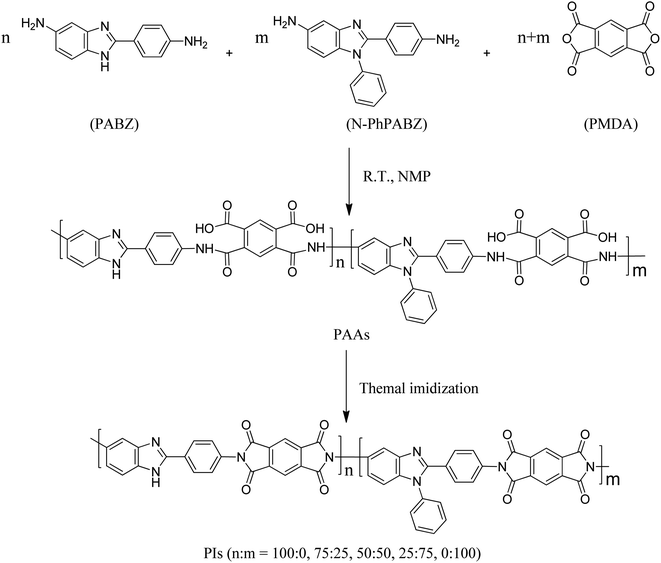
Open Access Article
Open Access ArticleN-Phenyl-substituted poly(benzimidazole imide)s with high glass transition temperature and low coefficient of thermal expansion
Dandan Lia,
Chengyang Wanga,
Xiaoying Yanb,
Shengqi Maa,
Ran Lu a,
Chunhai Chen
a,
Chunhai Chen a,
Guangtao Qian*b and
Hongwei Zhou
a,
Guangtao Qian*b and
Hongwei Zhou *a
*a
aCollege of Chemistry, Jilin University, Changchun, 130012, P. R. China. E-mail: zhw@jlu.edu.cn
bCenter for Advanced Low-Dimension Materials, State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Material Science and Engineering, Donghua University, Shanghai 201620, P. R. China. E-mail: qgt@dhu.edu.cn
First published on 2nd February 2022
Abstract
To obtain high thermostable materials for flexible display substrates, a series of copoly(benzimidazole imide)s was prepared using 5-amine-2-(4-aminobenzene)-1-phenyl-benzimidazole (N-PhPABZ) and 6(5)-amino-2-(4-aminobenzene)-benzimidazole (PABZ). Incorporating N-phenyl groups effectively healed the brittleness of the poly(benzimidazole imide)s (PBIIs) derived from pyromellitic dianhydride (PMDA), and the resultant homo- and copoly(benzimidazole imide)s displayed an outstandingly high glass transition temperature (Tg > 450 °C) and a low coefficient of thermal expansion (CTE < 10 ppm K−1). Furthermore, the influence of removing intermolecular hydrogen bonds on the properties of these poly(benzimidazole imide)s was systematically analyzed. These data provide a feasible method to prepare superheat-resistant poly(benzimidazole imide)s without H-bonding.
1. Introduction
Thermostable polymers, with their marked light weight and flexibility, and as potential alternatives to fragile inorganic glass, are the most popular candidates for reliable substrates in flexible image displays.1,2 The required substrate materials must possess both superheat resistance and outstanding dimensional stability to meet the harsh manufacturing requirements.3,4 For example, in the primary fabrication of next-generation displays, i.e., flexible active-matrix organic light-emitting-diode (AMOLED) devices, the processing temperature of low-temperature polysilicon thin-film transistors (LTPS TFTs) exceeds 450 °C and TFT fabrication is sensitive to dimensional changes caused by thermal or mechanical stress.5,6 Hence, alternative polymers are supposed to have superb thermal properties, including a high thermal decomposition temperature (Td > 500 °C), a high glass transition temperature (Tg > 450 °C) and a low coefficient of thermal expansion (CTE < 10 ppm K−1), and it is crucial to achieve higher heat resistance for plastic substrates.Poly(benzimidazole imide)s, as a class of polyimides containing heterocyclic rings, are some of the most reliable heat resistant materials used currently.7,8 They are characterized by incorporating imidazole rings. The electron-withdrawing imidazole moieties increase the nucleophilicity of the terminal amino groups in the diamine monomers, which improves the electron donation–acceptance interaction between chain segments in the related polymers.9,10 Besides, the unique NH protons in the imidazole units provide favorable conditions to form hydrogen bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯N–H and C
N⋯N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯N–H).11,12 Because of the high-strength physical interaction and conjugated rigid backbones, poly(benzimidazole imide)s usually exhibit excellent thermal stability, e.g., Tg > 400 °C, Td > 500 °C and CTE < 10 ppm K−1. Unfortunately, the benzimidazole-containing polyimides generally display a high water absorption (WA) because of the hydrogen-bonded water molecule formation, which may cause a series of application issues, such as package cracking, metal corrosion and degradation of dielectric properties.13,14 Additionally, their intermolecular H-bonding and rigid backbone reduce the flexibility of PBII films, resulting in the high brittleness of the series derived from fully rigid pyromellitic dianhydride (PMDA),7,15 whereas aromatic PMDA-based polyimides are usually superheat-resistant.16,17
O⋯N–H).11,12 Because of the high-strength physical interaction and conjugated rigid backbones, poly(benzimidazole imide)s usually exhibit excellent thermal stability, e.g., Tg > 400 °C, Td > 500 °C and CTE < 10 ppm K−1. Unfortunately, the benzimidazole-containing polyimides generally display a high water absorption (WA) because of the hydrogen-bonded water molecule formation, which may cause a series of application issues, such as package cracking, metal corrosion and degradation of dielectric properties.13,14 Additionally, their intermolecular H-bonding and rigid backbone reduce the flexibility of PBII films, resulting in the high brittleness of the series derived from fully rigid pyromellitic dianhydride (PMDA),7,15 whereas aromatic PMDA-based polyimides are usually superheat-resistant.16,17
In our previous discussion, it was pointed out that modified poly(benzimidazole imide)s without intermolecular H-bonding can achieve good comprehensive performance, e.g., lighter color and lower H2O-absorption.9,10,14,18 Incorporating a bulky N-phenyl unit can hinder hydrogen bond formation and loose chain packing; the method provides an effective way to reduce the segment packing density of poly(benzimidazole imide)s without compromising their high thermal property.18 Nevertheless, their Tg of less than 450 °C is the main hindrance facing their application as candidates in the flexible substrates of next-generation displays. Herein, homo- and copoly(benzimidazole imide)s were prepared by N-PhPABZ, PABZ and PMDA, as shown in Scheme 1, to investigate their properties with a reduction in H-bonding content. These data provide a feasible framework to prepare polymers with superb thermal stability.
2. Experimental
2.1 Materials
5-Amine-2-(4-aminobenzene)-1-phenyl-benzimidazole and 6(5)-amino-2-(4-aminobenzene)-benzimidazole were synthesized by our research team.18 Pyromellitic dianhydride was supplied by Sinopharm Chemical Reagent Shanghai Co., Ltd. N-Methyl-2-pyrrolidinone (NMP) was obtained from Shanghai Aladdin Biochemical Technology Co., Ltd, and was purified by vacuum distillation before use. All monomers were dried at 80 °C in a vacuum oven for 24 h to eliminate the effect of water before use.2.2 Characterization
Inherent viscosity (ηinh) was measured with an Ubbelohde viscometer at 25 ± 0.1 °C at a concentration of 0.5 g dL−1 in NMP. Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectra were conducted on a Nicolet 6700 infrared spectrometer (ThermoFisher, Waltham, MA) by averaging 32 scans within the range of 4000–400 cm−1. Wide-angle X-ray diffraction (WAXD) spectra were collected on a Rigaku Denki D/MAX-2500 diffractometer (Rigaku, Tokyo, Japan) with Cu Kα radiation (λ = 1.54 Å) at room temperature. Thermogravimetric analysis (TGA), dynamic mechanical analysis (DMA) and the coefficient of thermal expansion (CTE) were assessed using a Discovery TGA 550, DMA Q800 and TMA Q400 (TA Instrument, New Castle, DE), with a constant heating rate of 5 °C min−1, respectively. Mechanical properties of the polymer films were evaluated on an Instron 5966 universal testing machine (Instron, Boston, MA) at speed of 5 mm min−1, and the tensile modulus (E), tensile strength (σ), and elongation at break (ε) were demonstrated as the average of five strips.Water absorption (WA) of the films was confirmed by the weight differences before and after placing in distilled water. Typically, the film samples with dimensions of 50 × 50 mm were dried in a vacuum oven and weighed. After being placed in a water bath at 25 °C for 24 h, the specimens were wiped clean and weighed. The water absorption was determined by eqn (1):
 | (1) |
2.3 Synthesis of polymers
The poly(benzimidazole imide)s were prepared using a conventional two-stage procedure including polycondensation and thermal imidization (Scheme 1). Using the process of N-PhPABZ-PMDA as a representative example, PMDA (0.4362 g, 2.0 mmol) was added in a solution of N-PhPABZ (0.6007 g, 2.0 mmol) in NMP (5.9 g) at room temperature under a nitrogen atmosphere. After stirring at room temperature for 12 h, the viscous polyamic acid (PAA) solution was cast on a flat glass plate with a 300 μm deep blade, and dried at 80 °C for 4 h, 200 °C for 2 h, 300 °C for 2 h and 400 °C for 2 h. Finally, the PBII films were stripped off after immersion in deionized water after cooling. In general, the thickness of the obtained films was about 20 μm.3. Results and discussion
3.1 Polymer characteristics and intermolecular H-bonding
The inherent viscosity of the PAA precursors was in the range of 0.92–1.35 dL g−1 (Table 1), and the values showed an upward trend with increasing content of PABZ. The phenomenon could be explained as follows: the imidazole ring in PABZ increased the chances for H-bond formation, resulting in the enhanced physical interaction between the polymer chain segments. Following the trend, the PAA derived from PABZ and PMDA displayed the highest ηinh; however, the PI film was too brittle to form during the thermal imidization. This was in accordance with previous findings, which was attributed to its intermolecular hydrogen bonds and rigid backbones.7,15 Obviously, the brittleness of this series was healed by incorporating N-PhPABZ. The N-Ph group increased the steric hindrance between polymer chains and hindered the formation of H-bonds, which provided poly(benzimidazole imide)s with reduced chain orientation and sufficient chain entanglement, and so the PMDA-PBIIs containing N-PhPABZ with the necessary degree of flexibility possessed good film-forming capability.| PIs | Mole ratio (PABZ/N-PhPABZ) | ηinh(PAA) (dL g−1) | Film-forming (PBII) |
|---|---|---|---|
| PABZ-PMDA | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
1.35 | Incapable |
| CPIa | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
1.31 | Good |
| CPIb | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1.24 | Good |
| CPIc | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
1.19 | Good |
| N-PhPABZ-PMDA | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.92 | Good |
The anticipated chemical structures of the poly(benzimidazole imide)s were studied by ATR-FTIR (Fig. 1a). The characteristic bands at around 1776 cm−1 for imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric stretching, 1710 cm−1 for imide C
O asymmetric stretching, 1710 cm−1 for imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric stretching and 1367 cm−1 for imide C–N stretching confirmed the formation of the imide ring. The presence of peaks at 1306 cm−1 (imidazole breathing) was indicative of the successful incorporation of the imidazole ring.19,20 Additionally, the peak corresponding to C
O symmetric stretching and 1367 cm−1 for imide C–N stretching confirmed the formation of the imide ring. The presence of peaks at 1306 cm−1 (imidazole breathing) was indicative of the successful incorporation of the imidazole ring.19,20 Additionally, the peak corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the intermediate amic acid (1660 cm−1, gray band domain) was hardly observed, which explained the high imidization degree of all the PBII films.21,22
O in the intermediate amic acid (1660 cm−1, gray band domain) was hardly observed, which explained the high imidization degree of all the PBII films.21,22
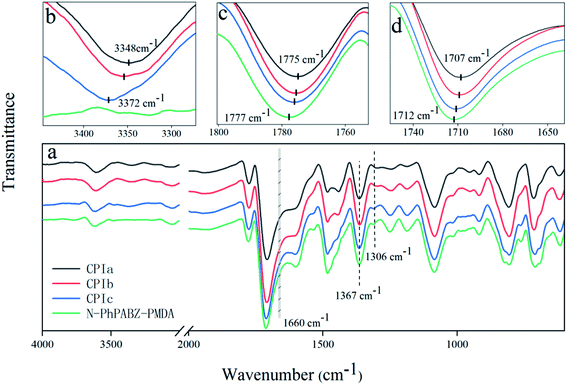 | ||
| Fig. 1 ATR-FTIR spectra of (a) (4000–600 nm); (b) (4000–3275 nm); (c) (1800–1750 nm); (d) (1750–1640 nm) the poly(benzimidazole imide) films. | ||
On the other hand, the FTIR spectra could be used to confirm the intermolecular hydrogen bonds between proton acceptors (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and proton donors (N–H) in the poly(benzimidazole imide)s.12,13,23 Generally, the locations of the N–H and imide C
O) and proton donors (N–H) in the poly(benzimidazole imide)s.12,13,23 Generally, the locations of the N–H and imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching bands displayed a displacement to higher frequencies with reducing H-bond content in the co-PBIIs.12,13,24 As the results depict in Fig. 1b, the peak of N–H stretching (∼3340 cm−1) was hardly observed in N-PhPABZ-PMDA and an up frequency shift occurred for the regions after the introduction of N-Ph-benzimidazole units. This is explained by the fact that the N-Ph substituent effectively declined the chance of H-bond formation through the placement on the imidazole nitrogen atoms. Further evidence could be found in the bonds of the asymmetric and symmetric stretching of imide carbonyl groups, which occurred in the vicinity of 1770 and 1710 cm−1 (Fig. 1c and d).
O stretching bands displayed a displacement to higher frequencies with reducing H-bond content in the co-PBIIs.12,13,24 As the results depict in Fig. 1b, the peak of N–H stretching (∼3340 cm−1) was hardly observed in N-PhPABZ-PMDA and an up frequency shift occurred for the regions after the introduction of N-Ph-benzimidazole units. This is explained by the fact that the N-Ph substituent effectively declined the chance of H-bond formation through the placement on the imidazole nitrogen atoms. Further evidence could be found in the bonds of the asymmetric and symmetric stretching of imide carbonyl groups, which occurred in the vicinity of 1770 and 1710 cm−1 (Fig. 1c and d).
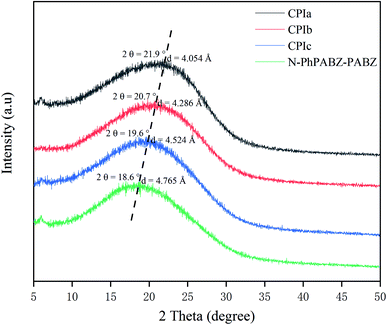
The polymer chain packing was estimated by wide-angle X-ray diffraction (WAXD, Fig. 2). All PBII films exhibited a broad diffraction halo at 2θ near 20°, suggesting their amorphous character. The phenomena were not in accordance with those of traditional PBIIs, which possess dense chain packing and highly ordered molecular arrangements with their unique H-bonding.9,25 Herein, the formation of such hydrogen bonds was completely forbidden by the N-substituents, and the bulky rigid side groups enhanced the difficulty of the tight molecular aggregation formation. As a result, the peaks at 2θ = ∼20° could be assigned to the intermolecular packing order of aromatic heterocyclic polymers and the calculated d-spacing values (4.054–4.765 Å) were close to the mean intermolecular distance.16 Obviously, the values increased as the content of N-PhPABZ increased, which confirmed the fact that the bulkiness of the N-Ph moieties reduced the chain packing density effectively.
3.2 Properties of polyimides
The H2O-absorption character was susceptible to the chain packing as well as the content of polar units and hydrophilic groups for the polymers. Benzimidazole moieties, as water-affinity-promoting structures, tended to form H-bonds with water molecules, and the benzimidazole-containing polymers generally displayed high water absorption values, i.e., the WA was up to 6% for the PBIIs derived from PABZ and 3,3′,4,4′-biphenyl tetracarboxylic dianhydride (BPDA).3,9 In the series shown, the result indicated that the N-Ph group effectively decreased the WA values of these poly(benzimidazole imide)s, which was evidenced by the reducing water absorption with increasing N-PhPABZ content (Table 2). The cause for the improved WA was that the N-substituent effectively blocked the formation of hydrogen-bonded water molecules, as described in our previous research.18| PIs | Tga (°C) | Td5%b (°C) | CTEc (ppm K−1) | E (GPa) | σ (MPa) | ε (%) | WA (%) |
|---|---|---|---|---|---|---|---|
| a Glass transition temperature was measured by DMA at a heating rate of 5 °C min−1 at 1 Hz.b 5% weight loss temperature measured by TGA in nitrogen at a heating rate of 5 °C min−1.c Coefficient of thermal expansion along the X–Y direction, measured in the range of 150–250 °C at a heating rate of 5 °C min−1. | |||||||
| CPIa | 465 | 552 | −0.6 | 8.1 | 197 | 5.0 | 4.8 |
| CPIb | 471 | 551 | 1.9 | 6.1 | 192 | 7.1 | 3.4 |
| CPIc | 478 | 553 | 4.9 | 5.6 | 173 | 7.9 | 1.9 |
| N-PhPABZ-PMDA | 486 | 554 | 9.7 | 4.7 | 158 | 8.9 | 1.2 |
As known, when polyimides experience high temperature, the rigid conjugated backbones facilitate heat conduction along the chains; therefore, polyimides with rigid chains usually possess higher thermal decomposition temperatures compared to those containing flexible bonds.26,27 According to the theory, the unaltered rigidity of these poly(benzimidazole imide)s with N-Ph groups led to the retention of the superheat-resistance of the polyimides containing benzimidazoles. Thus, these films had relatively good thermal stability, as suggested from the 5% weight loss values (Td5% = 551–554 °C), and the values did not change much with increasing N-PhPABZ content, which is summarized in Fig. 3 and Table 2.
As expected, these PBII films displayed a high Tg over 450 °C (Table 1), which was determined by the peak temperature of the tan delta curve (Fig. 4). Generally, the hydrogen bonds, due to strong intermolecular interaction, induced the occurrence of chain orientation by which the chain spacing decreased and the intermolecular interaction increased. In this case, the introduction of H-bonding in the polyimide backbones would result in an increase in their thermal properties; otherwise, the trend was contrary. Interestingly, when the H-bond content declined, the Tg of the resultant copoly(benzimidazole imide)s did not show a downward trend but rose instead. It could be explained by the fact that a large number of N-Ph units with obvious steric hindrance were able to limit the rotational freedom around the polymer backbone when polyimides experienced high temperature. The result exhibited that the poly(benzimidazole imide)s derived from N-PhPABZ achieved high Tg without the intermolecular hydrogen bonds and the value for N-PhPABZ-PMDA was as high as 486 °C.
The dimensional stability of these PBII films was estimated by their CTE values. As depicted in Table 2 and Fig. 5, the results showed a low level ranging −0.6–9.7 ppm K−1, which was superior to most reported polyimide films.28,29 The CTE was believed to be susceptible to the degree of in-plane orientation governed by interchain interactions and chain stiffness/linearity for polyimides.30,31 The N-Ph groups could undermine the regular arrangement, and the intermolecular spacing was enlarged with increasing content of N-Ph, which was confirmed by WAXD. Thus, increasing the content of N-Ph would reduce the intermolecular interactions and degree of chain orientation, resulting in a drop in the dimensional stability of these copoly(benzimidazole imide)s. As a consequence, the CTE values increased to some degree after the introduction of N-PhPABZ. However, CTEs below 10 ppm K−1 were sufficient for AMOLED application. Additionally, the copolymer containing 75 mol% PABZ displayed a slightly negative CTE, which was related to the high chain rigidity that came from the fully rod-like PMDA and PABZ.16,32
Mechanical analysis data of the PBII films are summarized in Fig. 6 and Table 2. It provided tensile moduli from 4.7 to 8.1 GPa depending on the content of N-PhPABZ. Young's modulus is the parameter for mechanical hardness, which is usually subject to molecular packing coefficients. Herein, it implied that the highly well-packed PABZ-PMDA would possess the highest modulus among these polyimides; however, the high brittleness resulting from the excessive rigidity reduced its film-forming ability. The incorporated N-Ph moieties caused the reduction of the modulus and enhanced the flexibility, as suggested from the elongation at break. The result indicated that the brittleness of PABZ-PMDA was healed by copolymerization with N-PhPABZ. This meant that the loose molecular packing caused by the bulky N-substituent enhanced the possibility of sufficient chain entanglement, which improved the flexibility and toughness of this series of films, for example, ε = 8.9% for N-PhPABZ-PMDA. On the other hand, the stresses of these polyimides were in the range of 158–197 MPa. When the N-PhPABZ content increased, the continuously expanding intermolecular distance would weaken the physical interaction between chain segments, and the stresses at break decreased as a result. However, the tensile strength (>150 MPa) was sufficient for further use.
4. Conclusions
A series of PMDA-based poly(benzimidazole imide)s were prepared by N-PhPABZ and PABZ. The introduction of the N-Ph group, leading to loose chain segment packing and hindered H-bonding, improved the flexibility and film-forming ability of the poly(benzimidazole imide)s derived from highly rigid PMDA. The chemical modification with significant steric hindrance avoided the degradation of thermostability coming from the H-bonding loss. Thus, the presented series showed an unconventional trend where the Tg values of these poly(benzimidazole imide)s continued to increase when the H-bond content decreased. Among them, N-PhPABZ-PMDA displayed an outstandingly high Tg of up to 486 °C. Although copolymerization with increasing N-PhPABZ content inevitably led to some degree of undermining of the dimensional stability and tensile strength, N-PhPABZ-PMDA still retained sufficient properties for use as a flexible substrate including CTE = 9.7 ppm K−1 and σ = 158 MPa. This study provides new poly(benzimidazole imide)s without hydrogen bonds to develop AMOLED flexible substrates.Conflicts of interest
There are no conflicts to declare.References
- M. C. Choi, Y. Kim and C. S. Ha, Prog. Polym. Sci., 2008, 33, 581–630 CrossRef CAS.
- P. Heremans, A. K. Tripathi, A. de Jamblinne de Meux, E. C. P. Smits, B. Hou, G. Pourtois and G. H. Gelinck, Adv. Mater., 2016, 28, 4266–4282 CrossRef CAS PubMed.
- M. Hasegawa, Y. Hoshino, N. Katsura and J. Ishii, Polymer, 2017, 111, 91–102 CrossRef CAS.
- G. Qian, F. Dai, H. Chen, M. Wang, M. Hu, C. Chen and Y. Yu, J. Polym. Sci., 2021, 59, 510–518 CrossRef CAS.
- X. Gao, L. Lin, Y. Liu and X. Huang, J. Disp. Technol., 2015, 11, 666–669 CAS.
- M. Lian, X. Lu and Q. Lu, Macromolecules, 2018, 51, 10127–10135 CrossRef CAS.
- S. Wang, H. Zhou, G. Dang and C. Chen, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2024–2031 CrossRef CAS.
- J. Liu, Q. Zhang, Q. Xia, J. Dong and Q. Xu, Polym. Degrad. Stab., 2012, 97, 1979–1987 CrossRef.
- G. Qian, H. Chen, G. Song, F. Dai, C. Chen and J. Yao, Polymer, 2020, 196, 122482 CrossRef CAS.
- H. Chen, F. Dai, M. Hu, C. Chen, G. Qian and Y. Yu, J. Polym. Sci., 2021, 59, 1942–1951 CrossRef CAS.
- L. Luo, J. Yao, X. Wang, K. Li, J. Huang, B. Li and X. Liu, Polymer, 2014, 55, 4258–4269 CrossRef CAS.
- G. Song, Y. Zhang, D. Wang, C. Chen, H. Zhou, X. Zhao and G. Dang, Polymer, 2013, 54, 2335–2340 CrossRef CAS.
- Y. Zhuang, X. Liu and Y. Gu, Polym. Chem., 2012, 3, 1517–1525 RSC.
- G. Qian, H. Chen, G. Song, J. Yao, M. Hu and C. Chen, J. Polym. Sci., 2020, 58, 969–976 CrossRef CAS.
- Q. Xia, J. Liu, J. Dong, C. Yin, Y. Du, Q. Xu and Q. Zhang, J. Appl. Polym. Sci., 2013, 129, 145–151 CrossRef CAS.
- S. I. Kim, T. J. Shin, S. M. Pyo, J. M. Moon and M. Ree, Polymer, 1999, 40, 1603–1610 CrossRef CAS.
- R. Moonhor, T. J. Shin and S. W. Lee, Macromol. Res., 2001, 9, 1–19 Search PubMed.
- G. Qian, F. Dai, H. Chen, M. Wang, M. Hu, C. Chen and Y. Yu, RSC Adv., 2021, 11, 3770–3776 RSC.
- P. Musto, F. E. Karasz and W. J. MacKnight, Polymer, 1993, 34, 2934–2945 CrossRef CAS.
- S. Qing, W. Huang and D. Yan, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 4363–4372 CrossRef CAS.
- Y. K. Xu, M. S. Zhan and K. Wang, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 2490–2501 CrossRef CAS.
- S. Diaham, M. L. Locatelli, T. Lebey and D. Malec, Thin Solid Films, 2011, 519, 1851–1856 CrossRef CAS.
- X. Liu, G. Gao, L. Dong, G. Ye and Y. Gu, Polym. Adv. Technol., 2009, 20, 362–366 CrossRef CAS.
- T. K. Ahn, M. Kim and S. Choe, Macromolecules, 1997, 30, 3369–3374 CrossRef CAS.
- L. Luo, Y. Dai, Y. Yuan, X. Wang and X. Liu, Macromol. Rapid Commun., 2017, 38, 1700404 CrossRef PubMed.
- Y. N. Sazanov, F. S. Florinsky and M. M. Koton, Eur. Polym. J., 1979, 15, 781–786 CrossRef CAS.
- J. Lin and D. C. Sherrington, Adv. Polym. Sci., 1994, 111, 177–219 CrossRef CAS.
- C. E. Sroog, Prog. Polym. Sci., 1991, 16, 561–694 CrossRef CAS.
- S. Numata, N. Kinjo and D. Makino, Polym. Eng. Sci., 1988, 28, 906–911 CrossRef CAS.
- J. H. Jou, P. T. Huang, H. C. Chen and C. N. Liao, Polymer, 1992, 33, 967–974 CrossRef CAS.
- M. Hasegawa, T. Matano, Y. Shindo and T. Sugimura, Macromolecules, 1996, 29, 7897–7909 CrossRef CAS.
- Y. Takeuchi, F. Yamamoto and Y. Shuto, Macromolecules, 1986, 19, 2059–2061 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |