Open Access Article
Open Access ArticleChemical profiles with cardioprotective and anti-depressive effects of Morus macroura Miq. leaves and stem branches dichloromethane fractions on isoprenaline induced post-MI depression†
Dalia I. Hamdana,
Samia S. Hafezb,
Wafaa H. B. Hassanb,
Mai M. Morsib,
Heba M. A. Khalil *c,
Yasmine H. Ahmedd,
Omar A. Ahmed-Faride and
Riham A. El-Shiekh
*c,
Yasmine H. Ahmedd,
Omar A. Ahmed-Faride and
Riham A. El-Shiekh *f
*f
aDepartment of Pharmacognosy and Natural Products, Faculty of Pharmacy, Menoufia University, Shibin Elkom, 32511, Egypt
bPharmacognosy Department, Faculty of Pharmacy, Zagazig University, Zagazig 44519, Egypt
cDepartment of Veterinary Hygiene and Management, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt. E-mail: heba.ali315@gmail.com; Tel: +201013666331
dDepartment of Cytology and Histology, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt
eDepartment of Physiology, National Organization for Drug Control and Research, Giza, Egypt
fDepartment of Pharmacognosy, Faculty of Pharmacy, Cairo University, Kasr El Aini St., Cairo, 11562, Egypt. E-mail: riham.adel@pharma.cu.edu.eg; Tel: +201064763764
First published on 26th January 2022
Abstract
This study was conducted to explore the potential cardioprotective and anti-depressive effects of dichloromethane (DCM) fractions of Morus macroura leaves (L) and stem branches (S) on post-myocardial infarction (MI) depression induced by isoprenaline (ISO) in rats in relation to their metabolites. The study was propped with a UPLC-ESI-MS/MS profiling and chromatographic isolation of the secondary metabolites. Column chromatography revealed the isolation of lupeol palmitate (6) that was isolated for the first time from nature with eight known compounds. In addition, more than forty metabolites belonging, mainly to flavonoids, and anthocyanins groups were identified. The rats were injected with ISO (85 mg kg−1, s.c) in the first two days, followed by the administration of M. macroura DCM-L and DCM-S fractions (200 mg kg−1 p.o) for 19 days. Compared with the ISO exposed rats, the treated rats displayed a reduction in cardiac biomarkers (LDH and CKMB), anxiety, and depressive-like behaviour associated with an increase in the brain defense system (SOD and GSH), neuronal cell energy, GABA, serotonin, and dopamine, confirmed by histopathological investigations. In conclusion, DCM-L and DCM-S fractions' cardioprotective and anti-depressive activities are attributed to their metabolite profile. Therefore, they could serve as a potential agent in amending post-MI depression.
1. Introduction
Myocardial infarction (MI) is a severe health illness affecting approximately 600 individuals per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people.1–3 Of the sequelae of MI is the depression that leads to high morbidities and mortalities globally.2 The relation between the heart and brain in the pathogenesis of depression associated with MI (post-MI depression) remains unclear.3 However, several medications have been used to treat either MI or depression but with no dual effect on the whole disease. Also, these medications have a high cost that leads to the discontinuation of the treatment protocol. Therefore, it is essential to investigate new medicines that have a dual effect on post-MI depression associated with studying possible mechanisms underlying this condition. Several traditional approaches have been adopted to use medicinal plants with various bioactive compounds to treat several diseases.
000 people.1–3 Of the sequelae of MI is the depression that leads to high morbidities and mortalities globally.2 The relation between the heart and brain in the pathogenesis of depression associated with MI (post-MI depression) remains unclear.3 However, several medications have been used to treat either MI or depression but with no dual effect on the whole disease. Also, these medications have a high cost that leads to the discontinuation of the treatment protocol. Therefore, it is essential to investigate new medicines that have a dual effect on post-MI depression associated with studying possible mechanisms underlying this condition. Several traditional approaches have been adopted to use medicinal plants with various bioactive compounds to treat several diseases.
Morus, commonly known as mulberries, is an economically and medically important genus in Moraceae. It has valuable bioactive compounds that showed many potential activities such as antidiabetic, hepatoprotective, antibacterial, anti-inflammatory, antihypertensive, antiarthritic, antiviral, and antioxidative.4
Among the plant species, M. macroura possesses many beneficial compounds with therapeutic activities. It includes phenolic compounds (stilbene and stilbene dimers, prenylated and geranylated flavanones, 2- arylbenzofurans), a variety of Diels–Alder adducts (prenylated benzaldehyde-chalcone, prenylated chalcone–chalcone, prenylated 2-arylbenzofuranchalcone, and prenylated stilbene-chalcone), and triterpenes.5
Therefore, the current study includes UPLC-ESI-MS/MS profiling and chromatographic isolation of the secondary metabolites using column chromatography for dichloromethane fraction of leaves (DCM-L) and dichloromethane fraction of stems (DCM-S) of M. macroura, then the evaluation of these fractions against post-MI depression induced by isoprenaline (ISO) in rats. Also, we assessed many underlying parameters, including depressive-like behavior, biochemical, and histopathological profile. It is worth noting that ISO is a widely used inducer for MI at high doses in preclinical studies and showed many metabolic and morphologic aberrations in experimental animals.6,7 Many studies induce post- MI depression surgically.8,9 However, in our study, we depend on a non-invasive method in the induction using ISO based on previous research,10 which demonstrates depressive-like behaviour and myocardial injury after administration of isopropyl adrenaline.
2. Material and methods
2.1. Plant material
Leaves and stem branches were collected from M. macroura Miq. tree cultivated on the farm of the Department of Pharmacognosy, Faculty of Pharmacy, Zagazig University 2020. A voucher specimen (MM100) kept at the department's herbarium was kindly identified by Prof. Dr Abdelhalem Abdelmogali, Taxonomy researcher, Ministry of Agriculture, Dokki- Cairo (Egypt). The leaves and stem branches of the plant under investigation were air-dried, ground separately into fine powders, and kept in a well-closed container until use.2.2. Extraction, fractionation, and chromatographic isolation of secondary metabolites
Air-dried powdered leaves (1.5 kg) and stem branches (2 kg) of M. macroura were separately extracted with 80% aqueous ethanol at room temperature. The ethanol extracts (leaves 350.0 g) and (stem branches 135.0 g) were subsequently partitioned against DCM and ethyl acetate. DCM fractions of the leaves and stems were 65.0 and 30.0 g, respectively.Thin layer chromatography (TLC) fingerprinting of DCM fractions obtained from leaves and stem branches revealed almost the same profiling; thus, a mixture of 40 g from the two fractions was mixed with 40 g of silica gel for the column to get a dry mixed initial zone, which was applied on the top of the silica gel column (6 × 150 cm, 800 g) packed using the wet method with n-hexane. The polarity was increased gradually using DCM then methanol and fractions (250 mL each) were collected, concentrated under reduced pressure, examined by TLC, and similar fractions were combined. Besides this, column fractions (9–13) eluted using 5% CH2Cl2/n-hexane revealed the presence of one major blue spot (using anisaldehyde–sulphuric) with Rf values of 0.69 and 0.85 (solvent systems I, 100% light petroleum and II, light petroleum: DCM 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), respectively. The crystallization of these fractions using DCM-methanol mixture (1
10), respectively. The crystallization of these fractions using DCM-methanol mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) afforded 500 mg of white waxy substance (compound 1). Additionally, the fraction numbers 17–23 were eluted with 10% CH2Cl2/n-hexane using light petroleum: DCM 75
