Open Access Article
Open Access ArticleRecovery of NH4+–N and PO43−–P from urine using sludge-derived biochar as a fertilizer: performance and mechanism†
Chaoyang Yu *ab
*ab
aCollege of Architecture and Environment, Sichuan University, Chengdu 610041, China. E-mail: wtu77_001@163.com
bSichuan-Tibet Railway Co., Ltd., Chengdu 610041, China
First published on 2nd February 2022
Abstract
Sludge-derived biochar (BS) was prepared by pyrolyzing municipal sludge at different temperatures and was used to recover NH4+–N and PO43−–P from urine. The effects of dosage, adsorption time, and urine concentration on the adsorption of NH4+–N and PO43−–P were investigated, and the adsorbed BS was used as a fertilizer to study its effect on the growth of pakchoi cabbage. The Elovich model was more consistent with the adsorption processes of NH4+–N and PO43−–P. Both the NH4+–N and PO43−–P adsorption isotherm model agreed with the Redlich–Peterson model. The Langmuir model showed that the largest adsorption capacity of BS600 for NH4+–N and PO43−–P could reach 114.64 mg g−1 and 31.05 mg g−1, respectively. The NH4+–N adsorption mechanism of BS may have complexation with O-containing functional groups and precipitation reactions, while the removal mechanism of PO43−–P was co-precipitation. The pot experiment demonstrated that adsorbed BS600 can better promote the growth of pakchoi cabbage with the same amount of addition. With the addition of 5% adsorbed BS600, the weight of cabbage was 64.49 g heavier than without the addition of BS600. This research provided theoretical support for the recovery of NH4+–N and PO43−–P from urine as a fertilizer.
1 Introduction
The rapid development of high-speed rail had brought convenient transportation for us, shortening people's travel time and making them more comfortable and convenient to travel. At the same time, it had also brought some problems. In the past, China's railway train toilet fecal sewage was directly discharged on the railway. However, the country paid more and more attention to the cause of environmental protection, and people had higher and higher requirements for the environment.1 Since the opening of high-speed rail, passengers' fecal sewage had been collected by the toilet collector. With the development of the high-speed railroad, the amount of high-speed railway fecal sewage was also rapidly increasing.2 According to the health department regulations, fecal sewage cannot be discharged directly along the railway line, and the sewage must be collected and then centralized. Hence, an economical and effective method to deal with high-speed railway fecal sewage was urgent.The existing urine treatment methods mainly included chemical precipitation, electrochemical, adsorption, and membrane separation.3–6 Among them, researchers had a great interest in the adsorption method to deal with urine because the adsorption method has the advantages of simple operation and high efficiency. Currently, there are also many materials used to treat urine, such as activated carbon, metal oxides, resins, and biochar.7–11 Biochar has a strong ion exchange capacity, large specific surface area, and pore volume.12,13 Besides, biochar is very stable owing to its aromatic structure. In recent years, many studies have shown that biochar has good adsorption properties for nitrogen and phosphorus.14–16 The study showed that the maximum adsorption capacities of cassava straw biochar and banana straw biochar were 24.04 mg g−1 (NH4+–N) and 31.15 mg g−1 (PO43−–P), respectively.14 The adsorption capacity of NH4+–N and PO43−–P was also improved by panda manure biochar (PMBC) modified by carboxymethyl cellulose (CMC) and nano zero-valent zinc (nZVZ), which could reach 40.31 mg g−1 (NH4+–N) and 154.30 mg g−1 (PO43−–P), respectively.17 Simultaneously, some scholars treated PO43−–P in urine with α-Fe2O3 synergistic activated carbon, and most of the PO43−–P in urine could be recovered.7 Additionally, Mg–biochar was used for simultaneous P and N recovery from sewage sludge ash (SSA) and food wastewater (FW) using ground coffee bean (GCB) and palm tree trunk (PTT) waste. PTT Mg–biochar could recover 92.2% of PO43−–P and 54.8% of NH4+–N, while GCB Mg–biochar could recover 79.5% of PO43−–P and 38.6% of NH4+–N.18 Notably, these modification methods were used to improve the NH4+–N and PO43−–P adsorption capacity on the original biochar by introducing metal oxides. In contrast, sludge-derived biochar contained a large number of inorganic compounds (e.g., Ca2+ and Mg2+), which can effectively improve the NH4+–N and PO43−–P adsorption capacity.19,20
In this study, sludge-derived biochar (BS) was prepared by pyrolyzing municipal sludge at different temperatures and recovered NH4+–N and PO43−–P from urine. The objectives were to (1) investigate the effects of dosage, adsorption time, and urine concentration on the NH4+–N and PO43−–P adsorption from the urine; (2) discuss the mechanism of NH4+–N and PO43−–P adsorption in urine by sludge-derived biochar; (3) record the effect of adsorbed BS on the growth of pakchoi cabbage. This study provided a new method and theoretical support for the treatment and resource recovery of NH4+–N and PO43−–P in urine.
2 Material and methods
2.1 Raw materials and reagents
Urine source: the experimental urine was fresh undiluted urine collected from healthy males. The concentrations of NH4+–N and PO43−–P in urine were 450.0 ± 14.3 mg L−1 and 168.0 ± 7.2 mg L−1, respectively, with a pH of 6.92 ± 0.3 and conductivity of (18.4 ± 1.8) mS cm−1.Reagents: amine molybdate (AR, Sinopharm Chemical Reagent Co., Ltd.) and nascent reagent (AR, Hash China). The water of the experiments was deionized water (DW).
