Open Access Article
Open Access ArticleHigh temperature structure evolution of SiBZrOC quinary polymer derived ceramics
Chen Liu *ab,
Changqing Hongb,
Xinwei Wang*a and
Jiecai Hanb
*ab,
Changqing Hongb,
Xinwei Wang*a and
Jiecai Hanb
aLaboratory for Space Environment and Physical Science, Harbin Institute of Technology, Harbin, 150001, PR China. E-mail: liuchen2016@hit.edu.cn; xinweiwang@hit.edu.cn
bNational Key Laboratory of Science and Technology on Advanced Composites in Special Environments, Harbin Institute of Technology, Harbin 150001, PR China
First published on 14th March 2022
Abstract
SiBZrOC quinary ceramics were obtained through the modification of a SiOC precursor with B(OH)3 and Zr(OnPr)4. The results showed that both B and Zr atoms were involved in the SiOC network through Si–O–B and Si–O–Zr bonds, respectively. The combined effects of B and Zr on the chemical structure and the thermal stability of the SiBZrOC system were investigated in detail. The sp3–C/Si ratio of SiBZrOC ceramics was between the values for SiZrOC and SiBOC. The presence of B promotes the crystallization of t-ZrO2, which precipitated at 1000 °C and transformed to m-ZrO2 at 1400 °C. At 1600 °C, ZrO2 reacted with the matrix and formed ZrSiO4, which consumed SiO2 and thus inhibited the carbothermal reaction. The very small I(D)/I(G) ratio of 0.13 in the Raman spectra indicated the high graphitization of free carbon in SiBZrOC ceramics, which was observed by TEM with 10–20 graphene layers. The SiBZrOC ceramics showed excellent thermal stability in argon at 1600 °C for 5 h with a mass loss of 6%. Both the formation of ZrSiO4 and the highly graphitized free carbon play important roles in inhibiting the carbothermal reaction and thus improving the thermal stability of SiBZrOC ceramics.
Introduction
Polymer-derived ceramics (PDCs) are attractive candidates for use under extreme conditions owing to their high strength, creep and thermal shock resistance, as well as stability in oxidative and corrosive environments. They are X-ray amorphous and comprised of nanodomains of different structures and compositions, obtained by the thermal decomposition of organic polymers. The most well-known classes of PDCs are the ternary systems SiCN and SiCO as well as the quaternary systems SiCNO, SiBCN, SiBCO, SiAlCN, and SiAlCO.1–4 Usually, quaternary ceramics exhibit improved mechanical and thermal properties as compared to those of the ternary materials. Recent investigations have shown that the high temperature stability in terms of decomposition for the SiBCN ceramics can reach 2200 °C.5The fabrication processes of these materials involve the preparation of precursors, pyrolysis and sintering. The modification of the precursor with B6–8 and metallic elements such as Al,9–11 Ti,12,13 or Zr14–16 Hf,17–20 allows the design and controlled synthesis of multifunctional PDC materials with tailored properties. They are typically prepared by modifying precursors with metal alkoxides and subsequent ceramization, leading to SiOC/SiCN nanocomposites containing a finely dispersed metal oxide nanodomains. The nanodomains may significantly influence the microstructure and physical properties of these ceramics on a large scale, especially when the domains differ in their chemistry, electrical conductivity, and mechanical properties.21–25
Though early work already strongly suggested the nanoscale heterogeneity both in SiCO- and SiCN-based materials, the detailed chemical and structural nature of the quinary PDCs remained elusive. In previous work,26,27 we prepared the B-modified and Zr-modified SiOC precursor through the chemical reactions of hybrid alkoxysilanes with boric acid and Zr(OnPr)4, respectively. The results showed that the effects of B and Zr are contrary on some cases, such as the precipitation of SiC and the graphitization of free carbon. In this work, we synthesized a SiBZrOC quinary precursor by using the same raw materials and compared with previous work, to study the combined effects of B and Zr on the polymer-to-ceramic transformation processes and the microstructure evolution of SiBZrOC materials. Various characterization techniques such as NMR and Raman spectroscopy, X-ray diffraction, as well as TEM were used to study the structure of the metallo-organic precursor and the nanocomposites.28–33
Experimental
Methyltriethoxysilane (MTES), methyldiethoxysilane (MDES), boric acid (B(OH)3) and Zr(OnPr)4 (Alfa Aesar, 70 wt% in propanol) were used as the starting raw materials. MTES, MDES and B(OH)3 was mixed with the molar ratio of MTES/MDES = 3 and B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si = 0.4. The mixture was stirred until complete dissolution. The appropriate amount of Zr(OnPr)4 was then added to the prehydrolyzed solution under fast stirring with the molar ratio of Zr
Si = 0.4. The mixture was stirred until complete dissolution. The appropriate amount of Zr(OnPr)4 was then added to the prehydrolyzed solution under fast stirring with the molar ratio of Zr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si = 0.2. The mixture was obtained and heated at 80 °C for 1 day for gelation. The SiBZrOC gel were dried at 80 °C for 2 days, grounded and finally sieved to <75 μm. The SiBZrOC precursor was finally obtained and pyrolyzed in a tube furnace with a heating rate of 5 °C min−1 in flowing argon gas, at 1000 °C, 1200 °C, 1400 °C, and 1600 °C for 1 h, followed by furnace cooling to ambient temperature.
Si = 0.2. The mixture was obtained and heated at 80 °C for 1 day for gelation. The SiBZrOC gel were dried at 80 °C for 2 days, grounded and finally sieved to <75 μm. The SiBZrOC precursor was finally obtained and pyrolyzed in a tube furnace with a heating rate of 5 °C min−1 in flowing argon gas, at 1000 °C, 1200 °C, 1400 °C, and 1600 °C for 1 h, followed by furnace cooling to ambient temperature.
TG were performed on the xerogels using a PerkinElmer differential scanning calorimeter from room temperature to 1400 °C at a heating rate of 10 °C min−1 under argon atmosphere. FTIR spectra of the precursor were recorded between 4000 and 400 cm−1 on a PerkinElmer Spectrum One FTIR spectrometer. NMR spectrum of precursor and ceramics was collected using a 4 mm Bruker CP MAS probe and a Bruker Ultrashield 300 MHz solid-state spectrometer. 29Si CP MAS Powder samples were taken in ZrO2 rotors and were spun at a rate of 5 kHz. The spectrum was collected using 30° pulses length of 4 μs and 2 s recycle delay. For each 29Si NMR spectrum, 4000–90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 transients were accumulated with a spectral width of 59 kHz. XRD patterns of ceramics were collected using a Philips X′Pert diffractometer with Cu Kα radiation. A 2θ range of 10–90° was scanned with a step size of 0.05°. Raman spectra were collected on a Raman Station (B&WTEK, BWS435-532SY) with a near infrared laser operating at 532 nm. The microstructure of the ceramics was observed using scanning electron microscopy (Helios Nanolab600i). A Philips-FEI Tecnai G2 F30 instrument was used to perform TEM investigations on the ceramic powders dispersed on copper grid.
000 transients were accumulated with a spectral width of 59 kHz. XRD patterns of ceramics were collected using a Philips X′Pert diffractometer with Cu Kα radiation. A 2θ range of 10–90° was scanned with a step size of 0.05°. Raman spectra were collected on a Raman Station (B&WTEK, BWS435-532SY) with a near infrared laser operating at 532 nm. The microstructure of the ceramics was observed using scanning electron microscopy (Helios Nanolab600i). A Philips-FEI Tecnai G2 F30 instrument was used to perform TEM investigations on the ceramic powders dispersed on copper grid.
Results and discussion
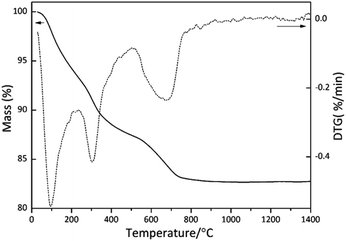
The TG/DTG curves of SiBZrOC precursor was shown in Fig. 1. The weight loss occurred mainly in three stages in the temperature intervals 50–200 °C, 200–500 °C, and 500–800 °C, respectively. The DTG curve shows an intense peak at 100 °C corresponding to a weight loss of 6 wt%, which is attributed to the loss of water and ethanol. The second weight loss of 7 wt% in the range of 200–500 °C, is probably related to the release of boric acid, H2, and CH4 as the predominant volatiles. In the third step, the weight loss of 4% is mainly due to the evolution of methane Si–CH3. At above 800 °C, the weight remains unchanged and the ceramic yield was found to be 83%.Fig. 2 shows the infrared spectra of SiBZrOC precursor and ceramics pyrolyzed at 1000 to 1600 °C. All the spectra show the characteristic peak of Si–O–Si bond between 1000 and 1130 cm−1, which is split into two bands at 1035 and 1125 cm−1, indicating the high degree of polymerization of the precursors. Si–O–Zr bond at 905 cm−1 was detected in the precursor formed through reactions (1) and (2), whilst disappeared in the ceramics owing to the redistribution reaction between Si–O and Zr–O bonds and thus formed Zr–O–Zr bonds. Si–O–B bond at 875 cm−1 was found in the ceramics, whilst absent in the precursor. This is probably because the Si–O–B bond has been formed through reaction (3) in the precursor, but covered up by the Si–O–Zr bond in the ceramics. The FTIR results confirmed that both B and Zr were involved in the siloxane network in the SiOC network in the ceramics.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–OH + Si–OH + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Zr–OPr → Zr–OPr → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O–Zr Si–O–Zr![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) + C3H7OH + C3H7OH
| (1) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–OH + Si–OH + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Zr–OH → Zr–OH → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O–Zr Si–O–Zr![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) + H2O + H2O
| (2) |
| Si–OH + B(OH)3 → Si–O–B + H2O | (3) |
Fig. 3 shows the 29Si NMR spectra of SiOC, SiBOC, SiZrOC and SiBZrOC precursor. The spectra of SiOC and SiBOC precursor present a sharp signal at −69 ppm assigned to SiCO3 units and another peak at −39 ppm due to HSiCO2 units, whilst the latter was absent in SiZrOC and SiBZrOC precursor, revealing that the Si–H bonds has been transformed into Si–O bonds through reactions (4) and (5) in Zr-containing precursor.26 Moreover, the SiCO3 peaks in SiZrOC and SiBZrOC precursor were simulated with two Gaussian peaks at −62.7 and −69 ppm, which were assigned to the SiCO3 units with and without Si–O–Zr bonds, respectively. The former unit accounts for 50% in SiBZrOC, which is obviously lower than the 74% in SiZrOC ceramics,26 suggesting that the SiCO3 units with Si–O–B bond in SiBZrOC accounts for 24% at most. The formation of Si–O–B bonds consumed a part of Si–O bonds and thus decreased the content of Si–O–Zr bonds. Combined with the FTIR results, it is confirmed that Zr and B was distributed uniformly in the SiOC network at atomic scale.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–OH + Si–OH + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Zr–OH → Zr–OH → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O–Zr Si–O–Zr![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) + H2 + H2
| (4) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–H + Si–H + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Zr–OPr → Zr–OPr → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O–Zr Si–O–Zr![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) + C3H8 + C3H8
| (5) |
 | ||
| Fig. 3 29Si MAS NMR spectra of SiOC, SiBOC, SiZrOC and SiBZrOC precursor.26,27 | ||
As comparison, TG curves of SiOC, SiBOC and SiZrOC26,27 precursor were shown in Fig. 4. The ceramic yield of SiBZrOC is 83%, much lower than the 93% of SiBOC, and slightly higher than the 81% of SiZrOC. This is probably because the formation of Si–O–B bond lead to a more interconnected gel network,34 and thus higher ceramic yield in B-containing precursors. Compared with SiZrOC, the ceramic yield of SiBZrOC was slightly improved owing to the Si–O–B bonds formed through the 24% Si–O bonds.
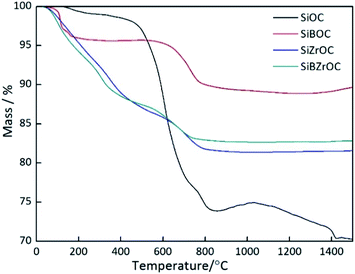 | ||
| Fig. 4 TG curves of SiOC, SiBOC, SiZrOC and SiBZrOC ceramics.26,27 | ||
The 29Si MAS NMR spectra of SiBZrOC ceramics pyrolyzed at various temperature are presented in Fig. 5. All the spectra show the resonance peaks of SiO4 (δ = −110 ppm) and SiC4 (δ = −15 ppm) units, resulting from the redistribution reaction between Si–O and Si–C bond. The sp3–C/Si ratio of the four ceramics were calculated according to the peak area of SiO4 and SiC4, listed in Table 1. It can be seen that the sp3–C/Si ratio of SiZrOC ceramics is 0.12, lower than the 0.16 of SiOC ceramics, which is attributed to the addition of Zr atom, leading to a decrease in the thermal stability of Si–C bond.15 The SiBOC ceramics is 0.27, much higher than that of SiOC ceramics.26 This is because the presence of Si–H groups in the precursor promotes the insertion of C into SiCO network26 and the redistribution reaction between B–C and Si–O bond could also lead to the increase of sp3–C.35 The sp3–C/Si ratio in SiBZrOC ceramics is 0.22, between the value of SiBOC and SiZrOC, resulting from the combined effects of B and Zr.
 | ||
| Fig. 5 29Si MAS NMR spectra of SiOC, SiZrOC, SiBOC and SiBZrOC ceramics pyrolyzed at 1200 °C.26,27 | ||
| Ceramics | sp3–C/Si |
|---|---|
| SiOC | 0.16 |
| SiZrOC | 0.12 |
| SiBOC | 0.27 |
| SiBZrOC | 0.22 |
XRD patterns of SiBZrOC ceramics pyrolyzed at various temperatures are presented in Fig. 6. At 1000 and 1200 °C, t-ZrO2 phase is found as the only crystalline phase in SiBZrOC ceramics. Small amounts of m-ZrO2 were detected at 1400 °C, which was formed through the phase transformation from t-ZrO2 during cooling down when the t-ZrO2 grew up to the critical size.26 It can be seen that the phase transformation temperature for SiBZrOC is 1400 °C, much lower than the 1600 °C for SiZrOC,26 revealing the growth of t-ZrO2 in SiBZrOC are faster than SiZrOC. Therefore, it can be concluded that the addition of B facilitates the growth of t-ZrO2 crystallites. Moreover, ZrSiO4 was found at 1600 °C, formed by the reaction of zirconia with amorphous silica in the matrix.
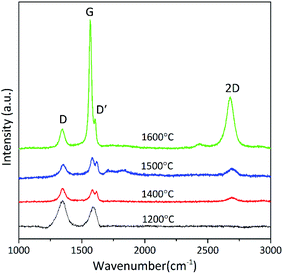
Fig. 7 shows the Raman spectra of SiBZrOC ceramics pyrolyzed at various temperatures. All the Raman spectra show two characteristic features of disordered graphitic forms of carbon: the D band at about 1350 cm−1 due to the defects and the G band at 1590 cm−1 related to in-plane bond stretching of sp2 carbon. The G band narrows from 47 cm−1 to 21 cm−1 and shifts to the lower frequencies, suggesting the increasing graphitization of free carbon with temperature.
The D band appears due to the A1g breathing mode and requires a defect for its activation. In our case, the D band is possibly related to carbon dangling bonds or sp3 carbon atoms. The intensity ratio of D and G bands, I(D)/I(G), reflects the graphitization of carbon materials, and it decreased with increasing graphitization. The diameter of free carbon cluster can be calculated using eqn (6),36,37 shown in Table 2. The intensity of G band increases dramatically at above 1600 °C, and accordingly the I(D)/I(G) rapidly decreases to 0.13 at 1600 °C, which leads to a large La of 38.1 nm.
 | (6) |
| Temperature (°C) | I(D)/I(G) | ωG (cm−1) | FWHM (cm−1) | La (nm) |
|---|---|---|---|---|
| 1200 | 1.3 | 1598 | 47 | 3.8 |
| 1400 | 1.2 | 1583 | 39 | 4.1 |
| 1500 | 0.69 | 1583 | 31 | 7.2 |
| 1600 | 0.13 | 1576 | 21 | 38.1 |
Usually, the value of I(D)/I(G) for SiBOC ceramics is more than 1 at below 1600 °C. In our case, the intensity of G band increases dramatically at above 1400 °C, and accordingly the I(D)/I(G) decreases to 0.13 at 1600 °C, which is far smaller than that in SiZrOC ceramics (I(D)/I(G) = 1.4). The results indicate that the addition of B significantly promotes the graphitization and the growth of free carbon cluster. This is probably owing to the partial substitution of C with B atoms, which could increase the crystallinity of carbon.37,38 The D′ bands at 1608 cm−1 is considered to be the fingerprint of BC3 site formed by the substitution of B for C atom.9 This substitution of C by B atom introduces a local distortion within the graphite layer planes, and thus formed crystalline defects. The electron deficiency of boron with respect to carbon causes a decrease in the repulsive interaction between the π-electron clouds of adjacent graphene layers, allowing these layers to come closer together, and thus lead to the increased graphitization degree of free carbon.37,38
Backscattered electron images of the fracture of SiBZrOC ceramics pyrolyzed at 1000 °C, 1200 °C, 1400 °C and 1600 °C are shown in Fig. 8. Combined with XRD results, the white grains precipitated at 1200 °C with a size of 30–80 nm were t-ZrO2. The spherical grains uniformly dispersed in the matrix and grew up to 200–350 nm at 1600 °C, which were supposed to be t-ZrO2, m-ZrO2 and ZrSiO4 crystallites, according to XRD results.
 | ||
| Fig. 8 SEM images of SiBZrOC ceramics pyrolyzed at (a) 1000 °C, (b) 1200 °C, (c) 1400 °C and (d) 1600 °C. | ||
Fig. 9 shows the TEM images and the corresponding diffraction patterns of SiBZrOC ceramics. The oval particle in the middle and the irregular particles in the upper are determined to be m-ZrO2 and the ZrSiO4, respectively, through the diffraction patterns in Fig. 9(b). They are well-crystallized as shown in the HRTEM images Fig. 8(c) and (d). Fig. 8(d) shows the typical turbostratic structure of free carbon with 10–20 graphene layers, which is rarely found in the SiZrOC ceramics.26 It is confirmed that the addition of B significantly promotes the graphitization of free carbon.
 | ||
| Fig. 9 TEM images of SiBZrOC ceramics pyrolyzed at 1600 °C. (a) Bright field image, (b) diffraction patterns, (c) and (d) high-resolution micrographs. | ||
Table 3 shows the mass loss of SiZrOC and SiBZrOC ceramics pyrolyzed at 1200 °C after annealing in argon at 1400 °C, 1500 °C and 1600 °C for 5 h. The SiBZrOC sample shows a mass loss of 6% compared with the SiZrOC sample of 10%, indicating the enhanced thermal stability in SiBZrOC. This can be attributed to the highly graphitized free carbon and the reaction of SiO2 and ZrO2, which both inhibit the carbothermal reaction between SiO2 and free carbon.
| Temperature (°C) | Mass loss | |
|---|---|---|
| SiZrOC | SiBZrOC | |
| 1400 | 1% | 1% |
| 1500 | 4% | 3% |
| 1600 | 10% | 6% |
Conclusions
SiBZrOC quinary ceramics was prepared through the chemical reactions of hybrid alkoxysilanes with boric acid and Zr(OnPr)4. Si–O–Zr and Si–O–B bonds were formed in the precursor and a high ceramic yield of 83% was achieved. The addition of B promoted the crystallization of ZrO2 and the graphitization of free carbon, as well as increased the sp3–C/Si ratio. t-ZrO2 precipitated at 1200 °C with a size of 30–80 nm, reacted with the matrix and thus formed ZrSiO4 at 1600 °C. Turbostratic graphite with 10–20 graphene layers was clearly observed in TEM. The SiBZrOC sample showed excellent thermal stability in argon at 1600 °C for 5 h with a mass loss of 6%. Both the formation of ZrSiO4 and the highly graphitized free carbon inhibited the carbothermal reaction and thus improved the thermal stability of SiBZrOC ceramics.Conflicts of interest
The authors confirm that the content of this article has no conflicts of interest.Acknowledgements
This research is supported by the Science Foundation of National Key Laboratory of Science and Technology on Advanced Composites in Special Environments, the National Natural Science Foundation of China (51602076) and China Postdoctoral Science Foundation Grant (2016M601426).Notes and references
- S. Fu, M. Zhu and Y. Zhu, J. Adv. Ceram., 2019, 8, 457–478 CrossRef CAS.
- G. Mera, A. Navrotsky and S. Sen, J. Mater. Chem. A, 2013, 1, 3826–3836 RSC.
- E. Erdem, V. Mass, A. Gembus, A. Schulz, V. Liebau-Kunzmann, C. Fasel, R. Riedelf and R. Eichel, Phys. Chem. Chem. Phys., 2009, 11, 5628–5633 RSC.
- I. Menapace, G. Mera, R. Riedel, E. Erdem, R. Eichel, A. Pauletti and G. A. Appleby, J. Mater. Sci., 2008, 43, 5790–5796 CrossRef CAS.
- P. Zhang, D. Jia, Z. Yang, X. Duan and Y. Zhou, J. Adv. Ceram., 2012, 1, 157–178 CrossRef CAS.
- G. D. Soraru, N. Dallabona, C. Gervais and F. Babonneau, Chem. Mater., 1999, 11, 910–919 CrossRef CAS.
- R. Pena-Alonso, G. Mariotto, C. Gervais, F. Babonneau and G. D. Soraru, Chem. Mater., 2007, 19, 5694–5702 CrossRef CAS.
- A. Schiavon, C. Gervais, F. Babonneau and G. D. Soraru, J. Am. Ceram. Soc., 2004, 87, 203–208 CrossRef.
- B. Julian, C. Gervais, E. Cordoncillo, P. Escribano, F. Babonneau and C. Sanchez, Chem. Mater., 2003, 15, 3026–3034 CrossRef CAS.
- X. Li and M. J. Edirisinghe, Chem. Mater., 2004, 16, 1111–1119 CrossRef CAS.
- T. Xu, Q. Ma and Z. Chen, Mater. Lett., 2012, 66, 364–366 CrossRef CAS.
- L. Crouzet, D. Leclercq, P. H. Mutin and A. Vioux, Chem. Mater., 2003, 15, 1530–1534 CrossRef CAS.
- S. Dire, R. Ceccato and F. Babonneau, J. Sol-Gel Sci. Technol., 2005, 34, 53–62 CrossRef CAS.
- E. Ionescu, C. Linck, C. Fasel, M. Müller, H. J. Kleebe and R. Riedel, J. Am. Ceram. Soc., 2010, 93, 241–250 CrossRef CAS.
- S. Dire, R. Campostrini and R. Ceccato, Chem. Mater., 1998, 10, 268–278 CrossRef CAS.
- S. Dire, R. Ceccato, S. Gialanella and F. Babonneau, J. Eur. Ceram. Soc., 1999, 19, 2849–2858 CrossRef CAS.
- E. Ionescu, B. Papendorf, H. Kleebe, F. Poli, K. Müller and R. Riedel, J. Am. Ceram. Soc., 2010, 93, 1774–1782 CrossRef CAS.
- E. Ionescu, B. Papendor, H. Kleebe, F. Poli, K. Müller and R. Riedel, J. Am. Ceram. Soc., 2010, 9, 1783–1789 Search PubMed.
- H. Kleebe, K. Nonnenmacher, E. Ionescu and R. Riedel, J. Am. Ceram. Soc., 2012, 95, 2290–2297 CrossRef CAS.
- K. Nonnenmacher, H. Kleebe, J. Rohrer, E. Ionescu and R. Riedel, J. Am. Ceram. Soc., 2013, 96, 2058–2060 CrossRef CAS.
- B. Papendorf, K. Nonnenmacher, E. Ionescu, H. Kleebe and R. Riedel, Small, 2011, 7, 970–978 CrossRef CAS PubMed.
- E. Ionescu, H. Kleebe and R. Riedel, Chem. Soc. Rev., 2012, 41, 5032–5052 RSC.
- F. Li, X. Huang, J. X. Liu and G. J. Zhang, J. Adv. Ceram., 2020, 9, 1–16 CrossRef CAS.
- P. Colombo, G. Mera, R. Riedel and G. D. Soraru, J. Am. Ceram. Soc., 2010, 93, 1805–1837 CAS.
- N. Srinivasan, S. Ravindran and K. Ravi, J. Am. Ceram. Soc., 2013, 2, 318–324 Search PubMed.
- C. Liu, R. Pan, C. Hong, X. H. Zhang, W. Han, J. Han and S. Du, J. Eur. Ceram. Soc., 2016, 36, 395–402 CrossRef CAS.
- X. Zhang, C. Liu, C. Hong, J. Han, W. Han and S. Du, Ceram. Int., 2015, 41, 15292–15296 CrossRef CAS.
- S. J. Widgeon, S. Sen, G. Mera, E. Ionescu, R. Riedel and A. Navrotsky, Chem. Mater., 2010, 22, 6221–6228 CrossRef CAS.
- S. J. Widgeon, G. Mera, Y. Gao, E. Stoyanov, S. Sen, A. Navrotsky and R. Riedel, Chem. Mater., 2012, 24, 1181–1191 CrossRef CAS.
- G. Mera, A. Navrotsky, S. Sen, H. Kleebe and R. Riedel, J. Mater. Chem. A, 2013, 1, 3826–3836 RSC.
- G. Hasegawa, K. Kanamori, K. Nakanishi and T. Hanada, J. Mater. Chem., 2009, 19, 7716–7720 RSC.
- G. Hasegawa, K. Kanamori, K. Nakanishi and T. Hanada, Chem. Mater., 2010, 22, 2541–2547 CrossRef CAS.
- Y. Shi, Y. Wan, Y. Zhai, R. Liu, Y. Meng, B. Tu and D. Zhao, Chem. Mater., 2007, 19, 1761–1771 CrossRef CAS.
- G. D. Soraru, R. Campostrini and S. Maurina, J. Am. Ceram. Soc., 1997, 80, 999–1004 CrossRef CAS.
- C. Gervais, F. Babonneau, N. Dallabonna and G. D. Sorarù, J. Am. Ceram. Soc., 2001, 84, 2160–2164 CrossRef CAS.
- T. Jiang, Y. Wang, Y. Wang and N. Orlovskaya, J. Am. Ceram. Soc., 2009, 92, 2455–2458 CrossRef CAS.
- M. Li, L. Cheng, F. Ye, C. Zhang and J. Zhou, J. Adv. Ceram., 2021, 10, 1256–1272 CrossRef CAS.
- Y. J. Lee, J. Nucl. Mater., 2004, 325, 174–179 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |