Open Access Article
Open Access ArticleResearch about the capacitance properties of ion-induced multilayer and self-assembled monolayer Ti3C2Tx†
Wenwen Maab,
Huaguo Tangac,
Tongyang Liad,
Lujie Wangad,
Lizhi Zhangab,
Yuan Yuad,
Zhuhui Qiao *abc and
Weimin Liua
*abc and
Weimin Liua
aState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, PR China. E-mail: zhqiao@licp.cas.cn; Tel: +86931-4968193
bCenter of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing, 100039, PR China
cShandong Laboratory of Yantai Advanced Materials and Green Manufacture, Yantai 264006, PR China
dYantai Zhongke Research Institute of Advanced Materials and Green Chemical Engineering, Yantai 264006, PR China
First published on 27th January 2022
Abstract
MXenes materials are two-dimensional inorganic materials with abundant surface sites as capacitors. Better control of its morphology and expression of surface groups helps to improve the performance of capacitors. Herein, we controlled the morphology of MXenes with HF, HCl–LiF etching conditions, alkali and metal ions inducing factors. Benefiting from the nanostructures, the capacitance of HCl–LiF-prepared self-assembled monolayer Ti3C2Tx soared to 370.96 F g−1 from 32.09 F g−1 of HF-etched multilaminate Ti3C2Tx. As a result of the introduction of ions, the surface termination group is replaced by –OH with –F. Profit from this, the alkalized single-deck plicated Ti3C2Tx exhibited a supernal capacitance up to 684.53 F g−1 because of the wrinkled morphology and more –OH terminal groups. Meanwhile, metal ion abduction brought some negative effects to electrochemical properties due to the oxidation of high-valent metal ions potentially.
1. Introduction
With the advent of graphene, two-dimensional (2D) layered materials have received increasing attention. MXenes, system compounds of two-dimensional metal carbides or nitrides, are insanely competitive in electrochemical energy storage because of their glorious pseudocapacitance properties,1,2 high metal conductivity,3,4 and modifiable termination.5 MXenes (written as Mn+1XnTx, where M is transition metals such as Ti, Cr, V, Zr, Mo, Nb, etc; X represents C or/and N; T is F, OH or/and O on behalf of surface groups. n = 1, 2, 3.) is synthesized by etching and stripping an “A” layer of MAX (A IIIA or IVA elements, such as Al, Si, Ga, and Ge, usually serve as the “A”.) phase with a stratified hexagon crystal structure.4,6–8To date, MXenes have been synthesized predominantly by wet-chemical etching in hydrofluoric acid (HF) or HF-forming etchants such as HCl–LiF.9–11 It is worth saying that MXenes prepared by HF etching directly are generally multilaminate, whereas those composited by HCl–LiF are few-layer or self-assembled monolayer nanosheets. These MXenes of the two sorts have different layer numbers and surface termination. This leads to different electrochemical properties. For instance, the capacitance of HF-prepared Ti3C2Tx only achieves less than 100 F g−1, while those of HCl–LiF-prepared MXenes can achieve 245 F g−1.4,12 To reveal the differences of the two etching mechanisms profoundly, we researched both the HF etching method and HCl–LiF etching method in detail.
Despite the excellent electrochemical performance of MXene, it is not enough to satisfy us due to the re-accumulation of ultrathin MXene nanosheets during the repeated insertion/extraction process. Therefore it cannot wait to prevent restacking. MXenes are layered inorganic functional materials with a two-dimensional space structure, according to this characteristic, making the molecules of different objects (including organic, inorganic and organic–metal compounds, etc.) into the interlayer could keep the layered structure remain the same in the meantime. This idea provides an important way to construct nanocomposites with supramolecular intercalation structures.4,13–16 An alkali-induced 3D porous network structure is designed to solve the restack problem of layers.17,18 With the change in the pH of the solution, NaOH induces MXenes to accelerate the flocculation, while abundant –OH with higher chemical reactivity can displace –F as surface groups. Gelation of MXenes induced by metal ions can also maintain an interconnected 3D network.19 Guided ions can serve as a bridge between nanoscale pieces and destroy the electrostatic repulsive-force between nanosheets, and then the 3D MXene network begins to take shape. As we can see in the paper, divalent metal ions (such as Co2+ and Cd2+) have a higher hydration energy and lower oxidation ability. However, the addition of too many hetero atoms may weaken the electrochemical reactivity, so we will remove the ions up to a point. To reveal the influence of ion induction on the structure and function of MXenes more comprehensively, two induction methods of alkali induction and metal ion induction were introduced.
Herein, we systematically compare of the differences between HF etching multilaminate Ti3C2Tx and HCl–LiF etching unilaminar Ti3C2Tx. To change the surface groups and destroy the interlayer repulsion, MXenes provided by HF portraying or HCl–LiF etching are induced in NaOH liquor or Co2+ metal ion solution or Cd2+ metal ion solution. Therefore, we can observe the variational electrochemical performance, which is beneficial for the changes in the surface functional groups and the destruction of the interlayer electrostatic repulsion.
2. Materials and experimental procedures
2.1. Synthesis of multilayer MXenes
Multilayer MXenes were prepared by etching Ti3AlC2 in hydrofluoric acid (HF) according to reported method.20 2 g of Ti3AlC2 was slowly added to 20 ml of HF solution and put into an oil bath electromagnetic stirring reaction pot for reaction 24 h at room temperature. The reaction liquid was placed in a 50 ml centrifuge tube and centrifuged 5 times approximately until it was nearly neutral. The ultrasonic separator with a power of 400 W was used for 4 h to remove impurities. Then, the precipitation was centrifuged at 3500 rpm at 20 °C for 60 min. Then, the multilayer MXenes were taken out for freeze-drying.2.2. Synthesis of monolayer or few-layer MXenes
Such Ti3C2Tx was made from Ti3AlC2 by reacting in HCl–LiF on the basis of the report.4,8 1 g of LiF was accurately weighed and slowly added to 9 M HCl. The solution was mixed in a magnetic agitator for 30 min, and then 2 g of Ti3AlC2 was deliberately added while stirring. Put it in a 45 °C oil bath environment and stir for 24 h. Put above liquid in 50 ml of centrifuge tube to wash by deionized water at 5000 rpm in centrifuge approximately 5 times till pH is increased to near neutral. The sediment was desiccated in vacuum and then put in three centrifuger tubes with 30 ml of DI water. The reaction system was stripped in an ultrasonic cleaning machine with a power of 400 W for 4 h. The mixture was placed in a centrifuge at 3500 rpm for 60 min to isolate the supernatant and sediment. Supernatant after separating was what we need. Then, the samples were freeze-dried and redissolved to prepare the MXene single-layer colloid solution at a concentration of 10 mg ml−1.2.3. Three-dimensional morphology construction of MXenes
Three copies of 3 ml of 10 mg ml−1 MXene solutions were removed by direct HF etching and by HCl–LiF preparation method, and then 3 ml of 1 M NaOH solution, 200 μl of 1 M Co(NO3)2 solution and 200 μl of 1 M CdCl2 solution were dropwise added. The reaction system was reacted for 2 h at room temperature, centrifuged at 8000 rpm at 20 °C for 10 min to remove the remaining doped ions and washed to near neutral in the alkali reaction scheme. Freeze drying to produce MXenes with wrinkles.2.4. Electrochemical measurements
MXenes powder, acetylene black and PVDF were evenly mixed at a mass ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, coated on copper foil, then vacuum dried at 120 °C for 10 h, and fixed with PET plate, then we made electrode with it. All the electrochemical measurements were conducted in 1 M H2SO4 on the electrochemical workstation (CHI 660E, Shanghai) at room temperature with a three-electrode system using Ag/AgCl and platinum electrodes as the reference and counter electrodes, respectively. Particular measurement items and specifications are as follows: the cyclic voltammetry (cyclic voltammetry curve was tested in the potential range −0.55 V to 0 V at a scan rate of 10 mV s−1), EIS (The A.C. Impedance was performed from 10 mHz to 100 kHz from the open circuit potential to the jarless applied voltage), charge–discharge curve (chronopotentiometry was measured at a current density of 1.0 A g−1).
1, coated on copper foil, then vacuum dried at 120 °C for 10 h, and fixed with PET plate, then we made electrode with it. All the electrochemical measurements were conducted in 1 M H2SO4 on the electrochemical workstation (CHI 660E, Shanghai) at room temperature with a three-electrode system using Ag/AgCl and platinum electrodes as the reference and counter electrodes, respectively. Particular measurement items and specifications are as follows: the cyclic voltammetry (cyclic voltammetry curve was tested in the potential range −0.55 V to 0 V at a scan rate of 10 mV s−1), EIS (The A.C. Impedance was performed from 10 mHz to 100 kHz from the open circuit potential to the jarless applied voltage), charge–discharge curve (chronopotentiometry was measured at a current density of 1.0 A g−1).
2.5. Materials
Titanium aluminum carbide (200 mesh) was purchased from 11 Technology Co. Ltd. Hydrofluoric acid (HF, ≥40.0%) was purchased from Nanjing Chemical Reagent Co., Ltd. Lithium fluoride (LiF, ≥98.0%), cadmium chloride (CdCl2·2.5H2O, 99.0%), cobalt nitrate hexahydrate (Co(NO3)2·6H2O, ≥98.5%) and hydrochloric acid (HCl, 36.0–38.0%) were purchased from Sinopharm Chemical Reagent Co. Ltd. Sodium hydroxide (NaOH, ≥96.0%) was purchased from Tianjin Guangcheng Chemical Reagent Co., Ltd.2.6. Experimental procedures
The three-dimensional morphology of MXene was observed by different methods. The morphology of multilayer and monolayer MXenes induced by different ions was characterized using SEM (JSM-7610F, Japan). The crystal structure of those composites was characterized by XRD (D8 ADVANCE, BRUKER, Germany) using Cu radiation. The surface chemistry of MXenes was measured by XPS (Thermo k-Alpha+) using Al K alpha radiation. The specific surface area (BET surface area) was determined on a Micromeritics ASAP 2460 instrument. All electrochemical tests were conducted on an electrochemical workstation (CHI 660E, Shanghai) at room temperature. Atomic force microscopy (AFM) images were acquired by a Multimode8 (Bruker) AFM system. The macro morphology photo was taken with Huawei p30.3. Results and discussion
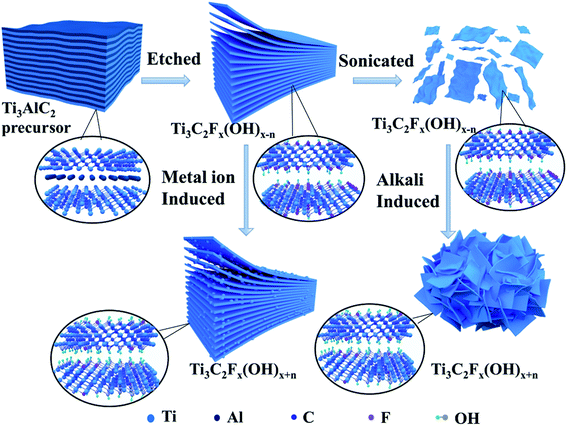
Fig. 1 shows the schematic diagram of preparing all kinds of Ti3C2Tx and induced-Ti3C2Tx which indicates the synthesis and modification of Ti3C2Tx. Al layers were selectively etched and removed from the Ti3AlC2 precursor by HF and HCl + LiF etching agents due to the weaker M–A bonds than M–X bonds, which shows a multilayer morphology similar to accordion. MXene by HCl + LiF prepared can be physically separated 2-D MXenes sheets after the sonication process. Metal ion induction and alkali induction changed the surface groups and morphologies of MXenes in different degrees. Usually, the introduction of metal ions makes the surface terminal group –F replaced by –OH, which destroys the surface electrostatic interaction of MXenes and concurrently increases the layer spacing concurrently. This could help to improve the chemical activity. There are some small protrusions on the surface of HF prepared MXenes, while the HCl + LiF prepared MXenes appeared to be in a folded state because of ion induction.3.1. Morphological characterization of the samples
As shown in Fig. 2a, the HCl–LiF prepared Ti3C2Tx was peeled into a single layer. AFM images of monolayer Ti3C2Tx scattered in water show a thickness of approximately 2 nm which proves the single-layer structure (Fig. S1†).21 However, the multi-layer Ti3C2Tx formed by HF etching are accordion-like and connected to a two-dimensional lamellar morphology (Fig. S2a†). The HCl–LiF prepared Ti3C2Tx was induced by ions, and the reaction produced flocculation (Fig. 2f). It can be seen that what alkali induced fold states are remarkable (Fig. 2c) greatly expands the surface area of two-dimensional Ti3C2Tx. Nevertheless, the folds generated by Co2+ and Cd2+ induction were lower (Fig. 2d and e). However, the various degrees of particles on the multilayer Ti3C2Tx surface (Fig. S2†) may affect the layer spacing to some extent. Meanwhile, the change in the XRD (002) peak position could also support this viewpoint.The low-temperature N2 adsorption/desorption isotherms (Fig. S3†) and BET surface area (Table 1) prove that ion-induced can inhibit layer restacking. The BET surface area of HCl–Ti3C2Tx is only 1.64 m2 g−1. Which of HCl–OH–Ti3C2Tx, HCl–Cd–Ti3C2Tx, HCl–Co–Ti3C2Tx is 1.79 m2 g−1, 6.50 m2 g−1, 16.43 m2 g−1. The BET surface area of HF–Ti3C2Tx is only 4.61 m2 g−1. Whom of HF–OH–Ti3C2Tx, HF–Cd–Ti3C2Tx, HF–Co–Ti3C2Tx is 5.56 m2 g−1, 6.87 m2 g−1, 8.27 m2 g−1. It is noted that metal ions-induced can greatly improve the specific surface area. HCl–LiF prepared Ti3C2Tx has a greater potency of restacking because of the monolayer structure, so the changes of this are more distinct than HF prepared Ti3C2Tx.
| Samples | BET surface area (m2 g−1) | Langmuir surface area (m2 g−1) |
|---|---|---|
| Ti3C2Tx–HCl | 1.64 | 2.19 |
| Ti3C2Tx–HCl–OH | 1.79 | 2.47 |
| Ti3C2Tx–HCl–Cd | 6.50 | 8.80 |
| Ti3C2Tx–HCl–Co | 16.43 | 22.49 |
| Ti3C2Tx–HF | 4.61 | 6.24 |
| Ti3C2Tx–HF–OH | 5.56 | 7.76 |
| Ti3C2Tx–HF–Cd | 6.87 | 9.45 |
| Ti3C2Tx–HF–Co | 8.27 | 11.40 |
3.2. Structural analysis of the samples
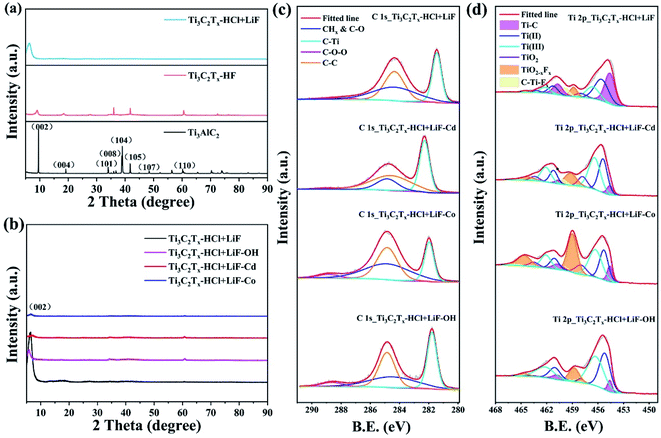
The X-ray diffraction patterns (XRD) of HCl–LiF-prepared Ti3C2Tx and HF-prepared Ti3C2Tx are shown in Fig. 3 The characteristic peak of 10° (002) which represents Ti3AlC2 is obviously weakened and shifted to a low angle, while the (104) peak significantly disappears, which means the dislodging of the Al atom layer in Ti3AlC2 and the production of Ti3C2Tx.4,5 The weakening and disappearance of a series of (001) peaks are caused by the disorder of the nanosheets. HF-etched Ti3C2Tx and HCl–LiF-prepared Ti3C2Tx have the same ultrasonic stripping process without the ion intercalation. The spectral peak of HF-prepared Ti3C2Tx has a smaller variation, which means the different orderliness of the two kinds of MXenes. The obvious spectrum peaks weakened after ion-induced represent the process of ion intercalation. However, it's hard to detect obvious induced ions in the graph, which means that intercalated ions are likely to fall off. The above phenomenon proves that the ions in this experiment only play the role of inducement. No phanerous (104) peak that belongs to MAX is observed in the XRD spectra of the two samples before and after induction, which indicates the stabilization of Ti3C2Tx. The (002) peak analysis shows that ion induction can change the c-lattice parameter to a certain extent. For the HCl–LiF-prepared Ti3C2Tx (Fig. 3b), the intermediate layer spacing increased visibly which varied from 9.2593 Å of Ti3AlC2 to 13.6425 Å for Ti3C2Tx, while which of alkali-induced and ion-induced are changed slightly compared with Ti3C2Tx. While the interplanar crystal spacing increases slightly of the HF-etched products (Fig. S4†), and obviously increases from Ti3AlC2 9.2593 Å to 12.1438 Å in alkali-induced Ti3C2Tx, while the tiny variation occurs on Co2+ and Cd2+ induced multilayer Ti3C2Tx. The variation in the c-lattice parameter which negatively correlates with the variation in layer spacing is also closely related to its performance.It can be seen in the full XPS spectrum peaks (Fig. S5†) that there is a small Cd peak of both HF-etched Ti3C2Tx and HCl–LiF-prepared Ti3C2Tx after the induction of Cd2+. The doping of Cd2+ may reduce the number of active sites on the Ti3C2Tx surface, thus affecting its electrochemical performance. This can be reflected in the CV curve and the charging–discharging performance in the electrochemical test. As shown in C 1s spectra, four peaks at 282.0, 284.6, 285.1 and 288.6 eV were corresponded to C–Ti, C–C, CHx & C–O, and C–O–O, respectively.22,23 By observing the C 1s energy spectrum (Fig. 3c and S6†) of different samples, it is manifest that the spectral changes are not obvious, indicating that ion induction has little effect on C 1s.
Both the 2p1/2 and the 2p3/2 spin–orbit split components of the Ti 2p spectrum (Fig. 3d and S5†) could be divided into six curves at 454.6 (460.7), 455.3 (461.1), 456.4 (462.1), 458 (463.4), 459 (464.6) and 460 (466.3) eV which were marked as Ti–C, Ti (II), Ti (III), TiO2, TiO2−xFx and C–Ti–Fx, respectively.24–26 Ti–C is in the core of layers whose intensity is associated with d-spacing. Thus, the weakening of Ti–C foreshadowed that the exfoliation of Al layers and the greater d-spacing of the HCl–LiF-prepared Ti3C2Tx. TiO2−xFx and C–Ti–Fx correspond to the surface terminal groups. The percentages of the peak areas of both TiO2−xFx and C–Ti–Fx are changed visibly after alkali induction and metal ion induction of Ti3C2Tx. For HCl–LiF-prepared Ti3C2Tx, the share of the peak field of C–Ti–Fx drops down to 1.12% of alkalized Ti3C2Tx, 2.48% of Co2+ induced Ti3C2Tx, and 2.99% of Cd2+ induced Ti3C2Tx from 5.73% of primary Ti3C2Tx (Table S1†). Whereas, the proportion of the peak area of TiO2−xFx of induced Ti3C2Tx is upward. For monolayer Ti3C2Tx, the proportion goes up obviously after alkalization and metal ion induced. Simultaneously, multilayer Ti3C2Tx has similar rulers (Table S2 and Fig. S6†). The changes of terminal oxygen–fluorine ratio reveal that the –F surface terminals decrease and the –O surface terminals increase.
3.3. Electrochemical properties of the samples
Fig. 4a and e show the EIS data and equivalent circuit (inserted in Fig. 4a), of the samples, the intermediate frequency curve with the semicircle represents the charge transfer resistance (Rct) between the electrolyte and the electrode.14 By comparing the curvature radius, it is clear that alkali-induced MXenes has smaller semicircle diameters, manifesting the faster charge transfer. The lower charge transfer resistance further proves that alkali-induced can supply more electronic pathways and elevate charge transfer kinetics. The low frequency display is determined by diffusion control, which related to the solution resistance (Rs) deeply, and influenced by the layer spacing. The more steep of the slope line, the more surface capacitance reactions are foreboded, and the smaller solution resistance is, which means the better electrochemical performance.27 Based on the slope of the low-frequency curve, the faster ions diffusion occurred on the alkali-induced MXenes system. However, metal ions induced MXenes have higher electrochemical impedance and concomitantly worse capacitive properties. This conclusion can also be seen in the CV curve (Fig. 4b and f).As we can see in Fig. S7,† we choose a 10 mV s−1 as the scan rate and choose −0.55 V to 0 V as the voltage of the CV and discharge–charge curves. Fig. 4c and g show the discharge–charge profiles of the electrode. According to the charge–discharge curve and C = It/mV, the capacitance of Ti3C2Tx–HF is 32.09 F g−1 and Ti3C2Tx–HF–OH is 124.61 F g−1. The capacitance of HCl–LiF-prepared monolayer Ti3C2Tx was 382.67 F g−1, and what of alkali-induced monolayer Ti3C2Tx reached 684.53 F g−1. This shows that the electrochemical performance could be greatly improved under alkali induction and electrochemical properties of monolayer Ti3C2Tx is more excellent than multilayer Ti3C2Tx. It is important to point out that the capacitance of the Co2+ and Cd2+ induced multilayer Ti3C2Tx is approximately 2 F g−1, which proves that ion doping has a negative influence on its electrochemical performance due to the oxidation of high-valent metal ions potentially.19 The capacitance of Co2+-induced monolayer Ti3C2Tx is 316.11 F g−1, which is slightly lower than that before induction. When induced by Cd2+, the capacitance performance decreases sharply to about 2 F g−1 due to a small amount of ion doping.
Fig. 4d shows the cycling stability of the alkali-induced monolayer Ti3C2Tx by discharge/charge curves in the voltage range of −0.55 to 0 V, at a current density of 1 A g−1. The discharge capacity of alkali-induced monolayer MXenes is 684.53 F g−1 in the first cycle. Notedly, there were no obvious changes about the capacitance in the fifth and later cycles. Better reproducibility means good reversibility of the electrode. No plateau region signifies that capacitors are most likely the charging mechanism.28 As shown in Fig. 4h, HCl–Ti3C2Tx has better charge and discharge reversibility and rate performance. The discharge capacity of HCl–Ti3C2Tx changes from 382.67 F g−1 to 373.92 F g−1 at a 1000 mA g−1 and 5000 mA g−1 current density. Apparently, the discharge capacity of the alkali-induced monolayer Ti3C2Tx changes from 684.53 F g−1 to 545.48 F g−1 at a 1000 mA g−1 and 5000 mA g−1 current density. We put this down to the more chemically active oxygen-containing groups on the surface of alkali-induced monolayer Ti3C2Tx. More reactive groups potentially mean more side reactions potentially, such as oxidation of MXenes.
Combined with various component and performance analyses, metal ions induced can improve the surface area to some extent, but this does not imply an improvement in electrochemical performance. We can infer that the BET surface area has no direct effect on the capacities. As I mentioned earlier, the –OH surface group has greater electrochemical activity than –F, and alkali induction and metal ions induction can increase the content of –OH surface group in some degree which means a higher capacitance. With largest BET surface area and larger content of –OH surface groups, metal ion-induced Ti3C2Tx has a worst capacitance performance. We speculate that the electrochemical performance is not strongly related to the BET surface area for metal ion-induced MXenes. The main reason is that metal ions have a small oxidation ability and can oxidize MXenes.19 Thus, the capacitance of metal ions induced MXenes decreased instead of growing up, and the alkali-induced MXenes had a better capacity performance.
4. Conclusions
In general, organ-like Ti3C2Tx, single-deck Ti3C2Tx and their revulsive Ti3C2Tx were studied to measure electrochemical performance. It is worthwhile to note that the pseudocapacitive performance of HCl–LiF-prepared monolayer Ti3C2Tx is better than organ-like Ti3C2Tx of HF-etched. Compared with Ti3C2Tx, an excellent capacitance of 684.53 F g−1 could be obtained for the alkalized single-deck Ti3C2Tx. The abduction of metal ions has some negative effects on electrochemical properties because of the oxidation of high-valent metal ions. However, whether the alkali-induced or metal ion induced methods can let –O terminal groups replace –F terminal groups completely, which means that the electrochemical performance can be further improved. Improving the electrochemical activity of metal ions induced MXenes with a higher surface area is a problem demanding prompt solution. There is shortened lifetime and poor rate performance due to factors such like oxidation and chemical activity. Those questions deserve further inquiry.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51775532), the project ZR2020ZD29 and ZR2021JQ20 supported by Shandong Provincial Natural Science Foundation, the Taishan Scholars Program of Shandong Province.References
- M. Boota, B. Anasori, C. Voigt, M. Q. Zhao, M. W. Barsoum and Y. Gogotsi, Pseudocapacitive Electrodes Produced by Oxidant-Free Polymerization of Pyrrole between the Layers of 2D Titanium Carbide (MXene), Adv. Mater., 2016, 28(7), 1517–1522 CrossRef CAS PubMed.
- X. Wang, S. Kajiyama, H. Iinuma, E. Hosono, S. Oro, I. Moriguchi, M. Okubo and A. Yamada, Pseudocapacitance of MXene nanosheets for high-power sodium-ion hybrid capacitors, Nat. Commun., 2015, 6, 6544 CrossRef CAS PubMed.
- Z. Ling, C. E. Ren, M. Q. Zhao, J. Yang, J. M. Giammarco, J. Qiu, M. W. Barsoum and Y. Gogotsi, Flexible and conductive MXene films and nanocomposites with high capacitance, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(47), 16676–16681 CrossRef CAS PubMed.
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Conductive two-dimensional titanium carbide clay with high volumetric capacitance, Nature, 2014, 516(7529), 78–81 CrossRef CAS PubMed.
- J. Li, X. Yuan, C. Lin, Y. Yang, L. Xu, X. Du, J. Xie, J. Lin and J. Sun, Achieving High Pseudocapacitance of 2D Titanium Carbide (MXene) by Cation Intercalation and Surface Modification, Adv. Energy Mater., 2017, 7(15), 1602725 CrossRef.
- W. M. Barsoum and T. El-Raghy, The MAX phases: Unique new carbide and nitride materials – Ternary ceramics turn out to be surprisingly soft and machinable, yet also heat-tolerant, strong and lightweight, Am. Sci., 2001, 89(4), 334–343 CrossRef.
- B. Anasori, Y. Xie, M. Beidaghi, J. Lu, B. C. Hosler, L. Hultman, P. R. C. Kent, Y. Gogotsi and M. W. Barsoum, Two-Dimensional, Ordered, Double Transition Metals Carbides(MXenes), ACS Nano, 2015, 9, 9507–9516 CrossRef CAS PubMed.
- M. Alhabeb, K. Maleski, B. Anasori, P. Lelyukh, L. Clark, S. Sin and Y. Gogotsi, Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene), Chem. Mater., 2017, 29(18), 7633–7644 CrossRef CAS.
- B. Anasori, M. R. Luhatskaya and Y. Gogotsi, 2D Metal carbides and nitrides (MXenes) for energy storage, Nat. Rev. Mater., 2017, 2(10), 16098 CrossRef CAS.
- Q. Tao, M. Dahlqvist, J. Lu, S. Kota, R. Meshkian, J. Halim, J. Palisaitis, L. Hultman, M. W. Barsoum, P. O. A. Persson and J. Rosen, Two-dimensional Mo1.33C MXene with divacancy ordering prepared from parent 3D laminate with in-plane chemical ordering, Nat. Commun., 2017, 8, 14949 CrossRef PubMed.
- J. Zhou, X. Zha, X. Zhou, F. Chen, G. Gao, S. Wang, C. Shen, T. Chen, C. Zhi, P. Eklund, S. Du, J. Xue, W. Shi, Z. Chai and Q. Huang, Synthesis and Electrochemical Properties of Two-Dimensional Hafnium Carbide, ACS Nano, 2017, 11(4), 3841–3850 CrossRef CAS PubMed.
- Y. Dall'Agnese, M. R. Lukatskaya, K. M. Cook, P. L. Taberna, Y. Gogotsi and P. Simon, High capacitance of surface-modified 2D titanium carbide in acidic electrolyte, Electrochem. Commun., 2014, 48, 118–122 CrossRef.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, 25th Anniversary article: MXenes: a new family of two-dimensional materials, Adv. Mater., 2014, 26(7), 992–1005 CrossRef CAS PubMed.
- X. Wang, J. Wang, J. Qin, X. Xie, R. Yang and M. Cao, Surface Charge Engineering for Covalently Assembling Three-Dimensional MXene Network for All-Climate Sodium Ion Batteries, ACS Appl. Mater. Interfaces, 2020, 12(35), 39181–39194 CrossRef CAS PubMed.
- M. R. Lukatskaya, O. Mashtalir, C. E. Ren, Y. Dall'Agnese, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide, Science, 2013, 341(6153), 1502–1505 CrossRef CAS PubMed.
- O. Mashtalir, M. Naguib, V. N. Mochalin, Y. Dall'Agnese, M. Heon, M. W. Barsoum and Y. Gogotsi, Intercalation and delamination of layered carbides and carbonitrides, Nat. Commun., 2013, 4, 1716 CrossRef PubMed.
- D. Zhao, M. Clites, G. Ying, S. Kota, J. Wang, V. Natu, X. Wang, E. Pomerantseva, M. Cao and M. W. Barsoum, Alkali-induced crumpling of Ti3C2Tx (MXene) to form 3D porous networks for sodium ion storage, Chem. Commun., 2018, 54, 4533–4536 RSC.
- D. Zhao, R. Zhao, S. Dong, X. Miao, Z. Zhang, C. Wang and L. Yin, Alkali-induced 3D crinkled porous Ti3C2 MXene architectures coupled with NiCoP bimetallic phosphide nanoparticles as anodes for high-performance sodium-ion batteries, Energy Environ. Sci., 2019, 12(8), 2422–2432 RSC.
- Y. Deng, T. Shang, Z. Wu, Y. Tao, C. Luo, J. Liang, D. Han, R. Lyu, C. Qi, W. Lv, F. Kang and Q. H. Yang, Fast Gelation of Ti3C2Tx MXene Initiated by Metal Ions, Adv. Mater., 2019, 31(43), e1902432 CrossRef PubMed.
- M. Naguib, O. Mashtalir, J. Carle, V. Presser, J. Lu, L. Hultman, Y. Gogotsi and M. W. Barsoum, Two-dimensional transition metal carbides, ACS Nano, 2012, 6(2), 1322–1331 CrossRef CAS PubMed.
- Q. Zhang, H. Lai, R. Fan, P. Ji, X. Fu and H. Li, High Concentration of Ti3C2Tx MXene in Organic Solvent, ACS Nano, 2021, 15(3), 5249–5262 CrossRef CAS PubMed.
- Z. Yang, A. Liu, C. Wang, F. Liu, J. He, S. Li, J. Wang, R. You, X. Yan, P. Sun, Y. Duan and G. Lu, Improvement of Gas and Humidity Sensing Properties of Organ-like MXene by Alkaline Treatment, ACS Sens., 2019, 4(5), 1261–1269 CrossRef CAS.
- X. Chen, Y. Zhu, M. Zhang, J. Sui, W. Peng, Y. Li, G. Zhang, F. Zhang and X. Fan, N-Butyllithium-Treated Ti3C2Tx MXene with Excellent Pseudocapacitor Performance, ACS Nano, 2019, 13(8), 9449–9456 CrossRef CAS PubMed.
- J. Halim, M. R. Lukatskaya, K. M. Cook, J. Lu, C. R. Smith, L.-Å. Näslund, S. J. May, L. Hultman, Y. Gogotsi, P. Eklund and M. W. Barsoum, Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films, Chem. Mater., 2014, 26(7), 2374–2381 CrossRef CAS PubMed.
- M. Benchakar, L. Loupias, C. Garnero, T. Bilyk, C. Morais, C. Canaff, N. Guignard, S. Morisset, H. Pazniak, S. Hurand, P. Chartier, J. Pacaud, V. Mauchamp, M. W. Barsoum, A. Habrioux and S. Célérier, One MAX phase, different MXenes: A guideline to understand the crucial role of etching conditions on Ti3C2Tx surface chemistry, Appl. Surf. Sci., 2020, 530, 147209 CrossRef CAS.
- J. Halim, K. M. Cook, M. Naguib, P. Eklund, Y. Gogotsi, J. Rosen and M. W. Barsoum, X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes), Appl. Surf. Sci., 2016, 362, 406–417 CrossRef CAS.
- C. Ding, F. Dong and Z. Tang, Construction of hollow carbon polyhedron supported Pt catalyst for methanol electrocatalytic oxidation, Electrochim. Acta, 2021, 390, 138790 CrossRef CAS.
- Y. Dall'Agnese, P. L. Taberna, Y. Gogotsi and P. Simon, Two-Dimensional vanadium carbide (MXene) as positive electrode for sodium-ion capacitors, J. Phys. Chem. Lett., 2015, 6, 2305–2309 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07771f |
| This journal is © The Royal Society of Chemistry 2022 |