Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Room temperature tunable multicolor phosphorescent polymers for humidity detection and information encryption†
Yulei Gao,
Xiang Di,
Fenfen Wang * and
Pingchuan Sun
* and
Pingchuan Sun *
*
Key Laboratory of Functional Polymer Materials of Ministry of Education and College of Chemistry, State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin 300071, P. R. China. E-mail: wff@mail.nankai.edu.cn; spclbh@nankai.edu.cn
First published on 14th March 2022
Abstract
Amorphous polymer-based room temperature phosphorescence (RTP) materials exhibiting tunable emission colors have received tremendous attention and are extremely challenging to prepare. Herein, polyacrylamide-based RTP materials with tunable multicolor emission were prepared via copolymerizing phosphor with concentration dependent luminescence colors and acrylamide with different molar ratios. The hydrogen bonding interactions and chemically crosslinked structures in these polymers effectively restrict the mobility of phosphors and activate efficient RTP emission. The molar ratio of phosphor and acrylamide has a significant influence on the photophysical properties of these polymers, which can be used to fabricate multicolor materials. In addition, the RTP intensity decreases with increasing humidity due to the disassociation of hydrogen bonding by adsorption of water, manifesting as a humidity sensor. Benefiting from the distinguishable RTP lifetimes and the responsiveness to humidity, triple encoding for information encryption is successfully realized.
1. Introduction
Room temperature phosphorescence (RTP) materials have become a research spotlight of chemists and materials scientists with their potential applications in the field of sensors,1,2 organic light-emitting diodes (OLED),3 bioimaging4 and document security5 owing to their unique photophysical properties. Generally, the strategies for designing efficient RTP materials include promoting the intersystem crossing (ISC) from the lowest excited singlet state (S1) to the excited triplet state (Tn), suppressing the nonradiative transition from the lowest excited triplet state (T1) to the ground state (S0) and shielding oxygen effectively which can easily quench T1. Conventional RTP materials are mainly coordination complexes containing noble metals such as platinum and palladium which can enhance spin–orbit coupling and facilitate the ISC process. However, the development of novel metal-free RTP materials is necessary due to drawbacks such as expensive, relatively toxic and limited resources for organometallic compounds. Based on the microscopic mechanism of RTP generation, several approaches have been put forward to prepare pure organic RTP materials, including crystallization-induced,6–10 embedding phosphors in a rigid matrix11,12 and H-aggregation13,14 etc. The nonradiative deactivation of triplet excitons is maximally suppressed due to the rigidity and oxygen barrier properties of crystals, while the complex growth process and poor processability limit their commercial applications. The past decade has witnessed the extensive investigation of polymer-based RTP materials owing to low cost, easy processing and low toxicity compared with crystalline RTP materials and coordination complexes containing noble metals.15–22 For example, Kim and co-workers devised Diels–Alder click chemistry as a method to covalent cross-linking between phosphors and polymer matrices to suppress molecular motions for enhanced RTP of metal-free organic materials.23 Tian and co-workers developed a facile way to construct pure organic amorphous polymers with high phosphorescent quantum yields and ultralong RTP lifetimes by the simple radical binary copolymerization of acrylamide and different phosphors without extra processing.24,25 Lu et al. proposed a reliable large-scale strategy for synthesizing the covalently efficient RTP materials via B–O click reaction between boronic acid-modified phosphors and polyhydroxy polymer matrix.26 Tang et al. presented wide-color ranged and persistent RTP from amorphous films by embedding electron-rich organic phosphor into electron-deficient matrix polyacrylonitrile.27 Despite numerous advances, developing advanced RTP materials still remains challenging in several aspects, for example, most of polymer-based RTP materials just emit a single color. If the luminescent color can vary with external stimulation, it will effectively expand the application range of materials and meet the new development requirements of organic functional materials.28,29Color-tunable luminescent materials, especially phosphorescence emission, have attracted tremendous interests due to their potential applications in sensing, information encryption and anti-counterfeiting.30,31 For instance, Zhao et al. achieved color-tunable ultralong RTP in single polymer through radical multicomponent luminophores cross-linked copolymerization.32 Besides, amorphous pure organic copolymers with multicolor RTP emission have been prepared by Zhang et al. via copolymerizing two phosphors (benzoic acid and 4-bromo-1,8-naphthalic anhydride) and acrylamide with different feeding ratios.33 Furthermore, multicolor phosphorescence signals were also observed in the doped binary luminescent copolymer systems.34 However, the incorporation of phosphors with divergent emission colors in a single polymer to generate multicolor RTP emission will complicate the luminescence system and make it difficult to control the homogeneity of each phosphor in polymer matrix.35 Accordingly, designing and developing single-phosphor RTP materials with multicolor luminescence for further broadening and optimizing the tunable RTP emission systems is highly desired.
