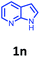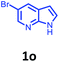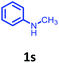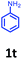Open Access Article
Open Access ArticleDMSO–allyl bromide: a mild and efficient reagent for atom economic one-pot N-allylation and bromination of 2°-aryl amines, 2-aryl aminoamides, indoles and 7-aza indoles†
Suresh Snoxma Smilea,
Motakatla Novannab,
Sathananthan Kannadasan b and
Ponnusamy Shanmugam
b and
Ponnusamy Shanmugam *a
*a
aOrganic and Bioorganic Chemistry Division, Council of Scientific and Industrial Research (CSIR)-Central Leather Research Institute (CLRI), Adyar, Chennai-600020, India. E-mail: shanmu196@rediffmail.com; Fax: +91-44-24911589; Tel: +91-44-24437130
bDepartment of Chemistry, School of Advanced Sciences, Vellore Institute of Technology, Vellore-632014, India
First published on 12th January 2022
Abstract
A mixture DMSO–allyl bromide has been developed as a reagent for an atom economic one-pot N-allylation and aryl bromination under basic conditions. Utilizing this reagent, N-allylation–bromination of a number of 2°-aryl amines, aryl aminoamides, indoles, and 7-aza indoles has been achieved. The scope of the substrates and limitations, the synthetic utility of the products, and a plausible reaction mechanism have been proposed.
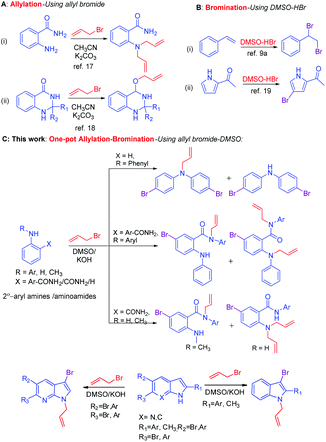
Bromination of aromatic compounds is an important transformation in organic synthesis.1 Aryl bromides are versatile synthons for functionalized aromatic compounds.2 Thus, a number of reagents have been developed for the bromination reaction include electrophilic substitution using molecular bromine,1,2 a dioxane–bromine complex for the direct bromination of aromatic amines,3 NBS,4 CuBr2,5 LiBr/O2,6 KBr/oxone,7 and NH4Br/H2O2.8 Nevertheless, the use of molecular bromine requires harsh conditions and is associated with hazardous waste and polybromination.1,2 In addition, the combination of the DMSO–aq. HBr system generates bromodimethyl sulfonium bromide (BDMS) species in situ,9 and acts as a versatile brominating reagent for olefins, alkynes, ketones and α-bromination of β-ketoesters.10,11 In addition to bromination, BDMS also catalyzes Mannich type reaction,12 dethiacetalization of thioacetals to carbonyls,13 oxidative coupling of thiols to disulfides,14 ring-opening of epoxides and aziridines,15 conversion of benzyl amines to benzoic acids via diazotization,16 oxidation of indoles to oxindoles17 and oxidative bromination and iodination of arenes and heteroarenes using DMSO–HX.18 Allyl bromide is a versatile reagent used for N- or O-allylation of aromatic amines19 and phenols.20 Recently, we have reported a microwave-assisted N-allylation of anthranilamide,21 and M. Guo et al.,22 reported the O-allylation of quinazolinone using allyl bromide/base (Scheme 1A). Primarily, the combination of DMSO–HBr system is well known for the bromination of olefins10a and electrophilic bromination of aromatic compounds23 (Scheme 1B). One-pot synthesis is a well-known method to conduct sequential multiple reactions in one reaction vessel24 and has advantages over the multistep synthesis in terms of atom economy,25 step economy,26 redox economy, thus reducing the number of steps.27 Minimization of waste production or loss of molecules without including in the products during a chemical reaction28 is necessary atom economy protocol,29 for example, the Diels–Alder reaction is an ideal chemical reaction in terms of atom economy and selectivities.30 Hence, utilization of one-pot atom economic reaction is of current interest of organic synthesis.
During a synthesis in one of our current projects, when the combination of DMSO–allyl bromide under basic condition has been used for allylation of a secondary aryl amide, an unforeseen atom economic one-pot N-allylation–bromination reaction has been observed. To the best of our knowledge, the combination of DMSO–allyl bromide reagent system has never been used as a dual source of one-pot allylation and bromination reactions. The serendipity observation emerges the DMSO–allyl bromides system as a reagent for atom economic one-pot N-allylation and bromination of 2°-aryl amines and other substrates include 2-aryl amino amides,31 indoles, and 7-aza indoles. The details are reported in this manuscript (Scheme 1C).
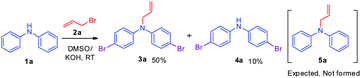
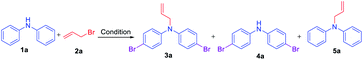
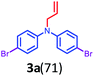
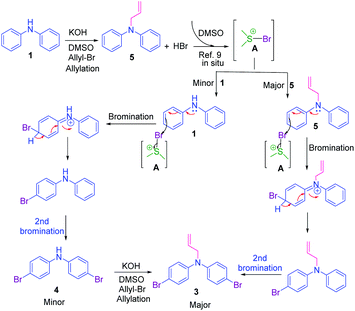
Initially, a reaction of diphenylamine 1a, allyl bromide 2a in DMSO (3 mL) and KOH at RT was investigated. The reaction provided the unexpected one-pot allylation–bromination product 3a along with dibrominated compound 4a in 50%, and 10% yields, respectively and found that the mixture of allyl bromide–DMSO/KOH emerged as a reagent system (Scheme 2, Table 1, entry 1). The N-allylated product 5a was not formed. The formation of both products 3a and 4a was confirmed from spectroscopic analysis (see ESI†).
| Entrya | 2a (equiv.) | Condition | Product(s) (% yield)b | ||
|---|---|---|---|---|---|
| 5a | 3a | 4a | |||
| a Substrate 1a was used from entries 1–17.b Isolated yield.c MW irradiation is not suitable.d Optimized condition.e 2.5 equiv. of CuBr2 was added as additive. | |||||
| 1 | 2a (2) | DMSO/KOH, RT, 72 h | — | 50 | 10 |
| 2 | 2a (2) | DMSO/KOH, MW, 5 min | —c | — | — |
| 3 | 2a (2) | DMSO/KOH, 70 °C, 6 h | — | 50 | 15 |
| 4 | 2a (2) | DMSO/KOH, 70 °C, 12 h | d | 71 | 16 |
| 5 | 2a (2) | DMSO/KOH, 70 °C, 1 h | 90 | — | — |
| 6 | 2a (2) | DMSO/KOH, 70 °C, 4 h | 40d | 35 | 8 |
| 7 | 2a (2) | DMSO/KOH, 70 °C, 24 h | — | 70 | 20 |
| 8 | 2a (2) | DMSO/NaH, 70 °C, 12 h | — | 60 | 15 |
| 9 | 2a (2) | DMSO/KOtBu, 70 °C, 12 h | — | 65 | 15 |
| 10 | 2a (4) | DMSO/KOH, 70 °C, 12 h | — | 56 | 10 |
| 11 | 2a (2) | DMSO/KOH, 70 °C, 12 he | — | 30 | 60 |
| 12 | CuBr2 | DMSO/KOH, 70 °C, 12 he | — | — | 10 |
| 13 | 2a (2) | DMSO/KOH, 70 °C, 24 he | — | 30 | 60 |
| 14 | 2a (2) | Dioxane/KOH, 70 °C, 12 h | 80 | — | — |
| 15 | 2a (2) | CH3CN/KOH, 70 °C, 12 h | 85 | — | — |
| 16 | 2a (2) | 2.5 equiv. DMSO, dioxane, KOH, 70 °C, 12 h | 52 | 14 | |
| 17 | 2a (2) | 2.5 equiv. DMSO, CH3CN, KOH, 70 °C, 12 h | 50 | 15 | |
To explore the optimum condition, reaction parameters such as temperature, time, base and equivalence of alkyl halide 2a and solvent were considered (Table 1). Besides, to speed up the reaction, under microwave (MW) irradiation, a mixture of 1 equiv. of diphenylamine 1a and 2.2 equiv. of allyl bromide 2a, and KOH in DMSO was MW irradiated for 5 min at 100 W, and no desired product was obtained (Table 1, entry 2). However, the reaction upon heating at 70 °C for 6 h afforded products 3a, and 4a in 50% and 15% yields, respectively (Table 1, entry 3). Extending the reaction time to 12 h and 24 h slightly improved the yields (Table 1, entries 4 & 7). To find out whether initially allylation or bromination takes place, repeating the above experiment for one hour afforded only allylated product 5a while another reaction stopped at 4 h afforded three products 5a, 3a, and 4a in 40%, 35%, and 8% yields respectively (Table 1, entries 5 & 6). This indicates initially allylation took place followed by bromination occurring via active species BDMS to yield the final product. Further, bases such as NaH and t-BuOK and mole equivalence of alkylating agent did not improve the yield (Table 1, entries 8 &10). On the other hand, the addition of the CuBr2 as an additive in the reaction completely reversed the yields of the products as compound 4a in 60% and compound 3a in 30% yield (Table 1, entry 11). The reaction of 1a with only CuBr2 in DMSO, KOH furnished traces of product 4a (Table 1, entry 12). Thus, CuBr2 alone is not sufficient and allyl bromide 2a is essential for the synthesis of dibromo compound 4a. However, CuBr2 improves the yield of brominated compound 4a over 3a. Evidently from the literature, this is due to CuBr2 also acting as a bromide source.32 The reactions in solvents such as dioxane, and CH3CN afforded only allylated product 5a in 80% and 85% yields, respectively (Table 1, entries 14 and 15). The absence of brominated products confirms the importance of DMSO solvent for the formation of BDMS species for bromination. To prove further that the formation of reactive species BDMS, experiments in dioxane and acetonitrile and several equivalents of DMSO were performed to yield the products minor 4a and major compound 3a without affecting the yield of the products (Table 1, entries 15 and 16). Thus, the condition shown in entry 4 of Table 1 was found to be optimum for allylated-brominated products and the condition shown in entry 9 was optimum for di/tetra brominated products. The additives were added to facilitate the bromination reaction.
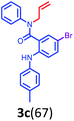
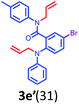
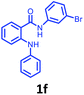
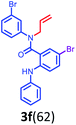
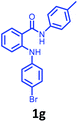
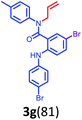
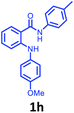
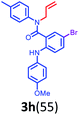
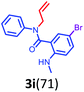
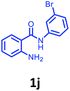
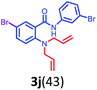
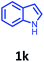
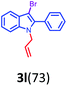
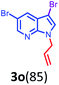
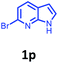
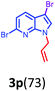
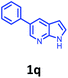
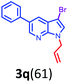
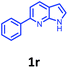
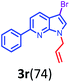
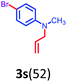
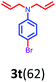
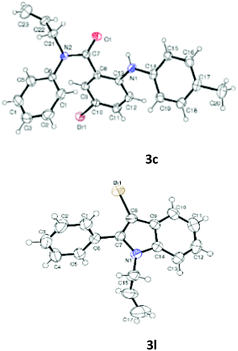
Having optimized condition in hand, the scope of the reaction DMSO–allyl bromide was explored to substituted aryl aminoamides 1b–j or indole 1k–m or 7-aza indole 1n–r to afford corresponding N-allylated-brominated aryl aminoamides 3b–j or indoles 3k–m or 7-aza indoles 3n–r in excellent yield (Table 2 and Fig. 1). It should be noted that in contrast to diphenylamine 1a, substrates 1b–r lead to products 3b–r. Whereas, N1-arylated/alkylated, N2-arylated amino amides 1b–r afforded the respective mono brominated/N1-mono allylated aryl aminoamides or indoles or 7-aza indoles 3b–r. However, substrates 1b–d afforded exclusively the mono brominated or N1, N2-diallylated aryl aminoamides 3b′–e′ in low yield. To widen the scope of the reaction, under optimized conditions, secondary amine with aryl alkyl substituents such as N-methyl aniline 1s and primary amine such as aniline 1t were also tested to afford products 3s and 3t in 52% and 62% yield, respectively. All the new compounds have been characterized by spectroscopic data and the representative compounds 3c and 3l were confirmed by XRD method (Fig. 1).34
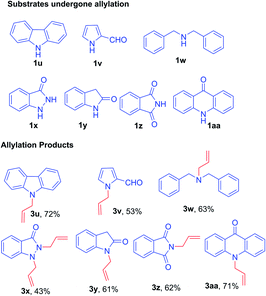
On the other hand, under optimized condition, substrates such as 9H-carbazole 1u, 2-formyl pyrrole 1v, dibenzyl amine 1w, pyrazolone 1x, 2-oxindole 1y, phthalimide 1z, and acridin-9(10H)-one 1aa afforded only the respective allylated products 3u–aa in excellent yield (Fig. 2). The subsequent bromination was not observed due to the substrates are not suitable for aromatic electrophilic reaction with the reactive BDMS species to provide allylated–bromination products.
Based on the optimization and control experiments shown in Table 1, entries 4–7, 16, and 17, a plausible mechanism for the formation of compounds 3a and 4a is shown in Scheme 3. The key intermediate bromodimethyl sulfoniumion (BDMS)9 plays a pivotal role for the brominated products via aromatic electrophilic substitution after initial allylation reaction of secondary amines. Thus, compound 3a is believed to form via initially the base abstracts the proton attached to nitrogen in compound 1a, and subsequent allylation leads to the formation of compound 5a. Then the first electrophilic attack of BDMS intermediate A on 5a leads to monobromo product followed by a second electrophilic attack on BDMS leads to N-allyl-bis(4-bromophenyl) amine 3a. Whereas the in situ formed BDMS intermediate A upon simultaneous competitive bromination with compound 1a affords compound 4a and subsequent base initiated allylation forms the major product 3a. However, upon completion of the reaction (TLC), a minor unreacted compound 4a is isolated along with major product 3a.
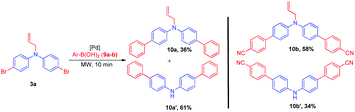
The synthetic utility of the compound 3a has been demonstrated by Suzuki coupling.25 Thus, a reaction between 3a and phenylboronic acid 9a, Pd(dppf)Cl2. DCM and K2CO3 as base in dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (3
MeOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent system was microwave (MW) irradiated (100 W) for 10 min to afford N-allyl-di([1,1′-biphenyl]-4-yl)amine 10a, along with the unexpected deallylated product di([1,1′-biphenyl]-4-yl)amine 10a′ in very good combined yield. Similarly, compounds 10b and 10b′ were synthesized from the reaction of 3a, 4-cyano phenylboronic acid 9b (Scheme 4).
1) solvent system was microwave (MW) irradiated (100 W) for 10 min to afford N-allyl-di([1,1′-biphenyl]-4-yl)amine 10a, along with the unexpected deallylated product di([1,1′-biphenyl]-4-yl)amine 10a′ in very good combined yield. Similarly, compounds 10b and 10b′ were synthesized from the reaction of 3a, 4-cyano phenylboronic acid 9b (Scheme 4).
To substantiate the formation of deallylation products (10a′–b′) formed during competitive Heck coupling,33 a model reaction was performed with allylated compound 5a under similar reaction conditions, deallylation product 1a was observed (Scheme 5).
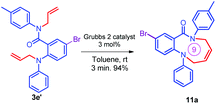
Further, the synthetic utility of the compound 3e′ has been tested through the RCM protocol.17 Thus, a solution of 3e′ in toluene, 3 mol% of Grubbs II catalyst afforded the (Z)-9-bromo-1-phenyl-6-(p-tolyl)-5,6-dihydro-1H-benzo[b][1,5]diazonin-7(2H)-one 11a in 94% yield (Scheme 6). It should be noted that the highly functionalized derivative 11a has been found in many natural products and could be synthesized via the short route reported herein.35
In conclusion, a mixture of DMSO–allyl bromide has emerged as a novel reagent for an atom economic one-pot method for the N-allylation/bromination of secondary aryl amines, aminoamides, indole, and 7-azaindole. A plausible mechanism has been proposed. The synthetic utility of the compound 3a has been demonstrated by synthesizing N-allyl-di([1,1′-biaryl]-4-yl) amine 10a–b along with the deallylated product 10a′–b′ via Suzuki coupling. A nine-membered diazonin-7(2H)-ones derivative 11a has also been constructed from compound 3e′ via RCM protocol using Grubbs II catalyst.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
P. S. thanks the Director, CSIR-CLRI for providing infrastructure facilities. The authors thank SAIF-IITM for single crystal XRD analysis and CSIR-IICB, Kolkata and VIT, Vellore for HRMS analysis. This manuscript bears CSIR-CLRI Communication No. 1601.Notes and references
- I. Saikia, A. J. Borah and P. Phukan, Chem. Rev., 2016, 11, 66837–67042 Search PubMed.
- J. Palou, Chem. Soc. Rev., 1994, 23, 357–361 RSC.
- G. M. Kosolapoff, J. Am. Chem. Soc., 1953, 75, 3596–3597 CrossRef CAS.
- B. Das, K. Venkateswarlu, A. Majhi, V. Siddaiah and K. R. Reddy, J. Mol. Catal. A Chem., 2007, 267, 30–33 CrossRef CAS.
- S. Bhatt and S. K. Nayak, Synth. Commun., 2007, 37, 1381–1388 CrossRef CAS.
- L. Menini, J. C. da Cruz Santos and E. V. Gusevskaya, Adv. Synth. Catal., 2008, 350, 2052–2058 CrossRef CAS.
- N. Narender, P. Srinivasu, M. R. Prasad, S. J. Kulkarni and K. V. Raghavan, Synth. Commun., 2002, 32, 2313–2318 CrossRef CAS.
- V. V. Krishna Mohan, N. Narender, P. Srinivasu, S. J. Kulkarni and K. V. Raghavan, Synth. Commun., 2004, 34, 2143–2152 CrossRef.
- G. Majetich, R. Hicks and S. Reister, J. Org. Chem., 1997, 62, 4321–4326 CrossRef CAS PubMed.
- (a) S. Song, X. Li, X. Sun, Y. Yuan and N. Jiao, Green Chem., 2015, 17, 3285–3289 RSC; (b) M. Karki and J. Magolan, J. Org. Chem., 2015, 80, 3701–3707 CrossRef CAS PubMed.
- T. Khan, M. A. Ali, P. Goswami and L. H. Choudhury, J. Org. Chem., 2006, 71, 8961–8963 CrossRef PubMed.
- T. Khan, T. Parvin and L. H. Choudhury, Eur. J. Org. Chem., 2008, 834–839 CrossRef.
- G. A. Olah, Y. D. Vankar, M. Arvanaghi and G. S. Prakash, Synthesis, 1979, 720–721 CrossRef CAS.
- G. A. Olah, M. Arvanaghi and Y. D. Vankar, Synthesis, 1979, 721–722 CrossRef CAS.
- B. Das, M. Krishnaiah and K. Venkateswarlu, Tetrahedron Lett., 2006, 47, 4457–4460 CrossRef CAS.
- R. Naik and M. A. Pasha, Synth. Commun., 2006, 36, 165–168 CrossRef CAS.
- K. Szabo-Pusztay and L. Szabo, Synthesis, 1979, 276–277 CrossRef CAS.
- S. Song, X. Sun, X. Li, Y. Yuan and N. Jiao, Org. Lett., 2015, 17, 2886–2889 CrossRef CAS PubMed.
- W. K. Walker, D. L. Anderson, R. W. Stokes, S. J. Smith and D. J. Michaelis, Org. Lett., 2015, 17, 752–755 CrossRef CAS PubMed.
- I. R. Baxendale, A.-L. Lee and S. V. Ley, Synlett, 2002, 3, 0516–0518 CrossRef.
- M. Novanna, S. Kannadasan and P. Shanmugam, ACS Omega, 2020, 5, 8515–8522 CrossRef CAS PubMed.
- M. Guo, L. Varady, D. Fokas, C. Baldino and L. Yu, Tetrahedron Lett., 2006, 47, 3889–3892 CrossRef CAS.
- C. Liu, R. Dai, G. Yao and Y. Deng, J. Chem. Res., 2014, 38, 593–596 CrossRef CAS.
- Y. Hayashi, Chem. Sci., 2016, 7, 866–880 RSC.
- B. M. Trost, Angew. Chem., Int. Ed., 1995, 34, 259–281 CrossRef CAS.
- P. A. Wender, M. P. Croatt and B. Witulski, Tetrahedron, 2006, 62, 7505–7511 CrossRef CAS.
- N. Z. Burns, P. S. Baran and R. W. Hoffmann, Angew. Chem., Int. Ed., 2009, 48, 2854–2867 CrossRef CAS PubMed.
- B. M. Trost and T. Schmidt, J. Am. Chem. Soc., 1988, 111, 1301 Search PubMed.
- B. M. Trost, Angew. Chem. Int. ed., 1995, 34, 259–281 CrossRef CAS.
- Comprehensive Organic Synthesis, ed. B. M. Trost, I. Fleming and L. A. Paquette, Pergamon, Oxford, 1991, vol. 5 Search PubMed.
- (a) M. Novanna, S. Kannadasan and P. Shanmugam, Dyes Pigm., 2020, 174, 108015 CrossRef CAS; (b) M. Novanna, S. Kannadasan and P. Shanmugam, Tetrahedron Lett., 2019, 60, 151163 CrossRef CAS; (c) M. Novanna, S. Kannadasan and P. Shanmugam, Tetrahedron Lett., 2019, 60, 201–206 CrossRef CAS.
- X. L. Li, W. Wu, X. H. Fan and L. M. Yang, RSC Adv., 2013, 3, 12091–12095 RSC.
- K. Manna, H. Mamataj Begam, K. Samanta and R. Jana, Org. Lett., 2020, 22, 7443–7449 CrossRef CAS PubMed.
- Compounds 3c (CCDC-2101632) and 3l (CCDC-2045826) contains the supplementary crystallographic data for this paper.†.
- (a) U. Nubbemeyer, Top. Curr. Chem., 2001, 216, 125 CrossRef CAS; (b) M. E. Maier, Angew. Chem., Int. Ed., 2000, 39, 2073 CrossRef CAS; (c) V. Magné, C. Lorton, A. Marinetti, X. Guinchard and A. Voituriez, Org. Lett., 2017, 19, 4794–4797 CrossRef PubMed; (d) E. M. Beck, R. Hatley and M. J. Gaunt, Angew. Chem., Int. Ed., 2008, 47, 3004–3007 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedure, characterization data and copies of all the spectra of all the new compounds have been provided. Crystallographic information of compounds 3c and 3l is provided. CCDC 2101632 and 2045826. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra08588c |
| This journal is © The Royal Society of Chemistry 2022 |