1) afforded 500 mg of white waxy substance (compound 1). Additionally, the fraction numbers 17–23 were eluted with 10% CH2Cl2/n-hexane using light petroleum: DCM 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (III) and 100% DCM (VI) revealed the presence of one major spot with Rf values of 0.55 and 0.73, respectively. The pooled fractions were purified by repeated crystallization from methanol as white crystalline scales (100 mg) compound 2. Furthermore, fractions (31–37) eluted with 25% DCM/n-hexane revealed the presence of two major violet spots with Rf values (0.50 and 0.42, III) and (0.64 and 0.54, IV). These fractions were pooled, concentrated, and chromatographed on a silica gel column packed with 100% n-hexane and the polarity was gradually increased by DCM. Sub-column fractions (9–12) and (14–18) that were eluted with 25% DCM in n-hexane, revealed the isolation of two major spots, which were designated as compound 3 (white amorphous, 400 mg) and compound 4 (white granules, 100 mg), respectively. Moreover, column fractions 47–51 eluted with 40% DCM/n-hexane showed the presence of one major violet spot with Rf values (0.22, III and 0.40, IV). Repeated crystallization from methanol resulted in the purification of compound 5 as white scales with m.p. 63–64 °C. Also, the pooled fractions (61–65) eluted with 50% DCM/n-Hexane showed one major violet spot with Rf values 0.78 and 0.85 using solvent system V (DCM: methanol, 99
25 (III) and 100% DCM (VI) revealed the presence of one major spot with Rf values of 0.55 and 0.73, respectively. The pooled fractions were purified by repeated crystallization from methanol as white crystalline scales (100 mg) compound 2. Furthermore, fractions (31–37) eluted with 25% DCM/n-hexane revealed the presence of two major violet spots with Rf values (0.50 and 0.42, III) and (0.64 and 0.54, IV). These fractions were pooled, concentrated, and chromatographed on a silica gel column packed with 100% n-hexane and the polarity was gradually increased by DCM. Sub-column fractions (9–12) and (14–18) that were eluted with 25% DCM in n-hexane, revealed the isolation of two major spots, which were designated as compound 3 (white amorphous, 400 mg) and compound 4 (white granules, 100 mg), respectively. Moreover, column fractions 47–51 eluted with 40% DCM/n-hexane showed the presence of one major violet spot with Rf values (0.22, III and 0.40, IV). Repeated crystallization from methanol resulted in the purification of compound 5 as white scales with m.p. 63–64 °C. Also, the pooled fractions (61–65) eluted with 50% DCM/n-Hexane showed one major violet spot with Rf values 0.78 and 0.85 using solvent system V (DCM: methanol, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and VI (DCM: methanol, 98
1) and VI (DCM: methanol, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), respectively. The residue of pooled fractions was crystallized from DCM/methanol as colorless needles (200 mg, compound 6) with m.p. 110–112 °C. Fractions (70–78) eluted with 100% DCM displayed one major spot with Rf values 0.65 (V) and 0.83 (VI). Purification was performed using repeated crystallization from the DCM/methanol mixture that yielded 500 mg of compounds 7 with Rf values 0.43 (V), 0.55 (VI), and m.p. 216–218 °C. Besides this, the fractions (90–95) eluted with 2.5% methanol/DCM revealed the presence of one major violet spot with Rf values 0.33 (V) & 0.45 (VI), A crystallized pooled fraction from methanol, afforded 500 mg of white needle-shaped crystals (compound 8) with m.p. 136–138 °C. Finally, TLC examination of fractions 101–108 eluted by 10% methanol/DCM showed the presence of one major violet spot with Rf value 0.33 (VII, DCM: methanol 95
2), respectively. The residue of pooled fractions was crystallized from DCM/methanol as colorless needles (200 mg, compound 6) with m.p. 110–112 °C. Fractions (70–78) eluted with 100% DCM displayed one major spot with Rf values 0.65 (V) and 0.83 (VI). Purification was performed using repeated crystallization from the DCM/methanol mixture that yielded 500 mg of compounds 7 with Rf values 0.43 (V), 0.55 (VI), and m.p. 216–218 °C. Besides this, the fractions (90–95) eluted with 2.5% methanol/DCM revealed the presence of one major violet spot with Rf values 0.33 (V) & 0.45 (VI), A crystallized pooled fraction from methanol, afforded 500 mg of white needle-shaped crystals (compound 8) with m.p. 136–138 °C. Finally, TLC examination of fractions 101–108 eluted by 10% methanol/DCM showed the presence of one major violet spot with Rf value 0.33 (VII, DCM: methanol 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and 0.48 (VIII, DCM: methanol 85
5) and 0.48 (VIII, DCM: methanol 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15). The residue of the pooled fractions was crystallized from hot DCM/methanol afford 2 gm of white amorphous powder (compound 9), as illustrated in Scheme S1.† All the isolated compounds were analyzed using EI/MS (Quadrupole mass analyzer in Thermo Scientific GC/MS model ISQ LT (USA) using Thermo Xcalibur software), 1H-NMR and 13C-NMR (Bruker (Switzerland)) at 400, 100 MHz, respectively. Chemical shifts are given in ppm with the TMS as an internal standard as well as, determination of m.p. (Digital, electro-thermal Ltd, England) for some isolated metabolites.
15). The residue of the pooled fractions was crystallized from hot DCM/methanol afford 2 gm of white amorphous powder (compound 9), as illustrated in Scheme S1.† All the isolated compounds were analyzed using EI/MS (Quadrupole mass analyzer in Thermo Scientific GC/MS model ISQ LT (USA) using Thermo Xcalibur software), 1H-NMR and 13C-NMR (Bruker (Switzerland)) at 400, 100 MHz, respectively. Chemical shifts are given in ppm with the TMS as an internal standard as well as, determination of m.p. (Digital, electro-thermal Ltd, England) for some isolated metabolites.
2.3. UPLC-ESI-MS-MS analysis of DCM-L and DCM-S fractions
The sample (100 μg mL−1) solutions were prepared using high-performance liquid chromatography (HPLC) analytical-grade methanol solvent, filtered using a membrane disc filter (0.2 μm), then subjected to analysis based on the procedure that was previously reported.112.4. In vivo evaluation of the cardioprotective, anxiolytic, and anti-depressant activity of DCM-L and DCM-S fractions
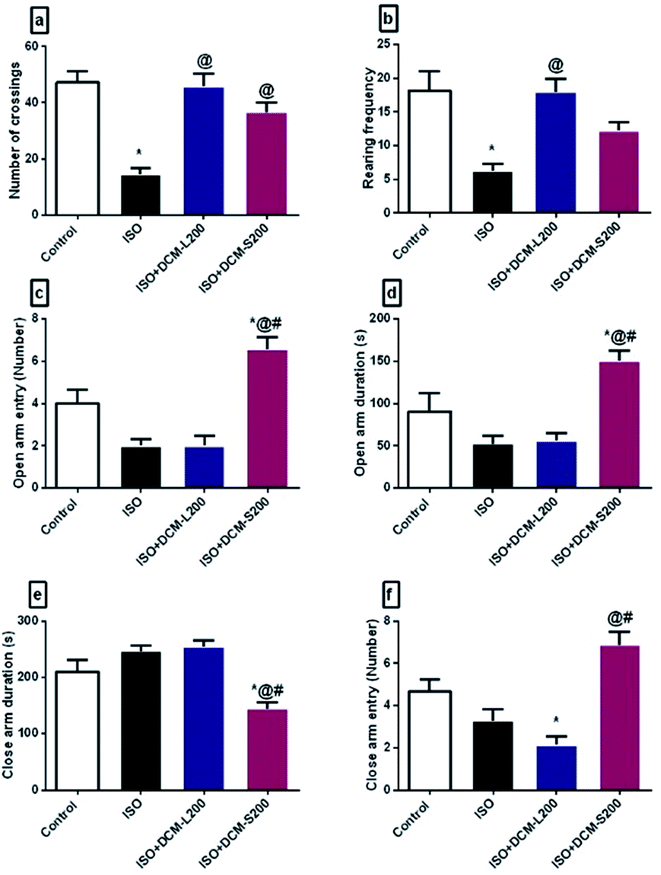
2.4.4.1. Anxiety like behaviour. Anxiety-like behaviour was determined using two behavioural tests. First, the open field test was used to measure the motor activity of rats. The measuring parameters were the number of crossings of the lines and frequency of rearing activity. The test duration was 3 min, and the procedures were conducted following the previous literature.13 Then, the elevated plus-maze was tested. It was dependent on the natural fear of rodents from heights. The recorded parameters were frequencies of entering the closed and open arms and durations for 5 min. The procedures and apparatus structure were following the previous ref. 11 and 14.
2.4.4.2. Depressive like behaviour. Depressive-like behaviour is performed using the forced swim test, which is a test of learned helplessness and used to screen the anti-depressant activity. Briefly, rats were tested in two sessions; the first is a training session for rats in a circular arena filled with warm water (27 °C) for 15 min. After 24 h, the second session (testing session) was performed where the mobility and immobility duration were recorded for 5 min.15
A scoring system to evaluate the myocardial injuries in rats caused by ISO was assessed.19 The findings were classified into the following degrees to compose a range of histologic myocardial injury considering (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) scores for the categories; edema, myocardial necrosis, and inflammatory cell infiltrates. The score (0
3) scores for the categories; edema, myocardial necrosis, and inflammatory cell infiltrates. The score (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was assigned to (0) no damage, (1) slight damage, (2) moderate damage, and (3) maximum damage. This method was used for estimating the HP index in the heart.
3) was assigned to (0) no damage, (1) slight damage, (2) moderate damage, and (3) maximum damage. This method was used for estimating the HP index in the heart.
2.5. Statistical analysis
All quantitative results were analyzed using the SPSS version 17.0 for Windows. Data were presented as mean ± SEM. Comparisons among multiple group means were performed using a one-way analysis of variance, followed by the Bonferroni test as a post hoc test. Statistical significance was set at p ≤ 0.05.3. Results and discussion
Our study aimed to investigate the possible cardioprotective and anti-depressant effects of DCM-L and DCM-S fractions of M. macroura. in an ISO induced post-MI depression-rat model. This study was reinforced by the chromatographic technique of these fractions, which afforded the isolation and identification of nine secondary metabolites belonging to fatty acids and their derivatives, steroid, and triterpenoid compounds. In addition, the UPLC-ESI-MS-MS profile of DCM-L and DCM-S fractions that were analyzed separately afforded the identification of 42 and 41 secondary metabolites, respectively. The flavonoids and anthocyanins are the major metabolites identified in M. macroura.3.1. Phytochemical study
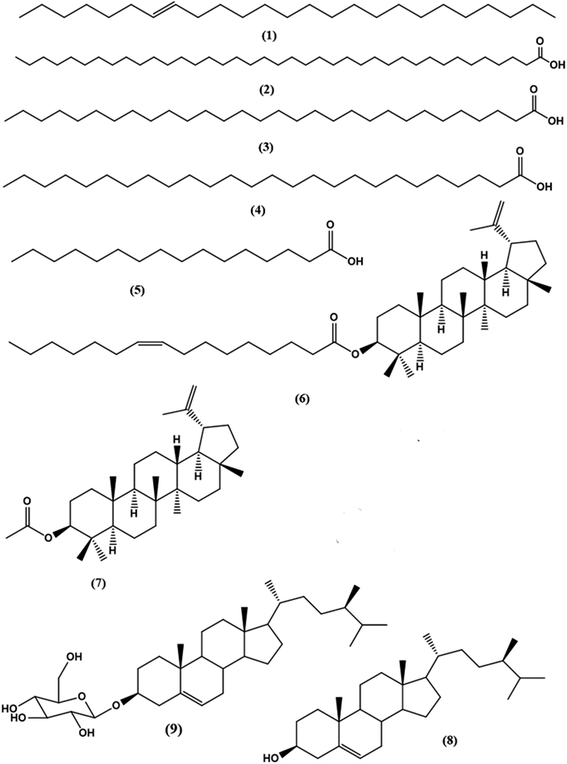
Column chromatography of the combined DCM fractions resulted in the isolation of nine compounds, namely; lupeol palmitate (6). It is isolated for the first time from nature with eight known compounds including pentacos-7-ene (1), nonatriacontanoic acid (2), melissic acid (3), cerotic acid (4), palmitic acid (5), lupeol acetate (7), β-sitosterol (8), β-sitosterol-3-O-β-D-glucopyranoside (9) (Fig. 1 and Schemes S1† and S texts 1–10). Where, fractions eluted with 50% CH2Cl2/n-hexane afforded 200 mg of colorless needles of compound 6 with m.p. 110–111 °C and Rf 0.78 & 0.85 using solvent system (DCM: methanol 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (DCM: methanol 98
1) and (DCM: methanol 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
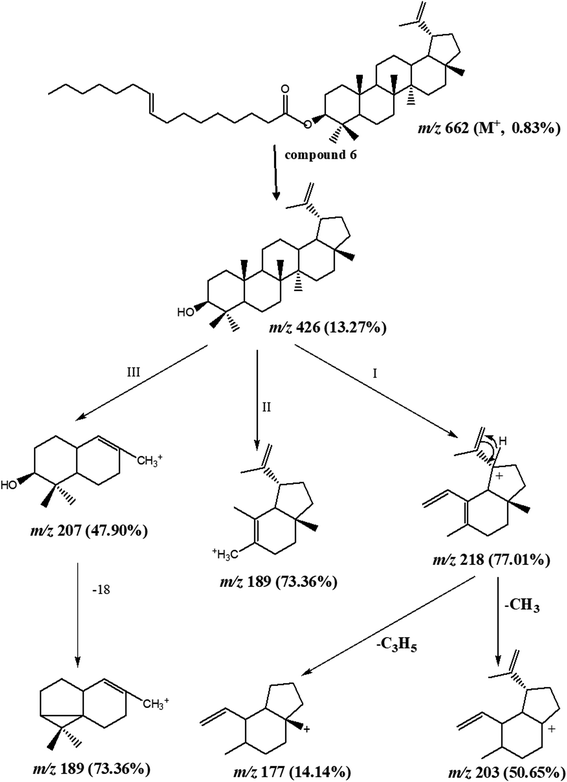
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Moreover, the physical and chemical characteristics of compound (6) suggested a steroidal or triterpenoidal skeleton. The EI-MS spectrum showed M+ at m/z 662 corresponding to molecular formula C46H78O2 and characteristic fragments at m/z 426 for [M+-236], where 218, 207, 203, 189, and 177 that are characteristic for triterpenoidal skeleton as illustrated in Fig. 2.20 1H-and 13C-NMR spectral data showed signals at δH 4.26, δC 79.17 for H-3 and C-3, respectively, and an exocyclic double bond at δH 4.69, 4.57 with corresponding olefinic carbons at δC 131.03 and 132.61 with other 1H-NMR signals confirmed the presence of lupeol as the triterpenoidal compound.21 The downfield shift of the proton at C-3 (δH 4.26 ppm) and the carbonyl group at δC 167.91 indicated the presence of an esterified hydroxyl group at this position. The 13C-NMR spectral data showed characteristic thirty carbons for lupeol nucleus with M+ 426 in addition to other sixteen signals for the acidic moiety [with m/z 236 (C16H30O)]. The double bond position is deduced from the loss of 26 amu between m/z 85 (C6H13) and m/z 111 (C8H15). This proved that the double bond is between C-9′ and C-10′. The consequent losses of 14 or 28 amu and the presence of the C
2). Moreover, the physical and chemical characteristics of compound (6) suggested a steroidal or triterpenoidal skeleton. The EI-MS spectrum showed M+ at m/z 662 corresponding to molecular formula C46H78O2 and characteristic fragments at m/z 426 for [M+-236], where 218, 207, 203, 189, and 177 that are characteristic for triterpenoidal skeleton as illustrated in Fig. 2.20 1H-and 13C-NMR spectral data showed signals at δH 4.26, δC 79.17 for H-3 and C-3, respectively, and an exocyclic double bond at δH 4.69, 4.57 with corresponding olefinic carbons at δC 131.03 and 132.61 with other 1H-NMR signals confirmed the presence of lupeol as the triterpenoidal compound.21 The downfield shift of the proton at C-3 (δH 4.26 ppm) and the carbonyl group at δC 167.91 indicated the presence of an esterified hydroxyl group at this position. The 13C-NMR spectral data showed characteristic thirty carbons for lupeol nucleus with M+ 426 in addition to other sixteen signals for the acidic moiety [with m/z 236 (C16H30O)]. The double bond position is deduced from the loss of 26 amu between m/z 85 (C6H13) and m/z 111 (C8H15). This proved that the double bond is between C-9′ and C-10′. The consequent losses of 14 or 28 amu and the presence of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group confirmed that we dealt with palmitoleic acid moiety.22 From the previous spectral data, compound (6) could be identified as lupeol palmitoleate (C46H78O2).
O group confirmed that we dealt with palmitoleic acid moiety.22 From the previous spectral data, compound (6) could be identified as lupeol palmitoleate (C46H78O2).
3.2. Characterization of metabolites from DCM-L and DCM-S analyzed by UPLC-ESI-MS-MS
The DCM fractions obtained from leaves and stem branches of M. macroura were profiled by UPLC- ESI-MS/MS using negative and positive ionization modes (Fig. 3 and 4). Altogether, 64 metabolites were identified depending on the MS–MS fragmentation pattern and data published in the literature. The compounds are summarized and ordered in Table 1 according to their relative retention time (RRT), calculated according to Euchrenone a7. The characterization of secondary metabolites identified in the DCM fraction of leaves and stem branches is explained in detail in ESI; S texts 11–23.†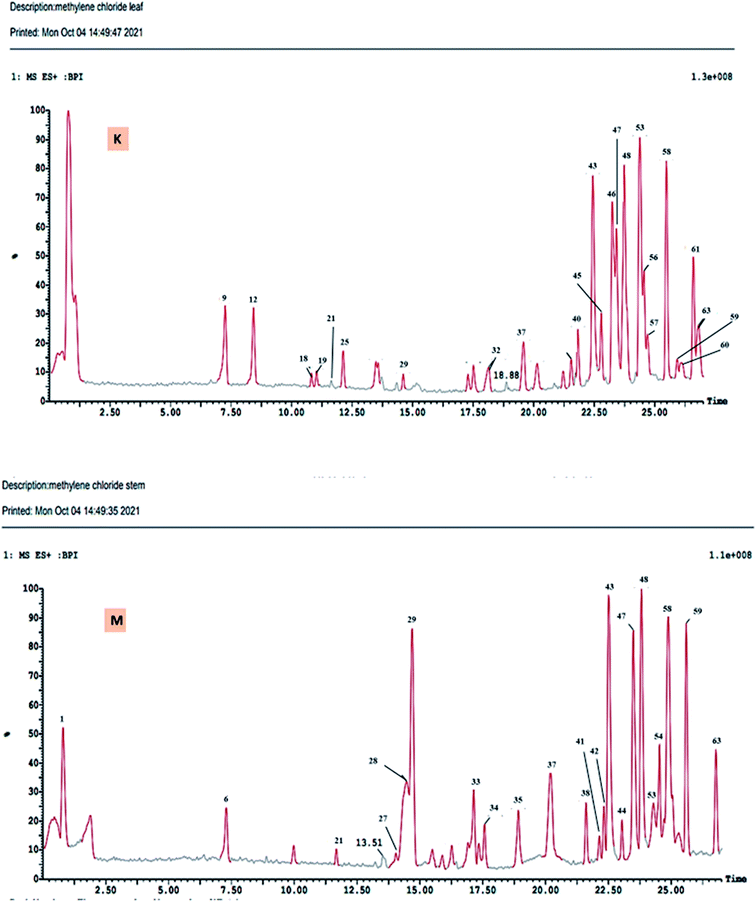 | ||
| Fig. 3 UPLC-ESI-MS chromatograms of DCM fractions of leaves (K), and stem branches (M) of M. macroura Miq in positive ionization mode. | ||
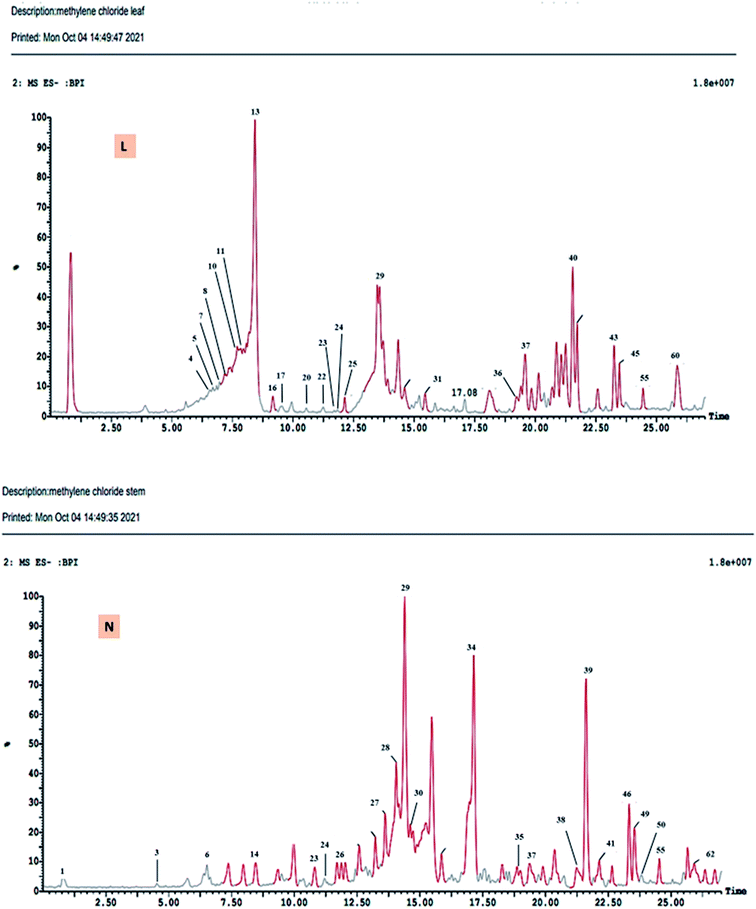 | ||
| Fig. 4 UPLC-ESI-MS chromatograms of DCM fractions of leaves (L), stem branches (N) of M. macroura Miq in negative ionization mode. | ||
| Peak no. | RT | RRT | MS1−/+ | MS2 | Leaves | Stem branches | Tentative assignment |
|---|---|---|---|---|---|---|---|
| a *Compounds identified for the first time in the family Moraceae. Bold with or without * means compounds isolated as pure metabolites from M. macroura. √ Minor compounds. — Compounds not detected. | |||||||
| 1 | 2.10 | 0.084 | 153/− | 109 | — | √ | Protocatecheic acid |
| 2 | 2.43 | 0.097 | 339/− | 177 (100%) | 0.95 | — | *Aesculin |
| 3 | 4.84 | 0.194 | 137/− | 93 (100%) | — | 0.51 | Hydroxybenzoic acid |
| 4 | 6.27 | 0.251 | 339/− | 177![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 175 175![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 161,135 161,135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 109 109 |
√ | — | Morachalcone A |
| 5 | 6.27 | 0.251 | 431/− | 269 (100%) | 0.95 | — | *Apigenin-7-O-β-glucoside |
| 6 | 6.73 | 0.269 | 163/165 | 119 (100%) | — | 0.24 | Coumaric acid |
| 7 | 6.92 | 0.277 | 463/− | 270.7 | 1.85 | — | *Naringenin derivatives |
| 8 | 7.26 | 0.291 | 447/− | 285,256.9, 242.5,198.8 | 0.47 | — | *Luteolin-7-O- glucoside |
| 9 | 7.46 | 0.299 | −/355 | 193 | 0.05 | — | Scopolin |
| 10 | 7.62 | 0.305 | 431/− | 341, 311, 269 | 1.09 | — | Apigenin-8-C-glucoside |
| 11 | 7.92 | 0.317 | 463/− | 301, 257 | 4.09 | — | *Morin-O-β-glucoside |
| 12 | 8.10 | 0.325 | −/449 | 287 | √ | — | Cyanidin-3-O-glucoside |
| 13 | 8.25 | 0.331 | 463/− | 301 (100%) | 7.33 | — | Quercetin-3-O-β-D-glucoside |
| 14 | 8.38 | 0.336 | 463/− | 301 | — | √ | Quercetin-3-O-β-galactoside |
| 15 | 8.96 | 0.359 | 431/− | 269 | 0.01 | — | *Apigenin-7-O- glucoside isomer |
| 16 | 9.24 | 0.370 | 447/− | 285 | 7.33 | — | Kaempferol-3-O-β-glucoside |
| 17 | 9.24 | 0.370 | 447/− | 343, 301 | 0.47 | — | Quercetin-8-C-rhamnoside |
| 18 | 10.95 | 0.439 | −/611 | 303, 328 | 0.80 | — | *Hesperidin |
| 19 | 10.95 | 0.439 | −/610 | 317.5, 256.8, 151.2 | √ | — | *Petunidin dirhamnoside |
| 20 | 11.14 | 0.446 | 447/− | 301![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 255 255 |
0.41 | — | Quercetin-3-O-β- rhamnoside |
| 21 | 11.18 | 0.448 | −/397 | 351 | 1.61 | 8.45 | Cerotic acid |
| 22 | 11.34 | 0.454 | 301/− | 139 (100%), 256 | 0.41 | — | Ellagic acid |
| 23 | 11.34 | 0.454 | 355/− | 193.3 (100%) | — | √ | *Ferulic acid-O-glucoside |
| 24 | 11.37 | 0.456 | 431/− | 285![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 284, 255 284, 255![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 227 227 |
— | √ | *Kaempferol-3-O-β- rhamnoside |
| 25 | 12.10 | 0.485 | 301/303 | 179, 151, 121 | 0.41 | — | Quercetin |
| 26 | 12.17 | 0.488 | 301/− | 256,151![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 107 107 |
— | √ | Morin |
| 27 | 14.06 | 0.563 | 467/469 | 305 | — | 5.18 | *Gallocatechin glycoside |
| 28 | 14.15 | 0.567 | 467/469 | 305 | — | 5.18 | *Epigallocatechin glycoside |
| 29 | 14.39 | 0.577 | 329/− | 287 [M–H–COCH3] | 2.30 | 13.36 | Phloracetophenone-4-O-glucoside |
| 30 | 14.68 | 0.588 | 575/− | — | — | 1.05 | β-Sitosterol-3-O-β-D-glucoside |
| 31 | 16.04 | 0.643 | 467/− | — | 0.27 | — | Lupeol acetate |
| 32 | 17.77 | 0.712 | −/579 | — | 1.78 | — | *Nonatriacontanoic acid |
| 33 | 17.83 | 0.715 | 353/355 | 203 | — | √ | *Epoxybergamottin |
| 34 | 17.83 | 0.715 | 353/355 | 299, 69 | — | 0.62 | Albanin A |
| 35 | 18.38 | 0.737 | 293/295 | 220.8 (100%) | — | 0.01 | *Hydroxy octadecatrienoic acid |
| 36 | 18.87 | 0.756 | 591/− | 287 | √ | — | *Cyanidin cinnamoyl glucuronide |
| 37 | 19.77 | 0.792 | 317/− | — | 0.86 | 1.73 | Unknown |
| 38 | 21.08 | 0.845 | 475/477 | 299.0 | — | 0.67 | *Chrysoeriol-uronic acid |
| 39 | 21.40 | 0.858 | 433/− | 271 | — | √ | *Naringenin-7-O-β-glucoside |
| 40 | 21.49 | 0.861 | 379/381 | 318, 163, 71, 69 | 1.61 | — | *Brassicasterol |
| 41 | 21.99 | 0.881 | 475/477 | 255 | — | 0.67 | Palmitic acid ester |
| 42 | 22.73 | 0.911 | −/683 | 331 | — | √ | *Malvidin diglucoronide |
| 43 | 23.02 | 0.922 | 451/- | 249.8390.5406.7435.1 | 0.80 | 16.23 | *Melissic acid |
| 44 | 23.06 | 0.924 | -/683 | 597, 435, 287 | — | 0.88 | *Cyanidin derivative |
| 45 | 23.24 | 0.931 | 255/− | — | 2.10 | 2.81 | Palmitic acid |
| 46 | 23.47 | 0.941 | 281/− | — | 1.08 | 2.14 | Oleic acid |
| 47 | 23.55 | 0.944 | −/391 | 279, 149 (100%) | 4.49 | 8.30 | *Bis (2-ethyl hexyl) phthalate |
| 48 | 23.72 | 0.951 | −/593 | 287![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 434, 595 434, 595 |
9.12 | 8.45 | Cyanidin rutinoside |
| 49 | 23.83 | 0.955 | 349/− | — | 1.08 | 2.14 | *Pentacos-7-ene |
| 50 | 23.86 | 0.956 | 395/− | 281 | — | √ | Oleic acid ester |
| 51 | 24.14 | 0.967 | −/607 | 286 | 0.23 | 2.48 | Australisine A |
| 52 | 24.44 | 0.979 | 283/− | 239 [M–H–COO] | 0.52 | 0.82 | Octadecanoic acid |
| 53 | 24.65 | 0.988 | −/535 | 317, 153 | 6.30 | 6.32 | *Isorhamnetin-3-O-acetyl glucuronide |
| 54 | 24.65 | 0.988 | −/536 | 317, 153 | — | 0.02 | *Petunidin 3-O-acetyl glucuronide |
| 55 | 24.95 | 1.000 | 339/− | 163 (100%) | 4.75 | 8.66 | Euchrenone a7 |
| 56 | 25.29 | 1.013 | −/397 | 254.9 | 3.90 | 2.99 | β-Sitosterol |
| 57 | 25.47 | 1.020 | −/535 | 331 | 6.30 | 6.32 | *Malvidin-3-O-acetylglucuronoide |
| 58 | 25.53 | 1.023 | −/683 | 597(loss of 86), 435 (loss of glucose), 287.7 | 10.15 | 12.42 | *Cyanidin-3-O-malonyl glucoside derivative |
| 59 | 25.84 | 1.035 | −/535 | 287 | 6.99 | 15.18 | *Cyanidin-3-O-malonyl glucoside |
| 60 | 26.34 | 1.055 | 661/663 | — | 0.30 | — | *Lupeol palmitate |
| 61 | 26.59 | 1.065 | −/611 | 287, 449 | 3.90 | 2.99 | *Cyanidin-3-O-hexosyl hexoside |
| 62 | 26.82 | 1.075 | 475/− | 299.8, 429 | — | 0.67 | *Peonidin-3-O-glucuronide |
| 63 | 27.68 | 1.109 | −/398 | 314 | 6.40 | 9.35 | *24-Methylene-ergosta-5-en-3β-Ol |
| 64 | 28.13 | 1.127 | 455/− | 400.5 | — | 8.66 | Oleanolic acid |
3.3. Biological study
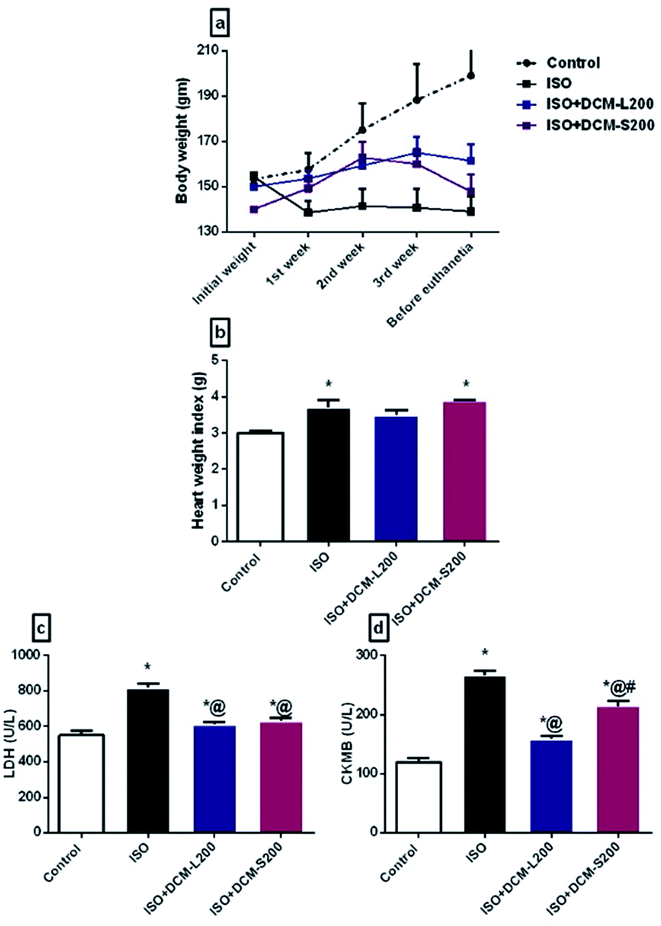
Concerning myocardial enzymes, ISO exposed rats displayed an increase in LDH and CK-MB levels compared to the control group. The increased serum myocardial enzyme levels upon ISO administration at a dose of 85 mg kg−1 reflect the degree of myocardial injury.26 The elevated myocardial enzyme levels could be attributed to the ability of ISO to produce free radicals via the adrenoceptor mechanism. Also, ISO affects the cell metabolism to such a degree that cytotoxic free radicals are formed, producing myocardial necrosis.27 On the other hand, the administration of DCM-L and DCM-S fractions at a dose of 200 mg kg−1 were able to reduce the CKMB and LDH levels with superiority to DCM-L in reducing CKMB levels compared to DCM-S (Fig. 5c and d).
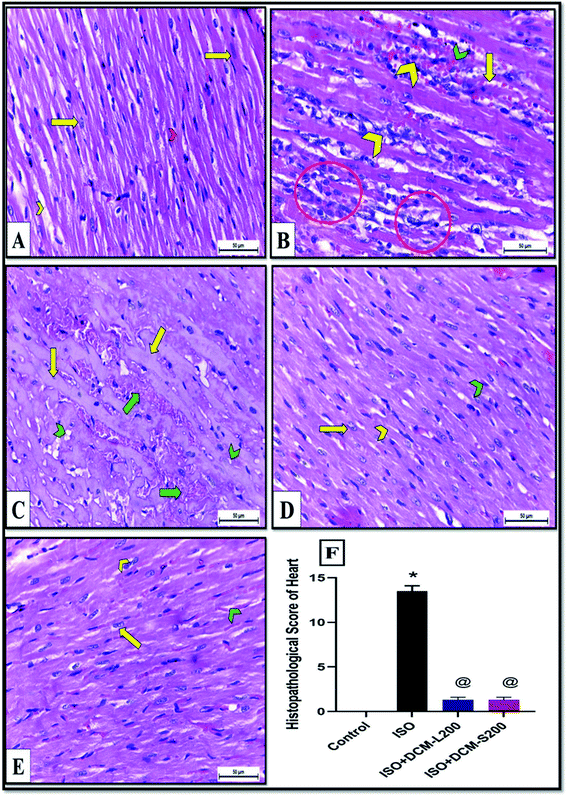
These enzymes leakage in the ISO exposed rats were associated with severe degenerative changes in cardiac myocytes, including lymphocytic infiltration, hemorrhage, wavy-shaped myofibers with unclear striation, some myofibers that appeared necrotic, and cytoplasmic vacuolation as shown in Fig. 6B and C. These findings are in agreement with the previously published works.28–30 Conversely, cardiac muscle sections of DCM-L200 and DCM-S200-treated rats showed significant recovery evidenced by nearly normal-shaped cardiac myofibers that appeared elongated, branched, striated, and contained oval central nuclei. However, few hemorrhages were observed at the interstices between myofibers (Fig. 6D and E). The cardiac muscle histopathological score was significantly higher in ISO exposed rats than in control rats. However, the score was significantly lower in rats treated with DCM-L200 and DCM-S200 compared to ISO exposed rats (Fig. 6F).
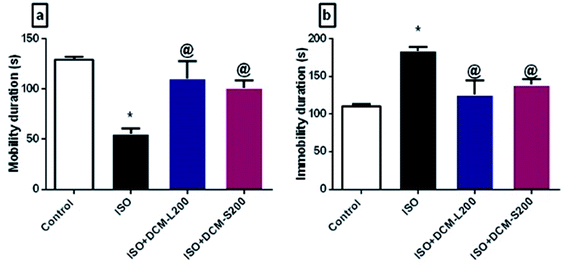
Concerning the behaviour of rats in the forced swim test, ISO exposed rats displayed a depressant-like behaviour in terms of decreasing the mobility duration and increasing the immobility duration. This was consistent with the previously published literature.35 However, administration of DCM-L and DCM-S fractions increased the mobility duration and reduced the immobility duration with superiority to DCM-L versus DCM-S, although the difference between both fractions is not significant (Fig. 8a and b).
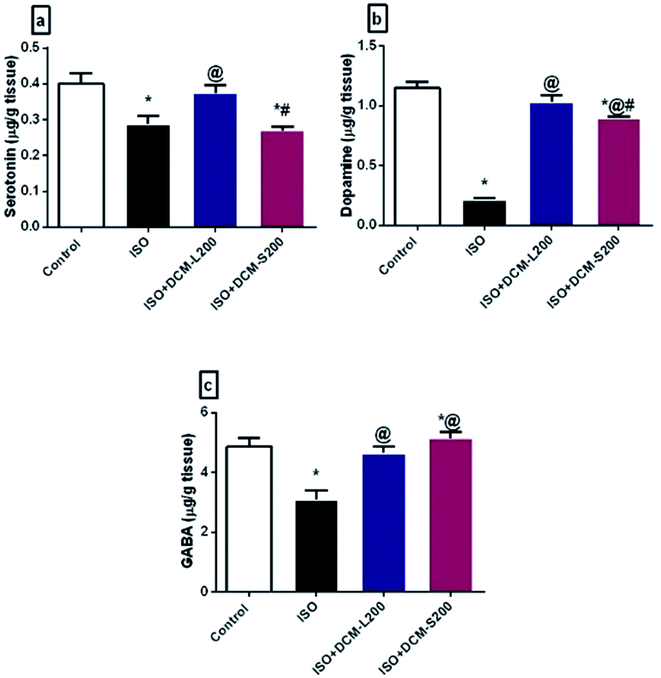
There are several possible explanations for these behavioural findings. First of all, the disturbance in the neurotransmitter system was associated with anxiety and depression.36 The decreased serotonin levels in the brain tissues are considered the major contributor to depression pathogenesis.37 In addition, the dopamine system contributes to some depression symptoms, such as decreasing motivation. Both serotonin and dopamine are responsible for the emotional well-being of humans.38 Herein, ISO exposed rats decreased the serotonin and dopamine brain levels due to endogenous homeostasis for adapted the inotropic effects and Ca2+-dependent mechanisms, as reported previously.39 However, the administration of DCM-L and DCM-S fractions boosted the serotonin and dopamine brain levels (Fig. 9a and b).
Another possible explanation for the behavioural alterations of ISO exposed rats is the disruption of GABA neurotransmitters. GABA is an inhibitory neurotransmitter responsible for the flow of information through the brain due to its extrinsic control.40 This finding is consistent with many clinical and pre-clinical studies that report the direct relationship between the reduced brain GABA levels and depression.40,41 However, administration of DCM-L and DCM-S fractions could increase the brain levels of GABA (Fig. 9c).
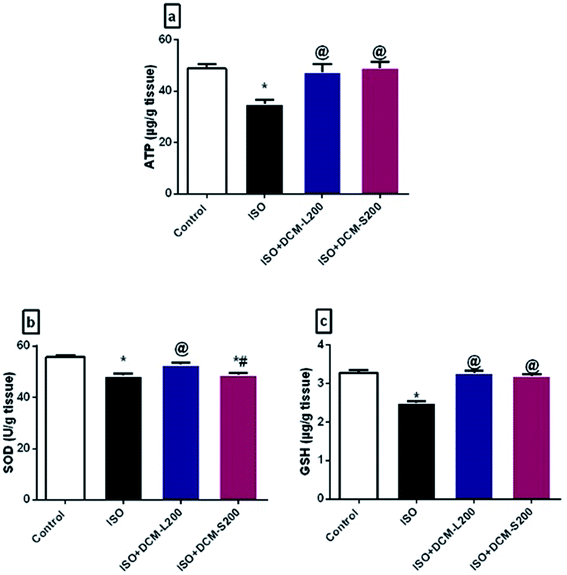
Moreover, a direct relationship exists between depletion of neuronal cell energy and depression.42 Energy requirements for neurotransmitter release are controlled by the levels of ATP.43 In our study, ISO exposed rats exhibited a decreased level of ATP. This finding agrees with previous literature and is confirmed by reducing serotonin and dopamine brain levels.44,45 However, the administration of DCM-L and DCM-S increased ATP brain levels (Fig. 10a).
Oxidative stress is involved in many brain disorders, including depression, due to the high susceptibility of the brain to the reactive oxygen species (ROS).46 In this study, ISO exposed rats displayed decreased SOD and GSH brain levels.47 While the administration of DCM-L and DCM-S increased the SOD and GSH brain levels (Fig. 10b and c).
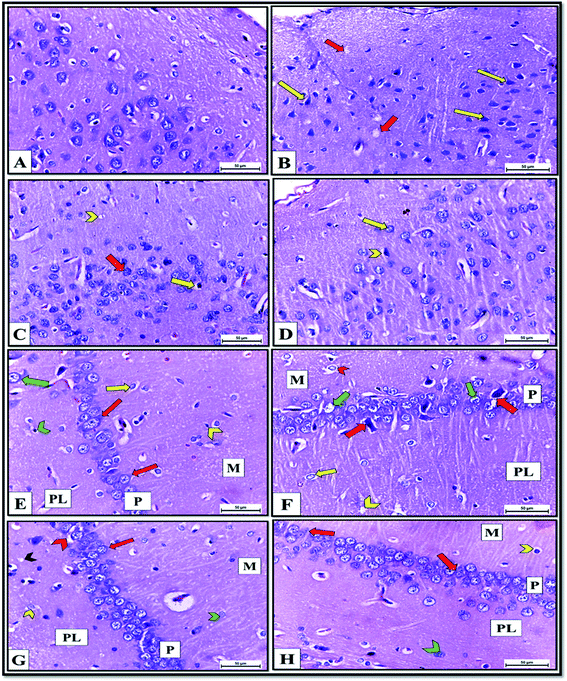
The histopathological picture of brain tissue, including the cerebral cortex and hippocampus, also confirmed the neurochemical disturbance in the brains of the ISO exposed rats. It revealed pyknosis and shrunken neurons with neuropil vacuolation (Fig. 11B). These structural abnormalities agree with previous findings,48 reporting that decreased neurogenesis and neurite outgrowth in the ventral hippocampus are associated with heart failure in rats. Meanwhile, cerebral cortex sections of DCM-L200 and DCM-S200-treated rats showed marked recovery, evidenced by a significant decrease in the neuropil vacuolation, pyramidal cells restored their normal shape with vesicular nuclei. However, few cells appeared pyknotic with pericellular space (Fig. 11C and D). Also, histological examination of H&E-stained hippocampus sections of control rats showed three layers; molecular, pyramidal, and polymorphic layers, respectively (Fig. 11E).
In contrast, hippocampus sections obtained from ISO exposed rats revealed histopathological changes such as chromatolysis of neurons surrounded by pericellular spaces in molecular and polymorphic layers. The pyramidal cell layer contained shrunken, pyknotic neurons, and some pyramidal neurons had karyolitic nuclei. Also, spongiform vacuolation of neuropil was observed (Fig. 11F). However, hippocampal sections of DCM-L200 and DCM-S200-treated rats exhibited great restoration of the shape of the neurons in the three layers, but few neurons appeared with pericellular space. However, Neuropil vacuolation was reduced to a greater extent (Fig. 11G and H).
In conclusion, Genus Morus is an enriched phytochemical plant and plays a significant role as a diet-based therapy to cure various disorders. The presence of phenolic compounds, including flavonoids, quercetin, and its derivatives, is responsible for the Morus cardioprotective potential,49 via several mechanisms: antioxidant, anti-apoptosis, anti-inflammatory, inhibition of mitochondrial dysfunction, and DNA damage.50 Additionally, the presence of fatty acids, including melissic acid and cerotic acid, played a role in the cardioprotective and anti-depressant actions of Morus.51,52 Also, β-sitosterol, an isolated compound, was reported as a cardioprotective drug by reducing myocardial infarcted size, inflammatory mediators, and apoptosis.53 Additionally, triterpenes, such as lupeol acetate, were reported to have cardioprotective effects by inhibiting lipid abnormalities and abnormal biochemical changes induced in MI in addition to anti-inflammatory actions.54,55
Moreover, anthocyanins, cyanidin, and their derivatives possess cardioprotective activity due to their antioxidant, anti-inflammatory actions, and inhibiting endothelial dysfunction and NO production.56 Chlorogenic acid and quercetin-O-glucoside also had a cardioprotective,57,58 and anti-depressant actions.59,60
4. Conclusions
We demonstrated that M. macroura DCM-S and DCM-L fractions harbor cardioprotective and antidepressive properties against ISO-induced post-MI depression as they persuade cardioprotection by decreasing myocardial enzymes and safeguarding the cardiac muscle histology. Likewise, they maintain emotional stability by boosting serotonin, dopamine, GABA, and ATP brain levels. Also, they restore the redox status of the brain by increasing SOD and GSH levels and protecting its architecture. Further studies in humans are necessitated to examine the clinical application of such observations, such as screening the ligands that support pharmacokinetics, as well as, in vivo dose determination, safety, and efficacy studies. The biological results may be attributed to the metabolite profile identified in DCM-L and DCM-S fractions and analyzed using UPLC- ESI-MS/MS.Conflicts of interest
The authors declared no conflict of interest.References
- D. K. Arnett, R. S. Blumenthal, M. A. Albert, A. B. Buroker, Z. D. Goldberger, E. J. Hahn, C. D. Himmelfarb, A. Khera, D. Lloyd-Jones, J. W. McEvoy, E. D. Michos, M. D. Miedema, D. Muñoz, S. C. Smith, S. S. Virani, K. A. Williams, J. Yeboah and B. Ziaeian, ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, Circulation, 2012, 140, 596–646 Search PubMed.
- A. Meijer, H. J. Conradi, E. H. Bos, M. Anselmino, R. M. Carney, J. Denollet, F. Doyle, K. E. Freedland, S. L. Grace, S. H. Hosseini, D. A. Lane, L. Pilote, K. Parakh, C. Rafanelli, H. Sato, R. P. Steeds, C. Welin and P. de Jonge, Adjusted prognostic association of depression following myocardial infarction with mortality and cardiovascular events: individual patient data meta-analysis, Br. J. Psychiatry, 2013, 203, 90–102 CrossRef CAS PubMed.
- F. Doyle, H. M. McGee, R. M. Conroy and M. Delaney, What predicts depression in cardiac patients: Sociodemographic factors, disease severity or theoretical vulnerabilities?, Psychol. Health, 2011, 26, 619–634 CrossRef CAS PubMed.
- S. J. Dai, Z. B. Ma, Y. Wu, R. Y. Chen, D. Q. Yu and F. -J. Guangsangons, Antioxidant and anti-inflammatory Diels–Alder type adducts, from Morus macroura Miq, Phytochemistry, 2004, 65, 3135–3141 CrossRef CAS PubMed.
- Y. Wang, L. Xu, W. Gao, L. Niu, C. Huang, P. Yang and X. Hu, Isoprenylated Phenolic Compounds from Morus macroura as Potent Tyrosinase Inhibitors, Planta Med., 2017, 84, 336–343 Search PubMed.
- P. M. Boarescu, I. Chirilă, A. E. Bulboacă, I. C. Bocșan, R. M. Pop, D. Gheban and S. D. Bolboacă, Effects of Curcumin Nanoparticles in Isoproterenol-Induced Myocardial Infarction, Oxid. Med. Cell. Longevity, 2019, 2019, e7847142 Search PubMed.
- C. Zhu, W. Li, X. Wang, J. Xue, L. Zhao, Y. Song, T. Zhou and M. Zhang, Phloroglucinol averts isoprenaline hydrochloride induced myocardial infarction in rats, Drug Dev. Res., 2019, 80, 453–460 CrossRef CAS PubMed.
- N. Sun, Y. Mei, Z. Hu, W. Xing, K. Lv, N. Hu, T. Zhang and D. Wang, Ghrelin attenuates depressive-like behavior, heart failure, and neuroinflammation in postmyocardial infarction rat model, Eur. J. Pharmacol., 2021, 901, 174096 CrossRef CAS PubMed.
- H. Wang, M. Ahmad, R. Jadayel, F. Najjar, D. Lagace and F. Leenen, Inhibition of inflammation by minocycline improves heart failure and depression-like behaviour in rats after myocardial infarction, PloS one, 2019, 14, e0217437 CrossRef CAS PubMed.
- Y. Hu, X. Liu, T. Zhang, C. Chen, X. Dong, Y. Can and P. Liu, Behavioral and Biochemical Effects of KXS on Postmyocardial Infarction Depression, Front. Pharmacol., 2020, 11, 561817 CrossRef CAS PubMed.
- D. Hamdan, R. A. El-Shiekh, M. El-Sayed, H. M. Khalil, M. Mousa, A. Al-Gendy and A. El-Shazly, Phytochemical characterization and anti-inflammatory potential of Egyptian Murcott mandarin cultivar waste (stem, leaves and peel), Food Funct., 2020, 11, 8214–8236 RSC.
- S. Ali, S. Mohamed, N. Rozalei, Y. Boon and S. Zainalabidin, Anti-fibrotic Actions of Roselle Extract in Rat Model of Myocardial Infarction, Cardiovasc. Toxicol., 2019, 19, 72–81 CrossRef CAS PubMed.
- H. M. A. Khalil, H. A. Eliwa, R. A. El-Shiekh, A. K. Al-Mokaddem, M. Hassan, A. M. Tawfek and W. H. El-Maadawy, Ashwagandha (Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-kB/MAPK signaling pathways, J. Ethnopharmacol., 2021, 277, 114141 CrossRef CAS PubMed.
- S. M. Zaki, G. H. A. Hussein, H. A. M. Khalil and W. A. A. Algaleel, Febuxostat ameliorates methotrexate-induced lung damage, Folia Morphol., 2020, 80, 392–402 CrossRef.
- D. A. Slattery and J. F. Cryan, Using the rat forced swim test to assess antidepressant-like activity in rodents, Nat. Protoc., 2012, 7, 1009–1014 CrossRef CAS PubMed.
- T. Teerlink, M. Hennekes, J. Bussemaker and J. Groeneveld, Simultaneous Determination of Creatine Compounds and Adenine Nucleotides in Myocardial Tissue by High-Performance Liquid Chromatography, Anal. Biochem., 1993, 214, 278–283 CrossRef CAS PubMed.
- P. Pagel, J. Blome and H. U. Wolf, High-performance liquid chromatographic separation and measurement of various biogenic compounds possibly involved in the pathomechanism of Parkinson's disease, J. Chromatogr. B Biomed. Appl., 2000, 746, 297–304 CrossRef CAS.
- J. D. Bancroft, and M. Gamble, Theory and practice of histology techniques, Churchill Livingstone Elsevier Lond., 2008, pp. 83–134 Search PubMed.
- C. Atkinson, S. He, K. Morris, F. Qiao, S. Casey, M. Goddard and S. Tomlinson, Targeted Complement Inhibitors Protect against Posttransplant Cardiac Ischemia and Reperfusion Injury and Reveal an Important Role for the Alternative Pathway of Complement Activation, J. Immunol. Res., 2010, 185, 7007–7013 CAS.
- H. C. Huang, C. C. Liaw, H. L. Yang, Y. C. Hseu, H. T. Kuo, Y. C. Tsai and Y. H. Kuo, Lanostane triterpenoids and sterols from Antrodia camphorate, Phytochemistry, 2012, 84, 177–183 CrossRef CAS PubMed.
- A. S. Kipkemei, Isolation of stigmasterol, α-amyrin acetate and lupeol acetate from Tabernaemontana Stapfiana Britten, Int. J. Sci. Eng. Res., 2017, 8, 1331–1335 Search PubMed.
- H. Li, Z. Ma, Y. Zhai, C. Lv, P. Yuan, F. Zhu, L. Wei, Q. Li and X. Qi, Trimetazidine Ameliorates Myocardial Metabolic Remodeling in Isoproterenol-Induced Rats Through Regulating Ketone Body Metabolism via Activating AMPK and PPAR α, Front. Pharmacol., 2020, 11, 1255 CrossRef CAS PubMed.
- K. Mnafgui, R. Hajji, F. Derbali, A. Gammoudi, G. Khabbabi, H. Ellefi, N. Allouche, A. Kadri and N. Gharsallah, Anti-inflammatory, Antithrombotic and Cardiac Remodeling Preventive Effects of Eugenol in Isoproterenol-Induced Myocardial Infarction in Wistar Rat, Cardiovasc. Toxicol., 2016, 16, 336–344 CrossRef CAS PubMed.
- M. Raish, A. Ahmad, M. Ansari, K. Alkharfy, A. Ahad, A. Khan, N. Ali and M. Ganaie, Beetroot juice alleviates isoproterenol-induced myocardial damage by reducing oxidative stress, inflammation, and apoptosis in rats, Biotech., 2019, 9, 147 Search PubMed.
- D. Mozaffarian, H. Cao, I. B. King, R. N. Lemaitre, X. Song, D. S. Siscovick and G. S. Hotamisligil, Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in US adults: a cohort study, Ann. Intern. Med., 2010, 153, 790–799 CrossRef PubMed.
- P. Akila, L. Asaikumar and L. Vennila, Chlorogenic acid ameliorates isoproterenol-induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes, Biomed. Pharmacother., 2017, 85, 582–591 CrossRef CAS PubMed.
- M. Sumitra, P. Manikandan, D. A. Kumar, N. Arutselvan, K. Balakrishna, B. M. Manohar and R. Puvanakrishnan, Experimental myocardial necrosis in rats: role of arjunolic acid on platelet aggregation, coagulation and antioxidant status, Mol. Cell. Biochem., 2001, 224, 135–142 CrossRef CAS PubMed.
- R. Zhou, P. Ma, A. Xiong, Y. Xu, Y. Wang and Q. Xu, Protective effects of low-dose rosuvastatin on isoproterenol-induced chronic heart failure in rats by regulation of DDAH-ADMA-NO pathway, Cardiovasc. Ther., 2017, 35, 12241 CrossRef PubMed.
- S. N. Fathima and S. V. Murthy, Cardioprotective effects to chronic administration of Rosa damascena petals in isoproterenol induced myocardial infarction: biochemical, histopathological and ultrastructural studies, Biomed. Pharmacol. J., 2019, 12, 1155–1166 CAS.
- M. Li, X. Li and L. Yang, Cardioprotective effects of garcinol following myocardial infarction in rats with isoproterenol-induced heart failure, AMB Express, 2020, 10, 1–7 CrossRef.
- K. Mal, I. D. Awan, J. Ram and F. Shaukat, Depression and Anxiety as a Risk Factor for Myocardial Infarction, Cureus, 2019, 11, e6064 Search PubMed.
- K. R. Lezak, G. Missig and W. A. Carlezon Jr, Behavioral methods to study anxiety in rodents, Dialogues Clin. Neurosci., 2017, 19, 181–191 Search PubMed.
- A. K. Kraeuter, P. C. Guest and Z. Sarnyai, The Forced Swim Test for Depression-Like Behavior in Rodents, Methods Mol. Biol., 2019, 1916, 75–80 CrossRef CAS PubMed.
- A. Frey, S. Popp, A. Post, S. Langer, M. Lehmann, U. Hofmann, A. L. Sirén, L. Hommers, A. Schmitt, T. Strekalova, G. Ertl, K. P. Lesch and S. Frantz, Experimental heart failure causes depression-like behavior together with differential regulation of inflammatory and structural genes in the brain, Front. Behav. Neurosci., 2014, 8, 376 CrossRef PubMed.
- N. Sun, Y. Mei, Z. Hu, W. Xing, K. Lv, N. Hu, T. Zhang and D. Wang, Ghrelin attenuates depressive-like behavior, heart failure, and neuroinflammation in postmyocardial infarction rat model, Eur. J. Pharmacol., 2021, 901, 174096 CrossRef CAS PubMed.
- M. Williams, Platelets and depression in cardiovascular disease: A brief review of the current literature, World J. Psychiatry, 2012, 2, 114–123 CrossRef PubMed.
- M. Naoi, W. Maruyama and M. Shamoto-Nagai, Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: from neurotransmitter imbalance to impaired neurogenesis, J. Neural Transm., 2018, 125, 53–66 CrossRef CAS PubMed.
- H. G. Ruhé, N. S. Mason and A. H. Schene, A Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies, Mol. Psychiatry, 2007, 12, 331–359 CrossRef PubMed.
- H. Zhang, Z. Ma, X. Luo and X. Li, Effects of mulberry fruit (Morusalba L.) consumption on health outcomes: A mini-review, Antioxidants, 2018, 7, 69 CrossRef PubMed.
- C. Fee, M. Banasr and E. Sibille, Somatostatin-Positive Gamma-Aminobutyric Acid Interneuron Deficits in Depression: Cortical Microcircuit and Therapeutic Perspectives, Biol. Psychiatry, 2017, 82, 549–559 CrossRef CAS PubMed.
- C. G. Abdallah, L. Jiang, H. M. De Feyter, M. Fasula, J. H. Krystal, D. L. Rothman, G. F. Mason and G. Sanacora, Glutamate Metabolism in Major Depressive Disorder, Am. J. Psychiatry., 2014, 171, 1320–1327 CrossRef PubMed.
- A. Moretti, A. Gorini and R. F. Villa, Affective disorders, anti-depressant drugs and brain metabolism, Mol. Psychiatry, 2003, 8, 773–785 CrossRef CAS PubMed.
- M. J. Devine and J. T. Kittler, Mitochondria at the neuronal presynapse in health and disease, Nat. Rev. Neurosci., 2018, 19, 63–80 CrossRef CAS PubMed.
- A. Karabatsiakis and C. Schönfeldt-Lecuona, Depression, mitochondrial bioenergetics, and electroconvulsive therapy: a new approach towards personalized medicine in psychiatric treatment - a short review and current perspective, Transl. Psychiatry, 2020, 10, 1–9 CrossRef PubMed.
- D. Martins-de-Souza, P. C. Guest, L. W. Harris, N. Vanattou-Saifoudine, M. J. Webster, H. Rahmoune and S. Bahn, Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients, Transl. Psychiatry, 2012, 2, 87 CrossRef PubMed.
- M. Maes, P. Galecki, Y. S. Chang and M. Berk, A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness, Prog. Neuropsychopharmacol. Biol. Psychiatry, 2011, 35, 676–692 CrossRef CAS PubMed.
- Y. Kurhe, M. Radhakrishnan, D. Gupta and T. Devadoss, QCM-4 a novel 5-HT3 antagonist attenuates the behavioral and biochemical alterations on chronic unpredictable mild stress model of depression in Swiss albino mice, J. Pharm. Pharmacol., 2014, 66, 122–132 CrossRef CAS PubMed.
- D. Arnone, S. McKie, R. Elliott, G. Juhasz, E. J. Thomas, D. Downey, S. Williams, J. F. Deakin and I. M. Anderson, State-dependent changes in hippocampal grey matter in depression, Mol. Psychiatry, 2013, 18, 1265–1272 CrossRef CAS PubMed.
- M. S. Butt, A. Nazir, M. T. Sultan and K. Schroën, Morus alba L. nature's functional tonic, Trends Food Sci. Technol., 2008, 19, 505–512 CrossRef CAS.
- K. Razavi-Azarkhiavi, M. Iranshahy, A. Sahebkar, K. Shirani and G. Karimi, The Protective Role of Phenolic Compounds Against Doxorubicin-induced Cardiotoxicity : A Comprehensive Review, Nutr. Cancer, 2016, 68, 892–917 CrossRef CAS PubMed.
- G. Grosso, A. Pajak, S. Marventano, S. Castellano, F. Galvano, C. Bucolo, F. Drago and F. Caraci, Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials, PLoS One, 2014, 9, e96905 CrossRef PubMed.
- L. Carnevali, F. Vacondio, S. Rossi, S. Callegari, E. Macchi, G. Spadoni, A. Bedini, S. Rivara, M. Mor and A. Sgoifo, Antidepressant-like activity and cardioprotective effects of fatty acid amide hydrolase inhibitor URB694 in socially stressed Wistar Kyoto rats, Eur. Neuropsychopharmacol., 2015, 25, 2157–2169 CrossRef CAS PubMed.
- F. Lin, L. Xu, M. Huang, B. Deng, W. Zhang, Z. Zeng and S. Yinzhi, β-Sitosterol Protects against Myocardial Ischemia/Reperfusion Injury via Targeting PPARγ/NF-κB Signalling, Evidence-Based Complementary Altern. Med., 2020, 28, 2679409 Search PubMed.
- V. Sudhahar, S. A. Kumar, P. T. Sudharsan and P. Varalakshmi, Protective effect of lupeol and its ester on cardiac abnormalities in experimental hypercholesterolemia, Curr. Vasc. Pharmacol., 2007, 46, 412–418 CrossRef CAS PubMed.
- S. Saha, E. Profumo, A. R. Togna, R. Riganò, L. Saso and B. Buttari, Lupeol Counteracts the Proinflammatory Signalling Triggered in Macrophages by 7-Keto-Cholesterol: New Perspectives in the Therapy of Atherosclerosis, Oxid. Med. Cell. Longevity, 2020, 2020, 1232816 Search PubMed.
- J. Liu, H. Zhou, L. Song, Z. Yang, M. Qiu, J. Wang and S. Shi, Anthocyanins: Promising Natural Products with Diverse Pharmacological Activities, Molecules, 2021, 26, 3807 CrossRef CAS PubMed.
- M. Li, Y. Jiang, W. Jing, B. Sun, C. Miao and L. Ren, Quercetin provides greater cardioprotective effect than its glycoside derivative rutin on isoproterenol-induced cardiac fibrosis in the rat, Can. J. Physiol. Pharmacol., 2013, 91, 951–959 CrossRef CAS PubMed.
- O. M. Agunloye, G. Oboh, A. O. Ademiluyi, A. O. Ademosun, A. A. Akindahunsi, A. A. Oyagbemi, T. O. Omobowale, T. O. Ajibade and A. A. Adedapo, Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats, Biomed. Pharmacother., 2019, 109, 450–458 CrossRef CAS PubMed.
- S. Park, Y. Sim, P. Han, J. Jin-Koo Lee and H. Suh, Antidepressant-like effect of chlorogenic acid isolated from Artemisia capillaris Thunb, Anim. Cells Syst., 2010, 4, 253–259 CrossRef.
- V. Singh, G. Chauhan and R. Shri, Anti-depressant like effects of quercetin 4'-O-glucoside from Allium cepa via regulation of brain oxidative stress and monoamine levels in mice subjected to unpredictable chronic mild stress, Nutr. Neurosci., 2021, 2, 35–44 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08320a |
| This journal is © The Royal Society of Chemistry 2022 |