Open Access Article
Open Access ArticleEncapsulation of bioactive peptides: a strategy to improve the stability, protect the nutraceutical bioactivity and support their food applications
J. E. Aguilar-Toaláa,
D. Quintanar-Guerrerob,
A. M. Liceaga c and
M. L. Zambrano-Zaragoza
c and
M. L. Zambrano-Zaragoza *a
*a
aLaboratorio de Procesos de Transformación y Tecnologías Emergentes de Alimentos-UIM, FES-Cuautitlán, Universidad Nacional Autónoma de México, Cuautitlán Izcalli, Estado de México 54714, Mexico. E-mail: luz.zambrano@unam.mx
bLaboratorio de Posgrado en Tecnología Farmacéutica, FES-Cuautitlán, Universidad Nacional Autónoma de México, Av. 1o de Mayo s/n, Cuautitlán Izcalli, Estado de México 54714, Mexico
cProtein Chemistry and Bioactive Peptides Laboratory, Department of Food Science, Purdue University, 745 Agriculture Mall Dr, West Lafayette, IN 47907, USA
First published on 23rd February 2022
Abstract
In recent decades, bioactive peptides have become an emerging field of interest in the scientific community as well as the food, pharmaceutical, and cosmetics industries. A growing body of research indicates that consumption of bioactive peptides may play a vital role in health through their broad spectrum of bioactivity such as antioxidant, antihypertensive, antimicrobial, anti-inflammatory, immunomodulatory, and anti-proliferative activities. In addition, bioactive peptides can be used as food preservatives due to their antimicrobial and antioxidant activities. However, some factors limit their nutraceutical and commercial applications, including easy chemical degradation (e.g., pH, enzymatic), food matrix interaction, low water-solubility, hygroscopicity, and potential bitter taste. Bearing that in mind, the encapsulation of bioactive peptides in different materials can help overcome these challenges. Studies have demonstrated that encapsulation of bioactive peptides increases their bioactivity, improves their stability, sensory properties, increases solubility, and decreases hygroscopicity. However, there is limited scientific evidence about the bioavailability and food matrix interactions of encapsulated peptides. Besides, the diverse colloidal systems used to encapsulate bioactive peptides have shown stability and good encapsulation efficiency. This review provides an overview of current advances in the encapsulation of bioactive peptides, considering the technology, developments, and innovations in the last lustrum.
1. Introduction
Nowadays, bioactive peptides have gained more attention from both the scientific community and the food, pharmaceutical, and cosmetics industries due to their broad spectrum of bioactivities, including antioxidant, antihypertensive, antimicrobial, anti-inflammatory, immunomodulatory, and anti-proliferative, among others.1–3 The above mentioned bioactivities are based on the amino acid composition and sequence of peptides, and they usually contain 2–20 amino acid residues. These peptides are inactive within the sequence of the parent protein, but are released by enzymatic, chemical and microbial hydrolysis.3 Currently, the main application of bioactive peptides is for the creating of functional foods and/or nutraceuticals in order to improve the human health.2 In addition, they can be used as food additives because of their techno-functional properties such as solubility, emulsifying, foaming, water/oil holding capacity.3Despite the above, it has been reported that in order to exhibit their bio-functionalities, the peptides' structural integrity should be maintained after ingestion and/or exhibit stability in the food.4,5 It has been reported that other factors that may affect the bio-functionalities of peptides and their commercial application are food matrix interactions,5 low water-solubility,6 hygroscopicity,7 and bitter flavour (Fig. 1).8
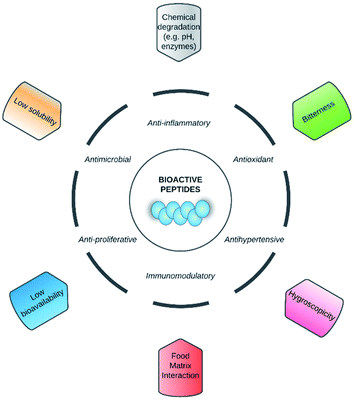 | ||
| Fig. 1 Factors and barriers that limited that the nutraceutical and food applications of bioactive peptides. | ||
Encapsulation methods are considered a promising strategy to improve stability, protect the functionalities, and control the release of bioactive peptides with nutraceutical and food applications. Further advantages of encapsulating peptides in nanocarriers include the improving of sensory properties by mask their bitter taste, increase their solubility, and decrease the incidence of hygroscopicity of peptides to ensure their storage preservation.5–8 Additionally, due to their antimicrobial and antioxidant properties, bioactive peptides are ideal candidates for natural food preservatives to extend the shelf-life of foods or by incorporating them into packaging material in the form edible films and coatings to improve food safety.9 This review provides new perspectives and current advances in the encapsulation of bioactive peptides considering the main methods used and the bioactivities of encapsulated peptides with focus on literature published over the last lustrum. Additionally, current trends, challenges and opportunities on the incorporation of encapsulated bioactive peptides in food and development of nutraceuticals forms, will be addressed.
2. Emerging use of encapsulation technologies in bioactive peptides area
Bioactive peptides need to overcome some challenges such as maintaining their structural integrity either at the physiological level or within foods, before exhibiting their bio-functionalities. The first challenge to address is the low bioavailability of bioactive peptides by their susceptibility to gastrointestinal degradation. Some studies reported that small peptides, mainly di- and tripeptides, pass through the intestinal membrane and reach the systemic circulation at nanomolar or even picomolar concentrations.5,10 Overall, when bioactive peptides are administered orally, they are subject to gastrointestinal digestion, mainly exposed to gastric, pancreatic, and small intestinal brush border membrane enzymes, and exposed to acidic conditions in the stomach that may hinder their bioactivity.4,5On the other hand, it has been reported that bioactive peptides can interact with food components, either when they are naturally developed within a food (e.g., fermented milk) or when they are generated from protein sources (e.g., protein hydrolysates). Foods can be carrier vehicles to the active peptides when added as an ingredient in a manufacturing step.11,12 Some peptides can react chemically due to their nucleophilic side chains of the amino acids that constitute them5,11 with diverse food components such as lipids, carbohydrates, phenolic compounds, and metals resulting in the formation of complexes (e.g., peptide-lipid, peptide-phenolic, peptide-metal)12,13 or peptide-derived products (e.g., Maillard reaction products).14
These last factors, their susceptibility to gastrointestinal degradation and food matrix interactions of bioactive peptides are mainly responsible for the fact that bioactive peptides evaluated using in vitro tests do not necessarily reflect their cellular or physiological effect in vivo.15–18
Besides, from a commercial point of view, the factors that affect the application of bioactive peptides in foods or pharmaceutical preparations are low water solubility,6 hygroscopicity,7 and potential bitter taste.8 This is because bioactive peptides are lyophilized to obtain powers for storage preservation, since peptides are susceptible to microbial contamination and can also be degraded by oxidation.7,19 In addition, peptides in powder form can be more easily incorporated into foods. Besides in mechanistic studies, bioactive peptides are primarily synthesized and delivered as a lyophilized powder.19,20
Commonly, in vitro, and in vivo studies require controlled and accurate peptide concentration; hence, peptide solubilization is a critical step for a successful assay. Thus, improper peptide solubilization can introduce experimental errors or lead to experimental failure.21 In this sense, the solubility of bioactive peptides depends on the length and the number of hydrophobic amino acids present in their sequence.19,22 Peptides with more hydrophobic amino acids (≥50%) are generally partly soluble in aqueous solutions.20,23 In the same way, the presence of hydrophobic amino acids in the sequence confers a bitter taste to these peptides.24,25 Further, salty off-flavours can result during the production bioactive peptides from protein hydrolysates; this is a result of the acids and alkalis used for neutralizing hydrolysis products.26 The great bitterness or off-flavours that bioactive peptides can impart to foods or nutraceutical preparations limit their sensory acceptability.7 Moreover, the high hygroscopicity of bioactive peptides produces physicochemical changes that affect the stability during storage, accelerate chemical and microbial degradation.27
These interactions can modify or affect their bioactivity and decrease the bioaccessibility of bioactive peptides. Given the above, encapsulation of bioactive peptides in different colloidal or nanostructured delivery systems are being designed to overcome these challenges so that bioactive peptides could display their bioactivity appropriately and can be successfully included in commercial products for their application. In this context, bioactive peptides have been encapsulated with different food-grade materials by using different techniques.
3. Encapsulation as a strategy to improve the bioactivities and stability of bioactive peptides
3.1 Main methods and vehicles used for encapsulation of bioactive peptides
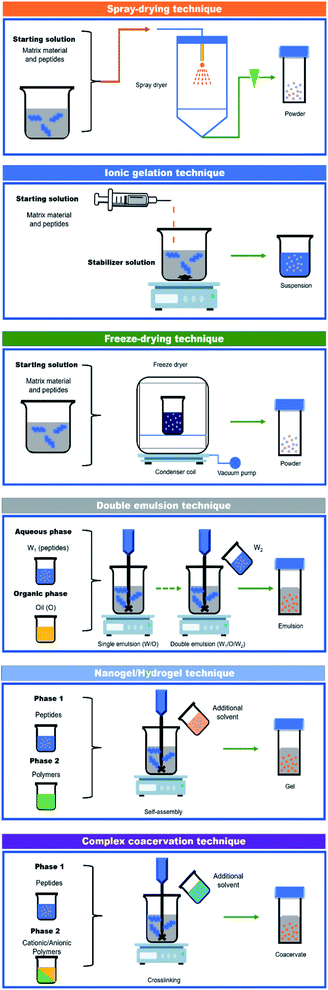
According to Table 1, the most common methods used to encapsulate bioactive peptides include liposomes, spray drying, double emulsion, freeze-drying, emulsification–gelation, and ionic gelation (Fig. 2). Among these methods, liposomes are a suitable option for the encapsulation of peptides due to their amphipathic characteristics, biocompatibility, non-immunogenic, and non-toxicity.28 Liposomes are made by phospholipid bilayers that aggregate when placed in an aqueous environment, forming sphere-shaped vesicles that include aqueous compartments where the peptides are enclosed.29 On the other hand, the advantages of using spray-drying methods include cost-effectiveness, short process time, and decrease in product weight/volume.7,30 Nevertheless, spray-drying can induce some peptide denaturation and aggregation by the increase of temperature during atomization;31 this is why carrier agents such as maltodextrin (MD) should be used for the protection of heat-sensitive compounds.32 In the case of freeze-drying, it is based on dehydration by sublimating a frozen sample, which is a suitable method for heat-sensitive compounds such as peptides.33,34 Additionally, ionic gelation is based on polyelectrolytes' ability to crosslink in the presence of counter ions, which is a low-cost method and does not require specialized equipment, high temperature, or the use of organic solvents.35 Lastly, the emulsification–gelation method involves a process that first stabilizes an emulsion of oil droplets in an aqueous protein solution (O/W) or an aqueous protein droplets in the oil phase (W/O), followed by the gelation of the protein, which is induced by heating, chemical or enzymatic crosslinking.36| Source of bioactive peptides | Encapsulation method/matrix material | Encapsulation efficiency | Biofunctionality | Principal findings | References |
|---|---|---|---|---|---|
| a ACE: angiotensin-converting enzyme; DPP-IV: dipeptidyl peptidase IV; DPPC: 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; PBS: phosphate buffered saline, DMEM: Dulbecco minimum essential medium, NR: not reported.b Reported in a previous study.59c Calculated as the average of different methods used in the cited study. | |||||
| Flaxseed (Linum usitatissimum) | Spray drying/maltodextrin | NR | Antioxidant | Spherical particles in various sizes (∼3–12 μm) | 41 |
| Antioxidant activity was >92% after encapsulation | |||||
| Sheep whey | Nanoliposomes/phosphatidylcholine | 48% | Antioxidant and ACE inhibitory activity | Particles with diameter 166 nm and zeta potential −17 mV | 29 |
| Antioxidant activity was 87% after 30 days of encapsulation | |||||
| Lima bean (Phaseolus lunatus) | Spray drying/maltodextrin–gum Arabic | 82% | ACE, α-glucosidase, α-amylase, and DPP-IV inhibition | All bio-functionalities were maintained after in vitro gastrointestinal digestion in microencapsulated peptides | 39 |
| Synthetized ACE inhibitory peptides (LKP and IPP) | Ionotropic gelation/chitosan | LKP: 65.1% | ACE inhibitory activity | Peptides retained their bioactivity (ca. 80%) after the encapsulation | 55 |
| IPP: 44.8% | |||||
| Rainbow trout (Oncorhynchus mykiss) skin gelatine | Nanoliposomes/phosphatidylcholine | 84.5% | Antioxidant | Peptides retained their bioactivity (100%) after the encapsulation | 43 |
| Particles with diameter 134–621.1 nm | |||||
| Jujube (Ziziphus jujube) seed | Ionic gelation/sodium alginate | 74.21% | Antioxidant | Peptides retained their bioactivity (ca. 74%) after the encapsulation | 46 |
| Encapsulated peptides showed storage stability of antioxidant activity (ca. 5.1% decreasec) | |||||
| Collagenous by-products of smoothhounds (Mustelus mustelus) | Emulsification–internal gelation method/alginate-whey protein isolate | NR | ACE inhibitory activity | Encapsulation improved ACE-I inhibitory activity (hydrolysate digested IC50 = 0.62 mg mL; hydrolysate-loaded capsules digested IC50 = 0.24 mg mL−1) | 45 |
| Atlantic salmon (Salmo salar) | Nanoliposomes/chitosan and milk fat globule membrane-derived phospholipids | 71.3% | Antioxidantb | Particles with diameter ∼200 nm | 44 |
| Particles with no significant size change after 4 weeks of storage | |||||
| Jumbo squid (Dosidicus gigas) collagen | Nanoliposomes/phosphatidylcholine | 53% | ACE inhibitory activity | Peptides retained their bioactivity (100%) after the encapsulation | 48 |
| Particles with an average diameter of 70.3 nm with a zeta potential of −59 mV | |||||
| Stable in the pH range 3–7 | |||||
| Goby fish (Zosterissessor ophiocephalus) | Ionic gelation/chitosan and tripolyphosphate | 58% | Antioxidant | Spherical particles (∼3.78 μm) with a zeta potential −50 mV | 49 |
| Peptides improved (ca. 57–92%) the antioxidant activity of chitosan microparticles | |||||
| Rainbow trout (Oncorhynchus mykiss) skin gelatine | Nanoliposomes/DPPC-cholesterol and chitosan | 80.2% | Antioxidant | Peptides retained their bioactivity (ca. 100%) after the encapsulation | 51 |
| Casein | Freeze-drying/maltodextrin–gum arabic | 87% | Antioxidant | Peptides retained their bioactivity (93%) after the encapsulation | 50 |
| Flaxseed | Nanoliposomes/phosphatidylcholine-cholesterol and chitosan | 90.73% | Antioxidant | Particles with an average diameter of 132.56 nm with a zeta potential of 29.67 mV | 27 |
| Peptides retained their bioactivity (ca. 92%) after the nanoencapsulation | |||||
| Soybean and lupin protein | Nanogels/PBS 1× (Ca2+/Mg2+ free) or DMEM | NR | DPP-IV inhibition | Particles increased bioavailability of encapsulated peptides and providing higher resistance against proteases | 56 |
| Hempseed (Cannabis sativa cv. Futura) | Hydrogels/nanofibers RADA16 and isotonic saline solution | NR | DPP-IV inhibition | Peptides loaded into hydrogels increased their bioactivity and stability | 57 |
| Tilapia viscera | Liposomes/soy-rapeseed lecithin-trehalose | 80.7–81.3% | Antioxidant | Peptides loading into liposomal increased storage stability and antioxidant capacity | 58 |
| Rice (Oryza sativa) husk | Nanoparticles/chitosan | 89% | Anticancer | Encapsulated peptides showed anticancer activities tested in A546 (human lung cancer) and MCF7 (human breast cancer) cell lines | 54 |
| Green algae (Chlorella pyrenoidosa) | Complex coacervation and ionotropic gelation/chitosan-sodium alginate | 30.1–74.5% | Anticancer | Encapsulated peptides showed anticancer activity against human liver cancer HepG2 and showed stability against gastric enzymatic degradation | 53 |
In the matter of materials used for encapsulation of bioactive peptides, the most common encapsulation materials used in the above mentioned methods include polysaccharides (e.g., MD, chitosan, and gum arabic), lipids (e.g., phosphatidylcholine, phospholipids), and to a lesser extent, proteins (e.g., whey protein isolate). The polysaccharides are generally recognized as safe (GRAS) established by the Food and Drug Administration (FDA)37,38 and have low cost, low viscosity at high solids ratios, and are biodegradable.39 Therefore, these characteristics make them suitable materials for encapsulation methods. For instance, the active use of chitosan is the availability of functional groups in the chemical structure of chitosan that enables it to react easily with other active compounds.40 On the other hand, it has been observed that the structure of bioactive peptides showed interaction with some functional groups of polysaccharide materials. For example, Fourier transform infrared (FTIR) spectroscopy of flaxseed-derived bioactive peptides encapsulated with MD after spray drying showed hydrogen bonds between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of peptides with O–H groups of MD evidenced by the shifter of amide I region of peptides from 1659 cm−1 to 1661 cm−1.41
O groups of peptides with O–H groups of MD evidenced by the shifter of amide I region of peptides from 1659 cm−1 to 1661 cm−1.41
Conversely, lipids are suitable for delivering bioactive compounds because of their similarity with cell membranes; however, some issues such as their poor stability in thermally processed foods and their susceptibility to oxidation limit their use.42 In this sense, phosphatidylcholine contains a positively charged choline group and negatively charged phosphate and carbonyl groups, which facilitate the interaction with proteins and bioactive peptides. For instance, Hosseini et al.43 observed a peak at 1754 cm−1 shift to a lower frequency for a peptide encapsulated into nanoliposome, indicating hydrogen bond interaction between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of phosphatidylcholine and the peptide. Also, there was a peak shift at 1670 cm−1 (–C
O group of phosphatidylcholine and the peptide. Also, there was a peak shift at 1670 cm−1 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) of the lipid to 1666 cm−1 in the peptide-loaded nanoliposome, which suggests a hydrophobic interaction between them. Similarly, phospholipids have been used to encapsulate bioactive peptides. For example, Li et al.44 used milk fat globule membrane phospholipids to encapsulate salmon-derived bioactive peptides that showed major stability in a range of pH conditions compared with soy lecithin and egg yolk lipids. Despite that, phospholipids are easily oxidizable during processing and storage, so the addition of chitosan as co-encapsulating material was used to avoid this issue.
C–) of the lipid to 1666 cm−1 in the peptide-loaded nanoliposome, which suggests a hydrophobic interaction between them. Similarly, phospholipids have been used to encapsulate bioactive peptides. For example, Li et al.44 used milk fat globule membrane phospholipids to encapsulate salmon-derived bioactive peptides that showed major stability in a range of pH conditions compared with soy lecithin and egg yolk lipids. Despite that, phospholipids are easily oxidizable during processing and storage, so the addition of chitosan as co-encapsulating material was used to avoid this issue.
As mentioned above, some proteins have been used to a lesser extent as encapsulating materials for bioactive peptides. Despite the above, showed some advantages such as nutritional benefits and good emulsification and gelation properties. Besides because their chemical similarity between them could increase their interactions providing structural stability.42 For example, whey protein isolate was used in the encapsulation of riboflavin because it increased hydrophobic interactions between them. In relation with bioactive peptides, microcapsules of alginate-whey protein containing collagen-derived peptides have been used, demonstrating that the bioactive peptides remained intact in gastric fluid, but were degraded in intestinal fluid.45
The type of encapsulating method and/or material has advantages and disadvantages that need to be considered based on the desired application for the bioactive peptide. Overall, the selection of the matrix material for encapsulation of bioactive peptides is based on properties such as biodegradability, non-toxicity, low cost, and preferably it should be edible.
3.2 Bioactivities of encapsulated bioactive peptides
One of the most important objectives of the encapsulation of peptides is maintaining their bioactivity after the encapsulation process and during storage.41 The most common bioactivities reported in studies revised in the present review were antioxidant, angiotensin-converting enzyme (ACE), α-glucosidase, α-amylase, and dipeptidyl peptidase-IV (DPP-IV) inhibition activities, and to a lesser extent, anticancer and immunomodulatory activities (Table 1).The most common bioactivity reported in studies of encapsulated peptides was antioxidant activity (Table 1). The antioxidant activity (e.g., reducing power, total antioxidant, and iron-chelating activities) of bioactive peptide powder derived from flaxseed hydrolysate was retained (>92%) after their encapsulation with MD through spray-drying.41 The authors also observed that increasing the carrier ratio (MD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) peptide 1
peptide 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) improved the retention of antioxidant activity of the spray-dried, encapsulated peptide. Similarly, whey peptides encapsulated in nanoliposomes of phosphatidylcholine retained ca. 65% and 68% of antioxidant and ACE-inhibitory activities, respectively, after the encapsulation process.29 Authors also reported 87% antioxidant activity after 30 days' storage. Kanbargi et al.46 reported that Jujube (Ziziphus jujube) seed peptides retained their bioactivity (ca. 74%) after the nanoencapsulation with alginate. The authors found through FTIR spectroscopy analysis that the peptides were cross-linked with sodium alginate. Sarabandi et al.47 reported that casein-derived peptides encapsulated with MD showed antioxidant activity retention from 77.5 to 91.6% and 90.4 to 98.5% for 1,1-diphenyl-2-picryl-hydrazyl (DPPH) and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) methods, respectively.
1 w/w) improved the retention of antioxidant activity of the spray-dried, encapsulated peptide. Similarly, whey peptides encapsulated in nanoliposomes of phosphatidylcholine retained ca. 65% and 68% of antioxidant and ACE-inhibitory activities, respectively, after the encapsulation process.29 Authors also reported 87% antioxidant activity after 30 days' storage. Kanbargi et al.46 reported that Jujube (Ziziphus jujube) seed peptides retained their bioactivity (ca. 74%) after the nanoencapsulation with alginate. The authors found through FTIR spectroscopy analysis that the peptides were cross-linked with sodium alginate. Sarabandi et al.47 reported that casein-derived peptides encapsulated with MD showed antioxidant activity retention from 77.5 to 91.6% and 90.4 to 98.5% for 1,1-diphenyl-2-picryl-hydrazyl (DPPH) and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) methods, respectively.
On the other hand, it was reported that the ACE inhibition (IC50 = 0.62 mg mL−1), after in vitro gastrointestinal digestion, of bioactive peptides derived from smoothhound (Mustelus mustelus) was improved after they were encapsulated with alginate-whey protein isolate (IC50 = 0.24 mg mL−1).45 This improvement of the bioactivity could be explained by the fact that some additional bioactive peptides were released from whey protein isolate during gastrointestinal digestion. Mosquera et al.48 reported that bioactive peptides from collagen of jumbo squid (Dosidicus gigas) encapsulated in phosphatidylcholine nanoliposomes, retained 100% of their bioactivity after the nanoencapsulation. Nasri et al.49 encapsulated goby fish peptides with antioxidant activity into chitosan microparticles. The authors found that those peptides significantly (P > 0.05) improved (ca. 57–92%) the antioxidant capacity of the microparticles. Rao et al.50 produced microcapsules loaded with peptides derived from casein, which retained 93% of their antioxidant activity after the encapsulation. Similarly, Sarabandi and Jafari27 found that peptides encapsulated into nanoliposomes based on phosphatidylcholine-cholesterol and chitosan retained their bioactivity (ca. 92%) after encapsulation. In other studies, Ramezanzade et al.51 found that nanoliposomes loaded with rainbow trout (Oncorhynchus mykiss) skin-derived antioxidant peptides retained bioactivity (ca. 100%) after the encapsulation. Similarly, Ma et al.52 reported that whey-derived peptides retained their in vitro immunomodulatory activity (ca. 100%) after encapsulation by spray- or freeze-drying with whey protein concentrate and sodium alginate. In a similar way, Wang and Zhang53 reported that peptides encapsulated into nanoparticles by complex coacervation and ionotropic gelation showed inhibitory activities against human liver cancer (HepG2) cell line. Besides, nanoparticles were against gastric enzymatic degradation after encapsulation and the slowly controlled release in the intestine could be potentially achieved. Meanwhile, Ilhan-Ayisigi et al.54 found that chitosan nanoparticles loaded with rice husk peptides possess anticancer activity towards human lung (A546) and breast (MCF7) cancer cell lines. Also, the authors reported that the anticancer mechanism of peptides was related with the stimulation of apoptosis in cell lines.
To the best of our knowledge, few studies have described the in vivo bioactivities and beneficial effects of encapsulated bioactive peptides. For instance, Auwal et al.60 found that bioactive peptides derived from stone fish (Actinopyga lecanora) encapsulated into chitosan nanoparticles showed significantly higher blood pressure lowering effect comparted with free peptides on spontaneously hypertensive rat's model. In contrast, Tkaczewska et al.61 reported that microencapsulation process of skin-derived peptides into furcellaran-coated microcapsules reduced the in vivo antioxidant effect of peptides in healthy rat's model. These results were consistent with the in vitro antioxidant results, being the peptides microencapsulated those with higher antioxidant activity compared with free-peptides. Other possible explanation of this results is that peptides were not released from the furcellaran-coated microcapsules during gastrointestinal digestion process of tested animals.
Lastly, the bioactivity stability or improvement of encapsulated peptides can be explained by several reasons. For example, in the spray-drying method, encapsulation takes place at high speed and very short time (few seconds), and because of the cooling effect by evaporation, the thermal stress can be low, which allows the retention and stability of bioactivity during the process. In contrast, it has been reported that some new peptides with bioactivity can be produced from matrix carriers during gastrointestinal digestion (e.g., whey protein isolate).44
3.3 Stability of encapsulated bioactive peptides
The stability of an encapsulated compound is determined by various factors related to their physicochemical and microbiological stability including moisture content, water activity, hygroscopicity, size, polydispersity index, and zeta potential.29,41 Generally, a powder with low moisture content (include a % value) and low water activity (<0.6) is considered stable because there is limited availability of free water for biochemical reactions and microbial activity.62 In contrast, the stability of the colloidal system is determined by the polydispersity index and zeta potential, being the former one an indicator of the broadness of the particle size distribution taking values from 0 to 1. In contrast, zeta potential shows the degree of repulsion between the charged nanoparticles taking values from +100 to −100 mV. Thus, low values of polydispersity index indicate homogeneity in particle size distribution,63 while values greater than +30 mV or less than −30 mV prevent aggregation of the particles.64 Finally, the stability of encapsulated bioactive peptides is reflected in their residual bioactivity, after their storage over time.41For instance, Akbarbaglu et al.41 reported that hygroscopicity of a bioactive peptide powder derived from flaxseed hydrolysate (39.2%) decreased significantly (17.4%) after encapsulation with MD. Authors also found that those encapsulated peptides showed a moisture content and water activity of 2.52% and 0.28, respectively. Similarly, Sarabandi et al.47 and Sarabandi and Jafari27 found that hygroscopicity of casein-peptides powder decreased (P < 0.05) after their encapsulation with MD. Encapsulated peptides showed an average moisture content and water activity of 3.1% and 0.26, respectively. Ma et al.52 reported that whey-derived peptides encapsulated with a mixture of whey protein concentrate and sodium alginate decreased significantly (P < 0.05) their hygroscopicity after spray-drying (from 29.7 to 20.9%) or freeze-drying (from 31.1 to 24.4%). The decrease in hygroscopicity of bioactive peptides powders could be attributed to the effect on wall material by increasing the total glass transition temperature and film-forming with low hygroscopicity around the peptides.30,34 Moreover, in the studies mentioned above, the reduction of hygroscopicity after the encapsulation process and their low moisture content and water activity of bioactive peptides powders suggest their potential microbiological stability.62
On the other hand, Li et al.44 produced nano-liposomes of chitosan and milk fat globule membrane-derived phospholipids with a particle size of ∼200 nm, and they showed no significant size change after 4 weeks of storage, which indicated those nano-liposomes had physical stability over time. Samples also had a polydispersity index and zeta potential of 0.33–0.45 and 0.11–8.65 mV, respectively. Corrêa et al.29 studied whey peptides encapsulated into phosphatidylcholine nanoliposomes and found a polydispersity index of 0.45, zeta potential of −16.7 mV, and stability that lasted over 30 days storage. Similarly, Sarabandi and Jafari27 observed that peptides encapsulated into nanoliposomes based on phosphatidylcholine-cholesterol and chitosan, showed an average size, polydispersity index, and zeta potential of 132.56 nm, 0.371, and 29.67 mV, respectively. Nasri et al.49 reported that microparticles of chitosan loaded with goby fish peptides had a zeta potential ranging from 35.4 to 50 mV and a diameter ranging from 3.78 to 4.81 μm. Finally, Ramezanzade et al.51 prepared nanoliposomes loaded skin gelatine peptides with a polydispersity index and zeta potential of 0.43 and 37.9 mV, respectively.
Likewise, Hosseini et al.43 prepared phosphatidylcholine nanoliposomes to encapsulate gelatine peptides from fish, reporting a polydispersity index and zeta potential of 0.33–0.45 and 0.11–8.65 mV, respectively. Similarly, Mosquera, et al.48 reported that liposomes containing ACE-inhibitory peptides showed an average of zeta potential and diameter of −59 mV and 70.3 nm, respectively. Next, after one week of storage (4 °C), those liposomes remained without significant differences (P > 0.05) on their zeta potential (−54.3 mV). Although such low zeta potential values of the above described nanoparticles, some authors mentioned that the presence of the hydrophobic compound in the liposomal system and its interaction with the hydrophobic core may have an effect on the charge of the nanoliposome partially masked it their real value.43,65 It is important to point out that studies that used polysaccharides as matrix materials do not report the zeta potential.
Regarding the residual activity of encapsulated peptides after storage, Kanbargi et al.46 reported that Jujube seed peptides encapsulated into sodium alginate particles showed storage stability of antioxidant activity, which decreased about 5.1% after 30 days. In a similar study, Corrêa et al.29 found that bioactive whey peptides encapsulated into liposomes retained 87% of their initial antioxidant activity (from 54% to 47% of ABTS scavenging activity) after 30 days. To the best of our knowledge, only a few literature reports mention the residual activity of encapsulated peptides after storage time. Despite this, it is encouraging that bioactivity was retained; however, it is possible to think that the preservation of the bioactivity is related to the matrix material used to encapsulate and the type of bioactive peptide being encapsulated.
4. Effect of encapsulation in solubility and sensory properties of bioactive peptides
It has been reported that the encapsulation process has a significant effect on the solubility, and sensory properties of encapsulated bioactive peptides. These two parameters are very important for the commercial application of bioactive peptides. Usually, bioactive peptides are encapsulated into a colloidal system to be added into a portion of food, functional food, or a nutraceutical preparation.The solubility of bioactive peptides is a key factor for their application in foods, particularly in beverages. Akbarbaglu et al.41 evaluated the effect of different ratios of the carrier (MD) to flaxseed-derived peptides (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) on the solubility of spray-dried encapsulated peptide powders. Higher carrier ratios resulted in increased solubility, with the 3
1 w/w) on the solubility of spray-dried encapsulated peptide powders. Higher carrier ratios resulted in increased solubility, with the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio being the one with the highest solubility (95.8%). On the other hand, Sarabandi et al.47 reported no differences in solubility of spray-dried encapsulated powders of casein-derived peptides, which showed solubilities ranging from 94.8 to 97.87%. The solubility of encapsulated bioactive peptides would depend on their particle size; commonly, if the nanoparticle has a high surface area it can increase their solubility.42 The common methods used to decrease particle size include jet- and ball-milling, high pressure homogenization, dialysis, and vacuum evaporation, among others.66
1 ratio being the one with the highest solubility (95.8%). On the other hand, Sarabandi et al.47 reported no differences in solubility of spray-dried encapsulated powders of casein-derived peptides, which showed solubilities ranging from 94.8 to 97.87%. The solubility of encapsulated bioactive peptides would depend on their particle size; commonly, if the nanoparticle has a high surface area it can increase their solubility.42 The common methods used to decrease particle size include jet- and ball-milling, high pressure homogenization, dialysis, and vacuum evaporation, among others.66
The negative sensory aspects of bioactive peptides are attributed to their bitterness resulting from their content of hydrophobic amino acids,24 and their salty off-flavours from the addition of acids or alkalis applied to neutralize protein hydrolysis products.26 Rao et al.50 reported that the encapsulation of casein peptides significantly (P > 0.05) decreased the bitterness of the free peptides. The authors found that a high concentration of the encapsulation material resulted in a lower perception of bitterness. Particularly, panellists perceived those encapsulated peptides at the different core to coat ratio (peptides: MD/gum arabic), had bitterness scores of 55 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), 37.5 (1
5), 37.5 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), and 15.5 (1
10), and 15.5 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) compared with free peptides (100). In a similar study, Sarabandi et al.47 found that microencapsulation process using spray-drying decreased the bitter taste of casein peptides added to a pastille formulation. The authors used a 7-point scale (1 = very bitter, 7 = very good taste) with eight trained evaluators, who perceived less bitter taste (3.2) and better total acceptance (3.4) in encapsulated peptides compared to free peptides (2.3 and 2.7, respectively). Likewise, Ma et al.52 evaluated the bitterness of encapsulated whey peptides using a taste dilution analysis with 15 trained panellists. Panellists detected a high score of bitterness in free peptides (32), while encapsulated peptides obtained by spray-drying and freeze-drying showed lower bitterness scores (4 and 8, respectively). Similarly, Subtil et al.67 found that casein peptides encapsulated with gum arabic by spray drying decreased their bitter taste. The authors compared three encapsulated peptides proportions of peptides
20) compared with free peptides (100). In a similar study, Sarabandi et al.47 found that microencapsulation process using spray-drying decreased the bitter taste of casein peptides added to a pastille formulation. The authors used a 7-point scale (1 = very bitter, 7 = very good taste) with eight trained evaluators, who perceived less bitter taste (3.2) and better total acceptance (3.4) in encapsulated peptides compared to free peptides (2.3 and 2.7, respectively). Likewise, Ma et al.52 evaluated the bitterness of encapsulated whey peptides using a taste dilution analysis with 15 trained panellists. Panellists detected a high score of bitterness in free peptides (32), while encapsulated peptides obtained by spray-drying and freeze-drying showed lower bitterness scores (4 and 8, respectively). Similarly, Subtil et al.67 found that casein peptides encapsulated with gum arabic by spray drying decreased their bitter taste. The authors compared three encapsulated peptides proportions of peptides![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gum arabic (10
gum arabic (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 20
90, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, and 30
80, and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70), and 17 trained panellists perceived that the sample of encapsulated peptides with the lowest peptide concentration (10%) obtained a rank of bitterness of 17 (less bitter), while the other proportions had a rank of >38 (more bitter). Similarly, Yang et al.68 reported that whey peptides had less bitterness after their encapsulation with MD or the MD/β-cyclodextrin mixture using spray-drying. According to their sensory evaluation, 15 trained panellists perceived a high score of bitterness in free peptides (32), while the encapsulated peptides showed a lower bitterness score (16 and 8, respectively).
70), and 17 trained panellists perceived that the sample of encapsulated peptides with the lowest peptide concentration (10%) obtained a rank of bitterness of 17 (less bitter), while the other proportions had a rank of >38 (more bitter). Similarly, Yang et al.68 reported that whey peptides had less bitterness after their encapsulation with MD or the MD/β-cyclodextrin mixture using spray-drying. According to their sensory evaluation, 15 trained panellists perceived a high score of bitterness in free peptides (32), while the encapsulated peptides showed a lower bitterness score (16 and 8, respectively).
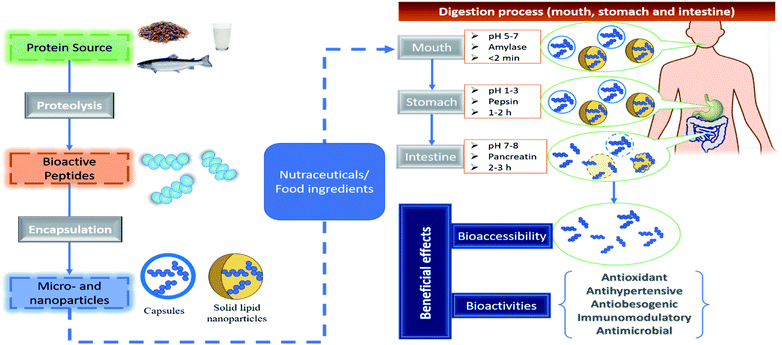
The evidence described above suggests that encapsulation technology can be used to overcome the challenges of bioactive peptides that limited their nutraceutical and commercial application by improving their sensory properties, increase solubility and decrease their hygroscopicity. On the other hand, more research is needed to elucidate the effect of encapsulation on bioavailability and food matrix interaction of bioactive peptides. In this context, the former is a crucial parameter so that bioactive peptides can move across the human intestinal barrier for it to be absorbed into the blood serum and reach their target organs at an effective amount (Fig. 3). There are few reports on the bioaccessibility of encapsulated peptides. For example, Cian et al.39 evaluated the effect of simulated gastrointestinal digestion on the ACE, α-glucosidase, α-amylase, and DPP-IV inhibition properties of Phaseolus lunatus peptides microencapsulated by spray drying using MD and gum arabic. These microencapsulated peptides maintained or improved their bioactivity compared with non-encapsulated peptides after in vitro gastrointestinal digestion, which is an indirect indicator of their bioaccessibility.
5. Challenges and opportunities on the incorporation of encapsulated bioactive peptides in food and development of nutraceuticals forms
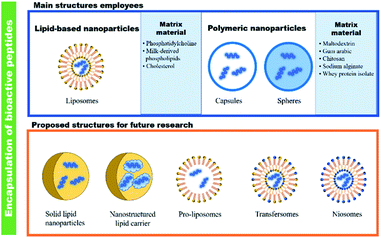
Bioactive peptides have been isolated and characterized providing many possibilities for the utilization of agricultural subproducts and other natural or microbial sources.69 However, in the development of food and nutraceutical products, bioactive peptides showed some limitations; therefore, studies on food matrix interactions, low water-solubility, hygroscopicity, and flavour-masking are necessary. In these aspect, micro ad nano-encapsulation have been demonstrated to increase biocompatibility, and bioactivity upon ingestion of bioactive peptides. Moreover, encapsulation has also improved the sensory properties, increased solubility, and assisted in the control of hygroscopicity. In addition, studies that address the interaction between biopolymers used as a wall component in micro- and nanoparticles are necessary. Some new strategies in the formation of nanoparticles such as Pickering emulsion formation, and techniques as spray dry and electrospinning or electrospray should be explored in order to improve the encapsulation efficiency, bioavailability, and controlled release of the bioactive peptides within in vitro and in vivo models.An important consideration to highlight is the need to explore novel methods to encapsulate bioactive peptides (Fig. 4). These methods can be useful to solve some issues of the current methods used for encapsulating bioactive peptides such as instability, poor biocompatibility with cell membranes, and improve the encapsulation efficiency of peptides. For example, it has been reported that peptide signalling molecules (e.g., hormones or growth factors) or synthetic peptides have been encapsulated successfully into solid-lipid nanoparticles;70,71 however, to the best of our knowledge, this method has not been used before on bioactive peptides. Similarly, pro-liposomes (dry, free-flowing particles that upon hydration form liposomal dispersion), transfersomes (ultra-deformable vesicles composed of phospholipids and edge activators), and niosomes (vesicles of non-ionic surfactant with phospholipids) could be suitable alternatives to encapsulate bioactive peptides because they are more stable than liposomes, which are prone to aggregation, sedimentation, and oxidation.72,73 Despite that the current methods used for the encapsulation of bioactive peptides have shown stability and good encapsulation efficiency, the proposed novel methods should be tested to increase these parameters (i.e., stability and good encapsulation efficiency). An important field of study in the encapsulation of bioactive peptides is determining the potential participation of matrix material and their possible interactions with bioactive peptides on their bioactive and food preservation properties. Besides, it is important to test the effect of encapsulated bioactive peptides in real food systems or appropriate food models. Alternatively, if there are more available methods for encapsulating peptides, more possibilities of applications can be development, not only for nutraceutical and food applications, but also pharmaceutical implementation.
An important research opportunity of encapsulated bioactive peptides is their use as natural food preservatives and include them into packaging materials with the aim of extend the shelf-life of foods. In both cases, bioactive peptides have demonstrated prevent lipid oxidation and inhibit the growth of pathogenic microorganisms due to its antioxidant and antimicrobial activities, respectively. For example, bioactive peptides derived from camel milk hydrolysates added to minced fish inhibited lipid peroxidation.74 Similarly, a hydrolysate derived from porcine blood was added to pork meat emulsion, which effectively inhibit the lipid oxidation and hinder microbial proliferation.75 Conversely, coatings holding bioactive peptides derived from gelatine hydrolysate significantly extended the shelf-life of whole shrimp by inhibiting lipid and protein oxidation.76 In a related study, an edible coating was prepared with fish protein hydrolysate and applied to bonito (Sarda sarda) fish in order to extend its shelf life. The authors found that edible coating showed higher antimicrobial activity against mesophilic, psychrophilic, and coliform bacteria and yeast and mold.77
Finally, most of the bioactive studies revised in the present manuscript were performed using in vitro assays. Nevertheless, there is a still a lack of sufficient in vivo studies to determine the effectiveness of encapsulated bioactive peptides with potential beneficial effect.
6. Concluding remarks and future directions
Encapsulation technology has emerged as a promising strategy in the field of bioactive peptides, to protect the integrity of bioactivity. Research demonstrates that encapsulation of bioactive peptides can protect and increase their bioactivity, improve their sensory properties, increase their solubility, and decrease the incidence of hygroscopicity in peptide powders. There is limited scientific evidence about the bioavailability and food matrix interactions of encapsulated peptides. Thus, this research area represents an opportunity to establish controlled delivery and release of bioactive peptides. The simulated digestions systems are commonly used to explain diverse aspects related with the metabolism and absorption of bioactive peptides, but new methods such as two-and dimensional cell cultures and three-dimensional structure living cells (e.g., organ-on-a-chip system) are recommended as being more physiologically relevant. In contrast, due to the generation of large amount of agricultural and food industry by-products (co-products) in the world, these can be used as protein sources, obtaining environmentally friendly and cost-effective manufacturing processes for the production of bioactive peptides. In this sense, new materials with appropriate bioadhesion properties should be explored because they provide a great opportunity as guided delivery system. Finally, due to the lack of in vivo studies on encapsulated bioactive peptides, well-designed and suitable animal models will be necessary to evaluate their effectiveness and the feasibility for a pilot plant process.Author contributions
J. E. Aguilar-Toalá: investigation, writing – original draft. D. Quintanar-Guerrero: writing – review & editing, visualization, supervision. A. M. Liceaga: writing – review & editing, visualization. M. L. Zambrano-Zaragoza: conceptualization, visualization, supervision, resources, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Aguilar-Toalá J. E. would like to thank DGAPA-UNAM for their scientific and financial support through the program of “Becas Posdoctorales”. Authors thanks financial support from to the projects PAPIIT IN222520 of DGAPA-UNAM, Investigation Internal Project-FESC PIAPI2060 and COMECYT FICDTEM-2021-074.References
- D. Bhandari, S. Rafiq, Y. Gat, P. Gat, R. Waghmare and V. Kumar, Int. J. Pept. Res. Ther., 2020, 26, 139–150 CrossRef CAS.
- A. Görgüç, E. Gençdağ and F. M. Yılmaz, Food Res. Int., 2020, 136, 109504 CrossRef PubMed.
- B. P. Singh, R. E. Aluko, S. Hati and D. Solanki, Crit. Rev. Food Sci. Nutr., 2021, 1–14, DOI:10.1080/10408398.2021.1877109.
- X. Sun, C. Acquah, R. E. Aluko and C. C. Udenigwe, J. Funct. Foods, 2020, 64, 103680 CrossRef CAS.
- C. C. Udenigwe and V. Fogliano, J. Funct. Foods, 2017, 35, 9–12 CrossRef CAS.
- S. F. Hosseini, L. Ramezanzade and D. J. McClements, Trends Food Sci. Technol., 2021, 109, 322–339 CrossRef CAS.
- K. Sarabandi, P. Gharehbeglou and S. M. Jafari, Drying Technol., 2020, 38, 577–595 CrossRef CAS.
- A. Iwaniak, M. Hrynkiewicz, J. Bucholska, P. Minkiewicz and M. Darewicz, J. Food Biochem., 2019, 43, e12500 CrossRef PubMed.
- J. Tkaczewska, Trends Food Sci. Technol., 2020, 106, 298–311 CrossRef CAS.
- E. C. Y. Li-Chan, Curr. Opin. Food Sci., 2015, 1, 28–37 CrossRef.
- J. P. Kamdem and A. Tsopmo, J. Food Biochem., 2019, 43, e12489 CrossRef PubMed.
- X. Sun and C. C. Udenigwe, J. Agric. Food Chem., 2020, 68, 12972–12977 CrossRef CAS PubMed.
- F. Van Lancker, A. Adams and N. De Kimpe, Chem. Rev., 2011, 111, 7876–7903 CrossRef CAS PubMed.
- Y. Fu, Y. Zhang, O. P. Soladoye and R. E. Aluko, Crit. Rev. Food Sci. Nutr., 2020, 60, 3429–3442 CrossRef CAS PubMed.
- H. Fujita, K. Yokoyama, R. Yasumoto and M. Yoshikawa, Clin. Exp. Pharmacol. Physiol., 1995, 22, S304–S305 CrossRef CAS PubMed.
- F. Jahandideh, S. Chakrabarti, S. T. Davidge and J. Wu, J. Agric. Food Chem., 2016, 64, 113–119 CrossRef CAS PubMed.
- Y. Nakashima, K. Arihara, A. Sasaki, H. Mio, S. Ishikawa and M. Itoh, J. Food Sci., 2002, 67, 434–437 CrossRef CAS.
- J. T. Ryan, R. P. Ross, D. Bolton, G. F. Fitzgerald and C. Stanton, Nutrients, 2011, 3(9), 765–791 CrossRef CAS PubMed.
- S. Sandhu, Peptide synthesis: Storage abd solubility, https://altabioscience.com/peptide-synthesis-storage-and-solubility/, 2020 Search PubMed.
- AnaSpec Inc., Peptide solubility guidelines, https://www.anaspec.com/content/pdfs/PeptidesolubilityguidelinesFinal.pdf, 2021 Search PubMed.
- Biotechnology, Peptide solubility guidelines, https://www.sb-peptide.com/support/solubility/, 2021 Search PubMed.
- W. H. Freeman, in Molecular Cell Biology, ed. H. Lodish, A. Berk and S. Zipursky, New York, USA, 2000 Search PubMed.
- P. Kumar, J. N. Kizhakkedathu and S. K. Straus, Biomolecules, 2018, 8, 4 CrossRef PubMed.
- G. B. Celli, A. Ghanem and M. S.-L. Brooks, Food Bioprocess Technol., 2015, 8, 1825–1837 CrossRef CAS.
- T. Lafarga and M. Hayes, Food Rev. Int., 2017, 33, 217–246 CrossRef CAS.
- B. Hernández-Ledesma, M. del Mar Contreras and I. Recio, Adv. Colloid Interface Sci., 2011, 165, 23–35 CrossRef PubMed.
- K. Sarabandi and S. M. Jafari, Food Chem., 2020, 310, 125951 CrossRef CAS PubMed.
- A. Akbarzadeh, R. Rezaei-Sadabady, S. Davaran, S. W. Joo, N. Zarghami, Y. Hanifehpour, M. Samiei, M. Kouhi and K. Nejati-Koshki, Nanoscale Res. Lett., 2013, 8, 102 CrossRef PubMed.
- A. P. F. Corrêa, D. Bertolini, N. A. Lopes, F. F. Veras, G. Gregory and A. Brandelli, LWT, 2019, 101, 107–112 CrossRef.
- Z. Fang and B. Bhandari, in Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals, ed. N. Garti and D. J. McClements, Woodhead Publishing, 2012, pp. 73–109 Search PubMed.
- A. Ajmera and R. Scherließ, Int. J. Pharm., 2014, 463, 98–107 CrossRef CAS PubMed.
- S. M. Jafari, E. Assadpoor, Y. He and B. Bhandari, Drying Technol., 2008, 26, 816–835 CrossRef.
- A. Rezvankhah, Z. Emam-Djomeh and G. Askari, Drying Technol., 2020, 38, 235–258 CrossRef CAS.
- Z. Fang and B. Bhandari, Food Res. Int., 2012, 48, 478–483 CrossRef CAS.
- L. E. Kurozawa and M. D. Hubinger, Curr. Opin. Food Sci., 2017, 15, 50–55 CrossRef.
- L. Chen, in Designing Functional Foods, ed. D. J. McClements and E. A. Decker, Woodhead Publishing, 2009, pp. 572–600 Search PubMed.
- K. Pycia, L. Juszczak, D. Gałkowska, M. Witczak and G. Jaworska, Carbohydr. Polym., 2016, 146, 301–309 CrossRef CAS PubMed.
- L. Wright, P. Joyce, T. J. Barnes, R. Lundmark, C. A. S. Bergström, M. Hubert and C. A. Prestidge, J. Pharm. Sci., 2021, 110, 217–227 CrossRef CAS PubMed.
- R. E. Cian, A. Campos-Soldini, L. Chel-Guerrero, S. R. Drago and D. Betancur-Ancona, Int. J. Food Sci. Technol., 2019, 54, 2002–2009 CrossRef CAS.
- J. Yu, D. Wang, N. Geetha, K. M. Khawar, S. Jogaiah and M. Mujtaba, Carbohydr. Polym., 2021, 261, 117904 CrossRef CAS PubMed.
- Z. Akbarbaglu, S. Mahdi Jafari, K. Sarabandi, M. Mohammadi, M. Khakbaz Heshmati and A. Pezeshki, Colloids Surf., B, 2019, 178, 421–429 CrossRef CAS PubMed.
- A. Mohan, S. R. C. K. Rajendran, Q. S. He, L. Bazinet and C. C. Udenigwe, RSC Adv., 2015, 5, 79270–79278 RSC.
- S. F. Hosseini, L. Ramezanzade and M. Nikkhah, Int. J. Biol. Macromol., 2017, 105, 1455–1463 CrossRef CAS PubMed.
- Z. Li, A. T. Paulson and T. A. Gill, J. Funct. Foods, 2015, 19, 733–743 CrossRef CAS.
- K. Lajmi, J. Gómez-Estaca, M. Hammami and O. Martínez-Alvarez, Food Biosci., 2019, 28, 99–108 CrossRef CAS.
- K. D. Kanbargi, S. K. Sonawane and S. S. Arya, Int. J. Food Prop., 2017, 20, 3215–3224 CrossRef CAS.
- K. Sarabandi, A. Sadeghi Mahoonak, H. Hamishekar, M. Ghorbani and S. M. Jafari, J. Food Eng., 2018, 237, 86–95 CrossRef CAS.
- M. Mosquera, B. Giménez, P. Montero and M. C. Gómez-Guillén, J. Sci. Food Agric., 2016, 96, 769–776 CrossRef CAS PubMed.
- R. Nasri, M. Hamdi, S. Touir, S. Li, M. Karra-Chaâbouni and M. Nasri, Int. J. Biol. Macromol., 2021, 167, 1445–1451 CrossRef CAS PubMed.
- P. S. Rao, R. K. Bajaj, B. Mann, S. Arora and S. K. Tomar, J. Food Sci. Technol., 2016, 53, 3834–3843 CrossRef CAS PubMed.
- L. Ramezanzade, S. F. Hosseini and M. Nikkhah, Food Chem., 2017, 234, 220–229 CrossRef CAS PubMed.
- J.-J. Ma, X.-Y. Mao, Q. Wang, S. Yang, D. Zhang, S.-W. Chen and Y.-H. Li, LWT–Food Sci. Technol., 2014, 56, 296–302 CrossRef CAS.
- X. Wang and X. Zhang, Biotechnol. Prog., 2013, 29, 681–687 CrossRef CAS PubMed.
- E. Ilhan-Ayisigi, G. Budak, M. S. Celiktas, C. Sevimli-Gur and O. Yesil-Celiktas, J. Ind. Eng. Chem., 2021, 103, 381–391 CrossRef CAS.
- M. K. Danish, G. Vozza, H. J. Byrne, J. M. Frias and S. M. Ryan, Innovative Food Sci. Emerging Technol., 2017, 44, 139–148 CrossRef CAS.
- R. Pugliese, C. Bollati, F. Gelain, A. Arnoldi and C. Lammi, J. Agric. Food Chem., 2019, 67, 3615–3623 CrossRef CAS PubMed.
- C. Lammi, C. Bollati, F. Gelain, A. Arnoldi and R. Pugliese, Front. Chem., 2019, 6, 670 CrossRef PubMed.
- C. T. Sepúlveda, A. Alemán, J. E. Zapata, M. P. Montero and M. C. Gómez-Guillén, Innovative Food Sci. Emerging Technol., 2021, 71, 102708 CrossRef.
- A. T. Girgih, C. C. Udenigwe, F. M. Hasan, T. A. Gill and R. E. Aluko, Food Res. Int., 2013, 52, 315–322 CrossRef CAS.
- S. M. Auwal, M. Zarei, A. Abdul-Hamid and N. Saari, J. Evidence-Based Complementary Altern. Med., 2017, 2017, 4765463 Search PubMed.
- J. Tkaczewska, E. Jamróz, E. Piątkowska, B. Borczak, J. Kapusta-Duch and M. Morawska, Nutrients, 2019, 11, 2502 CrossRef CAS PubMed.
- S. Y. Quek, N. K. Chok and P. Swedlund, Chem. Eng. Process., 2007, 46, 386–392 CrossRef CAS.
- N. Raval, R. Maheshwari, D. Kalyane, S. R. Youngren-Ortiz, M. B. Chougule and R. K. Tekade, in Basic Fundamentals of Drug Delivery, ed. R. K. Tekade, Academic Press, 2019, pp. 369–400 Search PubMed.
- E. Joseph and G. Singhvi, Nanomaterials for Drug Delivery and Therapy, ed. A. M. Grumezescu, William Andrew Publishing, 2019, pp. 91–116 Search PubMed.
- L. Bouarab, B. Maherani, A. Kheirolomoom, M. Hasan, B. Aliakbarian, M. Linder and E. Arab-Tehrany, Colloids Surf., B, 2014, 115, 197–204 CrossRef CAS PubMed.
- P. Khadka, J. Ro, H. Kim, I. Kim, J. T. Kim, H. Kim, J. M. Cho, G. Yun and J. Lee, Asian J. Pharm. Sci., 2014, 9, 304–316 CrossRef.
- S. F. Subtil, G. A. Rocha-Selmi, M. Thomazini, M. A. Trindade, F. M. Netto and C. S. Favaro-Trindade, J. Food Sci. Technol., 2014, 51, 2014–2021 CrossRef CAS PubMed.
- S. Yang, X.-Y. Mao, F.-F. Li, D. Zhang, X.-J. Leng, F.-Z. Ren and G.-X. Teng, Eur. Food Res. Technol., 2012, 235, 91–97 CrossRef CAS.
- J. E. Aguilar-Toalá, F. G. Hall, U. Urbizo-Reyes and A. M. Liceaga, in Biologically Active Peptides, ed. F. Toldrá and J. Wu, Academic Press, 2021, pp. 427–453 Search PubMed.
- C. Dumont, A. Beloqui, C. Miolane, S. Bourgeois, V. Préat, H. Fessi and V. Jannin, Int. J. Pharm., 2020, 586, 119581 CrossRef CAS PubMed.
- Y. Xu, Y. Zheng, L. Wu, X. Zhu, Z. Zhang and Y. Huang, ACS Appl. Mater. Interfaces, 2018, 10, 9315–9324 CrossRef CAS PubMed.
- S. Sharma, K. Jyoti, R. Sinha, A. Katyal, U. K. Jain and J. Madan, Mater. Sci. Eng., C, 2016, 67, 378–385 CrossRef CAS PubMed.
- B. Zheng, L. Teng, G. Xing, Y. Bi, S. Yang, F. Hao, G. Yan, X. Wang, R. J. Lee, L. Teng and J. Xie, Eur. J. Pharm. Sci., 2015, 77, 254–264 CrossRef CAS PubMed.
- K. A. Al-Shamsi, P. Mudgil, H. M. Hassan and S. Maqsood, J. Dairy Sci., 2018, 101, 47–60 CrossRef CAS PubMed.
- A. K. Verma, M. K. Chatli, N. Mehta and P. Kumar, LWT, 2018, 88, 71–79 CrossRef CAS.
- A. Mirzapour-Kouhdasht and M. Moosavi-Nasab, Food Sci. Nutr., 2020, 8, 214–223 CrossRef CAS PubMed.
- G. Balcik Misir and S. Koral, J. Aquat. Food Prod. Technol., 2019, 28, 999–1012 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |