoDTC and sulfurized MoDTC) over time. After 15 h of reaction, the percentage of sulfurized MoDTC increased gradually from 9% to 60% in the presence of secondary ZnDTP 2b and from 24% to 63% in the presence of di-isooctyl ZnDTP 2c. The higher proportion of sulfurized MoDTC already observed at T = 0 h in the presence of di-isooctyl ZnDTP 2c is likely due to the higher sulfurization efficiency of this reagent during the initial heating step as compared to the secondary ZnDTP 2b. Similar experiments involving primary 2-ethylhexyl ZnDTP 2a showed a much lower degree of sulfurization of MoDTC (<20% of sulfurized MoDTC; data not shown). These results are coherent with those from the experiments under concentrated conditions (see Section 3.1).
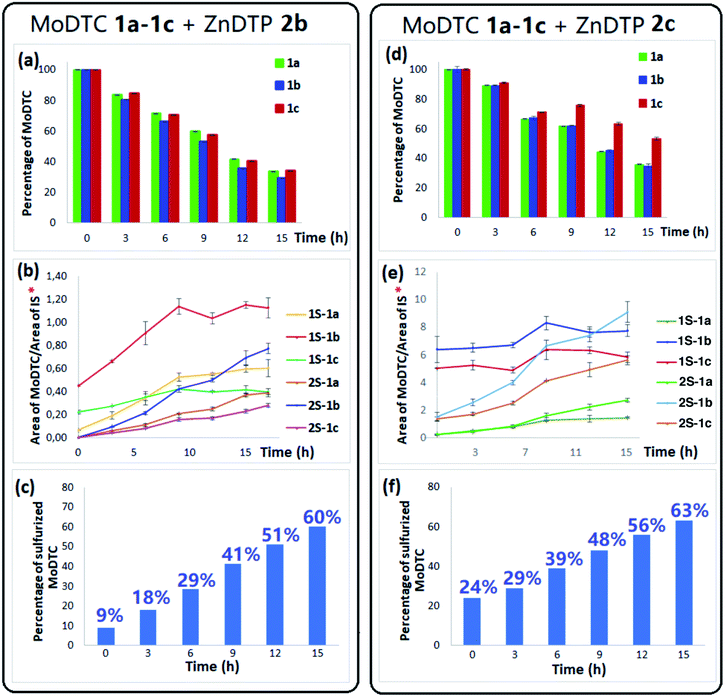 | ||
| Fig. 4 Evolution of the relative abundances of (a and d) MoDTC substrates 1a–1c; (b and e) sulfurized MoDTC 1S-1a–1c and 2S-1a–1c formed during the heating experiment involving MoDTC 1a–1c and ZnDTP 2b and 2c, respectively, in lubricant base oil at 135 °C under argon atmosphere; (c and f) evolution of the proportion of sulfurized MoDTC (sum of 1S-1a–1c and 2S-1b–1c) relative to the sum of the (regular plus sulfurized) MoDTC. IS: Internal standard. *Y-axis: arbitrary units. Error bars (Fig. 4a–e) correspond to triplicate HPLC-MS analyses for each sample. | ||
3.3 Synthesis of 2S-MoDTC
Since the formation of sulfurized MoDTC can be expected to favor the generation of MoS2 tribofilms, this process can be thought to contribute, at least to some extent, to the synergistic improvement of the tribological performances of MoDTC when used in combination with ZnDTP. In this respect, synthesis of 2S-MoDTC from MoDTC 1a–1c has been carried out in order to evaluate their tribological properties and compare them to those of the ‘‘classical’’ MoDTC. In addition, since sulfurization of MoDTC may potentially result in the increase of the functional lifetime of MoDTC during engine operation, the evolution of 2S-MoDTC under thermal and thermo-oxidative conditions was investigated.Lawesson's reagent (3) is a reagent able to induce the thionation of ketones and esters (e.g. Ozturk et al., 2007![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42). It has also been used to sulfurize the Mo
42). It has also been used to sulfurize the Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of Mo complexes in a context different to that related to the present study.43 We have, therefore, tested the capacity of this reagent to sulfurize MoDTC 1a–1c under the same conditions as those used for the sulfurization of MoDTC by ZnDTP under diluted conditions in a hydrocarbon base oil (see Section 2.2.2). Reaction of a mixture of MoDTC 1a–1c with Lawesson's reagent (3) at 135 °C under argon atmosphere led to the complete conversion of the starting MoDTC 1a–1c into their related disulfurized counterparts during the heating phase from room temperature to 135 °C as determined using HPLC-MS analysis of the crude mixture. The kinetic of the sulfurization reaction of MoDTC by Lawesson's reagent is thus obviously very fast under these conditions and this reagent can therefore be considered as an excellent sulfurization agent of MoDTC. Efficient sulfurization could also be observed when MoDTC (mixture of 1a–1c or pure 1d) were heated with Lawesson's reagent under reflux in toluene (i.e., at 110 °C). The latter procedure allowed synthesis in a quantitative yield of a mixture of 2S-1a–1c and of 2S-1d as determined by HPLC-MS analysis and NMR analysis (2S-1d, see Section 2.7).
O bond of Mo complexes in a context different to that related to the present study.43 We have, therefore, tested the capacity of this reagent to sulfurize MoDTC 1a–1c under the same conditions as those used for the sulfurization of MoDTC by ZnDTP under diluted conditions in a hydrocarbon base oil (see Section 2.2.2). Reaction of a mixture of MoDTC 1a–1c with Lawesson's reagent (3) at 135 °C under argon atmosphere led to the complete conversion of the starting MoDTC 1a–1c into their related disulfurized counterparts during the heating phase from room temperature to 135 °C as determined using HPLC-MS analysis of the crude mixture. The kinetic of the sulfurization reaction of MoDTC by Lawesson's reagent is thus obviously very fast under these conditions and this reagent can therefore be considered as an excellent sulfurization agent of MoDTC. Efficient sulfurization could also be observed when MoDTC (mixture of 1a–1c or pure 1d) were heated with Lawesson's reagent under reflux in toluene (i.e., at 110 °C). The latter procedure allowed synthesis in a quantitative yield of a mixture of 2S-1a–1c and of 2S-1d as determined by HPLC-MS analysis and NMR analysis (2S-1d, see Section 2.7).
3.4 The fate of 2S-MoDTC under thermal and thermo-oxidative conditions and evaluation of their tribological performances
3.4.1.1 Argon atmosphere. For the experiment under argon bubbling, HPLC-MS analyses of the oil samples showed a gradual decrease of the relative concentrations of sulfurized MoDTC substrates 2S-1a–1c with time, with ca. 50% of the initial MoDTC remaining after 18 h (Fig. 5). This decrease indicates that 2S-MoDTC are unstable at elevated temperature under an inert atmosphere. No thermal degradation products of 2S-MoDTC could, however, be detected under our HPLC-MS analytical conditions. Since it was shown previously that MoDTC 1a–1c in hydrocarbon base oil remained stable under the same conditions,41 it appears that sulfurized MoDTC 2S-1a–1c are less stable than their MoDTC 1a–1c counterparts and are susceptible to decompose by a yet unknown process under inert atmosphere conditions (no degradation products of Mo derivatives could be identified using our developed HPLC-MS analytical method). Despite being thermally unstable at high temperatures, 2S-MoDTC appeared, however, to be very stable at ambient temperature according to the NMR and HPLC-MS analysis of the synthesized 2S-MoDTC left at room temperature (i.e., between 20 °C and 35 °C) under air atmosphere for a period of seven months.
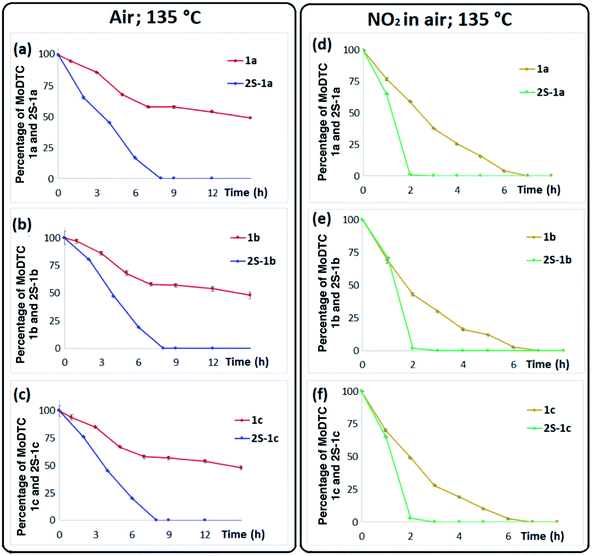
3.4.1.2 Air atmosphere. Fig. 6a–c show the evolution of sulfurized MoDTC 2S-1a–1c and of MoDTC 1a–1c during laboratory oil ageing experiments under air bubbling at 135 °C. The concentrations of both series of MoDTC decreased over time, the degradation rate of 2S-MoDTC (Fig. 6a–c, blue colour) being however much faster than that of the genuine MoDTC under these conditions (Fig. 6a–c, red colour), leading to total disappearance of the 2S-MoDTC substrates after 8 h whereas ca. 50% of the MoDTC species 1a–1c were still present after 15 h of ageing.
3.4.1.3 NO2 atmosphere. An even higher degradation rate was observed when 2S-MoDTC and regular MoDTC were subjected to ageing experiments under nitro-oxidation conditions at 135 °C as compared to air conditions. Substrates 2S-1a–1c were almost completely consumed after only 2 h of experiment (Fig. 6d–f, green colour) whereas MoDTC 1a–1c persisted for a period of ca. 7 h (Fig. 6d–f, yellow colour). As already pointed out in the case of the experiment under air atmosphere, these results clearly confirm that 2S-MoDTC are much less stable than their non-sulfurized counterparts and tend to decompose at a significantly faster rate under thermo-oxidative conditions.
It could however be determined by HPLC-MS analysis that the progressive degradation of 2S-MoDTC under thermo-oxidative conditions (i.e., under air and NO2 in air atmosphere) was accompanied by the formation of new MoDTC species corresponding to mono-sulfurized MoDTC 1S-1a–1c (Fig. 2: structure d and ESI Fig. S4† for the mass spectra of 1S-1a–1c) and of the “classical’’ MoDTC 1a–1c. These new species most likely originated from sulfur–oxygen substitution on the Mo2S4 core of the 2S-MoDTC. Thus, even though 2S-MoDTC tend to decompose very quickly under thermo-oxidative conditions, their detected transformation products (i.e., 1S-MoDTC and “classical” MoDTC) persist in the oil for a longer period of time (Fig. 7a–d) and could potentially continue to contribute to friction reducing capabilities of lubricants.
On the other hand, the degradation pathway of MoDTC 1a–1c under air and NO2 (2000 ppm in air) at 135 °C that ultimately leads to the formation of molybdenum oxides could involve the intermediate formation of oxygenated MoDTC species, which correspond to MoDTC derivatives with one or two sulfur atom(s) originally present in 1a–1c being replaced by oxygen atom(s) (see ESI Fig. S5 and S6† for the mass spectra and quantitative analysis of oxygenated MoDTC species).
The base oil containing 2S-MoDTC displayed low values of friction coefficients (≈0.07) during the first 5 h of nitro-oxidation, before showing a sharp decrease of the friction reducing capabilities (Fig. 8). Even though 2S-MoDTC were shown to be unstable and were completely degraded within 2 h under nitro-oxidation conditions (Fig. 6d–f), their oxidative transformation products (i.e. 1S-MoDTC and “classical” MoDTC) remained in the oil for longer periods than 2S-MoDTC (Fig. 7c and d) and were most likely still active as friction reducers accounting for the preservation of the friction reducing capabilities up to 5 h. Surprisingly, although MoDTC in the experiment involving “classical” MoDTC as well as MoDTC resulting from oxidative O–S exchanges in the experiments involving 2S-MoDTC have been extensively consumed, in both cases, after 7 h under nitro-oxidation conditions (cf. Fig. 6d–f, 7c and d, respectively), friction reducing capacities were preserved for a longer period of time in the case of the oil containing 2S-MoDTC (5 h vs. 4 h, Fig. 8). This result suggests that the use of 2S-MoDTC, although displaying a comparable friction coefficient than the “classical”’ MoDTC at T = 0 h, apparently results in a better retention of friction reducing capability with time under thermo-oxidative conditions.
4. Discussion
4.1 Mechanism of the sulfurization of MoDTC by ZnDTP and by Lawesson's reagent
The mechanism of sulfurization of MoDTC by ZnDTP additives is likely related to the affinity between phosphorus and oxygen atoms, which favours sulfur–oxygen exchange reactions on phosphorus. In this respect, sulfur-bearing phosphorus derivatives such as Lawesson's reagent 3 (e.g., Ozturk et al., 2007![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42) and P2S5 (e.g. Campaigne and Foye, 1952;44 Sakurai et al., 1971;45 Isomaya and Sakurai, 1974;46 Malik et al., 2008
42) and P2S5 (e.g. Campaigne and Foye, 1952;44 Sakurai et al., 1971;45 Isomaya and Sakurai, 1974;46 Malik et al., 2008![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47) are used in organic synthesis to induce sulfurization of oxygenated functions. In the case of the sulfurization of MoDTC by ZnDTP, a mechanism similar to that proposed by Partyka et al. (2003)43 and Moula et al. (2013)48 for the sulfurization of Mo
47) are used in organic synthesis to induce sulfurization of oxygenated functions. In the case of the sulfurization of MoDTC by ZnDTP, a mechanism similar to that proposed by Partyka et al. (2003)43 and Moula et al. (2013)48 for the sulfurization of Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds with Lawesson's reagent can be proposed (Fig. 9a).
O bonds with Lawesson's reagent can be proposed (Fig. 9a).
Although the formation of mixed Mo(DTC)(DTP) complexes is not favored in the case of mixtures comprising MoDTC and ZnDTP, they seemed, however, to be formed in small proportions as demonstrated by Kiw et al. (2020)41 and could act as intermediates during the sulfurization of MoDTC following the mechanism presented in Fig. 9b. Step II of this mechanism presents some analogies with the rearrangement reaction proposed by several authors to play a role in the tribochemical formation of MoS2 from MoDTC or in the thermo-oxidative degradation of MoDTC.27,49,50 It involves an exchange between a sulfur atom from the ligand and an oxygen atom on the MoDTC core. Another possible mode of formation of 2S-MoDTC could be the sulfurization of MoDTC by H2S resulting from the thermal decomposition of ZnDTP complexes described by Dickert and Rowe (1967)51 and Spikes (2004).31
4.2 Possible influence of the ZnDTP structures on their capacity to sulfurize MoDTC
According to the present study, the laboratory heating experiments involving MoDTC 1a–1c and ZnDTP (2b or 2c respectively), either in concentrated or diluted solutions in hydrocarbon base oils clearly showed that some ZnDTP species are more able to induce sulfurization of MoDTC, leading to the formation of 1S- and 2S-MoDTC. It appeared that the secondary ZnDTP 2b with C3 and C6 alkyl chains seem to sulfurize MoDTC more efficiently than the primary ZnDTP 2a bearing 2-ethylhexyl (C8) chains. This suggests that the chain length and structure of the side-chains and thus, the steric hindrance induced by these alkyl chains, might influence the kinetic of the sulfurization reaction. Nevertheless, the fact that ZnDTP 2c bearing isooctyl chains is even a more efficient sulfurization agent than ZnDTP 2a – both bearing C8 alkyl chains, but with different structures – rather seems to indicate that the length of the side chain is not the key structural feature of ZnDTP that controls their capacity to sulfurize MoDTC. Structural investigation of the nature of the ligands from ZnDTP 2c – no information being available for this commercial additive – showed that it bears DTP ligands with a great variety of isomeric C8 alkyl chains (cf. ESI Fig. S7† for the gas chromatogram of the dithiophosphate ligands of ZnDTP 2c analyzed as methylated derivatives). It therefore appears that the alkyl chains from ZnDTP 2c have most likely different modes of linkage to the dithiophosphate core (i.e., via C–O bonds at primary, secondary or even tertiary positions). Thus the capacity of ZnDTP 2c to sulfurize rapidly MoDTC is possibly related, as would be the case for ZnDTP 2b, to the occurrence of alkyl chains linked at secondary (and possibly also tertiary?) positions. This led us to propose that the modes of linkage of the alkyl chains to the dithiophosphate core play a key role in the efficiency of ZnDTP to sulfurize MoDTC. However, it is speculative at this stage to restrict the differences of reactivity of the various ZnDTP to a specific structural feature related to the nature of their ligands due to a lack of precise information on the structures of the isomeric C8 alkyl chains occurring on ZnDTP 2c and the absence of pure commercially available ZnDTP reference compounds bearing single secondary or tertiary alkyl chains (instead of isomeric mixtures).The kinetic of the formation of 2S-MoDTC from MoDTC 1a–1c showed, at least in the case of the reaction with ZnDTP 2c (Fig. 4), that MoDTC 1a and 1b bearing C8 alkyl chains underwent sulfurization more rapidly than MoDTC 1c which bears exclusively C13 alkyl chains (Fig. 4d). In parallel, the concentrations of sulfurized MoDTC 2S-1a and 2S-1b seemed to increase faster than that of the homologue 2S-1c (Fig. 4e). This could mean that the steric hindrance induced by the MoDTC alkyl chains also plays a role in their capacity to be sulfurized by ZnDTP. The apparently more rapid consumption of MoDTC with C8 alkyl chains relative to the MoDTC bearing exclusively C13 chains also implies that ligand exchange reactions occurring between the various MoDTC (sulfurized or not) are less rapid than the sulfurization reaction under the applied thermal conditions since, otherwise, no preferential consumption/formation of specific homologues would occur.
4.3 Effect of 2S-MoDTC on friction reduction efficiency
According to the tribological studies carried out with the synthesized 2S-MoDTC (see Section 3.4.2; Fig. 8), 2S-MoDTC additives do not appear to reduce more significantly the friction coefficient than the ‘‘classical’’ MoDTC do. Therefore, sulfurization of MoDTC by ZnDTP in solution (sulfurization in bulk) does most likely not contribute to the synergistic improvement of friction coefficients sometimes observed when MoDTC are used in combination with ZnDTP.34 Nonetheless, compared to the “classical” MoDTC species, 2S-MoDTC were shown to display extended periods of friction reducing capability in lubricants under thermo-oxidative conditions as compared to “classical” MoDTC. A possible explanation could be related to the presence of higher amounts of available sulfur for the formation of the MoS2 tribofilm due to the initial presence of 2S-MoDTC. Alternatively, it can be considered that the newly-formed active Mo species deriving from 2S-MoDTC and favoring the formation of the tribofilm were out of our analytical window. Whatever the reason is, our study indicates that sulfurization of MoDTC by ZnDTP could be considered as a process contributing to delay the decrease of the tribological performances of lubricants containing MoDTC.5. Conclusions
The present study involving thermal ageing experiments of MoDTC in the presence of ZnDTP species, combined with HPLC-MS monitoring of the evolution of the resulting MoDTC species, revealed the potential of ZnDTP additives to sulfurize MoDTC in lubricants. This process leads to substitution of Mo-bound oxygen atom(s) by sulfur atom(s) at temperatures and concentrations compatible with those undergone by lubricants under engine functioning conditions. A difference in the reactivity between ZnDTP additives towards sulfurization was observed, primary ZnDTP species leading to a lower degree of sulfurization as compared to secondary ZnDTP. A mechanism of sulfurization of MoDTC by ZnDTP was proposed, possibly involving mixed Mo(DTC)(DTP) intermediates. In addition, the capacity of Lawesson's reagent to (almost) quantitatively sulfurize MoDTC complexes was evidenced and was thus used to synthesize 2S-MoDTC reference compounds that were used for the tribological tests.Contrary to what could be expected based on structural considerations (i.e., enhanced capacity to form a MoS2 tribofilm), tribological measurements indicate that the use of 2S-MoDTC does not significantly improve the coefficients of friction when compared to their non-sulfurized MoDTC counterparts. Nevertheless, it appeared that under nitro-oxidation conditions, the use of synthetic 2S-MoDTC as friction modifiers in a hydrocarbon base oil during laboratory ageing experiments led to the retention of the friction reducing capacities for a longer period of time than the “classical” MoDTC, although the mechanism(s) involved remain(s) to be elucidated. Sulfurization of MoDTC might thus contribute to delay the decrease with time of the overall tribological properties of lubricants containing MoDTC when exposed to thermo-oxidative conditions. To further confirm this hypothesis, future work will be dedicated to study the implications of the sulfurization processes of MoDTC by ZnDTP on the tribological performances of fully formulated lubricants and on the persistence MoDTC derivatives under thermo-oxidative conditions. The possibility of using 2S-MoDTC instead of MoDTC (or a combination of both) as additives in formulated engine lubricants will also be investigated.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Total Marketing Services for funding this research work that was carried out at the University of Strasbourg and Total Solaize Research Center (CRES), and for supplying the base oils and the additives used in this study. The authors also thank E. Motsch for her help in the MS analyses. Y. M. K. thanks Total Marketing Services and the French “Ministère de l'Enseignement Supérieur, de la Recherche et de l'Innovation” for a doctoral fellowship (CIFRE).Notes and references
- S. Liu, W. Zheng, J. Fu, K. Alexopoulos, B. Saha and D. G. Vlachos, Molybdenum oxide-modified iridium catalysts for selective production of renewable oils for jet and diesel fuels and lubricants, ACS Catal., 2019, 9, 7679–7689 CrossRef CAS.
- Y. Mei, T. Li, J. Qian, H. Li, M. Wu and Y. Zheng, Construction of a C@MoS2@C sandwiched heterostructure for accelerating the pH-universal hydrogen evolution reaction, Chem. Commun., 2020, 56, 13393–13396 RSC.
- Y. Guo, Q. Huang, J. Ding, L. Zhong, T. Li, J. Pan, Y. Hu, J. Qian and S. Huang, CoMo carbide/nitride from bimetallic MOF precursors for enhanced OER performance, Int. J. Hydrogen Energy, 2021, 46, 22268–22276 CrossRef CAS.
- P. C. H. Mitchell, Oil-soluble MO-S compounds as lubricant additives, Wear, 1984, 100, 281–300 CrossRef CAS.
- H. Spikes, Friction modifier additives, Tribol. Lett., 2015, 60, 5 CrossRef.
- Y. Yamamoto and S. Gondo, Friction and wear characteristics of molybdenum dithiocarbamate and molybdenum dithiophosphate, Tribol. Trans., 1989, 32, 251–257 CrossRef CAS.
- H. H. Farmer and E. V. Rowan, Molybdenum oxysulfide dithiocarbamates and processes for their preparation, US Pat., 3356702, 1967.
- P. I. Sanin, G. N. Kuz'mina, Y. A. Lozovoi and T. A. Zaimovskaya, Molybdenum complexes as synthetic additives to lubricating oils, Pet. Chem., 1986, 26, 252–257 CrossRef.
- J. Graham, H. Spikes and S. Korcek, The friction reducing properties of molybdenum dialkyldithiocarbamate additives: Part I – factors influencing friction reduction, Tribol. Trans., 2001, 44, 626–636 CrossRef CAS.
- K. Yamamoto, K. Umehara, Y. Moriizumi, S. Lino and N. Tanaka, The effect of MoDTC for improving the fuel economy of diesel engine systems, SAE Tech. Pap. Ser., 2015, 2015-01-2032 Search PubMed.
- M. de Feo, C. Minfray, M. I. De Barros Bouchet, B. Thiébaut, T. L. Mogne, B. Vacher and J. M. Martin, Ageing impact on tribological properties of MoDTC-containing base oil, Tribol. Int., 2015, 92, 126–135 CrossRef CAS.
- G. Splengler and A. Webber, On the lubricating performance of organic molybdenum compounds, Chem. Ber., 1939, 92, 2163–2171 CrossRef.
- C. Grossiord, K. Varlot, J. M. Martin, T. Le Mogne, C. Esnouf and K. Inoue, MoS2 single sheet lubrication by molybdenum dithiocarbamate, Tribol. Int., 1998, 31, 737–743 CrossRef CAS.
- K. Kubo, N. Mitsuhiro, T. Shitamichi and K. Motoyama, The effect of ageing during engine running on the friction reduction performance of oil soluble molybdenum compounds, Proceedings of Japan International Tribology Conference, Yokohama, 1996, pp. 745–750 Search PubMed.
- D. N. Khaemba, F. Jarnias, B. Thiébaut, A. Neville and A. Morina, The role of surface roughness and slide-roll ratio on the decomposition of MoDTC in tribological contacts, J. Phys. D: Appl. Phys., 2017, 50, 85302–85310 CrossRef.
- Y. Yamamoto, S. Gondo, T. Kamakura and M. Konishi, Organoamine and organophosphate complexes as lubricant additives, Wear, 1987, 120, 51–60 CrossRef CAS.
- M. Kasrai, J. N. Culter, K. Gore, G. Canning and G. M. Bancroft, The chemistry of antiwear films generated by the combination of ZDDP and MoDTC examined by X-ray adsorption spectroscopy, Tribol. Trans., 1998, 41, 69–77 CrossRef CAS.
- H. Isomaya and T. Sakurai, The lubricating mechanism of di-μ-thio-dithio-bis(diethyldithiocarbamate) dimolybdenum during extreme pressure lubrication, Tribol. Int., 1974, 7, 151–160 Search PubMed.
- N. T. McDevitt, M. S. Donley and J. S. Zabinski, Utilization of Raman-spectroscopy in tribochemical studies, Wear, 1993, 166, 65–72 CrossRef CAS.
- P. A. Willermet, R. O. Carter, P. J. Schmitz, M. Everson, D. J. Scholl and W. H. Weber, Formation, structure and properties of lubricant-derived antiwear films, Lubr. Sci., 1997, 9, 325–348 CrossRef CAS.
- T. A. Coffey, G. D. Forster and G. Hogarth, Molybdenum(VI) imidodisulfur complexes formed via double sulfur–carbon bond cleavage of dithiocarbamates, J. Chem. Soc., Dalton Trans., 1996, 54, 183–193 RSC.
- D. N. Khaemba, A. Neville and A. Morina, New insights on the decomposition mechanism of molybdenum dialkyldithiocarbamate (MoDTC): a Raman spectroscopic study, RSC Adv., 2016, 6, 38637–38646 RSC.
- M. Shekarriz, An investigation of antioxidant properties of zinc and molybdenum dithiocarbamates in hydrocarbons, J. Petrol. Sci. Technol., 2016, 6, 109–113 Search PubMed.
- M. I. De Barros Bouchet, J. M. Martin, T. Le Mogne, P. Bilas, B. Vacher and Y. Yamada, Mechanisms of MoS2 formation by MoDTC in presence of ZnDTP: effect of oxidative degradation, Wear, 2005, 258, 1643–1650 CrossRef CAS.
- M. I. De Barros Bouchet, J. M. Martin, C. Oumahi, O. Gorbatchev, P. Afanasiev, C. Geantet, R. Iovine, B. Thiébaut and C. Heau, Booster effect of fatty amine on friction reduction performance of Mo-based additives, Tribol. Int., 2018, 119, 600–607 CrossRef CAS.
- M. De Feo, Impact of thermo-oxidative degradation of MoDTC additive on its tribological performance for steel–steel and DLC-steel contacts, PhD thesis, Université de Lyon, 2015.
- M. De Feo, C. Minfray, M. I. De Barros Bouchet, B. Thiébaut and J. M. Martin, MoDTC friction modifier additive degradation: Correlation between tribological performance and chemical changes, RSC Adv., 2015, 5, 93786–93796 RSC.
- J. Igarashi, Y. Yamada, M. Ishimaru and M. Kagaya, Degradation of friction modifiers, Proceedings of Japan International Tribology Conference, Japanese Society of Tribologists, Nagoya, Tokyo, 1990, pp. 421–426 Search PubMed.
- R. K. Jensen, M. D. Johnson, S. Korcek and M. J. Rokosz, Friction reducing and antioxidant capabilities of engine oil additive systems under oxidative conditions. I. Effects of ligand exchange between molybdenum dialkyldithiocarbamate and zinc dialkyldithiophosphate in hexadecane, Lubr. Sci., 1998, 10, 99–120 CrossRef CAS.
- K. Arai, M. Yamada, S. Asano, S. Yoshizawa, H. Ohira, K. Hoshino, F. Ueda and K. Akiyama, Lubricant technology to enhance the durability of low friction performance of gasoline engine oils, SAE Trans., 1995, 104, 1964–1972 Search PubMed.
- H. Spikes, The history and mechanisms of ZDDP, Tribol. Lett., 2004, 17, 469–489 CrossRef CAS.
- K. Kubo, Y. Hamada, Y. Moriki and M. Kibukawa, Friction behaviour of lubricants containing organo molybdenum compounds. Part 2. Application to the lubricants containing zinc dialkyl dithiophosphate, J. Jpn. Soc. Tribol., 1989, 34, 185–192 CAS.
- M. Muraki and H. Wada, Frictional properties of organomolybdenum compounds in the presence of ZDTPs under sliding conditions, Tribol. Ser., 1995, 30, 409–422 CAS.
- M. Muraki, Y. Yanagi and K. Sakaguchi, Synergistic effect on frictional characteristics under rolling-sliding conditions due to a combination of molybdenum dialkyldithiocarbamate and zinc dialkyldithiophosphate, Tribol. Int., 1997, 30, 69–75 CrossRef CAS.
- F. Rounds, Effects of organic molybdenum compounds on the friction and wear observed with ZDP-containing lubricant blends, Tribol. Trans., 1990, 33, 345–354 CrossRef CAS.
- A. B. Greene and T. J. Risdon, The effect of Mo-containing oil soluble friction modifiers on engine fuel economy and gear oil efficiency, SAE Tech. Pap. Ser., 1981, 811187 Search PubMed.
- Y. Sogawa, N. Yoshimura and O. Iwasaki, R&D on new friction modifier for lubricant for fuel economy improvement, Japan Petroleum Energy Centre Report, E1.1.5, 1999 Search PubMed.
- K. Umehara, Y. Tatsumi and N. Tanaka, MoS2 production mechanism of MoDTC, in Proceedings of the FISITA 2012 World Automotive Congress, Lecture Notes in Electrical Engineering 189, ed. SAE-China and FISITA, Springer-Verlag Berlin Heidelberg, 2013, vol. 1, pp. 525–531 Search PubMed.
- J. Q. Hu, X. Y. Wei, G. L. Dai, Y. W. Fei, F. Xie and Z. M. Zong, Tribological behaviours and mechanism of sulfur-and phosphorus-free organic molybdate ester with zinc dialkyldithiophosphate, Tribol. Int., 2008, 41, 549–555 CrossRef CAS.
- W. Huai, X. Chen, F. Lu, C. Zhang, L. Ma and S. Wen, Tribological properties of sulfur- and phosphorus-free organic molybdenum compound as additive in oil, Tribol. Int., 2020, 141, 105944 CrossRef CAS.
- Y. M. Kiw, P. Schaeffer, P. Adam, B. Thiébaut, C. Boyer and G. Papin, Ligand exchange processes between molybdenum and zinc additives in lubricants: evidence from NMR (1H, 13C, 31P) and HPLC-MS analysis, RSC Adv., 2020, 10, 37962–37973 RSC.
- T. Ozturk, E. Ertas and O. Mert, Use of Lawesson's reagent in organic synthesis, Chem. Rev., 2007, 107, 5210–5278 CrossRef CAS PubMed.
- D. V. Partyka, R. J. Staples and R. H. Holm, Nucleophilic reactivity and oxo/sulfido substitution on MVIO3 groups (M= Mo, W), Inorg. Chem., 2003, 42, 7877–7886 CrossRef CAS PubMed.
- E. Campaigne and W. O. Foye, The Synthesis of 2,5-Diarylthiophenes, J. Org. Chem., 1952, 17, 1405–1412 CrossRef CAS.
- T. Sakurai, H. Okabe and H. Isomaya, The synthesis of Di-μ-thio-dithio bis(dialkyldithiocarbamates)-dimolybdenum(V) and their effects on boundary lubrication, J. Jpn. Pet. Inst., 1971, 13, 243–249 CAS.
- H. Isomaya and T. Sakurai, Inhibition of autoxidation by di-μ-thio-dithio-bis(dialkyldithiocarbamate)dimolybdenum, J. Jpn. Pet. Inst., 1974, 16, 112–117 Search PubMed.
- M. A. Malik, P. O'Brien, A. Adeogun, M. Helliwell and J. Raftery, Synthesis and X-ray single crystal structures of [Mo(S2CNnBu2)4] and [Mo2S4(S2CNnBu2)2]1/2H2O, J. Coord. Chem., 2008, 61, 79–84 CrossRef CAS.
- G. Moula, M. Bose, H. Datta and S. Sarkar, Photoluminescent Mo(IV) and W(IV) bis-dithiolene complexes with bidentate phosphonoditioato ligand derived from Lawesson's reagent, Polyhedron, 2013, 52, 900–908 CrossRef CAS.
- T. Onodera, Y. Morita, A. Suzuki, R. Sahnoun, M. Koyama, H. Tsuboi, N. Hatakeyama, A. Endou, H. Takaba, C. A. Del Carpio, M. Kubo, T. Shin-yoshi, N. Nishino, A. Suzuki and A. Miyamoto, A theoretical investigation of the dynamic behavior of molybdenum dithiocarbamate molecule in the engine oil phase, Tribol. Online, 2008, 3, 80–85 CrossRef.
- S. Peeters, P. Restuccia, S. Loehlé, B. Thiébaut and M. C. Righi, Characterization of molybdenum dithiocarbamates by first-principles calculations, J. Phys. Chem. A, 2019, 123, 7007–7015 CrossRef CAS.
- J. J. Dickert Jr and C. N. Rowe, Thermal decomposition of metal O,O-dialkyl phosphorodithioates, J. Org. Chem., 1967, 32, 647–653 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08657j |
| This journal is © The Royal Society of Chemistry 2022 |