Open Access Article
Open Access ArticleGoethite/montmorillonite adsorption coupled with electrocoagulation for improving fluoride removal from aqueous solutions†
Jiali Kanga,
Junfeng Li *a,
Chengxiao Maa,
Lijuan Yib,
Tiantian Gub,
Jiankang Wanga and
Shenglin Liuc
*a,
Chengxiao Maa,
Lijuan Yib,
Tiantian Gub,
Jiankang Wanga and
Shenglin Liuc
aSchool of Water Conservancy and Architectural Engineering, Shihezi University, Shihezi, 832000, PR China. E-mail: ljfshz@126.com
bKey Laboratory for Green Process of Chemical Engineering of Xinjiang Bingtuan, School of Chemistry and Chemical Engineering, Shihezi University, Xinjiang 832003, PR China
cXinjiang Western Eclogue Agricultural Science and Technology Co. Ltd, Shihezi 832000, PR China
First published on 7th March 2022
Abstract
With the increasing problem of fluoride pollution, it is urgent to find an efficient method to remove fluoride (F−). In this study, a new material goethite–montmorillonite-sorbent (GMS) was prepared and added into the electrocoagulation (EC) reaction to form a new pathway (EC/GMS) for the removal of fluoride. Scanning electron microscopy (SEM), Brunauer–Emmett–Teller (BET), Fourier-transform infrared (FT-IR), X-ray photoelectron spectroscopy (XPS) and other characterization methods were used to analyze the properties of GMS. The fluoride removal performance and mechanism of EC/GMS was studied. The results showed that GMS could provide numerous adsorption sites. EC/GMS could achieve a high removal efficiency of 95.98% and lower energy consumption of 0.58 kW h m−3 for 60 min. EC/GMS could achieve a removal efficiency of 99.47% after optimization by single-factor experiments and RSM-BBD optimal experiments. Meantime, the removal rate of the EC/GMS still reached over 87% after six cycles. The kinetic analysis indicated that the degradation pathways could also achieve a high removal rate for high fluoride-containing concentration solutions within a short time. The stretching vibration of C–F and C–O and the existence of F− revealed that the electrophoresis of the electrodes, adsorption of GMS, and co-precipitation of flocs were the main removal pathways, and the accelerating effect between the electrocoagulation and adsorption process was addressed. This study provides a new pathway for removing fluoride from aqueous environments.
1. Introduction
Fluoride (F−) is an anion that occurs naturally in groundwater, and commonly originates from weathered rocks that contain fluorine-containing minerals, such as fluorite, fluorspar, cryolite, apatite, and wollastonite.1 However, the erosion and weathering of fluoride minerals and the discharge of industrial wastewater exacerbate fluoride pollution.2 The intake of F− within the allowable range is beneficial to human health and life activities, and can maintain the health of teeth and bones.3 However, prolonged exposure to an environment containing excessive F−can cause tooth and bone fluorosis,4 muscle fibrosis, low haemoglobin levels, thirst and structural changes in DNA.2,4,5 More than 260 million people worldwide use water in which the F−content exceeds the limit.6 In the remote areas of north-western China and some rural areas without a centralised water supply, fluoride pollution is more prominent and difficult to solve. Therefore, the demand for improved water quality and guaranteed safety is fundamental for developing water treatment science and technology.7Currently, the main technologies that remove fluorides, include reverse osmosis,8,9 nanofiltration,10–12 precipitation-coagulation,13,14 electrocoagulation,15–17 adsorption5,18,19 and ion-exchange.20,21 The first three methods are rarely used for fluoride removal because of unmet discharge requirements, membrane pollution, and cumbersome treatment. Comparatively, electrocoagulation performs well for the removal of pollutants.22,23 Several studies have used continuous electrochemical reactions to investigate the removal of fluoride, achieving a removal efficiency of 80% or more.17,24,25 However, electrocoagulation has limitations, such as high-energy consumption and cumbersome sludge treatment.26–29 As pollution worsens and the standards of sewage discharge become stricter, electrocoagulation often needs to be combined with other technologies to meet the treatment requirements.30 In previous studies, electrocoagulation and membrane separation were combined.9,31 However, membrane fouling has restricted the popularization and application of this method. Based on comparison of factors such as the site, operation, and operating costs, adsorption is considered to be the most economical and effective technology for the decentralised reduction of fluoride pollution.19,32,33 Many studies have evaluated the efficacy of adsorption for the removal of fluoride, and the results showed that new composite adsorbents, prepared by various methods, had good adsorption capacity for fluoride, indicating that adsorption can effectively remove fluoride from water.5,15,32,34 Narayanan N. V. et al. used batch stirring electro-flocculation reactor, the feasibility of Fe-aluminium electrode combined with granular activated carbon adsorption to remove hexavalent chromium in synthetic wastewater.35 Malakootian M. et al. investigated the differences in the decolourization ability and operating cost of indigo carmine in aqueous solution between four different commercial activated carbon combined with electrocoagulation technology and traditional electrocoagulation technology. Under lower current density and working time, the energy consumption of traditional electrocoagulation is 3.41 kW h kg−1, while the energy consumption of EC/GAC strengthening technology is only 1.35 kW h kg−1 for 90 minutes.36 Although the synthesised adsorbents have a large specific surface area, the high cost of these materials limits their practical application.37 Compared with these synthetic materials, goethite is a low-cost ore mineral that is available in large quantities in China. Studies have shown that although natural goethite is a promising adsorbent for removing pollutants from water,38–40 it has a low specific surface area, resulting in a relatively low adsorption rate. Considering these limitations, namely the cost and adsorption capacity, herein, natural goethite, montmorillonite and soluble starch were used to develop a new adsorbent (GMS) with a higher specific surface area. There are few studies about the removal of fluoride from water by electrocoagulation and adsorption coupling method. So, the GMS were put into the EC reaction to produce a new fluoride removing pathway (EC/GMS). With this approach, electrocoagulation and adsorption can be coupled to improve the removal efficiency and accelerate the reaction velocity.
In this study, a batch reactor integrated electrocoagulation and adsorption is set up and an adsorbent is prepared from modified particulate goethite. The removal capacity of the electrocoagulation (EC) and electrocoagulation–adsorption (EC/GMS) systems is compared, and response surface methodology is used to analyse the optimal operating conditions for EC/GMS. To provide a theoretical reference for future research, the mechanism of fluoride removal via EC/GMS is clarified and the interaction between electrocoagulation and adsorption is also discussed.
2. Materials and methods
2.1 Raw materials
Goethite was purchased from the Hubei Wande Chemical Co., Ltd (the content of goethite ≥99%). The main materials used were montmorillonite (MMT), soluble starch, sodium fluoride (NaF), sodium chloride (NaCl), hydrochloric acid (HCl), and sodium hydroxide (NaOH). And the above reagents are all analytical pure.2.2 Preparation of adsorbent
To prepare the absorbent, goethite was mixed with montmorillonite in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, after which soluble starch was added to approximately half of the total dose. The mixture was rubbed into spherical particles with a uniform size after adding deionised water. The particles were placed in a muffle furnace at 700 °C for 60 min to obtain the goethite–montmorillonite sorbent (GMS).
1, after which soluble starch was added to approximately half of the total dose. The mixture was rubbed into spherical particles with a uniform size after adding deionised water. The particles were placed in a muffle furnace at 700 °C for 60 min to obtain the goethite–montmorillonite sorbent (GMS).
2.3 Experimental device
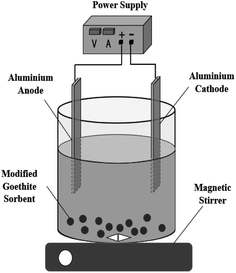
A reactor in which electrocoagulation and adsorption were combined was used to investigate the effect of EC/GMS for treating fluorinated water (Fig. 1). The device consisted of a plastic measuring cup with an effective volume of 1000 mL and a clear water tank. In the reaction cell, two Al electrodes were used as the anode and cathode, separated by a distance of 1 cm, and the adsorbent was placed at the bottom. In the experiment, the anode and cathode were connected to a DC power supply model (MS-155D). Sodium fluoride and deionised water were used to prepare fluorinated water (300 mL) for treatment, while NaCl was used as the electrolyte in the reaction cell for the removal of fluoride. The experiments were performed at room temperature.2.4 Characterization method
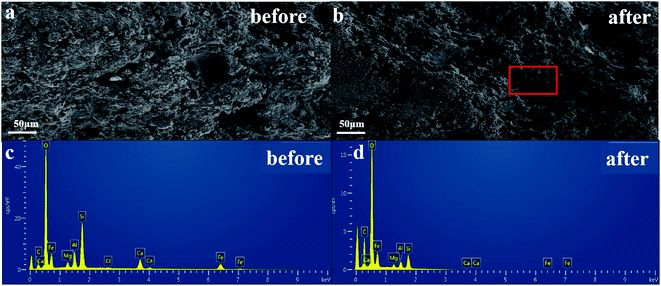
Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX) (FEI Quanta 650, FEI Company, U. S. A.) were used to analyse the surface morphology and elemental compositions of the particles before and after sintering. X-ray diffraction (XRD) (Ultima-IV, Rigaku, Japan), the Brunauer–Emmett–Teller (BET) method (Micromeritics Instrument Ltd, Corp., USA) and Fourier-transform infrared spectroscopy (FT-IR) (Thermo Fisher, USA) were used to record the crystalline texture, specific surface area, and surface properties of GMS. X-ray photoelectron spectroscopy (XPS) (Thermo Scientific Escalab250Xi) and Fourier-transform infrared spectroscopy (FT-IR) (Thermo Fisher, USA) were used to analyse the elements, valence states, and other properties on the surface of the EC-flocs, EC/GMS-flocs, electrodes, and adsorbent after the reaction.2.5 Response Surface Methodology (RSM)
To optimise the combination with the best removal rate, the data processing software, Design-Expert11.0, was used to design the response surface method. The RSM-BBD method was used to evaluate the effects of three factors, namely the electrolysis time, initial pH, and current density on the rate of fluoride removal from fluorinated water. Seventeen experiments were designed, and the central point was repeated five times. Three levels were set: high (–1), medium (0), and low (−1). The encoded variables and the level of response of the surface method were shown in Table S1.† The experimental data were fitted and analysed by using the software.2.6 Experimental methods
NaCl (1 M) and the electrodes were placed in the configured fluoride-containing solution (300 mL). Samples were taken every 10 min after the power supply was switched on. The reaction was performed in two modes: EC (without adsorbent) and EC/GMS (add adsorbent). The electrolysis time, initial pH (5, 6, 7, 8, and 9, respectively), current density (6, 12, and 24 mA cm−2, respectively), adsorbent dosage (5, 10, and 15 g, respectively), and initial fluoride concentration (5, 10, and 15 mg L−1, respectively) were controlled to evaluate the efficacy of EC and EC/GMS for removing fluoride. A WS100 portable fluoride ion concentration meter was used to measure the concentration of fluoride in the sample. HCl and NaOH (1 mol L−1, respectively) were used to adjust the pH of the solution. The removal rate of fluoride was calculated from eqn (1).| R = (C0 − Ct)/C0 × 100% | (1) |
EEC = 1000Pt/(60V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(C0/Ct)) log(C0/Ct))
| (2) |
To determine the removal mechanism and reaction rate of the pollutant, kinetic analysis was performed with different initial concentrations of fluoride. Based on this analysis, the removal of fluoride followed a pseudo-first-order model, as shown in eqn (3).
| ln(C0/Ct) = −kt | (3) |
3. Results and discussion
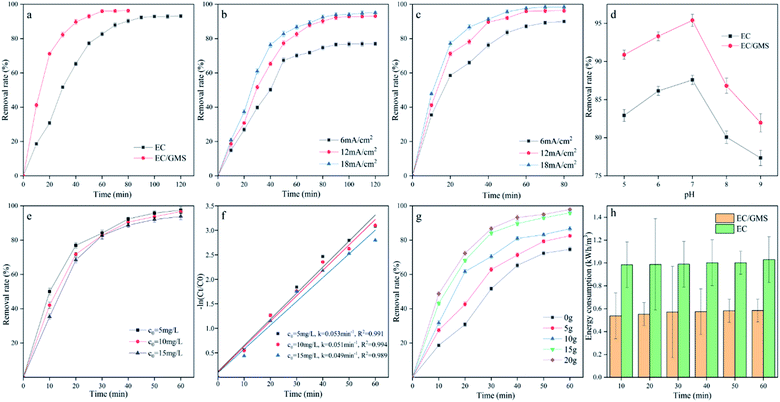
3.1 Comparison of EC and EC/GMS for the removal of fluoride
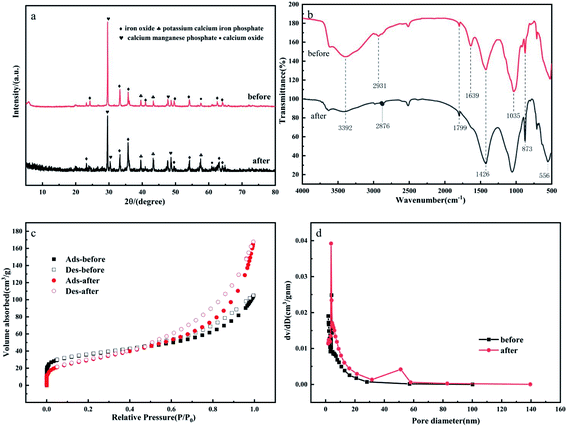
The XRD pattern of GMS in Fig. 3a shows that the main crystalline phases are goethite, montmorillonite and nontronite.43 The characteristic peaks represent iron oxide, potassium calcium iron phosphate, calcium manganese phosphate, and calcium oxide, respectively. The XRD pattern of GMS show low-intensity peaks and broadening of the diffraction peaks after modification. These changes correspond to the small particulate sizes and low crystallinity of the modified goethite. The FT-IR spectra of GMS before and after modification are shown in Fig. 3b. The peak at 3392 cm−1 is related to the hydroxyl group on the surface of the GMS, which become weaker after firing, possibly due to decomposition of water and some hydroxides during the high-temperature calcination. The peaks at 2931 cm−1 and 2876 cm−1, corresponding to the asymmetric stretching vibration of the sp3 hybridised –CH3 and –CH2– groups, indicate that there are few aliphatic hydrocarbons.44 The peaks at 1799 cm−1 and 1639 cm−1 correspond to the stretching vibrations of the C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.45 The peak at 1426 cm−1 corresponds to the bending vibration of the C–H bond in the vinyl group C
O bonds, respectively.45 The peak at 1426 cm−1 corresponds to the bending vibration of the C–H bond in the vinyl group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2.46 The peaks at 1035 cm−1 and 873 cm−1 correspond to the symmetric stretching vibrations of the Si–O and Si–O–Si bonds, respectively. The peak at 556 cm−1 corresponds to the vibration of the Fe–O bond (haematite).47 The adsorption of nitrogen and the pore size distribution curve are used to characterise the specific surface areas of GMS before and after modification. Fig. 3c shows that the N2 adsorption–desorption isotherms are type-II, which confirm that GMS is a multilayer adsorbent. The data in Fig. 3d indicate that the sorbent has a mesoporous structure on the nanoscale, where the pore size distribution is centred between 2 and 30 nm. Table 1 shows that the surface area and pore volume of GMS after modification are 128.1656 m2 g−1 and 0.2475 cm3 g−1, respectively, indicating that GMS should provide numerous adsorption sites and should be effective for fluoride adsorption.
CH2.46 The peaks at 1035 cm−1 and 873 cm−1 correspond to the symmetric stretching vibrations of the Si–O and Si–O–Si bonds, respectively. The peak at 556 cm−1 corresponds to the vibration of the Fe–O bond (haematite).47 The adsorption of nitrogen and the pore size distribution curve are used to characterise the specific surface areas of GMS before and after modification. Fig. 3c shows that the N2 adsorption–desorption isotherms are type-II, which confirm that GMS is a multilayer adsorbent. The data in Fig. 3d indicate that the sorbent has a mesoporous structure on the nanoscale, where the pore size distribution is centred between 2 and 30 nm. Table 1 shows that the surface area and pore volume of GMS after modification are 128.1656 m2 g−1 and 0.2475 cm3 g−1, respectively, indicating that GMS should provide numerous adsorption sites and should be effective for fluoride adsorption.
 | ||
| Fig. 3 (a) XRD patterns, (b) FT-IR spectra, (c) N2 adsorption–desorption isotherms, and (d) pore size distribution of GMS before and after modification. | ||
| GMS | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore diameter (nm) |
|---|---|---|---|
| Before modification | 107.4174 | 0.1577 | 7.9557 |
| After modification | 128.1656 | 0.2475 | 6.2204 |
| nAl3− − (3n − m)OH− − mF− → AlnFm(OH)3n−m | (4) |
| Aln(OH)3n − mF− → AlnFm(OH)3n−m − mOH− | (5) |
| Fe(OH)3 − 3F− → FeF3 − 3OH− | (6) |
3.2 RSM for optimization of the key parameters
To further explore the interaction of various factors and develop an optimal combination scheme, the Box–Behnken design (BBD) was used to evaluate the correlation between different factors and the removal of aqueous fluoride during EC/GMS; to implement the BBD, the results of the single factor test were used. The data processing software, Design-Expert 11.0, was used to design the response surface method. In this method, the removal rate of fluoride was the response variable, while time, current density, and initial pH were the three independent operating factors (Table S1†). The test scheme and results are listed in Table S2.†Based on the BBD, the removal of fluoride ranged from 57.5 to 97.42% for the experimental combination. A regression model equation (eqn (7)) was used to fit the experimental data, and was used to analyse and predict the optimal level for improving the removal rate.
| Y = 95.51 − 12.39A − 5.48B − 5.57C − 1.94AB − 0.087AC − 0.48BC − 9.73A2 − 4.42B2 − 10.48C2 | (7) |
The variance (ANOVA) of the quadratic response surface model is shown in Table S3.† In this model, the value of P < 0.0001 indicates that the obtained level was extremely significant, while the missing term, P = 0.5249 > 0.05, was not significant, which indicates that the simulation was good.60 The adjusted regression coefficient (R2adj = 0.9947) was close to R2, which indicates that the model explained 99.47% of the change in the response values. The fit of the equation was good and could be used to optimise the analysis and predict the test results. The F-value revealed that the influence of the three operational factors on the removal rate of fluoride decreased in the order, time > initial pH > current density.61
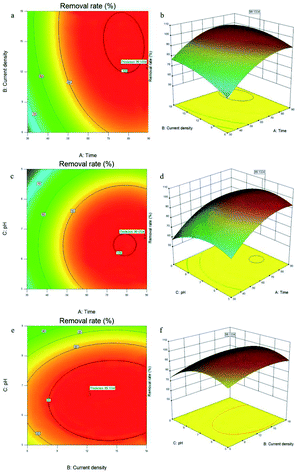
The response surface diagram shows the optimal level of a single operating parameter, which was derived from the interaction between two independent factors (Fig. 5a–f). Elliptical shapes were generated due to perfect interactions between the optimal predicted values and various parameters.62 Fig. 5a and b show the effects of the time and current density on the removal of fluoride. The removal rate increased and then stabilised, but the surface had a greater degree of curvature for time than for the current density, which indicates that time had a greater impact on the removal rate than the current density. Comparatively, the contour maps of these variables became elliptical, which indicates that the interaction of these variables influenced the removal rate.63 The effect of the time and initial pH on the removal is shown in Fig. 5c and d. The relatively flat surfaces indicate that both of these variables largely influenced the removal rate. The variation of the removal rate was similar to that in the single-factor experiment. The contour maps confirmed that the interaction of these variables influenced the removal rate. Fig. 5e and f shows that the initial pH and current density first increased the removal rate, which then decreased as the initial pH and current density increased. The contour maps similarly confirmed that the interaction of these variables influenced the removal rate. All the plots showed an optimal removal rate of 99.1334%.
The BBD was used to predict the optimal experimental conditions for the removal of fluoride using EC/GMS. These optimal experimental conditions (79.919 min, 6.724, and 11.303 mA cm−2) afforded a removal efficiency of 99.47%. To check the accuracy of the BBD, three groups of parallel experiments were conducted to predict the optimal conditions. The average percentage of fluoride removed was 98.19%, which differed from the predicted value by 1.28%. Therefore, the RSM-BBD model showed good predictive power, which indicates that it was useful for optimising the parameters.
3.3 Analysis of mechanism and reusability of method
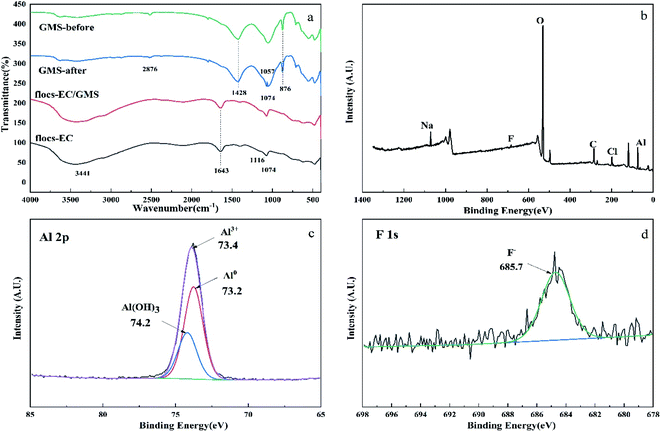
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–F bonds, respectively. These spectra showed that the hydroxyl group is the main functional group responsible for the removal of fluoride and the existence of fluoride on the flocs.
O and C–F bonds, respectively. These spectra showed that the hydroxyl group is the main functional group responsible for the removal of fluoride and the existence of fluoride on the flocs.
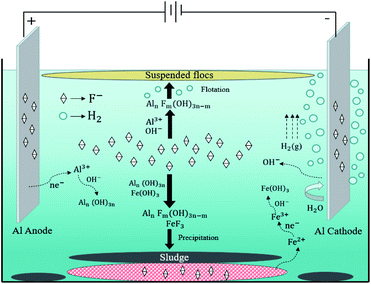
XPS was used to identify the elemental composition of the surface of the flocs after EC/GMS process (Fig. 6b–d). In addition to the C 1s, O 1s, Na 1s, Cl 2p, and Al 2p peaks of the solution, the full-spectrum scan of the flocs showed an obvious F 1s peak around 680 eV, indicating the removal of F− (Fig. 6b).66 The Al 2p XPS profile of the flocs displayed three characteristic peaks at 73.2, 73.4, and 74.2 eV, which indicated the presence of Al, Al3–, and Al(OH)3, respectively67 (Fig. 6c). Specifically, the presence of Al3– and Al(OH)3 suggests that the removal of fluoride depended on chemical adsorption and co-precipitation. The peak at 685.7 eV showed that ionic fluoride was the only fluoride species that was removed68 (Fig. 6d). The flocs were removed when F− replaced the OH− group of [Aln(OH)3n]. Additionally, the fluoride and hydroxide ions co-precipitated with Al3– ions to form [AlnFm(OH)3n−m]. XPS was used to analyse the Al electrodes and GMS after defluoridation. The results of this analysis proved that ionic fluoride existed on the surficial layers (Table 2). The results also demonstrate that electrophoresis of the electrodes and adsorption of the GMS remove fluoride.52 The FT-IR and XPS results proved some of these fluoride removal pathways as shown in figure: (i) chemical adsorption and co-precipitation with other ions; (ii) adsorption of GMS; (iii) electrophoresis of the electrodes. Combined with the previous experimental results that obtained from the influence of electrolysis time and mechanism analysis, it is believed that the addition of electrophoresis, adsorption and other processes may accelerate the electrocoagulation process (Fig. 7).
| Atomic concentration (%) | Cl 2p | F 1s | Fe 2p | Na 1s | Al 2p | C 1s |
|---|---|---|---|---|---|---|
| Anode | 3.62 | 1.58 | 0.80 | 2.88 | 52.57 | 38.56 |
| Cathode | 2.77 | 0.80 | 1.11 | 1.45 | 50.91 | 42.96 |
| GMS | 2.54 | 3.05 | 8.42 | 1.80 | 25.75 | 58.43 |
4. Conclusions and perspectives
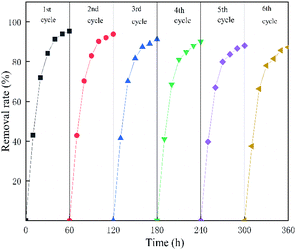
EC/GMS, a coupled method, was developed and used for the removal of fluoride from water. SEM-EDX, FT-IR, BET, and XRD analyses confirmed that modified GMS had good fluoride adsorption and removal properties. The addition of GMS can reduce costs when compared to the simple EC, which requires more energy consumption and a longer electrolysis time. EC/GMS could achieve a faster removal efficiency when compared to EC at the same electrolysis time, pH, and current density. Single-factor experiments and RSM-BBD optimization experiments revealed that EC/GMS achieved a removal efficiency of 99.47%. Evaluation of the reaction kinetics with different fluoride concentrations showed that the removal rate of the device was quick for solutions that contained a high and low concentration of fluoride. The electrodes, GMS, and flocs were also characterised. Electrophoresis of the electrodes, adsorption of GMS, and co-precipitation of flocs were the main processes by which fluoride was removed. The reuse performance of this method was also good.Nowadays, one of the most significant challenges is to develop adequate technologies for fluoride treatment. Therefore, it is essential to guarantee an appropriate operation with high volume and flow rate for aqueous solutions to expand the EC/GMS technology for the welfare of humans and the environment.
Author contributions
J. L. Kang: conceptualization, methodology, software, data curation, writing–original draft preparation; T. T. Gu, C. X. Ma, J. K. Wang and S. L. Liu: visualization, investigation; J. F. Li: supervision, involved in conceptualization, methodology, software and english revising; L. J. Yi: involved in software and validation.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
Financial support from the National Natural Science Foundation of China (22109106, 42107414) and the Key Production Innovative Development Plan of the Southern Bingtuan (2019DB007, 2020AB024) are gratefully acknowledged.References
- K. K. Yadav, N. Gupta, V. Kumar, S. A. Khan and A. Kumar, Environ. Int., 2018, 111, 80–108 CrossRef CAS PubMed.
- Meenakshi and R. C. Maheshwari, J. Hazard. Mater., 2006, 137, 456–463 CrossRef CAS PubMed.
- G. Biswas, M. Kumari, K. Adhikari and S. Dutta, Curr. Pollut. Rep., 2017, 3, 104–119 CrossRef CAS.
- A. M. Gbadebo, Environ. Geochem. Health, 2012, 34, 597–604 CrossRef CAS PubMed.
- U. S. Rashid and A. N. Bezbaruah, Chemosphere, 2020, 252, 126639 CrossRef CAS PubMed.
- M. Amini, K. Mueller, K. C. Abbaspour, T. Rosenberg, M. Afyuni, K. N. Moller, M. Sarr and C. A. Johnson, Environ. Sci. Technol., 2008, 42, 3662–3668 CrossRef CAS PubMed.
- N. Mumtaz, G. Pandey and P. K. Labhasetwar, Crit. Rev. Environ. Sci. Technol., 2015, 45, 2357–2389 CrossRef CAS.
- V. N. Karbasdehi, S. Dobaradaran, A. Esmaili, R. Mirahmadi, F. F. Ghasemi and M. Keshtkar, Data Brief, 2016, 8, 867–870 CrossRef PubMed.
- K. Bani-Melhem and R. Elektorowicz, Environ. Sci. Technol., 2010, 44, 3298–3304 CrossRef CAS PubMed.
- W.-F. Ma and W.-J. Liu, Huanjing Kexue, 2009, 30, 787–791 CAS.
- M. Tahaikt, R. El Habbania, A. A. Haddou, I. Acharya, Z. Amora, M. Takya, A. Alamil, A. Boughriba, A. Hafsil and A. Elmidaoui, Desalination, 2007, 212, 46–53 CrossRef CAS.
- M. Valentukeviciene, R. Zurauskiene and Y. A. Boussouga, Ecol. Chem. Eng. S, 2019, 26, 133–147 CAS.
- R. P. Liu, B. Liu, L. J. Zhu, Z. He, J. W. Ju, H. C. Lan and H. J. Liu, J. Environ. Sci., 2015, 32, 118–125 CrossRef CAS PubMed.
- W.-X. Gong, J.-H. Qu, R.-P. Liu and H.-C. Lan, Colloids Surf., A, 2012, 395, 88–93 CrossRef CAS.
- Y. Ye, Y. Wei, Y. Gu, D. Kang, W. Jiang and J. Kang, J. Alloys Compd., 2020, 838, 155528 CrossRef CAS.
- B. Palahouane, A. Keffous, M. W. Naceur, M. Hecini, O. Bouchelaghem, A. Lami, K. Laib, A. Manseri and N. Drouiche, Desalin. Water Treat., 2020, 183, 240–247 CrossRef CAS.
- L. F. Castaneda, O. Coreno, J. L. Nava and G. Carreno, Chemosphere, 2020, 244, 125417 CrossRef CAS PubMed.
- Z. He, R. Liu, J. Xu, H. Liu and J. Qu, Sep. Purif. Technol., 2015, 148, 68–75 CrossRef CAS.
- F. Zhu, Z. Guo and X. Hu, J. Hazard. Mater., 2020, 397, 122789 CrossRef CAS PubMed.
- T. Tanigami, H. Iwata and T. Mori, J. Appl. Polym. Sci., 2007, 103, 2788–2796 CrossRef CAS.
- R. J. Won and C. O. Park, Membr. J., 2017, 27, 84–91 CrossRef.
- D. Das and B. K. Nandi, J. Environ. Chem. Eng., 2020, 8, 103643 CrossRef CAS.
- M. Lopez-Guzman, M. T. Alarcon-Herrera, J. R. Irigoyen-Campuzano, L. A. Torres-Castanon and L. Reynoso-Cuevas, Sci. Total Environ., 2019, 678, 181–187 CrossRef CAS PubMed.
- V. F. Mena, A. Betancor-Abreu, S. Gonzalez, S. Delgado, R. M. Souto and J. J. Santana, J. Environ. Manage., 2019, 246, 472–483 CrossRef CAS PubMed.
- M. Alimohammadi, A. Mesdaghinia, M. H. Shayesteh, H. J. Mansoorian and N. Khanjani, Int. J. Environ. Sci. Technol., 2019, 16, 8239–8254 CrossRef CAS.
- J. F. A. Silva, N. S. Graça, A. M. Ribeiro and A. E. Rodrigues, Sep. Purif. Technol., 2018, 197, 237–243 CrossRef CAS.
- V. L. Dhadge, C. R. Medhi, M. Changmai and M. K. Purkait, Chemosphere, 2018, 199, 728–736 CrossRef CAS PubMed.
- B. K. Nandi and S. Patel, Arabian J. Chem., 2017, 10, S2961–S2968 CrossRef CAS.
- K. S. Hashim, A. Shaw, R. Al Khaddar, M. O. Pedrola and D. Phipps, J. Environ. Manage., 2017, 189, 98–108 CrossRef CAS PubMed.
- S. V. Jadhav, E. Bringas, G. D. Yadav, V. K. Rathod, I. Ortiz and K. V. Marathe, J. Environ. Manage., 2015, 162, 306–325 CrossRef CAS PubMed.
- W. Zuo, G. Zhang, M. Qin and H. Zhang, Pollut. Control Technol., 2004, 222, 187–196 Search PubMed.
- M. Kim, C. E. Choong, S. Hyun, C. M. Park and G. Lee, Chemosphere, 2020, 253, 126580 CrossRef CAS PubMed.
- S. Huang, M. Hu, D. Li, L. Wang, C. Zhang, K. Li and Q. He, Fuel, 2020, 279, 118486 CrossRef CAS.
- W. Li, T. Zhang, L. Lv, Y. Chen, W. Tang and S. Tang, Colloids Surf., A, 2021, 624, 126791 CrossRef CAS.
- N. V. Narayanan and M. Ganesan, J. Hazard. Mater., 2009, 161, 575–580 CrossRef CAS PubMed.
- M. Malakootian, H. J. Mansoorian and M. Moosazadeh, Desalination, 2010, 255, 67–71 CrossRef CAS.
- T. Wang, L. Y. Zhang, H. Y. Wang, W. C. Yang, Y. C. Fu, W. L. Zhou, W. T. Yu, K. S. Xiang, Z. Su, S. Dai and L. Y. Chai, ACS Appl. Mater. Interfaces, 2013, 5, 12449–12459 CrossRef CAS PubMed.
- M. G. Goren, R. M. Bolanz, S. Parry, J. Goettlicher, R. Steininger and J. Majzlan, Clays Clay Miner., 2021, 69, 188–204 CrossRef.
- D. Bhatt, N. Gururani, A. Srivastava and P. C. Srivastava, Environ. Earth Sci., 2021, 80, 273 CrossRef CAS.
- T. L. Wu, Q. Sun, G. D. Fang, P. X. Cui, C. Liu, M. E. Alves, W. X. Qin, D. M. Zhou, Z. Q. Shi and Y. J. Wang, Chem. Eng. J., 2019, 369, 414–421 CrossRef CAS.
- Y. Wen, Y. Ji, S. Zhang, J. Zhang and G. Cai, Polymers, 2019, 11, 711 CrossRef CAS PubMed.
- X. Teng, J. Li, J. Wang, J. Liu, X. Ge and T. Gu, Sep. Purif. Technol., 2021, 267, 118661 CrossRef CAS.
- V. P. Ponomar, Miner. Eng., 2018, 127, 143–152 CrossRef CAS.
- J. Xu, L. K. Koopal, M. Wang, J. Xiong, J. Hou, Y. Li and W. Tan, Environ. Sci.: Nano, 2019, 6, 3625–3637 RSC.
- Y.-y. Ma, F.-y. Ma, W.-l. Mo and Q. Wang, Fuel, 2020, 266, 117039 CrossRef CAS.
- S. Salustro, A. M. Ferrari, F. S. Gentile, J. K. Desmarais, M. Rerat and R. Dovesi, J. Phys. Chem. A, 2018, 122, 594–600 CrossRef CAS PubMed.
- B. B. Zviagina, V. A. Drits and O. V. Dorzhieva, Minerals, 2020, 10, 153 CrossRef CAS.
- U. T. Un, A. S. Koparal and U. B. Ogutveren, Chem. Eng. J., 2013, 223, 110–115 CrossRef.
- M. M. Emamjomeh and M. Sivakumar, J. Environ. Manage., 2009, 90, 1204–1212 CrossRef CAS PubMed.
- M. Bennajah, B. Gourich, A. H. Essadki, C. Vial and H. Delmas, Chem. Eng. J., 2009, 148, 122–131 CrossRef CAS.
- C. Y. Hu, S. L. Lo, W. H. Kuan and Y. D. Lee, Water Res., 2005, 39, 895–901 CrossRef CAS PubMed.
- J. Zhu, H. Zhao and J. Ni, Sep. Purif. Technol., 2007, 56, 184–191 CrossRef CAS.
- U. T. Un, A. S. Koparal, U. B. Ogutveren and A. Durucan, Fresenius Environ. Bull., 2010, 19, 1906–1910 CAS.
- U. T. Un, A. S. Koparal and U. B. Ogutveren, J. Environ. Manage., 2009, 90, 428–433 CrossRef CAS PubMed.
- S. Vasudevan, S. K. Balasingam, J. Lakshmi, S. Mohanraj and G. Sozhan, J. Chem. Technol. Biotechnol., 2011, 86, 428–436 CrossRef CAS.
- H. Zhao, H. Liu and J. Qu, J. Colloid Interface Sci., 2009, 330, 105–112 CrossRef CAS PubMed.
- R. Zhou, F. Liu, N. Wei, C. Yang, J. Yang, Y. Wu, Y. Li, K. Xu, X. Chen and C. Zhang, J. Water Proc. Eng., 2020, 37, 101387 CrossRef.
- D. Ghosh, C. R. Medhi and M. K. Purkait, Chemosphere, 2008, 73, 1393–1400 CrossRef CAS PubMed.
- S. Elabbas, N. Adjeroud, L. Mandi, F. Berrekhis, M. N. Pons, J. P. Leclerc and N. Ouazzani, Int. J. Environ. Anal. Chem., 2020, 1761963 Search PubMed.
- G. I. Danmaliki, T. A. Saleh and A. A. Shamsuddeen, Chem. Eng. J., 2017, 313, 993–1003 CrossRef CAS.
- J. Yang, C. Ma, J. Tao, J. Li, K. Du, Z. Wei, C. Chen, Z. Wang, C. Zhao and M. Ma, Carbohydr. Polym., 2020, 245, 116542 CrossRef CAS PubMed.
- M. Zhu, X. Yin, Q. Liu and Z. Feng, Pol. J. Environ. Stud., 2020, 29, 2493–2502 CrossRef CAS.
- A. Witek-Krowiak, K. Chojnacka, D. Podstawczyk, A. Dawiec and K. Pokomeda, Bioresour. Technol., 2014, 160, 150–160 CrossRef CAS PubMed.
- Z. Chen, Y. Liu, L. Mao, L. Gong, W. Sun and L. Feng, Ceram. Int., 2018, 44, 6002–6009 CrossRef CAS.
- J. He, K. Chen, X. Cai, Y. Li, C. Wang, K. Zhang, Z. Jin, F. Meng, X. Wang, L. Kong and J. Liu, J. Colloid Interface Sci., 2017, 490, 97–107 CrossRef CAS PubMed.
- J. T. Feng, Z. Y. Wang, W. L. Zhang, X. Y. Zhao, J. T. Zhang, Y. P. Liu and W. Yan, Environ. Sci. Pollut. Res., 2021, 28, 1–13 CrossRef PubMed.
- Y. Wang, L. Yu, R. Wang, Y. Wang and X. Zhang, J. Colloid Interface Sci., 2020, 564, 392–405 CrossRef CAS PubMed.
- C. J. L. Silwood, I. Abrahams, D. C. Apperley, N. P. Lockyer, E. Lynch, M. Motevalli, R. M. Nix and M. Grootveld, J. Mater. Chem., 2005, 15, 1626–1636 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08503d |
| This journal is © The Royal Society of Chemistry 2022 |