Luminescence color tuning is essentially the alteration of the transition energy levels of the phosphor molecules.36 Research reveals that for certain polar phosphors, different phosphor–phosphor interactions regulated by concentration can affect the ground state or excited state energies of light-emitting cores, which analogous to solvatochromism.37 This implies that covalent bonding of phosphors with concentration-dependent luminescent colors into a polymer matrix may be a reasonable strategy to prepare color-tunable RTP emission materials. Difluoroboron β-diketonates (BF2bdks) are a classic polar fluorescent dyes and have emerged as a class of functionalized luminescent materials because of their large extinction coefficient, sensitivity to the surrounding environment, high quantum yields and chemical stability.38,39 After Fraser's group first reported the RTP of BF2bdks induced by coupling with poly(lactic acid),40 emission color tuning for RTP materials based on BF2bdks has been investigated, while persistent RTP is restricted to inert conditions which limits the application of BF2bdks.41–44
Herein, we proposed a new strategy to prepare a single-phosphor amorphous polymer (abbreviated as SPAP) with efficient RTP emission under ambient conditions via combining BF2bdks (named as BF2bad) and acrylamide monomer (Scheme 1a). Where the chemical crosslinking structures and high-density hydrogen bonds between polyacrylamide (PAM) chains are devised as the effective method to restrict molecular motion (Scheme 1b) of BF2bdks and polymer chains. It is demonstrated that the molar ratio of two comonomers (BF2bad and acrylamide) has a significant effect on the photophysical properties of SPAP which can be used for fabricating tunable multicolor materials. Besides, since hydrogen bonding interactions can be broken by water, the moisture content is quantitatively detected by the change in RTP emission intensity, and information encryption is also realized.
 | ||
| Scheme 1 (a) Synthetic routes of BF2bad and SPAP. (b) Rational design strategy of tunable multicolor RTP in SPAP. | ||
2. Experimental
2.1 Materials
Methyl 4-methoxybenzoate, 4′-methoxyacetophenone, potassium carbonate (K2CO3) and sodium hydride (NaH, 60% dispersed in mineral oil) were supplied by Aladdin, while magnesium sulfate (MgSO4), boron tribromide (BBr3) and 3-bromopropene were obtained from Innochem. Boron trifluoride-diethyl etherate (BF3·Et2O) was purchased from Shanghai Merrill Chemical Technology Co., Ltd. Acrylamide and 2,2′-azobis(2-methylpropionitrile) (AIBN) were obtained from TCI. AIBN was recrystallized from anhydrous ethanol before use. All solvents were commercially available and used as received.2.2 Synthesis of difluoroboron-1,3-bis(4-(allyloxy)phenyl)propane-1,3-dione (BF2bad)
Under magnetic stirring, difluoroboron-1,3-bis(4-hydroxyphenyl)propane-1,3-dione (H1, 1.0 g, 3.29 mmol, see Scheme S1 (ESI†) for the synthetic route), 3-bromopropene (1.49 g, 12.35 mmol) and K2CO3 (1.136 g, 8.22 mmol) were added into acetone (20 mL), and the reaction mixture was heated to 60 °C for 10 h (monitored by TLC). After cooled to room temperature, the deionized water (20 mL) was added and extracted with CH2Cl2 (3 × 20 mL). The organic phase was collected, dried with MgSO4, filtered and concentrated under reduced pressure to yield the BF2bad as a yellow solid (1.07 g, 84.9% yield). 1H NMR (Fig. S4 (ESI†), 400 MHz, in CDCl3): δ 8.11 (d, 4H, Ha), 7.03 (d, 4H, Hb), 7.01 (s, 1H, Hc), 6.06 (ddd, 2H, Hd), 5.45 (d, 2H, He), 5.36 (d, 2H, He’), 4.66 (d, 4H, Hf); 13C NMR (Fig. S5 (ESI†), 101 MHz, in CDCl3, δ ppm): 180.93, 164.45, 132.20, 131.36, 124.75, 118.73, 115.36, 91.69, 69.31.2.3 Typical procedures for the preparation of SPAP
Taking polymer SPAP(1/50) as a typical example, SPAP(1/50) was prepared by free radical copolymerization of compound BF2bad (1 eq.) and acrylamide (50 eq.) by AIBN (1.4 wt% of acrylamide) as radical initiator at 70 °C under nitrogen atmosphere in 4.5 mL DMSO for 17 h. After cooled to room temperature, the mixture was added dropwise to methanol to precipitate and the resulting solid was washed 3–5 times with methanol, to give a SPAP(1/50).2.4 Instruments and methods
3. Results and discussion
3.1 Preparation of SPAP with different molar ratios
The copolymerization between phosphors and polymer matrix is identified as a common method to achieve RTP under ambient conditions by suppressing thermal motions and shielding quenchers.20,25,46 The abundant hydrogen bonds in the PAM can further effectively immobilize phosphors and greatly enhance the RTP emission intensity, which prompts us to explore whether the fluorescent dye BF2bdks with multifaceted optical properties can also inflict RTP in this system. Therefore, SPAP was synthesized through thermal initiated radical copolymerization of BF2bad and acrylamide. The characterizations of 1H NMR and 13C NMR are described in the supplementary information† (Fig. S4 and S5, ESI†). To evaluate the influence of the molar ratio of BF2bad and acrylamide on the photophysical properties of materials (e.g., emission wavelength and phosphorescence lifetime), SPAPs were synthesized whose molar ratios of BF2bad and acrylamide were 1/10, 1/25, 1/50, 1/75 and 1/100, respectively (Table S1, ESI†).3.2 Photophysical properties of BF2bad
Photophysical properties of monomer BF2bad in different solvents with varied polarities were first investigated by UV-vis absorption and fluorescence spectra. As shown in Fig. 1a, the maximum absorption peak of BF2bad in the diluted dichloromethane solution was observed at 411 nm with a molar extinction coefficient as high as 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 M−1 cm−1 (Fig. S6, ESI†), which was ascribed to the typical π–π* transitions.47 The higher extinction coefficient indicates that BF2bad has a stronger light-harvesting ability compared with other BF2bdks.48,49 Besides, due to the electron-donating ability of allyloxy substituent and the electron-accepting ability of difluoroboron β-diketone moieties, BF2bad also exhibited an intramolecular charge-transfer (ICT) transition, which could be supported by the solvent polarity-dependent fluorescence emission spectra.50,51 As we can see in Fig. 1a, the absorption spectra were slightly influenced as increasing solvent polarity (dichloromethane < tetrahydrofuran < acetone < acetonitrile < dimethyl sulfoxide), while the emission of BF2bad manifested positive solvatochromism. For example, the emission spectra showed a red shift from 429 nm in tetrahydrofuran to 450 nm in dimethyl sulfoxide. In addition, owing to the interaction between molecules, the maximum emission wavelength bathochromic shifted to 501 nm in the solid state with a fluorescence lifetime of 5.49 ns (Fig. 1b and c). The above-mentioned results demonstrate that BF2bad is an excellent stimuli-responsive phosphor and can be used for the preparation of color-tunable luminescent materials, which will be further discussed subsequently. However, due to the quenching effect and nonradiative relaxation process, phosphorescence emission was not observed for BF2bad monomer in either solution or solid state.
700 M−1 cm−1 (Fig. S6, ESI†), which was ascribed to the typical π–π* transitions.47 The higher extinction coefficient indicates that BF2bad has a stronger light-harvesting ability compared with other BF2bdks.48,49 Besides, due to the electron-donating ability of allyloxy substituent and the electron-accepting ability of difluoroboron β-diketone moieties, BF2bad also exhibited an intramolecular charge-transfer (ICT) transition, which could be supported by the solvent polarity-dependent fluorescence emission spectra.50,51 As we can see in Fig. 1a, the absorption spectra were slightly influenced as increasing solvent polarity (dichloromethane < tetrahydrofuran < acetone < acetonitrile < dimethyl sulfoxide), while the emission of BF2bad manifested positive solvatochromism. For example, the emission spectra showed a red shift from 429 nm in tetrahydrofuran to 450 nm in dimethyl sulfoxide. In addition, owing to the interaction between molecules, the maximum emission wavelength bathochromic shifted to 501 nm in the solid state with a fluorescence lifetime of 5.49 ns (Fig. 1b and c). The above-mentioned results demonstrate that BF2bad is an excellent stimuli-responsive phosphor and can be used for the preparation of color-tunable luminescent materials, which will be further discussed subsequently. However, due to the quenching effect and nonradiative relaxation process, phosphorescence emission was not observed for BF2bad monomer in either solution or solid state.
3.3 Multicolor fluorescence and RTP of SPAP
After copolymerization with acrylamide, SPAP(1/50) (BF2bad![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acrylamide = 1
acrylamide = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, molar ratio) was obtained. SPAP(1/50) exhibited green persistent phosphorescence at 518 nm with a lifetime of 16.8 ms after turning off the UV irradiation under ambient conditions, as shown in Fig. 2a and b. The afterglow could be observed by the naked eye (Fig. 2c). Moreover, larger Stokes shift (approximately 140 nm) and red-shifted emission peak compared to fluorescence emission (λem = 486 nm) further indicate that the delayed luminescence is derived from phosphorescence rather than possible delayed fluorescence.
50, molar ratio) was obtained. SPAP(1/50) exhibited green persistent phosphorescence at 518 nm with a lifetime of 16.8 ms after turning off the UV irradiation under ambient conditions, as shown in Fig. 2a and b. The afterglow could be observed by the naked eye (Fig. 2c). Moreover, larger Stokes shift (approximately 140 nm) and red-shifted emission peak compared to fluorescence emission (λem = 486 nm) further indicate that the delayed luminescence is derived from phosphorescence rather than possible delayed fluorescence.
The fluorescence emission and RTP emission spectra of SPAP were further investigated (Fig. 3a and b) and the corresponding photophysical data was summarized in Table 1. In general, solvatochromism is related to the difference in the polarity of the surrounding liquid or solid-state medium;52 however, the polarity of the medium may also be influenced by polar dyes.53 In terms of materials containing BF2bdks, this is reflected in the different phosphor loadings.44,54 Clearly, just as the fluorescence emission of BF2bad red-shifted with increasing solvent polarity, the fluorescence emission wavelength of SPAP also showed a bathochromic shift from 468 nm (SPAP(1/100)) to 523 nm (SPAP(1/10)) as the BF2bad content increased which accompanied by the luminescence color turned from blue to green under environment conditions, and the spectra became broader. The RTP emission wavelength of SPAP showed the similar trend as the fluorescence emission. The corresponding Commission Internationale de l'Éclair-age (CIE) coordinates were calculated in Fig. 3c and d. According to previous reports, BF2bdks have a larger dipole moment38 and their emission is sensitive to the polarity of the surrounding medium.55 Higher concentration of BF2bad will result in a greater probability of BF2bad–BF2bad interactions in the system. When the distance between BF2bad molecules is about 3 Å,56,57 ground–state association or excimers will be formed, which resulting in lower energy emission.37 Conversely, the reduction of BF2bad–BF2bad interactions is responsible for the short-wave emission at lower BF2bad content. The results indicate that varying the feed amount of BF2bad is a simple but efficient approach to generate color tunability for PAM-based RTP systems.
| Sample | λF [nm] | λP [nm] | τP [ms] | ΦF [%] | ΦP [%] |
|---|---|---|---|---|---|
| SPAP(1/100) | 468 | 502 | 9.81 | 30.27 | 3.90 |
| SPAP(1/75) | 474 | 504 | 9.71 | 32.87 | 3.29 |
| SPAP(1/50) | 486 | 518 | 16.81 | 33.33 | 2.31 |
| SPAP(1/25) | 494 | 522 | 8.07 | 31.39 | 4.11 |
| SPAP(1/10) | 523 | 534 | 4.24 | 22.04 | 3.24 |
Furthermore, both phosphorescence lifetime (τP) and phosphorescence quantum yield (ΦP) of SPAP were also measured and calculated (Table 1 and Fig. 3e–h). τP and ΦP are important physical quantities to describe the properties of phosphorescence, among which, τP refers to the time required for the phosphorescent intensity to decay to 1/e of the initial value. ΦP is a measure of the utilization efficiency of absorbed photons during the phosphorescent process, and is defined as the ratio of the number of emit ted phosphorescent photons and the number of absorbed photons. The equations are shown below:
| τP = 1/(kP + kts + kq) | (1) |
| ΦP = ΦisckP/(kP + kts + kq) | (2) |
From eqn (1), the key to obtaining long τP is to minimize kts and kq. The abundant hydrogen bonding interactions among the PAM chains restrict the molecular motion of phosphors associated with kts and effectively shield quenchers like oxygen associated with kq.25 Accordingly, longer τP can be observed at lower content of BF2bad. In contrast, higher BF2bad content will reduce the number of hydrogen bonds and weaken the shielding effect of quenchers, thus is thought to cause a shorter τP. As shown in Table 1, τP shortens from 16.8 ms (SPAP(1/50)) to 4.2 ms (SPAP(1/10)) as the BF2bad content increases. Even so, very low contents will also lead to a shortening of τP because enough BF2bad contact is necessary to achieve RTP.17,58,59 Besides, ΦP varies slightly with the molar ratio of SPAP, which is influenced by the competition between phosphorescence emission and other nonradiative relaxations, such as vibrational dissipation and oxygen-mediated quenching.17,18,60
To verify the important role of copolymerization of BF2bad and acrylamide for realizing RTP emission, we tested the delayed photoluminescence spectra of mechanical mixture of BF2bad and PAM (SPAP′(1/50)) as a control experiment. As shown in Fig. S8 (ESI†), SPAP′(1/50) has no emission peak at 518 nm, indicating that simple mixing cannot bind BF2bad to PAM tightly and thus the thermal motion of BF2bad cannot be inhibited effectively. As mentioned above, the copolymerization of BF2bad with acrylamide is the key to active RTP emission. The abundant hydrogen bonding interactions and the inherent crosslinked structure in SPAP can effectively promote RTP emission by locking phosphors and shielding quenchers.
3.4 Microstructure characterization of SPAP
Since the microstructure of polymers, such as crystalline or amorphous state, have a great influence on the corresponding luminescence properties,24,25,46 XRD analysis was carried out to determine the structure of SPAP, among which PAM was also studied as a reference polymer. As shown in Fig. 4a, PAM was amorphous from the only broad characteristic diffraction peak appeared at 2θ = 20–23°. SPAP also showed an amorphous structure similar to PAM, indicating that the amorphous state of these polymers is related to their luminescence properties.The microscopic morphology of SPAP also was observed by SEM and the corresponding porosity was estimated (Fig. S9, ESI†). As shown in Fig. 4b, SPAP(1/100) showed a distinct porous network structure which became progressively denser with increasing BF2bad content (see SPAP(1/10)). Understandably, the chemical crosslinking density of SPAP increased as the phosphor content increased due to the modification of the end group double bond functionalization which made BF2bad act as a chemical crosslinker, resulting in a more dense microscopic morphology. Meanwhile, the denser structure of SPAP promoted BF2bad–BF2bad interactions and facilitated the formation of ground-state association or excimers, which was consistent with previous results.
3.5 Humidity responsiveness of RTP intensity
The rigid environment consisting of chemical crosslinking and interchain interactions via hydrogen bonding can immobilize BF2bad, shield quenchers and enhance the RTP emission. Therefore, we speculated that the presence of water would reduce the rigidity of polymer network thus weaken the RTP emission intensity of SPAP to a certain extent because the hydrogen bonding interactions can be broken by water (Fig. 4c and d). Since PAM is only soluble in water, we prepared seven suspension samples of 1.5 mg mL−1 by dispersing SPAP(1/50) in H2O/DMF mixed solutions to explore the effect of humidity on the RTP emission intensity. As shown in Fig. 4e, the RTP emission intensity of SPAP(1/50) at 518 nm gradually decreased with increasing the volume fraction of water, and was totally quenched when the water fraction reached 15%. It is noteworthy that the phosphorescence quenching efficiency followed a good linear correlation with water content (R2 = 0.9914) (Fig. 4f) and the LOD was calculated as 0.014%, which is comparable to other fluorescent sensors for water detection,61 which makes SPAP(1/50) a potential candidate for moisture content detection in organic solvents.3.6 Information encryption application
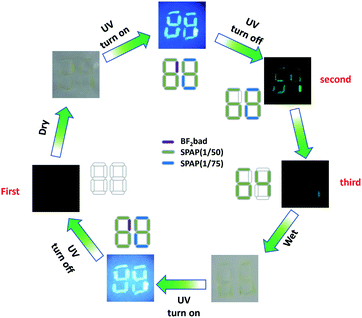
Benefiting from the diversified RTP lifetimes and the humidity responsiveness, these polymers can be applied to multiple encodings for information encryption. As an example shown in Fig. 5, the digits were written by different inks in a piece of non-fluorescent paper, where the number “64” was written by long-lived SPAP(1/50) (dissolved in water, 20 mg mL−1), the blue part of “88” was marked by SPAP(1/75) (dissolved in water, 20 mg mL−1) and the purple part was coated with BF2bad (dissolved in dichloromethane, 20 mg mL−1). Number “88” could be found under the daylight in both the wet and dry state due to the fluorescence properties of BF2bad. In the dry state, the bright number “88” appeared under the irradiation of UV lamp and disappeared immediately after turning off the irradiation due to no RTP emission of BF2bad, leaving the long-lived SPAP(1/50) and SPAP(1/75) emitted a green luminescence of “68”. Thenceforth, the RTP of SPAP(1/75) decayed to invisibility and the residual phosphorescence of SPAP(1/50) emitted “64” (Movie 2, ESI†). Under moist state, number “88” was still visible under UV light, but nothing could be found after turning off the UV light due to the quenching of RTP. As a result, the triple encoding of digital encryption was realized, and this process was completely reversible and could be repeated more than once.4. Conclusions
In summary, we have successfully designed and synthesized concentration-dependent luminescent metal-free amorphous RTP materials with color-tunable through radical copolymerization of the single phosphor BF2bad and acrylamide. The results indicate that the chemical crosslinking structures and the hydrogen bonding interactions between polymer chains effectively restrict the molecular motion of phosphors and shield the quenching of the triplet excitons by oxygen, creating the necessary conditions for observing the persistent RTP with the naked eye under environmental conditions. Multicolor phosphorescence signals are observed in this polymer system which can be effectively regulated by changing the feed amount of phosphor. The phosphorescence quenching mechanism is attributed to the disassociation of hydrogen bonds in the presence of water. Furthermore, triple encoding of information encryption is achieved by utilizing the humidity response and the different RTP lifetimes of SPAP. This work is expected to have applications in the fields of moisture content detection and document security as well as will further contribute to the development of tunable multicolor materials.Author contributions
Yulei Gao: conceptualization, data curation, writing original draft, and visualization. Xiang Di: methodology and investigation. Fenfen Wang: conceptualization, resources, writing – review & editing. Pingchuan Sun: resources, writing – review & editing, supervision, project administration, and funding acquisition.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22003028 and 21534005).References
- C. A. DeRosa, S. A. Seaman, A. S. Mathew, C. M. Gorick, Z. Fan, J. N. Demas, S. M. Peirce and C. L. Fraser, ACS Sens., 2016, 1, 1366–1373 CrossRef CAS PubMed.
- M. Fan, T. Gan, G. Yin, F. Cheng and N. Zhao, RSC Adv., 2021, 11, 27845–27854 RSC.
- G. Zhan, Z. Liu, Z. Bian and C. Huang, Front. Chem., 2019, 7, 305 CrossRef CAS PubMed.
- A. S. Mathew, C. A. DeRosa, J. N. Demas and C. L. Fraser, Anal. Methods, 2016, 8, 3109–3114 RSC.
- X. Q. Bi, Y. G. Shi, T. Peng, S. W. Yue, F. Wang, L. Y. Zheng and Q. E. Cao, Adv. Funct. Mater., 2021, 31, 2101312 CrossRef CAS.
- W. Z. Yuan, X. Y. Shen, H. Zhao, J. W. Y. Lam, L. Tang, P. Lu, C. L. Wang, Y. Liu, Z. M. Wang, Q. Zheng, J. Z. Sun, Y. G. Ma and B. Z. Tang, J. Phys. Chem. C, 2010, 114, 6090–6099 CrossRef CAS.
- O. Bolton, K. Lee, H.-J. Kim, K. Y. Lin and J. Kim, Nat. Chem., 2011, 3, 205–210 CrossRef CAS PubMed.
- Y. Gong, Y. Tan, J. Mei, Y. Zhang, W. Yuan, Y. Zhang, J. Sun and B. Z. Tang, Sci. China Chem., 2013, 56, 1178–1182 CrossRef CAS.
- Y. Xie, Y. Ge, Q. Peng, C. Li, Q. Li and Z. Li, Adv. Mater., 2017, 29, 1606829 CrossRef PubMed.
- X. Fang and D. Yan, Sci. China Chem., 2018, 61, 397–401 CrossRef CAS.
- X. Ma, J. Wang and H. Tian, Acc. Chem. Res., 2019, 52, 738–748 CrossRef CAS PubMed.
- T. Zhang, X. Ma and H. Tian, Chem. Sci., 2020, 11, 482–487 RSC.
- Z. An, C. Zheng, Y. Tao, R. Chen, H. Shi, T. Chen, Z. Wang, H. Li, R. Deng, X. Liu and W. Huang, Nat. Mater., 2015, 14, 685–690 CrossRef CAS PubMed.
- L. Tang, J. Zan, H. Peng, X. Yan, Y. Tao, D. Tian, Q. Yang, H. Li, Q. Chen, W. Huang and R. Chen, Chem. Commun., 2020, 56, 13559–13562 RSC.
- K. T. Kamtekar, A. P. Monkman and M. R. Bryce, Adv. Mater., 2010, 22, 572–582 CrossRef CAS PubMed.
- S. Hirata, K. Totani, J. Zhang, T. Yamashita, H. Kaji, S. R. Marder, T. Watanabe and C. Adachi, Adv. Funct. Mater., 2013, 23, 3386–3397 CrossRef CAS.
- D. Lee, O. Bolton, B. C. Kim, J. H. Youk, S. Takayama and J. Kim, J. Am. Chem. Soc., 2013, 135, 6325–6329 CrossRef CAS PubMed.
- M. S. Kwon, D. Lee, S. Seo, J. Jung and J. Kim, Angew. Chem., Int. Ed., 2014, 53, 11177–11181 CrossRef CAS PubMed.
- H. Chen, L. Xu, X. Ma and H. Tian, Polym. Chem., 2016, 7, 3989–3992 RSC.
- N. Gan, H. Shi, Z. An and W. Huang, Adv. Funct. Mater., 2018, 28, 1802657 CrossRef.
- S. Cai, H. Ma, H. Shi, H. Wang, X. Wang, L. Xiao, W. Ye, K. Huang, X. Cao, N. Gan, C. Ma, M. Gu, L. Song, H. Xu, Y. Tao, C. Zhang, W. Yao, Z. An and W. Huang, Nat. Commun., 2019, 10, 4247 CrossRef PubMed.
- X. Qin, S. Wang, L. Luo, G. He, H. Sun, Y. Gong, B. Jiang and C. Wei, RSC Adv., 2018, 8, 31231–31236 RSC.
- M. S. Kwon, Y. Yu, C. Coburn, A. W. Phillips, K. Chung, A. Shanker, J. Jung, G. Kim, K. Pipe, S. R. Forrest, J. H. Youk, J. Gierschner and J. Kim, Nat. Commun., 2015, 6, 8947 CrossRef PubMed.
- H. Chen, X. Yao, X. Ma and H. Tian, Adv. Opt. Mater., 2016, 4, 1397–1401 CrossRef CAS.
- X. Ma, C. Xu, J. Wang and H. Tian, Angew. Chem., Int. Ed., 2018, 57, 10854–10858 CrossRef CAS PubMed.
- R. Tian, S. M. Xu, Q. Xu and C. Lu, Sci. Adv., 2020, 6, eaaz6107 CrossRef CAS PubMed.
- H. Z. Wu, D. L. Wang, Z. Zhao, D. Wang, Y. Xiong and B. Z. Tang, Adv. Funct. Mater., 2021, 31, 2101656 CrossRef CAS.
- X. Lin, J. Wang, B. Ding, X. Ma and H. Tian, Angew. Chem., Int. Ed., 2021, 60, 3459–3463 CrossRef CAS PubMed.
- L. Zou, X. Qin, H. Sun, S. Wang, W. Ding, Y. Liu, C. Wei, B. Jiang and Y. Gong, RSC Adv., 2019, 9, 36287–36292 RSC.
- R. Gao and D. Yan, Chem. Commun., 2017, 53, 5408–5411 RSC.
- Y. Su, Y. Zhang, Z. Wang, W. Gao, P. Jia, D. Zhang, C. Yang, Y. Li and Y. Zhao, Angew. Chem., Int. Ed., 2020, 59, 9967–9971 CrossRef CAS PubMed.
- L. Gu, H. Wu, H. Ma, W. Ye, W. Jia, H. Wang, H. Chen, N. Zhang, D. Wang, C. Qian, Z. An, W. Huang and Y. Zhao, Nat. Commun., 2020, 11, 944 CrossRef CAS PubMed.
- T. Zhang, Y. Wu and X. Ma, Chem. Eng. J., 2021, 412, 128689 CrossRef CAS.
- J. Wang, X. Y. Lou, J. Tang and Y. W. Yang, Macromol. Rapid Commun., 2021, 42, 2100544 CrossRef CAS PubMed.
- X. Dou, T. Zhu, Z. Wang, W. Sun, Y. Lai, K. Sui, Y. Tan, Y. Zhang and W. Z. Yuan, Adv. Mater., 2020, 32, 2004768 CrossRef CAS PubMed.
- A. A. Shoustikov, Y. Yujian and M. E. Thompson, IEEE J. Sel. Top. Quantum Electron., 1998, 4, 3–13 CrossRef CAS.
- G. Q. Zhang, S. E. Kooi, J. N. Demas and C. L. Fraser, Adv. Mater., 2008, 20, 2099–2104 CrossRef CAS.
- P.-Z. Chen, L.-Y. Niu, Y.-Z. Chen and Q.-Z. Yang, Coord. Chem. Rev., 2017, 350, 196–216 CrossRef CAS.
- N. Liu, P. Z. Chen, J. X. Wang, L. Y. Niu and Q. Z. Yang, Chinese Chem. Lett., 2019, 30, 1939–1941 CrossRef CAS.
- G. Q. Zhang, J. B. Chen, S. J. Payne, S. E. Kooi, J. N. Demas and C. L. Fraser, J. Am. Chem. Soc., 2007, 129, 15728 CrossRef CAS.
- G. Zhang, G. M. Palmer, M. W. Dewhirst and C. L. Fraser, Nat. Mater., 2009, 8, 747–751 CrossRef CAS PubMed.
- C. Kerr, C. A. DeRosa, M. L. Daly, H. Zhang, G. M. Palmer and C. L. Fraser, Biomacromolecules, 2017, 18, 551–561 CrossRef CAS PubMed.
- M. Zhuang, A. Perkins, C. A. DeRosa, T. Butler, J. N. Demas and C. L. Fraser, Macromol. Chem. Phys., 2018, 219, 1800240 CrossRef.
- C. A. DeRosa, S. Hiroto and C. L. Fraser, J. Phys. Chem. C, 2019, 123, 20488–20496 CrossRef CAS.
- C. Chan, H. Liu and Z. Xue, Microchem. J., 2021, 166, 106247 CrossRef CAS.
- Z. Y. Zhang, W. W. Xu, W. S. Xu, J. Niu, X. H. Sun and Y. Liu, Angew. Chem., Int. Ed., 2020, 59, 18748–18754 CrossRef CAS PubMed.
- S. Xu, R. E. Evans, T. Liu, G. Zhang, J. N. Demas, C. O. Trindle and C. L. Fraser, Inorg. Chem., 2013, 52, 3597–3610 CrossRef CAS PubMed.
- M. L. Daly, C. Kerr, C. A. DeRosa and C. L. Fraser, ACS Appl. Mater. Interfaces, 2017, 9, 32008–32017 CrossRef CAS PubMed.
- H. Zhang, P. Z. Chen, L. Y. Niu and Q. Z. Yang, Mater. Chem. Front., 2020, 4, 285–291 RSC.
- C. Qian, K. Cao, X. Liu, X. Zhang, D. Xu, P. Xue and R. Lu, Chinese Sci. Bull., 2012, 57, 4264–4271 CrossRef CAS.
- C. Qian, M. Liu, G. Hong, P. Xue, P. Gong and R. Lu, Org. Biomol. Chem., 2015, 13, 2986–2998 RSC.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- V. Bulović, R. Deshpande, M. E. Thompson and S. R. Forrest, Chem. Phys. Lett., 1999, 308, 317–322 CrossRef.
- G. Q. Zhang, T. L. S. Clair and C. L. Fraser, Macromolecules, 2009, 42, 3092–3097 CrossRef CAS.
- E. Cogné-Laage, J.-F. Allemand, O. Ruel, J.-B. Baudin, V. Croquette, M. Blanchard-Desce and L. Jullien, Chem. Eur. J., 2004, 10, 1445–1455 CrossRef PubMed.
- T. Förster, Angew. Chem., Int. Ed., 1969, 8, 333–343 CrossRef.
- J. N. Pitts, G. S. Hammond, K. Gollnick and D. J. Eustace, J. Electrochem. Soc., 1977, 124, 433C CrossRef.
- C. A. DeRosa, J. Samonina-Kosicka, Z. Y. Fan, H. C. Hendargo, D. H. Weitzel, G. M. Palmer and C. L. Fraser, Macromolecules, 2015, 48, 2967–2977 CrossRef CAS PubMed.
- X.-F. Wang, H. Xiao, P.-Z. Chen, Q.-Z. Yang, B. Chen, C.-H. Tung, Y.-Z. Chen and L.-Z. Wu, J. Am. Chem. Soc., 2019, 141, 5045–5050 CrossRef CAS PubMed.
- T. Zhang, H. Chen, X. Ma and H. Tian, Ind. Eng. Chem. Res., 2017, 56, 3123–3128 CrossRef CAS.
- Y. Gao, M. Li, X. Tian, S. Gong, Y. Zhang, Y. Yang, Z. Wang and S. Wang, Microchem. J., 2021, 169, 106631 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2ra00294a |
| This journal is © The Royal Society of Chemistry 2022 |