2.2 Experimental materials
Preparation of sludge-derived biochar (BS): the dewatered municipal sludge was taken from a wastewater treatment plant in Chengdu (Sichuan Province, China), and a certain amount of sludge was dried in an oven at 90 °C to a constant weight. The dried sludge was crushed through a mortar and sieved through 80 mesh and the sieved sludge powder was collected. Then, the sludge powder was weighed into a crucible and compacted with a tight lid. The crucible was placed in a muffle furnace with a heating rate of 10 °C min−1 and pyrolyzed at different temperatures (300–600 °C) for 3 h. The black solid obtained from the crucible was biochar, and biochar was denoted as BS300, BS400, BS500, and BS600 depending on the pyrolysis temperature.2.3 Adsorption experiment
25 mL of fresh urine was reacted in a 50 mL conical flask with a certain amount of adsorbent, and the conical flask was placed in a thermostatic shaker (IS-RDD3, Crystal Technology & Industries, Inc, USA) at 25 °C and 150 rpm for 24 h. After reaching the adsorption equilibrium, the supernatant was passed through a 0.45 μm nylon membrane and the remaining concentration of NH4+–N and PO43−–P in the filtrate was analyzed. Three parallel experiments were performed for each group. The removal efficiency and adsorption capacity were calculated by eqn (1) and eqn (2), respectively.
 | (1) |
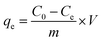 | (2) |
| qt = qe(1 − e−k1t) | (3) |
 | (4) |
 | (5) |
| qt = kdt1/2 + Ci | (6) |
 | (7) |
| qe = KfCe1/n | (8) |
 | (9) |
2.4 Pot experiment
The soil was taken from the campus of Sichuan University (Chengdu, Sichuan Province, China) and its basic properties are shown in Table S1.† The stones in the soil were removed before planting. The adsorbed BS was mixed with 1 kg soil in proportions (0%, 0.5%, 1%, 2%, and 5%) and then planted with pakchoi cabbage in the pots (10 cm in diameter and 15 cm in height). When the pakchoi cabbage was mature, the fresh weight and heavy metal content of the pakchoi cabbage were measured.2.5 Detection method
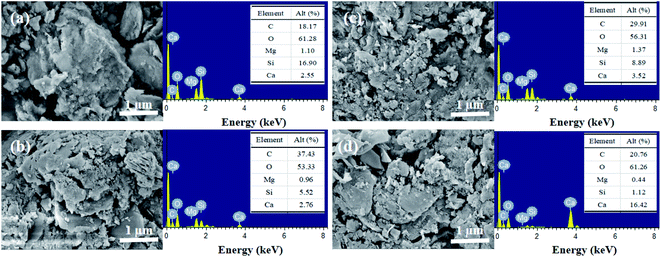
Conductivity and pH were determined with a portable multi-parameter water quality analyzer (SX731, Shanghai San-Xin Instrumentation Factory, China); NH4+–N and PO43−–P concentrations were determined with a double-beam UV spectrophotometer (TU-1901, Beijing Pu-analysis General Instrument Co., Ltd.). The specific surface area and pore volume were measured with a specific surface area and pore size analyzer (BET, Tristar II Plus 2.02, USA). The changes of functional groups of BS were analyzed using Fourier Transform Infrared Spectroscopy (FTIR, Nicolet-460, Thermo Fisher, USA). The crystalline structure and physical composition were analyzed using an X diffraction analyzer (XRD, D8X, Bruker, Germany). The surface morphological features and elemental composition were analyzed by Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM-EDS, Zeiss SUPRA40, Germany). The elemental composition and valence state of biochars were analyzed using an X-ray photoelectron spectrometer (XPS, ESCALAB 250Xi, Thermo Fisher Scientific, USA). An elemental analyzer (Flash 2000, Thermo Fisher Scientific, USA) was used to determine C, H, O, and N contents. Heavy metals in the plants were determined by dry ashing-flame atomic absorption spectrophotometry.21 The mixture was mixed with 1 g of biochar and 20 mL of DW at a solid–liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. The pH of the mixture solution was determined with a pH meter (PHS-3C, Shanghai Rez, China), which was the pH of the BS. The ash content of the BS was determined by the scorching method.22 Available P and NH4+–N of BS were measured by the colorimetric method.23 The zeta potential of BS was determined at pH = 6.92 ± 0.3 using a potential analyzer (Nano-ZS90, Malvern, England). Leaching experiments were conducted on the soil after pot experiments using the acetic acid leaching method.24
20. The pH of the mixture solution was determined with a pH meter (PHS-3C, Shanghai Rez, China), which was the pH of the BS. The ash content of the BS was determined by the scorching method.22 Available P and NH4+–N of BS were measured by the colorimetric method.23 The zeta potential of BS was determined at pH = 6.92 ± 0.3 using a potential analyzer (Nano-ZS90, Malvern, England). Leaching experiments were conducted on the soil after pot experiments using the acetic acid leaching method.24
3 Results and discussion
3.1 Characterization analysis
| BS300 | BS400 | BS500 | BS600 | |
|---|---|---|---|---|
| C (%) | 18.34 | 16.84 | 13.49 | 11.09 |
| H (%) | 4.34 | 3.78 | 2.47 | 1.69 |
| O (%) | 23.68 | 19.70 | 15.33 | 12.17 |
| N (%) | 2.01 | 1.48 | 1.02 | 0.67 |
| Ca (%) | 10.38 | 13.98 | 17.19 | 19.35 |
| Mg (%) | 2.36 | 4.71 | 5.08 | 6.11 |
| H/C | 0.237 | 0.224 | 0.183 | 0.152 |
| O/C | 1.291 | 1.170 | 1.136 | 1.097 |
| O + N/C | 3.301 | 2.650 | 2.156 | 1.767 |
| Ash (%) | 51.63 | 58.2 | 67.69 | 74.38 |
| pH | 8.14 | 8.87 | 9.35 | 9.68 |
| Specific surface area (m2 g−1) | 12.35 | 42.57 | 67.68 | 102.35 |
| Pore volume (cm3 g−1) | 0.15 | 0.24 | 0.36 | 0.54 |
| Proe size (nm) | 14.35 | 16.51 | 17.69 | 27.21 |
| NH4+–N (mg g−1) | 1.237 | 0.394 | 0.085 | 0.033 |
| Available-P (mg g−1) | 0.581 | 0.426 | 0.409 | 0.398 |
| Zeta potential (mV) | −8.67 | −14.37 | −19.36 | −25.18 |
The increase in pyrolysis temperature from 300 °C to 600 °C increased the ash content by 22.75%. This phenomenon was caused by the high content of minerals in municipal sludge (e.g. silica and other inorganic compounds).24,25 In addition, the increase in ash content was related to the decrease in C, H, O, and N content. This phenomenon was consistent with the results of many researchers.21 The pH of the BS gradually increased with increasing pyrolysis temperature. The aliphatic compounds in the municipal sludge underwent condensation/polymerization reactions during pyrolysis and this reaction caused an increase of alkaline substances in the BS.24,26
The pore volume, specific surface area, and pore size of BS increased with the increase of pyrolysis temperature (Table 1). The pore volume, specific surface area, and pore size of sludge-derived biochar increased from 0.15 cm3 g−1, 12.35 m2 g−1, and 14.35 nm to 0.54 cm3 g−1, 102.35 m2 g−1, and 27.21 nm, respectively. It is suggested that high temperatures can expose more active sites to improve the removal ability of biochar.27 The literature showed that high temperature was able to produce more volatiles from sludge, and the richer pore structure on the biochar surface was produced.28 It was also possible that the high temperature caused the original pore structure to collapse and form larger pores.24 The content of NH4+–N and available-P in BS decreased from 1.237 mg g−1 and 0.581 mg g−1 to 0.033 mg g−1 and 0.398 mg g−1, respectively, with the increase of pyrolysis temperature. The zeta potential of BS was all negative, which indicated that BS had a higher adsorption capacity for cations than for anions.14,19,29
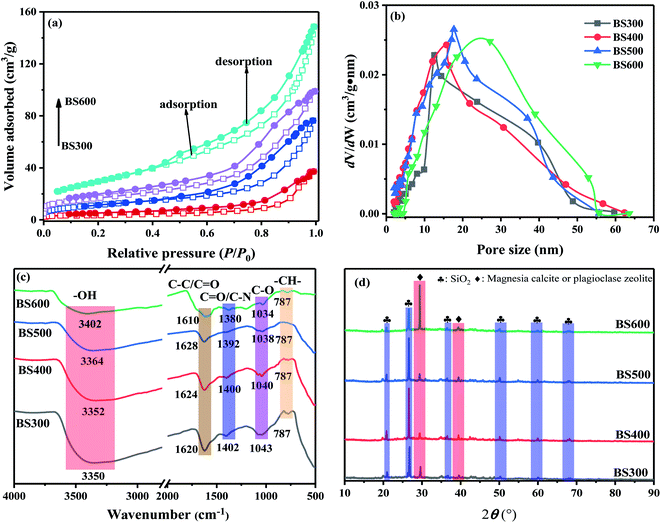
The N2 adsorption–desorption isotherm of BS belonged to type IV (Fig. 1a). When P/P0 < 0.4, the gas entered into the pores, and the N2 adsorption capacity increased sharply at this time. With the increase of P/P0, the adsorption capacity gradually increased. At this time, the adsorption type of BS changed from single-molecular layer adsorption to multi-molecular layer adsorption, and multi-molecular layer adsorption was dominant. Moreover, adsorption–desorption curves in the second half of the isotherm did not overlap, which was caused by the multi-layer filling of capillary pores, indicating the existence of both micropores and mesopores in sludge-derived biochar.17 The pore distribution (Fig. 1b) of the BS was mainly in the mesoporous range (2 nm < pore size < 50 nm).17 The analysis of the N2 adsorption–desorption isotherm and pore distribution indicated that the BS was a mesoporous material.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O structures were indicated by the vibrational peaks at 1620 cm−1 (BS300), 1624 cm−1 (BS400), 1628 cm−1 (BS500) and 1610 cm−1 (BS600).26,32 The intensity of the C
O structures were indicated by the vibrational peaks at 1620 cm−1 (BS300), 1624 cm−1 (BS400), 1628 cm−1 (BS500) and 1610 cm−1 (BS600).26,32 The intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak was the highest in BS600, indicating that BS600 was the most stable. The broad bands at 1402 cm−1, 1400 cm−1, 1492 cm−1, and 1480 cm−1 corresponding to BS300, BS400, BS500, and BS600, respectively, were due to the stretching vibration of C
O peak was the highest in BS600, indicating that BS600 was the most stable. The broad bands at 1402 cm−1, 1400 cm−1, 1492 cm−1, and 1480 cm−1 corresponding to BS300, BS400, BS500, and BS600, respectively, were due to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C–N.33 The peak around 1043 cm−1 corresponded to the C–O stretching vibration, which became smaller and shifted to 1034 cm−1 as the pyrolysis temperature increased. The peak at 787 cm−1 represents the unsaturated alkane group (–CH–) in aromatic or isoaromatic compounds.24 Notably, the peak of –CH– was still visible at 600 °C, indicating that the C in aromatic or heteroaromatic compounds was relatively stable.
O/C–N.33 The peak around 1043 cm−1 corresponded to the C–O stretching vibration, which became smaller and shifted to 1034 cm−1 as the pyrolysis temperature increased. The peak at 787 cm−1 represents the unsaturated alkane group (–CH–) in aromatic or isoaromatic compounds.24 Notably, the peak of –CH– was still visible at 600 °C, indicating that the C in aromatic or heteroaromatic compounds was relatively stable.The XRD analysis of the sludge-derived biochar is shown in Fig. 1d. The main component in the sludge-derived biochar was SiO2,24 followed by inorganic compounds containing Ca, mostly in the form of compounds containing Mg calcite and plagioclase zeolite.24
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–O.34 As the pyrolysis temperature increased, the binding energy and relative content of the three peaks changed. The content of O–H and C–O decreased to 6.61% and 9.03%, respectively. However, the content of C
O, and C–O.34 As the pyrolysis temperature increased, the binding energy and relative content of the three peaks changed. The content of O–H and C–O decreased to 6.61% and 9.03%, respectively. However, the content of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O increased from 26.35% to 84.36%. The C 1s spectrum was divided into three peaks of C–C/C–H, C–O, and C
O increased from 26.35% to 84.36%. The C 1s spectrum was divided into three peaks of C–C/C–H, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.30 Notably, the binding energy of the C–C/C–H spectral peak (284.8 eV) has remained constant with the increase of the pyrolysis temperature, while the binding energies of C–O and C
O.30 Notably, the binding energy of the C–C/C–H spectral peak (284.8 eV) has remained constant with the increase of the pyrolysis temperature, while the binding energies of C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moved from 286.0 eV and 289.2 eV to 286.3 eV and 288.4 eV, respectively. And then, the relative contents of C–O and C
O moved from 286.0 eV and 289.2 eV to 286.3 eV and 288.4 eV, respectively. And then, the relative contents of C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O changed from 33.45% and 10.52% to 17.46% and 15.07%, respectively. The relative content analysis of C–O and C
O changed from 33.45% and 10.52% to 17.46% and 15.07%, respectively. The relative content analysis of C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O illustrated that the content of C–O groups in BS decreased with increasing pyrolysis temperature, while the content of C
O illustrated that the content of C–O groups in BS decreased with increasing pyrolysis temperature, while the content of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O increased with increasing temperature. This result was also consistent with the variation of C–O and C
O increased with increasing temperature. This result was also consistent with the variation of C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group contents in FTIR.
O group contents in FTIR.
3.2 Effect of dosage on adsorption
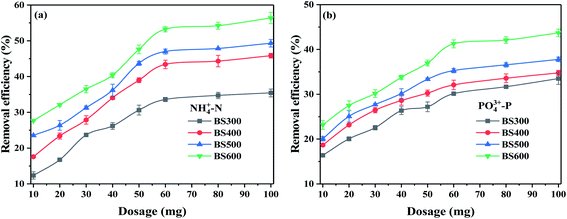
The results of adsorbent dosage (10–100 mg L−1) on the NH4+–N and PO43−–P removal at a temperature of 25 °C and adsorption time of 24 h are shown in Fig. 4. When the dosage was increased from 10 mg to 60 mg, the removal efficiency of NH4+–N and PO43−–P by BS gradually increased, presumably because the adsorption sites increased at the beginning with the increase of the dosage. The adsorption efficiency increased slowly when the dosage was greater than 60 mg, presumably because the increase in adsorbent content caused agglomeration among the adsorbents and thus reduced the number of adsorption sites, resulting in a slow increase in the removal efficiency.7 Notably, the adsorption efficiency increased with increasing pyrolysis temperature, which was presumed to be related to its inorganic content, due to the reaction of inorganic substances (e.g. Ca or Mg) with NH4+–N and PO43−–P to produce precipitation.19 Therefore, 60 mg was chosen as the appropriate dosage for this experiment.3.3 Adsorption kinetics
The effect of adsorption time on the NH4+–N and PO43−–P removal at a temperature of 25 °C and dosage of 60 mg is shown in Fig. 5. Within 180 min, this process was a fast adsorption process for NH4+–N and PO43−–P, and the adsorption capacity increased at a significant rate. In 180–600 min, the adsorbent adsorbed slowly, and then the adsorption reached equilibrium after 600 min. In the initial stage of adsorption, the adsorption rate was very fast due to the abundance of binding sites and pores on the adsorbent surface.8,35 However, with the increase of time, the available binding sites and pores decreased, and then the adsorption gradually reached equilibrium. This phenomenon indicated that the reaction proceeded on the surface of the BS at the adsorption beginning. And then, the NH4+–N and PO43−–P gradually penetrated from the surface to the inner surface of the BS, which led to a slower adsorption rate owing to the active sites on the inner surface being less than those on the outer surface.29,36To evaluate the adsorption characteristics of BS on NH4+–N and PO43−–P, the pseudo-first order kinetic model, the pseudo-second order kinetic model, the Elovich model, and the intra-particle diffusion model were applied to the adsorption data, and the results are shown in Fig. 5 and Table 2. By comparing the correlation coefficients R2 of the pseudo-first order kinetic model and the pseudo-second order kinetic model, the result showed that the pseudo-second order kinetic model could better describe the kinetic processes of NH4+–N and PO43−–P adsorption by BS, suggesting that the adsorption process was dominated by chemisorption.7,34,37,38 In addition, the pseudo-second order model constant k2 increased with the increase of pyrolysis temperature, which was presumed to be due to the formation of a richer pore structure on the surface of the biochar, thus exposing more adsorption sites.35 The correlation coefficient R2 of the Elovich model can reach above 0.95, indicating that the adsorption processes were mainly influenced by chemisorption, and the activation energy varies greatly.7 Moreover, this result indicated that the adsorption process was multilayer adsorption on an inhomogeneous interface, including not only its diffusion at the adsorbent interface but also the activation and de-activation of the surface.7,9,37
| Pseudo-first-order | Pseudo-second-order | Elovich | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qm | k1 | R2 | qm | k2 | R2 | α | β | R2 | ||
| NH4+–N | BS300 | 60.30 | 0.017 | 0.821 | 64.42 | 3.34 × 10−4 | 0.910 | 10.31 | 0.109 | 0.967 |
| BS400 | 73.06 | 0.020 | 0.811 | 78.63 | 3.71 × 10−4 | 0.914 | 11.55 | 0.087 | 0.983 | |
| BS500 | 77.72 | 0.021 | 0.770 | 83.02 | 4.01 × 10−4 | 0.890 | 15.73 | 0.085 | 0.985 | |
| BS600 | 93.32 | 0.027 | 0.725 | 98.78 | 4.44 × 10−4 | 0.869 | 27.28 | 0.074 | 0.986 | |
| PO43−–P | BS300 | 20.00 | 0.007 | 0.866 | 22.01 | 0.52 × 10−3 | 0.924 | 1.31 | 0.296 | 0.953 |
| BS400 | 20.60 | 0.014 | 0.791 | 22.20 | 1.02 × 10−3 | 0.888 | 2.88 | 0.310 | 0.969 | |
| BS500 | 23.27 | 0.049 | 0.692 | 24.55 | 3.03 × 10−3 | 0.869 | 17.40 | 0.333 | 0.979 | |
| BS600 | 26.64 | 0.065 | 0.603 | 28.23 | 3.14 × 10−3 | 0.820 | 29.02 | 0.302 | 0.979 | |
The kinetics data were fitted by the intra-particle diffusion model, and the results are displayed in Fig. S1 and Table S2.† The fitting analysis of the intraparticle diffusion model revealed that the adsorption process can be divided into three stages. In the first stage of diffusion, the adsorbate was adsorbed from the aqueous phase to the outer surface of the adsorbent.7,26 At this time, the mass transfer resistance was small, and the adsorption rate was fast. Subsequently, the available adsorption sites on the outer surface of BS decreased and the adsorption process entered the second stage.7,9 At this point, the NH4+–N and PO43−–P entered the inner pore of BS and the adsorption rate became slower. Finally, the adsorption reached the third stage of adsorption–desorption dynamic equilibrium. In addition, the fitted straight line did not pass through the origin, indicating that the adsorption process was controlled by the boundary layer to some extent, and the intra-particle diffusion was not the only rate-limiting step.7,26 From Table S2,† the intra-particle diffusion rate constants kd1 > kd2 > kd3 and the boundary layer constants C1 < C2 < C3 indicated that the first stage played a dominant role, which was because the BS can provide a large number of available active sites in the initial stage.35 After the surface available active sites were gradually saturated, the NH4+–N and PO43−–P adsorption was mainly controlled by the intra-particle diffusion rate.
3.4 Adsorption isotherm
The effect of fresh urine at different dilution levels on the NH4+–N and PO43−–P removal was investigated at the adsorption temperature of 25 °C, the dosage of 60 mg, and the adsorption time of 24 h. The results are shown in Fig. 6. The adsorption capacity of NH4+–N and PO43−–P increased rapidly, and then the trend of increasing adsorption decreased. Notably, the adsorption capacity of NH4+–N and PO43−–P by BS increased with the increase of pyrolysis temperature.The results were fitted by the Langmuir model, Freundlich model, and Redlich–Peterson model as shown in Fig. 6 and Table 3. The Langmuir model, Freundlich model, and Redlich–Peterson adsorption isotherm model can well describe the adsorption process (R2 > 0.9), confirming that the NH4+–N and PO43−–P adsorption process on BS was a combination of monolayer and multilayer adsorption.19,34 Nevertheless, the R2 of the Redlich–Peterson model was higher, showing that the Redlich–Peterson model can better describe the adsorption process of NH4+–N and PO43−–P by BS. These results also confirmed that the adsorption process of NH4+–N and PO43−–P belonged to a combination of multiple mechanisms.38,39 The characteristic coefficient Kb in the Langmuir model was correlated with the adsorption performance. The value of Kb increased with the increase of BS pyrolysis temperature in this experiment, which also confirmed that the high-temperature pyrolysis was favorable for the adsorption of NH4+–N and PO43−–P by BS.40 The NH4+–N and PO43−–P adsorption capacity by sludge-derived biochar increased with the increase of pyrolysis temperature, which also indicated that higher pyrolysis temperature was beneficial to the adsorption.29 Simultaneously, the maximum adsorption of NH4+–N and PO43−–P by BS600 was 114.71 mg g−1 and 30.29 mg g−1, respectively. This also suggested that higher pyrolysis temperatures were beneficial to the recovery of NH4+–N and PO43−–P from urine by BS. This result was considered to be that high-temperature pyrolysis can produce larger specific surface area and wider pore structure and provide more active sites; thus, the H4+–N and PO43−–P adsorption capacity by BS was improved.41 The 1/n in the Freundlich model was less than 1, indicating that the NH4+–N and PO43−–P adsorption was easy to occur.35 Comparing the adsorption capacity of different types of adsorption for NH4+–N and PO43−–P (Table S3†), the adsorption capacity of BS600 for NH4+–N was much higher than that of other types of adsorbents. However, the adsorption capacity of BS600 for PO43−–P was lower than that of bamboo leaf biochar and panda manure biochar. This may be caused by the lower concentration of PO43−–P in urine and the negatively charged surface of biochar.20,29 It may also be caused by the difference in raw materials of biochar.19 However, sludge-derived biochar was also capable of being an excellent NH4+–N and PO43−–P adsorbent.
| Model | BS300 | BS400 | BS500 | BS600 | |||||
|---|---|---|---|---|---|---|---|---|---|
| NH4+–N | PO43−–P | NH4+–N | PO43−–P | NH4+–N | PO43−–P | NH4+–N | PO43−–P | ||
| Langmuir | Kb | 0.014 | 0.108 | 0.015 | 0.292 | 0.020 | 0.323 | 0.054 | 0.387 |
| Qmax | 79.64 | 23.13 | 97.60 | 23.38 | 109.16 | 26.90 | 114.71 | 30.29 | |
| R2 | 0.986 | 0.993 | 0.980 | 0.993 | 0.982 | 0.986 | 0.985 | 0.988 | |
| Freundlich | Kf | 5.245 | 4.85 | 6.57 | 7.08 | 9.83 | 8.55 | 20.21 | 10.08 |
| n | 2.22 | 3.01 | 2.18 | 3.70 | 2.40 | 3.77 | 3.05 | 3.81 | |
| R2 | 0.982 | 0.962 | 0.998 | 0.944 | 0.986 | 0.942 | 0.956 | 0.952 | |
| Redlich–Peterson | Kr | 1.71 | 2.99 | 15.98 | 8.78 | 11.27 | 12.67 | 6.757 | 16.67 |
| a | 0.072 | 0.176 | 1.993 | 0.497 | 0.753 | 0.660 | 0.069 | 0.769 | |
| g | 0.797 | 0.934 | 0.575 | 0.936 | 0.657 | 0.922 | 0.969 | 0.919 | |
| R2 | 0.988 | 0.995 | 0.999 | 0.996 | 0.988 | 0.991 | 0.987 | 0.993 | |
The literature has reported that PO43−–P adsorption was not favored under weakly acidic or alkaline conditions.14 The reason speculated that the surface of sludge-derived biochar was negatively charged and generated electrostatic repulsion with PO43−–P, which reduced the adsorption of phosphate.7,10 In this work, the main reason for PO43−–P adsorption was the release of Ca2+ from the sludge-derived biochar into the solution to react with PO43−–P to form a precipitate (eqn (10)).26,42 And the NH4+–N adsorption might be related to the complexation reaction of O-containing functional groups in the BS and the precipitation reaction with inorganic substances.7,43,44 Many researchers had shown that NH4+, PO43− combined with Mg2+ to form struvite (eqn (11)), which was presumed to be the main mechanism for the decrease in NH4+–N and PO43−–P concentrations in urine.39,41,45 Adsorption and co-precipitation were presumed to be the mechanisms for the decrease in urinary NH4+–N and PO43−–P concentrations.
| 3PO43− + 5Ca2+ + OH− ⇌ Ca5(OH)(PO4)3 ↓ (pKs = 57.5, 25 °C) | (10) |
| Mg2+ + PO43− + NH4+ + 6H2O → MgNH4PO4·6H2O ↓ (pKs = 12.6, 25 °C) | (11) |
3.5 Adsorption mechanism
The FTIR of BS600 before and after adsorption of NH4+–N and PO43−–P is shown in Fig. S2a.† The peak at 3402 cm−1 was attributed to the stretching vibration of –OH, and the wave peak was enhanced and shifted to 3422 cm−1 after adsorption, indicating that –OH was involved in the adsorption of NH4+–N and PO43−–P.12,14,38 Meanwhile, the vibrational peak of C–C/C–O at the wavenumber of 1610 cm−1 after adsorption was shifted to 1642 cm−1, which indicated that the C–C/C–O group was involved in the adsorption of NH4+–N and PO43−–P.14,30,46 The characteristic peak of phosphate was found at 1041 cm−1, and the wave peak was sharper after adsorption, indicating that BS600 successfully adsorbed PO43−–P.14,30,47 Meanwhile, a new peak appeared at 1428 cm−1, attributed to the stretching vibration peak of N–H.9,44 In summary, –OH and C–C/C–O played an important role in the adsorption of NH4+–N and PO43−–P by BS.The strong diffraction peaks at 2θ 14.98°, 15.82°, 16.50°, 20.10°, 30.63°, 31.80°, 33.28°, and 33.69° indicated that BS600 combined with NH4+–N and PO43−–P in urine to form granite crystals (Fig. S2b†).12,14,22,32,47 A new diffraction peak appeared at 2θ of 38.71°, which was considered as Ca5(OH)(PO4)3 precipitation, suggesting that Ca reacted with PO343−–P in BS600 to form a precipitate.46 The above analysis indicated that co-precipitation was the main mechanism for the removal of NH4+–N and PO43−–P from urine by BS.
In summary, the mechanism of NH4+–N and PO43−–P adsorption in urine by BS may include co-precipitation, in which the main role was played by the generation of granite crystalline precipitation. In addition, NH4+–N also complexed with the O-containing functional group on BS.
3.6 Pot experiments
As shown in Fig. 7, the fresh weight of pakchoi cabbage showed an increasing trend when the addition amount of BS was gradually increased from 0% to 5%. The fresh weight of pakchoi cabbage increased significantly when the addition amount was increased from 0% to 2%, whereas the fresh weight of pakchoi cabbage increased greatly at the addition amounts of 2% and 5%. When no BS was added, the fresh weight of pakchoi cabbage was 37.57 g. However, when unadsorbed BS was added, the fresh weight of pakchoi cabbage was all enhanced. Notably, BS after adsorption can improve the fresh weight of pakchoi cabbage better than BS. Additionally, the highest fresh weight of pakchoi cabbage was obtained by adding adsorbed BS600 at the same addition amount, presumably due to the ability of BS600 to adsorb more NH4+–N and PO43−–P in the urine. The addition of 5% adsorbed BS600 made the weight of pakchoi cabbage 64.49 higher than that of no BS600, indicating that the adsorbed BS was effective in promoting the growth of pakchoi cabbage. | ||
| Fig. 7 Effect of sludge-derived biochar (a) and adsorbed sludge-derived biochar (b) dosing on the fresh weight of pakchoi cabbage. | ||
Meanwhile, the Pb, Cr, Cd, As, Ni, Zn, and Cu contents in pakchoi cabbage were also analyzed (Table S4†). The heavy metal contents of Pb, Cr, Cd, As, Ni, Zn, and Cu in pakchoi cabbage were lower than the limit value (Standard for the Detection of Heavy Metal Contents in Vegetables), which indicated that the sludge-derived biochar could effectively become a source of fertilizer for vegetables after adsorption of NH4+–N and PO43−–P from urine, and also provided a basis for the reuse of resources. In addition, the soil in the pot experiment was analyzed for leaching experiments (Table S5†). The results showed that the leaching concentrations of heavy metals in the soil were much lower than the leaching toxicity identification criteria values, which confirmed the feasibility of BS for the recovery of NH4+–N and PO43−–P from urine as a fertilizer.
In this paper, sludge-derived biochar was prepared by pyrolysis of municipal sludge at different temperatures and applied to recover N and P from urine. The results showed that the maximum adsorption of NH4+–N and PO43−–P by BS600 could reach 114.71 mg g−1 and 30.29 mg g−1, respectively. In addition, the heavy metal content of chard was analyzed separately, and the results showed that the concentrations of many heavy metals were very low. Then, the leaching experiments were performed on the soil after planting, and the results showed that the heavy metal leaching concentrations were all within the standard range. The above analysis showed that the adsorbed sludge-derived biochar could be a potential fertilizer. It also provided a new idea for the treatment of municipal sludge and high-speed railway fecal sewage.
4 Conclusion
Sludge-derived biochar (BS) was prepared by pyrolysis of municipal sludge and used for NH4+–N and PO43−–P adsorption from urine. Kinetic studies showed that the adsorption of both NH4+–N and PO43−–P could reach adsorption equilibrium within 12 h, and the kinetic adsorption was more consistent with the Elovich model, which was multilayer adsorption at inhomogeneous interfaces and controlled by multiple diffusion rates during the adsorption process. The adsorption isothermal model of both NH4+–N and PO43−–P was consistent with the Redlich–Peterson model, which indicated that the adsorption was a combination of monolayer adsorption and multilayer adsorption. The maximum adsorption of BS600 for NH4+–N and PO43−–P could reach 114.71 mg g−1 and 30.29 mg g−1, respectively. The adsorption mechanism of BS on NH4+–N may have complexation with O-containing functional groups and precipitation reactions, while the removal mechanism of PO43−–P was co-precipitation. Simultaneously, the addition of adsorbed BS600 at 5% can better promote the growth of pakchoi cabbage. This work presented a new way and basis for resource utilization and recycling of municipal sludge and urine.Ethical statement
All experiments were performed in accordance with the ethical guidelines, and approved by the ethics committee at Sichuan university. Informed consents were obtained from human participants of this study.Funding
This work was supported by National Key R&D Program (2018YFC1505404) and Sichuan Provincial Science and Technology Department Science and Technology Plan Project (No. 2021YJ0033).Conflicts of interest
The authors declare that they have no competing financial interests.References
- W. M. Chen and Y. X. Zheng, Railway Stand. Des., 2012, 9, 123–126 Search PubMed.
- M. Hu, B. Fan, B. Qu and S. K. Zhu, China Water Wastewater, 2016, 32, 20–26 CAS.
- Y. Gao, W. Zhang, B. Gao, W. Jia, A. Miao, L. Xiao and L. Yang, Water Res., 2018, 139, 301–310 CrossRef CAS PubMed.
- Y. J. Dai, W. Wang, L. Lu, L. Yan and D. Yu, J. Cleaner Prod., 2020, 257, 120573 CrossRef CAS.
- Q. Q. Yin, Z. B. Dong, R. K. Wang and Z. H. Zhao, Environ. Sci. Pollut. Res., 2017, 24, 26297–26309 CrossRef CAS PubMed.
- Z. Zeng, S. D. Zhang, T. Q. Li, F. L. Zhao and M. T. Rafiq, J. Zhejiang Univ., Sci., B, 2013, 14, 1152–1161 CrossRef CAS PubMed.
- Y. Y. Jiao, S. K. Zhou, L. C. Zhang, W. D. Ai, S. Kang, C. L. Li, L. B. Zheng and Y. S. Wei, Chem. Ind. Eng. Prog., 2021, 40, 2347–2356 Search PubMed.
- X. T. Jiang and J. Chi, J. Agric. Environ. Sci., 2014, 33, 1817–1822 CAS.
- Y. H. Jiang, L. I. An-Yu, F. Yan and H. Deng, J. Agric. Resour. Environ., 2018, 35, 559–567 Search PubMed.
- W. D. Wang, H. Liu, Y. T. Zhang and S. J. Yang, Environ. Sci., 2016, 37, 3186–3191 Search PubMed.
- J. J. Xu, X. H. Wu, N. W. Zhu, Y. W. Shen and H. P. Yuan, Sci. Total Environ., 2020, 748, 141367 CrossRef CAS PubMed.
- M. Yi, T. T. Li, H. H. Li, H. Qiao and Z. M. Yang, Environ. Sci., 2019, 40, 1318–1327 Search PubMed.
- L. F. Yue, Y. Xie, L. Shi, X. L. Li, F. R. Li and J. F. Wang, Chin. J. Environ. Eng., 2015, 9, 1221–1226 Search PubMed.
- Y. H. Jiang, A. Y. Hua, H. Deng, C. H. Ye, Y. Q. Wu, Y. D. Linmu and H. L. Hang, Bioresour. Technol., 2019, 276, 183–189 CrossRef CAS PubMed.
- S. H. Mood, M. Ayiania, H. L. Cao, O. Marin-Flores and M. Garcia-Perez, Biomass Convers. Biorefin., 2021, 14, 1–20 Search PubMed.
- Y. Gao, C. Yan, R. Wei, W. Zhang and L. Yang, Ecol. Eng., 2019, 138, 71–78 CrossRef.
- M. J. Wang, S. K. Hu, Q. G. Wang, Y. Liang, C. R. Liu, H. Xu and Q. Ye, J. Cleaner Prod., 2020, 291, 125221 CrossRef.
- M. Zin and D. J. Kim, J. Hazard. Mater., 2020, 403, 123704 Search PubMed.
- W. F. Cheng, H. Li, Y. Q. Yang, B. Yin and H. B. Liu, CIESC J., 2016, 67, 1541–1548 CAS.
- L. Zhang, F. Deng, Z. K. Liu and L. X. Ai, J. Environ. Manage., 2021, 282, 111970 CrossRef CAS PubMed.
- Z. Khanmohammadi, M. Afyuni and M. R. Mosaddeghi, Waste Manage. Res., 2015, 33, 275 CrossRef CAS PubMed.
- A. D. Abdelhafid, S. X. Chang, Y. S. Ok, A. U. Rajapaksha and A. Anyia, Environ. Sci. Pollut. Res., 2018, 25, 25799–25812 CrossRef PubMed.
- M. K. Hossain, V. Strezov, K. Y. Chan, A. Ziolkowski and P. F. Nelson, J. Environ. Manage., 2011, 97, 223–228 CrossRef PubMed.
- J. W. Jin, Y. A. Li, J. Y. Zhang, S. C. Wu, Y. C. Cao, P. Liang, J. Zhang, M. H. Wong, M. Y. Wang and S. D. Shan, J. Hazard. Mater., 2016, 320, 417–426 CrossRef CAS PubMed.
- T. Chen, Z. Y. Zhou, H. Rong, R. H. Meng, H. T. Wang and W. J. Lu, Chemosphere, 2015, 134, 286–293 CrossRef CAS PubMed.
- M. Chen, F. Wang, D. L. Zhang and W. M. Yi, Environ. Sci., 2019, 40, 5421–5429 Search PubMed.
- T. Chen, Z. Y. Zhou, X. Sai, H. T. Wang and W. J. Lu, Bioresour. Technol., 2015, 190, 388–394 CrossRef CAS PubMed.
- T. Chen, Z. Zhou, R. Han, R. Meng, H. Wang and W. Lu, Chemosphere, 2015, 134, 286–293 CrossRef CAS PubMed.
- X. Liu, H. Y. Nan and Q. An, J. Agric. Resour. Environ., 2017, 35, 66–73 CrossRef PubMed.
- A. Y. Li, S. L. Li, B. G. Yu, A. Y. Ma, X. L. Zhou, J. H. Xie, Y. H. Jiang and H. Deng, CIESC J., 2020, 71, 1683–1695 CAS.
- Y. H. Du, E. C. Jiang, Z. Y. Li, S. J. Zhang and M. F. Wang, Trans. Chin. Soc. Agric. Mach., 2016, 47, 193–199+214 CAS.
- N. Cheng, B. Wang, Q. Feng, X. Zhang and M. Chen, Bioresour. Technol., 2021, 340, 125696 CrossRef CAS PubMed.
- T. Chen, Y. X. Zhang, H. T. Wang, W. J. Lu, Z. Y. Zhou, Y. C. Zhang and L. L. Ren, Bioresour. Technol., 2014, 164, 47–54 CrossRef CAS PubMed.
- J. Li, B. Li, H. Huang, N. Zhao and L. Cao, Sci. Total Environ., 2020, 714, 136839 CrossRef CAS PubMed.
- F. F. Ma, B. W. Zhao, J. R. Diao, J. K. Zhong and A. B. Li, Environ. Sci., 2015, 36, 1678–1685 CAS.
- H. Y. Luo, Y. J. Wang, X. P. Wen, S. L. Cheng, J. Li and Q. T. Lin, Sci. Total Environ., 2020, 766, 142618 CrossRef PubMed.
- B. T. Li, F. Y. Jing, Z. Q. Hu, Y. X. Liu and D. B. Guo, J. Saudi Chem. Soc., 2021, 25, 101213 CrossRef CAS.
- J. Q. Liang, Y. Lv, Y. Lu, X. H. Wang, M. Zheng and K. N. Xu, Environ. Eng., 2020, 38, 89–94 Search PubMed.
- M. Li, Z. Zhang, Z. Li and H. Wu, Ecol. Eng., 2020, 149, 105792 CrossRef.
- D. Liu, Z. B. Huang, S. H. Men, H. Zhen and C. R. Wnag, Water Sci. Technol., 2019, 79, 2175–2184 CrossRef CAS PubMed.
- P. M. Melia, R. Busquets, P. S. Hooda, A. B. Cundy and S. P. Sohi, Sci. Total Environ., 2019, 675, 623–631 CrossRef CAS PubMed.
- J. K. Njuguna, R. B. Zhang, Y. Li, X. J. Wang and J. F. Zhao, Acta Sci. Circumstantiae, 2018, 38, 4383–4390 CAS.
- Y. H. Shen, L. L. Zhuang, J. Zhang, J. L. Fan, T. Yang and S. Sun, Chem. Eng. J., 2019, 359, 706–712 CrossRef CAS.
- L. Y. Shi, A. L. Wei, K. D. Xue, X. Q. Zheng and H. Gao, Environ. Pollut. Control, 2017, 39, 1077–1081 Search PubMed.
- M. M. Zhi, P. F. Wang, Z. Y. Hou, J. Cao and Y. Z. Yang, Environ. Sci., 2019, 40, 669–676 Search PubMed.
- Y. Zhu, Q. B. Xiao, Y. L. Xi, D. Gao, Y. X. Wang, J. Du and X. M. Ye, Ecol. Environ. Sci., 2020, 29, 1897–1903 Search PubMed.
- O. C. Hai, X. S. Guo, B. Y. Xiao, X. Wang and J. X. Liu, Acta Sci. Circumstantiae, 2017, 37, 213–218 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08558a |
| This journal is © The Royal Society of Chemistry 2022 |