Open Access Article
Open Access ArticleMunicipal solid waste incineration residues recycled for typical construction materials—a review
Dan Chen *a,
Yingying Zhanga,
Yao Xub,
Qing Niec,
Zhanbin Yangc,
Wenyu Shenga and
Guangren Qiana
*a,
Yingying Zhanga,
Yao Xub,
Qing Niec,
Zhanbin Yangc,
Wenyu Shenga and
Guangren Qiana
aSchool of Environmental and Chemical Engineering, Shanghai University, No. 99 Shangda Road, Shanghai 200444, China
bHuahui Engineering Design Group Co., Ltd, No. 177 Jiefang Avenue, Shaoxing, Zhejiang Province 312000, China
cChina Building Materials Academy Co., Ltd, No. 1 Guanzhuang Dongli, Beijing 100024, China
First published on 23rd February 2022
Abstract
Focusing on the great potential of municipal solid waste incineration (MSWI) residues in the construction sector, the applications of recycling MSWI residues in construction materials are discussed in this review. Incineration is a promising method for managing the great quantity of municipal solid waste (MSW). Careful handling of incineration residues including fly ash, air pollution control (APC) residues, and bottom ash is required for this approach. The yield of these residues is large, and they contain many toxic and harmful substances. On the other hand, these residues contain valuable components such as SiO2, CaO, Al2O3, MgO, which are important components of building materials. Therefore, MSWI residues present huge opportunities for potential recycling and reuse in the construction and building industry. This paper summarized and discussed the application of MSWI residues in four typical building materials including cast stone, glass-ceramic, cement, and concrete. Before utilization, three types of pretreatment methods can be used to reduce the toxicity of the residues and improve the performance of the products. In addition, the current issues and the prospects of this field, and the environmental impacts of this application were discussed. It was concluded that MSWI residues can be used to prepare building materials after proper treatment which can improve the mechanical and chemical properties of the residues. The recycling can gain significant economic and environmental benefits at the same time. However, further researches on treatment methods for fine particles are needed.
1 Introduction
Nowadays, the yield of municipal solid waste (MSW) is enormous and the growth rate is high. It has been estimated that the quantities of MSW may markedly increase to 2200 million tonnes per year by 2025 because of the rapid urbanization progress and economic growth.1,2 Incineration is a promising alternative approach to landfill as a treatment method for the huge number of MSW. It does not only reduce the volume and weight of solid waste by 90% and 70% respectively3 but also generate energy and heat that could be used for other purposes. On average, over 10% of the generated MSW globally are incinerated at present.4 The percentage is up to over 50% in many European countries such as Sweden, France, the Netherlands and Denmark.1,5 Many countries, like China, have been rapidly developing incineration facilities in recent years. Statistics further suggested the amount of city-generated solid waste in China was 228 million tons in 2018, approximately 102 million tons (45% of the total quantity) were incinerated.6 The prominent application ways of municipal solid waste incineration (MSWI) residues in these countries are showed in Table 1.7–9| Continent | Country | Application ways of MSWI residues |
|---|---|---|
| Europe | United Kingdom | Road construction, structural platforms |
| Sweden | Road construction, landfill cover | |
| Netherlands | Construction material | |
| Italy | Road construction, cement | |
| German | Road base | |
| Denmark | Subbase layer | |
| Belgium | Construction material | |
| Poland | Road construction | |
| Spain | Embankments, road subbase, cement and concrete | |
| Czech Republic | Soil surface | |
| France | Road construction | |
| Norway | Landfill construction | |
| Asia | China | Road construction |
| Japan | Cement clinker |
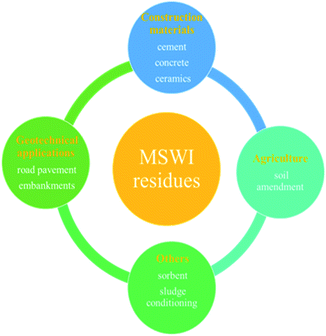
The biggest concern of incineration, however, is the vast number of residues generated from the process, including fly ash, air pollution control (APC) residues and bottom ash. Studies have found that the incineration of per ton MSW produces 250–300 kg of bottom ash and 25–30 kg of APC residues and fly ash.10 These residues are poisonous and harmful because of containing heavy metals, salts and organic pollutants such as dioxins,11 therefore treatment and disposal methods are needed for safe handling and management. Two basic ways of handling MSWI residues are recycling after appropriate treatment and landfilling.12,13 These residues contain SiO2, CaO, Al2O3, MgO, etc. valuable composites, therefore many researchers have explored the recycling and reuse of them in different fields. In general, these applications can be summarized into four fields, including construction materials, geotechnical applications, agriculture and other application ways, as summarized in Fig. 1.1,14 MSWI residues often contain elements of Si, Ca, Al and Fe, like the building materials composition. They are therefore considered to be an alternative raw material for producing building materials,3 such as glass-ceramic,15 cement,16 concrete,17,18 etc. 50 percent of MSWI bottom ashes mixed with metakaolin were used to prepare mixed alkali-activated binders successfully.19 Alkali-activated MSWI fly ash was used blending with 10% metakaolin to manufacture cementitious material.20 MSWI fly ash and bottom ash were used as substitutes for Portland cement to prepare concrete for 3D printing.21 Rotary kiln sintering and non-sintering methods were used to prepare ceramists with MSWI fly ash.22 The second way of using MSWI residues is geotechnical engineering such as roads, embankments, etc.23 MSWI residues used in agriculture as soil amendment has also been studied. Moreover, one of the main components of MSWI residues, CaO is a candidate for thermochemical energy storage. MSWI fly ash was a promising thermochemical energy storage material.24 Many other application ways have been studied as well. In short, MSWI residues have wide potential for many purposes, and a large number of studies have investigated specific applications for MSWI residues. Compared with landfilling, the recycling of MSWI residues can obtain both economic and environmental benefits. Landfilling of MSWI residues is likely to cause additional environmental impacts such as climatic change and acidification,25 whereas the recycling of MSWI residues can save more natural resources and energy than landfilling,25,26 save available landfilling sites and costs at the same time.27 Therefore, the adequate treatment of residues and their reutilization is preferred rather than landfilling.27
Among the four types of application ways, construction materials have an important place. In some European countries, nearly 50% or more of bottom ash has been used for road construction or used as raw material for manufacturing building materials.1,28 A large number of studies showed that the utilization of MSWI residues as an alternative for producing construction materials has broad potential and prospects.
Herein, the possibilities and methods of preparing four typical building materials including cast stone, glass-ceramic, cement, and concrete from MSWI residues were summarized and discussed. In order to better reuse the residues, their physical and chemical properties ought to be well recognized and appropriate treatment methods should be chosen. The current challenges and the prospects, as well as the environmental impacts of recycling MSWI residues in construction materials were also discussed.
2 Characteristics of MSWI residues
2.1 Definition
Different types of MSWI residues are named according to their collection locations in the incinerator.4 The raw particulate matter collected from the flue gas before the addition of any sorbent material is typically called fly ash.4,11 The mixture of fly ash and fine particulate solids collected in scrubbers and fabric filters can be called APC residues.29 That means APC residues include all particulate materials captured before discharging the gases to stack. In some studies, APC residues and fly ash are regarded as one type of wastes to be discussed. Bottom ash is usually found on the grate or collected from the bottom of the furnace.2.2 Characteristics
Some characteristics like major elements, particle size, density, mineralogical composition and toxicity are various among different types of MSWI residues. Examples of chemical compositions of MSWI fly ash, APC residues and bottom ash are shown in Table 2 for comparison.17,30 However, the composed oxides, treatment methods, potential applications and factors affecting characteristics are same or similar among all kinds of MSWI residues.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1). The primary compositions of MSWI fly ash are CaO, SiO2, Al2O3, MgO, etc. As for mineralogical compositions, MSWI fly ash mainly consists of amorphous, sulfate and chlorides minerals.32 Besides, MSWI fly ash often consists of toxic organic compounds such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCB), and polychlorinated dibenzo-p-dioxins and furans (PCDD/Fs).33 In addition, alkali chlorides and soluble metals salts may lead to the leaching of heavy metals.32
000 mg kg−1). The primary compositions of MSWI fly ash are CaO, SiO2, Al2O3, MgO, etc. As for mineralogical compositions, MSWI fly ash mainly consists of amorphous, sulfate and chlorides minerals.32 Besides, MSWI fly ash often consists of toxic organic compounds such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCB), and polychlorinated dibenzo-p-dioxins and furans (PCDD/Fs).33 In addition, alkali chlorides and soluble metals salts may lead to the leaching of heavy metals.32Six main methods were summarized for managing MSWI fly ash and APC residues, including backfilling, landfilling, detoxification, product manufacturing, practical applications and recovery of materials.34 Among them, the last four methods presented the potential for recycling and utilization.
MSWI bottom ashes exhibit remarkable potential to be recycled as resources. For example, scrap metals can be recovered from bottom ash.26 Furthermore, MSWI bottom ash has been proved to be an important alternative to natural aggregates and other natural materials used in construction industry.26,37 Bottom ashes can be commonly recycled as sub-grade materials, ceramics raw materials, mineral admixtures and concrete aggregates36 as well as glass-ceramic preparation or cobblestones for street paving.38
2.3 Factors influencing characteristics
The properties of MSWI residues are not immutable. Many factors make a difference to the characteristics and can be divided into two aspects.39,40 One is incineration conditions including types of fuel, operating parameters, types of furnaces and the APC system design,1,4,25 and the other is the incinerated waste compositions,39 which are usually affected by human habits, local economic policies and the recycling system before incineration.39,40Variation in physical and chemical compositions of MSWI residues caused by the heterogeneous nature of MSW and incineration factors is a fundamental barrier for reusing MSWI residues. Therefore, proper treatments are necessary before recycling to reduce the impact of the variation of residues on the properties of products.
3 Treatment methods of MSWI residues
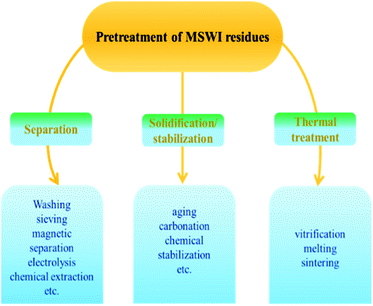
The treatment of MSWI residues before commercial application is significant. In general, the treatment methods are summarized into three categories.1,11 As shown in Fig. 2,41 one category is physical or chemical separation such as washing, chemical extraction, etc., another is solidification/stabilization such as chemical stabilization, aging, etc., and the third category is thermal treatment such as vitrification, etc.423.1 Separation
The goal of separation is to improve the quality of MSWI residues and to enhance their utilization potential through separating specific components thereby minimizing their leaching risk.1,41 The main drawback is the generation of waste which becomes the secondary pollutant.43 Separation process includes sieving, magnetic separation, mechanical resizing, washing/leaching process, etc.1,4,41 Among all the separation methods, washing is the simplest, and water washing can decrease the number of soluble salts and reduce the leaching risk of heavy metals.32 Through systematic investigation of MSWI APC residues, it was found that a large proportion of chlorides (over 70%) and nearly 25% of sulfates could be removed after water washing treatment.35 Similar conclusion was drawn that water washing removed 23% dry mass of soluble salts.44As an efficient pretreatment method, water washing was usually used combining other methods to improve the treatment effect and conducive to the large-scale application of products. The MSWI boiler fly ash was treated with the combination of wet sieving and water washing and reduced the leachability of the ash.45 On the other hand, more combined reagent washing has been used which has been proved more efficient. Combined reagent washing involves stepwise reactions. Usually, washing out oxyanions such as Cr and Mo under alkaline condition. Mobilizing of cations such as Zn, Cd, and Pb under acid condition follows. The pH can be lowered by adding acidic or through intermediate storage in contact with air.46 The MSWI fly ash in Sweden was treated by a two-step washing process with acid and base solutions respectively.46 A three-step combined washing47 was used for the MSWI fly ash and the efficiency was increased by 10–1000 fold for different heavy mental elements compared with water-only treatment.
3.2 Solidification/stabilization
Solidification/stabilization process mainly aims at minimizing the solubility, leachability and toxicity of pollutants.41 Physical strength and the durability of the materials can be enhanced through most of the solidification/stabilization methods. It also benefits the reuse process of the residues.1 Solidification/stabilization process includes weathering/aging, carbonization, accelerated carbonization, binder stabilization and solidification.1,4,41 Among them, binder stabilization is one of the main solidification/stabilization treatments. Various binders include polymeric binders, bitumen and cement-based binders, etc. When it comes to binder stabilization, the cost is often considered in binder selection, and cement is the most adaptable binder commonly used.13.3 Thermal treatment
Thermal treatment mainly includes vitrification, melting and sintering.1,11 They are distinguished by the characteristics of the products generated by the process. Thermal treatment methods aim at destroying toxic organic and inorganic pollutants, stabilizing the heavy metals, reducing the leachability of harmful constituents, reducing the volume of residues and producing materials with good environmental stability.1,41 However, the high temperature during the process also leads to high energy consumption and high treatment costs, which are the main disadvantages of thermal treatment.11After thermal treatment, the MSWI residues exhibit great application potential. Foremost, the obtained environmentally stable materials can be used as raw or alternative materials in construction or other applications such as the production of concrete, ceramic tiles, bricks and different type of glass or glass-ceramic.11 Moreover, the thermal-treated MSWI residues can even be used as fertilizer material in agriculture industry, owing to the separating of poisonous heavy metals.11
Amongst all the thermal treatment methods, vitrification is considered a promising approach and has been widely studied. Typical vitrification temperature is 1000–1500 °C.1,11 The vitrification of boiler ash (a kind of APC residues) from one MSWI company in the Netherlands was studied.40 The uniform vitrified slag was formed at 1400 °C, and most elements in vitrified slag exhibited lower leachability than original ash. In another study regarding electrostatic precipitator ash (another kind of APC residues collected after the boiler ash collection by electrostatic precipitators), similar conclusions were reached.48 It was found that the glass made by vitrification of the bottom ash had good mechanical and chemical properties and could be used for many purposes.49 Sand (SiO2), limestone (CaCO3), soda ash (Na2CO3), boric acid (H3BO3), etc. are typical raw materials of vitrification.11 In addition, the vitrified glassy materials can be further modified with additives (such as the compound of alkali metals and alkaline earth metals) to change melting temperature, chemical resistance, water solubility, and devitrification tendency, etc.11 In one study, the formation of glass phase of MSWI fly ash was promoted and the melting temperature was reduced with B2O3 (decomposed from H3BO3 when the temperature was over 160 °C) as a fluxing agent.50
3.4 Comparison of treatment methods
In general, the contents of toxic substances in MSWI residues can be reduced through treatment, therefore increasing the application potential of these residuals.32 However, the mechanism, target toxic substance and treatment cost varies among different treatment methods and each of these methods has advantages and disadvantages, as shown in Table 3.32,33 It should be noted that the characteristics of the treatment methods include many dimensions, and three categories of treatments are difficult to be evaluated quantitatively. Therefore, only the qualitative comparison for pretreatment methods have been provided. Among these methods, the most commonly used is solidification/stabilization treatment,43 whilst thermal treatment is considered to be the most promising approach for removing hazardous substances and reusing the residues.42 In order to improve the quality and to make better use of the MSWI residues, proper treatment can be adopted selectively before recycling in accordance with the purpose and economical efficiency. Among various application fields, preparing construction materials from proper-treated MSWI residues saves natural resources and reduces costs, having good social and economic benefits. Therefore, it has attracted widespread attention and research.| Category | Specific methods | Advantages | Disadvantages |
|---|---|---|---|
| Separation | Water washing | Simple | Secondary pollution possible increase leaching of heavy metals |
| High dioxins degradation | |||
| High soluble salts removal | |||
| Wet grinding | Simple | Secondary pollution | |
| No additional chemicals | |||
| Electrodialysis | Good heavy metals removal efficiency | High technical requirement | |
| High cost | |||
| Secondary pollution | |||
| Solidification/stabilization | Chemical stabilization | No secondary pollutant | Unable to destruct organic pollutants |
| Carbonation | Utilizing CO2 from stack gas | Not very fast | |
| Reducing off-gas emission | |||
| Carbon sequestration | |||
| Cement stabilization | Stabilizing heavy metals | Unable to destruct organic pollutants | |
| Low technical requirement | |||
| Low cost | |||
| Thermal treatment | Vitrification | High removal efficiency of chlorine and organics | High energy consumption |
| Melting | High cost | ||
| Sintering | Formation of low volatilization of oxides or alumina/silicates |
3.5 Roles of treatment methods
One of the roles of treatment for MSWI residues is improving the mechanical and chemical properties of the original residues. Ebert et al.51 indicated that electrodialysis remediation improved the performance of MSWI fly ash in Portland cement-based materials. Cement solidification methods can decrease the amount of the considerable mobile heavy metals contained in MSWI residues,52 thus improving the chemical properties of the residues. The thermal treatment can reduce the toxicity of raw ash effectively, thereby achieve residues detoxified.53Another significant roles of the three categories of treatment methods is to reduce environmental risk of reusing MSWI residues.41 For MSWI fly ashes and APC residues, washing treatment makes a great importance.35 The simple water-washing can not only effectively remove soluble chlorides such as NaCl, KCl and CaCl2 thereby improving compressive strength of prepared products,54 but also partially remove some heavy metals such as Pb and Zn. In short, using washed residues can eliminate the corrosion and leaching problem.35 For MSWI bottom ash, magnetic separation and stabilization are usual methods for reducing the environmental impact. Two main methods were proposed to reduce the leaching of metals and one of them was the recovery of ferrous and non-ferrous metals.39 Solidification/stabilization is also an efficient method to reduce the environmental risk of MSWI bottom ash.55 The solidification of MSWI bottom ashes in the autoclave led to over 99% of total heavy metals being immobilized in the obtained matrices, and the leaching concentrations were below the limits.56 The effect of accelerated carbonation on MSWI bottom ash with incinerator chimney gas was studied.57 After accelerated carbonation, the whole sample was carbonized after only one week and the quality of bottom ash was improved. Either for MSWI fly ash and APC residues or MSWI bottom ash, thermal treatment is a good way to reduce environmental risk of leaching because of destroying toxic organic pollutants and stabilizing heavy metals.58 The toxicity characteristic leaching procedure (TCLP) was used to evaluate the emission of Pb and PAHs from thermal treated MSWI fly and bottom ash. Results showed that the thermal treatment reduced the toxicity of raw fly ash and thus reduced the impacts on the environment.53 Cheng et al.59 used vitrified MSWI scrubber ash and fly ash at a mixing ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to prepare glass-ceramic with desirable properties. The leachability characteristics for heavy metals were low because the heavy metal ions were locked within the framework of glass.
3 to prepare glass-ceramic with desirable properties. The leachability characteristics for heavy metals were low because the heavy metal ions were locked within the framework of glass.
In short, proper treatments play important roles on reducing the environmental risk of recycling MSWI residues and improving the product's engineering properties. However, studies have also shown that not all pretreatment methods are effective,41 the choice of the pretreatment methods is significant. It is worth noting that contaminants in the fine particles (0–2 mm) barely change after treatment,55 therefore the roles of treatment for reducing environmental risk on fine MSWI residues particles are not obvious enough.
4 MSWI residues recycled as building materials
Herein, the possible use of MSWI residues as raw or substitute materials for manufacturing building materials (cast stone, glass-ceramic, cement, and concrete) was discussed and the process of each product was described.4.1 Cast stone
Cast stone is a kind of crystalline silicate material that is prepared from four processes named melting, cast molding, crystallization and annealing.60–62 Cast stone is characterized by resistance to abrasion and corrosion, and it has excellent insulation properties as well as high compressive strength. Because of its significant benefit, cast stone is widely used in metallurgy, thermal power, mining, chemical, building materials and other industrial sectors.60 Natural minerals and solid waste residues are two main categories of raw materials for cast stone production.60,62At present, the majority of the researches has focused on the use of natural rock or industrial waste residues including tailings or metal smelting slags to produce cast stone, such as igneous rock.61 However, preparing cast stone with this method has the problem of high energy consumption. To achieve the cost-effective and environment-friendly purpose, the liquid slag from furnace was used directly to prepare cast stone as decorative building material.62
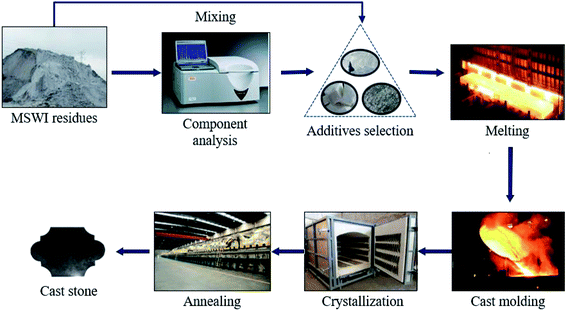
The main reason for preparing cast stone from natural rock or waste residues is that these raw materials contain the oxides that are needed in cast stone preparation such as SiO2, CaO, Fe2O3, Al2O3, MgO, etc. It is not difficult to find that these oxides are abundant in the MSWI residues. The components of MSWI residues belong to the SiO2–CaO–Al2O3 ternary-component phase which is imperative in the compositions of raw materials for cast stone production. Therefore, it can be deduced that MSWI residues have great potential in cast stone production. Fig. 3 (the picture of cast stone is from ref. 63) shows the design process for preparation of cast stone from MSWI residues. The composition of MSWI residues was analyzed first, then the appropriate additives (such as SiO2, CaO, Al2O3, etc.) were selected to mix with it. The mixture was melted at high temperature, cast in the mold, and then subjected to heat treatment named crystallization and annealing. At last, the cast stone products were obtained.
In short, the use of MSWI residues to prepare cast stone with excellent performance instead of expensive metal materials or synthetic materials can save metal materials, reduce production costs, extend the service life of equipment and reduce maintenance.
4.2 Glass-ceramic
Glass-ceramic has excellent mechanical performance and outstanding stabilization efficiency of heavy metals therefore has a good application prospect.64,65During the process of glass-ceramic production, the composition and phase design of the mixture take great importance because they will determine the physicochemical and mechanical properties of final product.64 As glass-based products, [SiO4] tetrahedral units are widespread in internal structure of glass-ceramic. Si and O elements are covalently linked to form a stable glass network structure.66 In terms of elemental composition, oxides that act as network formers such as SiO2 and P2O5, network-modifier (Na2O, K2O, MgO and CaO) and intermediates such as Al2O3, Fe2O3, etc. are required.64
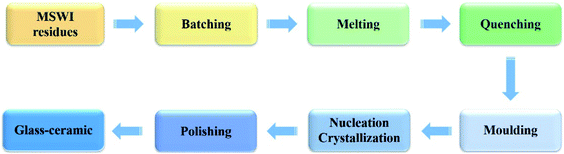
What excites people is that these required oxides all exist in MSWI residues. In other words, MSWI residues contain CaO, SiO2, A12O3, MgO etc., which are consistent with the raw materials composition needed for the glass-ceramic production, therefore, MSWI residues can be used as the raw materials to produce glass-ceramic. SiO2 and CaO are glass network former and modifier. The presence of Al2O3 is conducive to form stable glass skeleton structure. MgO and Na2O serving as network modifier make for reducing the softening temperature of materials.67 Therefore, MSWI residues have great potential for recycling and being utilized in glass-ceramic production. The process of preparing glass-ceramic from MSWI residues by sintering method is shown in Fig. 4. The suitable batched MSWI residues were melted and quenched and then molded. After nucleation and crystallization heat treatment, the products were polished finally, and then the glass-ceramic could be obtained. During the process of glass-ceramic production, the used residues have usually been pre-treated by immobilization, vitrification, alkali activation, or adding additives and other materials. Pre-stabilized fly ash mixed with clay and recycled soda-lime glass has been used to prepare glass-ceramic by direct and inexpensive viscous flow sintering method.66 The glass-ceramic had excellent properties and was proved to be an environmentally safe product. Sintered glass-ceramic material was prepared from vitrified iron-rich MSWI bottom ash powder.68 When sintering temperature was increased to 1120–1130 °C, the glass-ceramic material with zero water absorption, low closed cell percentage and high crystallinity was produced. In another study, four different types of glass-ceramic were prepared successfully with vitrified MSWI bottom ash as raw material by the combination of alkali activation and sintering.2 Samples A and B used vitrified MSWI bottom ash (100%) as raw material and the sintering temperature is 900 °C with different concentrations of alkali solutions (A: 1 M NaOH; B: 1.5 M NaOH). Samples C and D used vitrified MSWI bottom ash (90%) and soda-lime glass (10%) as raw materials with the same alkali solution concentration (1 M NaOH), however, the sintering temperature are 800 °C and 900 °C, respectively. The high porosity and high strength of the products were achieved with low production cost.
In addition to using single kind of residues to prepare glass-ceramic, the use of mixed residues has also been investigated. Low-cost glass-ceramic composites were prepared successfully with MSWI bottom ash and oil shale fly ash and the best content ratio of vitrified ash to oil shale fly ash was 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.67 Similar conclusions could be reached in another study.69 The mixture of MSWI bottom ash and fly ash was the raw material, and the petrurgic method was used. The petrurgic method is a controlled and very slow process, during which the parent glass was cooled from the molten state directly without heat preservation process at an intermediate temperature. The optimal mass ratio of bottom ash to fly ash was also 8
2.67 Similar conclusions could be reached in another study.69 The mixture of MSWI bottom ash and fly ash was the raw material, and the petrurgic method was used. The petrurgic method is a controlled and very slow process, during which the parent glass was cooled from the molten state directly without heat preservation process at an intermediate temperature. The optimal mass ratio of bottom ash to fly ash was also 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. An additive-free glass-ceramic was prepared with MSWI bottom ash and coal fly ash as raw materials.70 The glass-ceramic products had the best compressive strength and corrosion resistance when the basicity (CaO/SiO2 ratio) was 0.25. It could be concluded that the preparation of glass-ceramic with MSWI residues as raw materials was a feasible and promising method.
2. An additive-free glass-ceramic was prepared with MSWI bottom ash and coal fly ash as raw materials.70 The glass-ceramic products had the best compressive strength and corrosion resistance when the basicity (CaO/SiO2 ratio) was 0.25. It could be concluded that the preparation of glass-ceramic with MSWI residues as raw materials was a feasible and promising method.
4.3 Cement
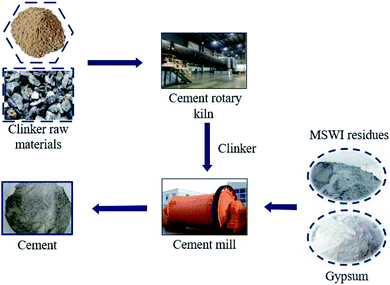
Cement is a building material widely used all over the world. However, cement manufacturing industry is energy-intensive and emits a considerable amount of CO2 and other greenhouse gases, which is considered to be a challenge to cement industry.71,72 It is estimated that 850![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kcal of energy and 1.7 tons of natural raw materials were consumed as well as 0.85 tons of CO2 released approximately for the production of one ton of cement clinker.17 It is a fact that cement industry has large pressure on reducing its CO2 emission. Using cement substitution is a good choice for cement industry.71,73 Fig. 5 describes the process of preparation of Portland cement from MSWI residues. The limestone and other raw materials were broken and batched first. The raw material powder was ground and homogenized and then put into the cement rotary kiln and fired at high temperature to transform into cement clinker. The clinker is mixed with MSWI residues, gypsum and other raw materials and then put into the cement mill to grind into fine powder. After that the cement can be obtained.
000 kcal of energy and 1.7 tons of natural raw materials were consumed as well as 0.85 tons of CO2 released approximately for the production of one ton of cement clinker.17 It is a fact that cement industry has large pressure on reducing its CO2 emission. Using cement substitution is a good choice for cement industry.71,73 Fig. 5 describes the process of preparation of Portland cement from MSWI residues. The limestone and other raw materials were broken and batched first. The raw material powder was ground and homogenized and then put into the cement rotary kiln and fired at high temperature to transform into cement clinker. The clinker is mixed with MSWI residues, gypsum and other raw materials and then put into the cement mill to grind into fine powder. After that the cement can be obtained.
It was studied the effect of large-scale application of MSWI fly ash in Portland cement production on the heavy metal content in cement.74 They found that the total content of Cd, Hg, Pb and Zn in cement increased after the addition of untreated MSWI fly ash from grate incinerator, but the content was still lower than the threshold stated by the Austrian regulations for cement industry. The feasibility of using energy, ash and carbon dioxide generated from MSW incineration to produce cement was studied.75 With fly ash and APC waste-lime serving as main sources of silica and lime respectively, cement material was synthesized successfully, and carbon dioxide activated the product rapidly. With waste materials accounting for 85% of the raw material, this clean cement material had a similar binding ability to ordinary Portland cement. Stabilized MSWI bottom ash was used in Italy as aggregates in cement-bound mixes and asphalt concrete for road surface with a maximum content of 10%.76 30% of the cement was replaced by ground MSWI bottom ash in cement composites production.77 Results of leaching tests demonstrated that the cement composite had good durability under ocean conditions, and the concentration of most heavy metals was within the prescribed range except for As in saline water. The maximum replacement ratio of Portland cement of 30% was concluded by Cristelo et al. as well.78 The recommended content of MSWI bottom ash in the blended cement was also less than 30%.79
However, some studies showed that the application of MSWI residues could retard the cement hydration due to the low hydraulic activity of the residues. Hydraulic activity is represented by hydraulic ratio (HR), one of the parameters used to define the characteristics of clinker. HR can be calculated as eqn (1):80
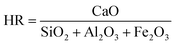 | (1) |
The bottom ash fines (<2 mm) from two different waste-to-energy plants in the Netherlands were used as cement replacement.80 It demonstrated that the bottom ashes from both factories could be used as a substitute material for cement. However, the bottom ashes have very low hydraulic activity, which retarded the cement hydration. Similar conclusions were reached in Li's study.79 MSWI bottom ash was used as supplementary cementitious material to prepare blended cement. They found the addition of MSWI bottom ash may retard cement hydration because of its lower reactivity than that of Portland cement, and the mechanical properties gradually decreased with the increase of MSWI bottom ash addition. The pre-washed MSWI fly ash and bottom ash were used as supplementary cementitious materials to prepare blended cement.30 It was found that the lower activity of washed MSWI fly ash and bottom ash led to a negative impact on the mechanical strength of cement composites.
It was further found the MSWI residues could not be applied directly on large scale under some conditions. The statistical entropy of the distribution of Cd, Pb and Cu as selected metals was evaluated after mixing MSWI fly ash or bottom ash with cement clinker respectively.81 The author used this method to quantify the statistical entropy of the distribution of selected substances. Statistical entropy analysis is a method of evaluating the ability of material flow systems to concentrate or dilute a substance throughout its entire life cycle.82 It can quantify changes in the substance distribution pattern throughout a system in which materials undergo transitions. During the processes, the statistical entropy of substances may increase, decrease or unchanged.83 The results showed that neither MSWI fly ash nor bottom ash should be used in cement on a large scale without any pretreatment.
In short, MSWI residues are promising alternative raw materials in cement production, however, there's a limitation on the maximum additional amount to decrease the effect of MSWI residues on cement properties.
4.4 Concrete
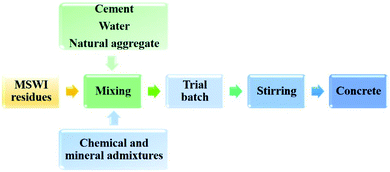
Concrete is also a kind of widely used building material all around the world.84 The most common components of concrete include cement, water and aggregates which consist of fine and coarse natural aggregates and sometimes chemical and mineral admixtures. Due to consisting of cement and wide use, concrete has a significant impact on environment as well. Therefore, making the concrete industry more sustainable has important significance. One of the most popular methods is to use recycled materials as substitution of concrete components. Industrial by-products such as fly ash, blast furnace slag, silica fume as well as recycled aggregate are commonly replacement materials for cement.85,86 The products with these alternative materials always have good properties. Moreover, the utilization of recycled materials decreased the emission of CO2 related to cement production, thereby decreasing the environmental impacts of concrete.85,86MSWI residues are also competitive materials that can be used in concrete. Fig. 6 shows the process of MSWI residues recycled for concrete production. MSWI residues serve as cement or aggregate partial substitute materials. Cement, natural aggregate, other additives and water are added and mixed. The mixture is fully stirred, and then concrete products can be obtained.
MSWI residues serving as cement replacement is one of the common applications. MSWI bottom ash was used partially replacing cement.87 The equivalent substitution of bottom ash for cement reduced the compressive strength of concrete. The maximum ratio of replacing cement with bottom ash was 15% and this binder was suitable for application in low strength concrete.88 When MSWI bottom ash and recycled aggregate were added, the consistency measured by slump was reduced by more than 50%.
In addition, MSWI residues can serve as fine or coarse aggregates substitution. 100% MSWI bottom ash (0–2 mm) was used to replace natural sand.89 They found that the upgraded MSWI bottom ash had the same properties as the natural sand, except the higher water absorption. Adding some mineral additives as superplasticizers could reduce the water adsorption and improve the workability of the mixture, proving the possibility of MSWI bottom ash for replacing natural sand in concrete. MSWI fly ash and foundry residues were used as recycled aggregates in concrete production after treatment of water washing, inorganic reagents stabilization–solidification and grinding.54 The recycled concretes had good structural and environmental quality. Exhausted sand and bottom ash from fluidized bed incinerators were used to partially replace natural aggregate and found that the average compressive strength and material reliability of concrete containing recycled aggregate were comparable to that of reference concrete.90 For different types of concrete, the replacement ratio of MSWI residues for aggregate is variable. The upgraded MSWI bottom ash could replace up to 20% sand and/or gravel in reinforced concrete, however, the ratio could rise to 50% in plain (non-structural) concrete, whereas, the bottom ash was not recommended for prestressed concrete, as prestressed steels may present a higher risk of stress corrosion.91
In summary, the MSWI residues could be used as both fine and coarse aggregate replacement in concrete.92 The prepared concrete always had the performance of low density, high consistency and lower compressive strength. Therefore, the additive quantity has a limitation.
4.5 Summary
Take four typical building materials (cast stone, glass-ceramic, cement and concrete) as examples, the characteristics of using MSWI residues to produce each product are compared in Table 4.34 Each production process has benefits or challenges on products properties, resources, environment and economy.| Building material | Type of MSWI residues | Pretreatment | Role of MSWI residues | Waste generation | Leaching | Benefits | Challenges |
|---|---|---|---|---|---|---|---|
| a Inference results derived from the preparation of cast stone from metallurgical waste slag. | |||||||
| Cast stonea | Fly ash, bottom ash, mixture | Not required | Raw material | None | Low | Good performance | High treatment temperature |
| Glass-ceramic | Fly ash, bottom ash, mixture | Not required | Raw material | None | Low/medium | Natural resources protection | Possible leaching |
| Economic viability | |||||||
| Cement | Fly ash, bottom ash, mixture | Advised | Partial substitute for raw materials | Off-gas | Low | Easy to implement | Possible leaching |
| CO2 emission reduction | |||||||
| Concrete | Fly ash, bottom ash | Advised | Substitute for cement or aggregate | Off-gas | Low | Resource conservation | Possible leaching |
| Early cracking in structured concrete | |||||||
| Chemical corrosion of product | |||||||
In general, in the field of building materials, MSWI residues exhibit wide application potential and great economic and environmental benefits because of recycled as supplementary materials in construction materials, thereby returning the waste materials to the economic cycle and saving natural resources. In Europe, the stabilized MSWI fly ashes can be sold as a filler, thereby saving calcite and talc natural resources. It was estimated that, in Europe alone, recycling MSWI fly ashes instead of landfilling may save approximately 1.6 million euros per day. The saved carbon dioxide emission into the atmosphere is approximately 960 kilotons per year.27 In China, the biggest cement production country around the world, about 72 million tons of cement can be saved per year according to calculation if the maximum replacement of MSWI fly ash and bottom ash (40 wt% and 20 wt% respectively) for cement composites can be achieved. It means large quantities of raw materials (about 131 million tons of limestone, 23 million tons of clay, 8 million tons of sand and 2 million tons of hematite) can be saved per year.30 Therefore, the recycling of pretreated MSWI residues in construction materials, especially in the production of cement, is a cost-effective and eco-friendly application.
Meanwhile, studies have shown that the properties of the building materials containing MSWI residues can meet relevant standards. When considering the use of MSWI residues as building materials, besides the mechanical properties of the products, the environmental risk of the residues is another major problem concerned.
5 Environmental impact of recycling MSWI residues
5.1 LCA method
When MSWI residues are recycled, the leaching of toxic and harmful composition to soil and water as well as other environmental impact are important points of concern.93 Many approaches94 can be used to assess environmental impacts such as life cycle assessment (LCA), cost-benefit analysis and statistical entropy analysis. Among them, LCA is an effective method for assessing comprehensive environmental impacts95 and has been widely used in waste management field.25When recycling MSWI residues as raw materials or aggregate alternatives in building materials production, using LCA method can not only obtain long-term impacts of the recycling but also verify the validity of reuse as well as the environmental and economic benefits. Fig. 7 (the upper left and upper right pictures of the “building materials” in the figure are from ref. 62 and 63) describes the materials and energy flowing into and out of the system from a life cycle perspective during the process of preparing building materials with MSWI residues. LCA was used to compare the environmental impacts of lightweight aggregates with and without APC residues.96 Results showed that in all categories, a reduction of environmental impacts could be found when 3% of natural clay was replaced by APC residues.
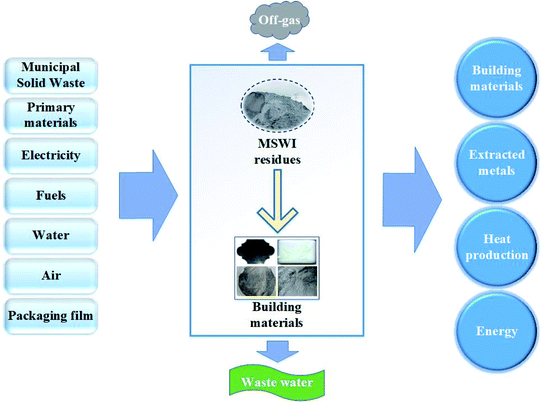 | ||
| Fig. 7 Materials and energy flowing into and out of the system during the preparation of building materials with MSWI residues. | ||
5.2 The environmental impacts
From LCA perspective, recycling MSWI residues in building materials production has both benefits and drawbacks. Foremost, this application reduces the production and depletion of primary materials compared with the management of landfilling.25 However, the environmental risk exists deriving from the potential leaching of toxic substances,97 for the residues contain enriched concentrations of heavy metals and salts.98 Using MSWI residues may negatively affect the products' quality and performance as well. For example, the presence of metallic Al or Zn in MSWI residues may cause early cracking in structured concrete, and the high concentration of Cl may cause corrosion of reinforcement steel.99 When 20% MSWI fly ash were mixed into hardened cement paste, the specimens expanded and microcracks appeared because of the metallic aluminum in MSWI fly ash. The cement strength was reduced as well.100Among the disadvantages, the possible leaching of contaminants has drawn the most attention.25 It has been concluded that ash properties, pH and liquid to solid ratio (L/S) are the main factors accounting for the leaching from MSWI residues, besides, solubility and sorption are the two major leaching mechanisms.41 Usually, solubility control occurs when a solution touching with the solid is saturated in regard to the constituent species of interest. On the other hand, if the elements show sorptive affinity to the solid surface's active sites, sorption control occurs. The release properties of selected metals (Cd, Cr, Cu, Ni, Pb, and Zn) from MSWI bottom ash were investigated. The results showed that precipitation/dissolution reactions make a great importance on the leaching of Cu, Pb and Zn, while the leaching of Cr, Cd, and Ni were mainly controlled by adsorption.101 However, the leaching of pollutants from MSWI residues is complicated and still a research hotspot. The leaching of 15 types of elements from fresh and aged MSWI fly ashes was investigated under different pH ranges from 2 to 14.102. For a majority of the heavy metal elements, the leached concentration decreased when pH increased. Furthermore, the potential leaching from products containing MSWI residues has also attracted wide attention. The successive leaching concentration of heavy metals from cement with MSWI fly ash was studied and found that the leaching concentrations and leaching time had strong positive relationships.103 In the circumstances of factual utilization, leaching is a very slow and gradually diluting process. The element leaching of Cd, Cr, Hg, Pb, etc. was investigated from an ecological cement prepared with MSWI residues.10 The concentrations of all the heavy metals in the leachate were below regulatory limits, and the leaching tests validated the stability of the product. To assess the environmental impact of MSWI bottom ash contained in permeable asphalt mixture, the leaching behaviors of four heavy metals (Zn, Pb, Cu and Cr) were studied. Result showed that the leachate has very little negative impact on surrounding surface and underground water quality and the permeable asphalt mixture containing MSWI bottom ash would be safe for the environment.104 It can be concluded that the leaching concentration of poisonous substances was usually below the relevant limitation when recycling MSWI residues for different purposes. However, the environmental impact from leaching is still worth focusing on. Before using MSWI residues, it is important to pre-treat them to reduce the environmental impacts.
6 Conclusion and perspective
6.1 Conclusion
The composition of the huge number of MSWI residues is like raw materials used for construction sector, having been studied extensively. This paper reviews and discusses the possibility and benefits of recycling MSWI residues as raw material or aggregate replacement in four construction materials (cast stone, glass-ceramic, cement and concrete) production. A few conclusions can be drawn.(1) Before reusing MSWI residues, the ingredient and characteristics should be well learnt because of the heterogeneity of the residues from diverse sources and incineration conditions. Usually, fly ash and APC residues are more harmful than bottom ash.
(2) The residues can be treated by separation, solidification/stabilization or thermal treatment selectively before recycling, thereby reducing the content of toxic substances, decreasing the environmental impact and increasing the properties of products.
(3) For the four typical building materials discussed in this review, MSWI residues were usually used as supplementary material and the prepared products have better performance meeting relevant standards. Besides, little waste was produced during the preparation process.
(4) In general, the residues have broad application potential in construction field serving as raw materials or aggregate substitutes. The application way has economic and environmental benefits. However, the additional proportion of the residues in construction materials has a threshold to limit its negative impacts on the property and quality of the products.
(5) The environmental impacts of preparing building materials from MSWI residues are usually lower than the limit value of relevant standards, but the hazards caused by possible leaching are still concerns.
(6) Management methods should vary according to size fractions of the residues. Proper treatments can reduce the environmental impact, but the effect on fine residues particles below 2 mm is not obvious, thereby more attention should be paid to the fine particles when reusing MSWI residues.
6.2 Challenge and opportunity
To decrease the environmental risk of utilizing MSWI residues, the combination of multiple treatments and new treatment methods should be investigated extensively, especially the effective treatment for the fine particles.Besides, in order to use MSWI residues on a large scale in construction sector, more attempts ought to be made to use MSWI residues as primary raw material rather than additives. Also, in-depth studies could be conducted to explore broader application potential of MSWI residues to reduce the consumption of energy and natural resources.
At the same time, attention should be drawn to the long-term leaching behavior and the leaching mechanism of the contaminants. Ultimately, it is also important to further improve performance of the products prepared from MSWI residues. The development of standardized quality-control protocols for utilizing MSWI residues would be significant.
Author contributions
Dan Chen contributed to the conceptualization of the work, gave the ideas and goals. Yingying Zhang summarized the articles and wrote the manuscript. Yao Xu and Wenyu Sheng helped to summarize the published articles. Qing Nie and Zhanbin Yang helped to collect and analyze the previous research articles. Guangren Qian managed the work and helped with the preparation of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by National Key Research and Development Program of China (2019YFC1906900). We thank Shanghai University for the support of this work. We also appreciate Xiang Cheng (University of Cambridge, UK) for providing language help.Notes and references
- X. M. Dou, F. Ren, M. Q. Nguyen, A. Ahamed, K. Yin, W. P. Chan and V. W. C. Chang, Renewable Sustainable Energy Rev., 2017, 79, 24–38 CrossRef CAS.
- P. R. Monich, F. Dogrul, H. Lucas, B. Friedrich and E. Bernardo, Detritus, 2019, 8, 101–108 Search PubMed.
- B. Y. Chen and K. L. Lin, J. Hazard. Mater., 2006, 136, 741–746 CrossRef CAS PubMed.
- A. M. Joseph, R. Snellings, P. Van den Heede, S. Matthys and N. De Belie, Materials, 2018, 11, 141–170 CrossRef PubMed.
- H. Ecke and A. Aberg, Sci. Total Environ., 2006, 362, 42–49 CrossRef CAS PubMed.
- Y. Zhu, Y. Zhao, C. Zhao and R. Gupta, Environ. Sci. Pollut. Res., 2020, 27, 14184–14197 CrossRef CAS PubMed.
- S. Kumar and D. Singh, Innov. Infrastruct. Solut., 2021, 6, 201–216 CrossRef.
- X. M. Dou, F. Ren, M. Q. Nguyen, A. Ahamed, K. Yin, W. P. Chan and V. W. Chang, Renewable Sustainable Energy Rev., 2017, 79, 24–38 CrossRef CAS.
- M. Margallo, M. B. M. Taddei, A. Hernández-Pellón, R. Aldaco and Á. Irabien, Clean Technol. Environ. Policy, 2015, 17, 1333–1353 CrossRef CAS.
- M. S. Ashraf, Z. Ghouleh and Y. Shao, Resour., Conserv. Recycl., 2019, 149, 332–342 CrossRef.
- D. Lindberg, C. Molin and M. Hupa, Waste Manage., 2015, 37, 82–94 CrossRef CAS PubMed.
- M. J. Quina, J. C. Bordado and R. M. Quinta-Ferreira, Waste Manage., 2008, 28, 2097–2121 CrossRef CAS PubMed.
- F. Huber, D. Laner and J. Fellner, Waste Manage., 2018, 73, 392–403 CrossRef PubMed.
- C. Ferreira, A. Ribeiro and L. Ottosen, J. Hazard. Mater., 2003, B96, 201–216 CrossRef.
- J. Zhang, B. Liu, S. Zhao, H. Shen, J. Liu and S. Zhang, Constr. Build. Mater., 2020, 262, 120781 CrossRef CAS.
- K. A. Clavier, J. M. Paris, C. C. Ferraro and T. G. Townsend, Resour., Conserv. Recycl., 2020, 160, 104888 CrossRef.
- S. Zhang, Z. Ghouleh and Y. Shao, Cem. Concr. Compos., 2020, 113, 103725 CrossRef CAS.
- S. Zhang, Z. Ghouleh, Z. He, L. Hu and Y. Shao, Constr. Build. Mater., 2021, 266, 120890 CrossRef CAS.
- S. Manzi, I. Lancellotti, G. Masi and A. Saccani, Front. Mater., 2020, 7, 583400 CrossRef.
- J. Liu, L. Hu, L. P. Tang and J. Ren, J. Hazard. Mater., 2021, 402, 123451 CrossRef CAS PubMed.
- A. Ur Rehman, S. M. Lee and J. H. Kim, Process Saf. Environ. Prot., 2020, 142, 219–228 CrossRef.
- H. L. Zhao, F. Liu, H. Q. Liu, L. Wang, R. Zhang and Y. Hao, Waste Manage., 2020, 113, 447–455 CrossRef CAS PubMed.
- D. Singh, T. Kumar, B. Emmanuel James and M. Hanifa, Environ. Geotech., 2019, 22, 229–236 Search PubMed.
- S. S. Jahromy, C. Jordan, M. Azam, A. Werner, M. Harasek and F. Winter, Energy Fuels, 2019, 33, 5810–5819 CrossRef.
- S. Toller, E. Karrman, J. P. Gustafsson and Y. Magnusson, Waste Manage., 2009, 29, 2071–2077 CrossRef CAS PubMed.
- E. Allegrini, C. Vadenbo, A. Boldrin and T. F. Astrup, J. Environ. Manage., 2015, 151, 132–143 CrossRef CAS PubMed.
- A. Assi, F. Bilo, A. Zanoletti, J. Ponti, A. Valsesia, R. La Spina, A. Zacco and E. Bontempi, J. Clean. Prod., 2020, 245, 118779–118791 CrossRef CAS.
- J. Giro-Paloma, V. Ribas-Manero, A. Maldonado-Alameda, J. Formosa and J. M. Chimenos, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 251, 012126 Search PubMed.
- M. J. Quina, J. C. Bordado and R. M. Quinta-Ferreira, Waste Manage., 2011, 31, 1984–1991 CrossRef CAS PubMed.
- Z. Z. Yang, R. Ji, L. L. Liu, X. D. Wang and Z. T. Zhang, Constr. Build. Mater., 2018, 162, 794–801 CrossRef CAS.
- B. H. Cho, B. H. Nam, J. An and H. Youn, Materials, 2020, 13, 3143 CrossRef CAS PubMed.
- A. Ferraro, I. Farina, M. Race, F. Colangelo, R. Cioffi and M. Fabbricino, Rev. Environ. Sci. Biotechnol., 2019, 18, 453–471 CrossRef.
- Z. H. Phua, A. Giannis, Z. L. Dong, G. Lisak and W. J. Ng, Environ. Sci. Pollut. Res., 2019, 26, 16974–16997 CrossRef CAS PubMed.
- M. J. Quina, E. Bontempi, A. Bogush, S. Schlumberger, G. Weibel, R. Braga, V. Funari, J. Hyks, E. Rasmussen and J. Lederer, Sci. Total Environ., 2018, 635, 526–542 CrossRef CAS PubMed.
- Z. Z. Yang, S. C. Tian, R. Ji, L. L. Liu, X. D. Wang and Z. T. Zhang, Waste Manage., 2017, 68, 221–231 CrossRef CAS PubMed.
- C. H. Chen and I. J. Chiou, J. Hazard. Mater., 2007, 148, 346–352 CrossRef CAS PubMed.
- A. T. Ahmed and H. A. Khalid, Waste Manage., 2011, 31, 2431–2439 CrossRef PubMed.
- P. Stabile, M. Bello, M. Petrelli, E. Paris and M. R. Carroll, Waste Manage., 2019, 95, 250–258 CrossRef CAS PubMed.
- A. Vaitkus, J. Gražulytė, O. Šernas, V. Vorobjovas and R. Kleizienė, Constr. Build. Mater., 2019, 212, 456–466 CrossRef.
- Y. Yang, Y. Xiao, J. H. L. Voncken and N. Wilson, J. Hazard. Mater., 2008, 154, 871–879 CrossRef CAS PubMed.
- H. W. Luo, Y. Cheng, D. Q. He and E. H. Yang, Sci. Total Environ., 2019, 668, 90–103 CrossRef CAS PubMed.
- Y. Min, C. Qin, P. Shi, C. Liu, Y. Feng and B. Liu, ISIJ Int., 2017, 57, 1955–1961 CrossRef CAS.
- E. Atanes, B. Cuesta-Garcia, A. Nieto-Marquez and F. Fernandez-Martinez, J. Environ. Manage., 2019, 240, 359–367 CrossRef CAS PubMed.
- A. A. Bogush, J. A. Stegemann and A. Roy, J. Hazard. Mater., 2019, 361, 187–199 CrossRef CAS PubMed.
- A. De Boom and M. Degrez, Waste Manage., 2015, 39, 179–188 CrossRef PubMed.
- D. Nordmark and A. Lagerkvist, Waste Manage., 2018, 76, 727–733 CrossRef CAS PubMed.
- E. Loginova, M. Proskurnin and H. J. H. Brouwers, J. Environ. Manage., 2019, 235, 480–488 CrossRef CAS PubMed.
- Y. Yang, Y. Xiao, N. Wilson and J. H. L. Voncken, J. Hazard. Mater., 2009, 166, 567–575 CrossRef CAS PubMed.
- R. C. C. Monteiro, S. J. G. Alendouro, F. M. L. Figueiredo, M. C. Ferro and M. H. V. Fernandes, J. Non-Cryst. Solids, 2006, 352, 130–135 CrossRef CAS.
- J. Gao, C. Q. Dong, Y. Zhao, X. Y. Hu, W. Qin, X. Q. Wang, J. J. Zhang, J. J. Xue and X. M. Zhang, Waste Manage., 2020, 102, 932–938 CrossRef CAS PubMed.
- B. A. R. Ebert, M. R. Geiker, W. Kunther and G. M. Kirkelund, Constr. Build. Mater., 2021, 309, 125193–125206 CrossRef CAS.
- J. Li, M. Zeng and W. Ji, Environ. Sci. Pollut. Res., 2018, 25, 36736–36744 CrossRef CAS PubMed.
- J. D. Chou, M. Y. Wey and S. H. Chang, J. Hazard. Mater., 2008, 150, 27–36 CrossRef CAS PubMed.
- C. Collivignarelli and S. Sorlini, Waste Manag. Res., 2001, 19, 539–544 CrossRef CAS PubMed.
- D. X. Xuan, P. Tang and C. S. Poon, Constr. Build. Mater., 2018, 167, 890–898 CrossRef CAS.
- P. Rożek, M. Król and W. Mozgawa, Constr. Build. Mater., 2019, 202, 603–613 CrossRef.
- S. Arickx, T. Van Gerven and C. Vandecasteele, J. Hazard. Mater., 2006, 137, 235–243 CrossRef CAS PubMed.
- K. Xie, H. Hu, S. Xu, T. Chen, Y. Huang, Y. Yang, F. Yang and H. Yao, Waste Manage., 2020, 103, 334–341 CrossRef CAS PubMed.
- T. W. Cheng, C. C. Tu, M. S. Ko and T. H. Ueng, Ceram. Int., 2011, 37, 2437–2444 CrossRef CAS.
- V. A. Krenev, E. N. Pechenkina and S. V. Fomichev, Inorg. Mater., 2019, 55, 1189–1194 CrossRef.
- S. E. Barantseva, A. I. Poznyak, Y. A. Klimosh and N. N. Gundilovich, Glass Ceram., 2019, 76, 265–269 CrossRef CAS.
- J. Zhou, Z. Shu, X. Hu and Y. Wang, Constr. Build. Mater., 2010, 24, 811–817 CrossRef.
- A. M. Ignatova, M. M. Chernykh and M. N. Ignatov, Glass Ceram., 2011, 68, 198–202 CrossRef.
- D. M. A. Valderrama, J. A. G. Cuaspud, J. A. Roether and A. R. Boccaccini, Materials, 2019, 12, 2032 CrossRef CAS PubMed.
- L. Liu, H. Yu, Y. Li and Z. Zhang, J. Clean. Prod., 2020, 269, 122417 CrossRef CAS.
- I. Ponsot, E. Bernardo, E. Bontempi, L. Depero, R. Detsch, R. K. Chinnam and A. R. Boccaccini, J. Clean. Prod., 2015, 89, 224–230 CrossRef CAS.
- Z. K. Zhang, L. Zhang and A. Li, Waste Manage., 2015, 38, 185–193 CrossRef CAS PubMed.
- A. Karamanov, L. M. Schabbach, E. Karamanova, F. Andreola, L. Barbieri, B. Ranguelov, G. Avdeev and I. Lancellotti, J. Non-Cryst. Solids, 2014, 389, 50–59 CrossRef CAS.
- D. H. Vu, K. S. Wang, J. H. Chen, B. X. Nam and B. H. Bac, Waste Manage., 2012, 32, 2306–2314 CrossRef CAS PubMed.
- Z. Zhang, J. Wang, L. Liu, J. Ma and B. Shen, Constr. Build. Mater., 2020, 254, 119345 CrossRef CAS.
- D. B. Jiang, X. G. Li, Y. Lv, M. K. Zhou, C. H. He, W. G. Jiang, Z. L. Liu and C. J. Li, Constr. Build. Mater., 2020, 232, 117228 CrossRef CAS.
- M. Zabihi-Samani, S. P. Mokhtari and F. Raji, J. Appl. Eng. Sci., 2018, 8, 35–40 Search PubMed.
- K. Ohenoja, M. Körkkö, V. Wigren, J. Österbacka and M. Illikainena, Waste Biomass Valorization, 2019, 10, 3525–3534 CrossRef CAS.
- J. Lederer, V. Trinkel and J. Fellner, Waste Manage., 2017, 60, 247–258 CrossRef CAS PubMed.
- Z. Ghouleh and Y. X. Shao, J. Clean. Prod., 2018, 195, 268–279 CrossRef CAS.
- E. Toraldo, S. Saponaro, A. Careghini and E. Mariani, J. Environ. Manage., 2013, 121, 117–123 CrossRef PubMed.
- Z. Z. Yang, S. C. Tian, L. L. Liu, X. D. Wang and Z. T. Zhang, Waste Manage., 2018, 78, 841–848 CrossRef CAS PubMed.
- N. Cristelo, L. Segadaes, J. Coelho, B. Chaves, N. R. Sousa and M. de Lurdes Lopes, Waste Manage., 2020, 104, 60–73 CrossRef CAS PubMed.
- X. G. Li, Y. Lv, B. G. Ma, Q. B. Chen, X. B. Yin and S. W. Jian, J. Clean. Prod., 2012, 32, 96–100 CrossRef CAS.
- P. Tang, M. V. A. Florea, P. Spiesz and H. J. H. Brouwers, Constr. Build. Mater., 2015, 83, 77–94 CrossRef.
- H. Rechberger, Waste Manag. Res., 2001, 19, 262–268 CrossRef CAS PubMed.
- D. Laner, O. Zoboli and H. Rechberger, Ecol. Indic., 2017, 83, 232–242 CrossRef CAS.
- P. Nimmegeers, A. Parchomenko, P. De Meulenaere, D. R. D’hooge, P. H. M. Van Steenberge, H. Rechberger and P. Billen, Sustainability, 2021, 13, 3553 CrossRef CAS.
- R. Kurda, J. de Brito and J. D. Silvestre, J. Clean. Prod., 2019, 226, 642–657 CrossRef.
- V. W. Y. Tam, K. N. Le, A. C. J. Evangelista, A. Butera, C. N. N. Tran and A. Teara, Front. Eng. Manag., 2019, 6, 395–405 CrossRef.
- Y. C. Ersan, S. Gulcimen, T. Nur Imis, O. Saygin and N. Uzal, Eur. J. Environ. Civ. Eng., 2020, 1–14, DOI:10.1080/19648189.2020.1767216.
- Y. Cheng, Y. Dong, J. Diao, G. Zhang, C. Chen and D. Wu, Appl. Sci., 2019, 9, 5091 CrossRef CAS.
- B. Juric, L. Hanzic, R. Ilic and N. Samec, Waste Manage., 2006, 26, 1436–1442 CrossRef CAS PubMed.
- J. R. Minane, F. Becquart, N. E. Abriak and C. Deboffe, Procedia Eng., 2017, 180, 1213–1220 CrossRef.
- A. Abbà, M. C. Collivignarelli, S. Sorlini and M. Bruggi, Composites, Part B, 2014, 58, 502–509 CrossRef.
- G. van der Wegen, U. Hofstra and J. Speerstra, Waste Biomass Valorization, 2013, 4, 737–743 CrossRef CAS.
- C. J. Lynn, R. K. Dhir Obe and G. S. Ghataora, Constr. Build. Mater., 2016, 127, 504–517 CrossRef CAS.
- H. A. van der Sloot, D. S. Kosson and O. Hjelmar, Waste Manage., 2001, 21, 753–765 CrossRef CAS PubMed.
- F. Huber and J. Fellner, Resour., Conserv. Recycl., 2018, 139, 17–26 CrossRef.
- T. Y. Huang, P. T. Chiueh and S. L. Lo, Resour., Conserv. Recycl., 2017, 123, 255–260 CrossRef.
- M. J. Quina, R. Garcia, A. S. Simões and R. M. Quinta-Ferreira, J. Mater. Cycles Waste Manage., 2020, 22, 1922–1931 CrossRef CAS.
- E. Allegrini, S. Butera, D. S. Kosson, A. Van Zomeren, H. A. Van der Sloot and T. F. Astrup, Waste Manage., 2015, 38, 474–485 CrossRef CAS PubMed.
- V. Bruder-Hubscher, F. Lagarde and M. J. F. Leroy, Waste Manag. Res., 2001, 19, 557–566 CrossRef CAS PubMed.
- B. Verbinnen, P. Billen, J. Van Caneghem and C. Vandecasteele, Waste Biomass Valorization, 2017, 8, 1453–1466 CrossRef CAS.
- X. G. Li, Z. Q. Yu, B. G. Ma and B. Wu, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2010, 25, 312–315 CrossRef CAS.
- H. Zhang, P. J. He, L. M. Shao and X. J. Li, J. Mater. Cycles Waste Manage., 2008, 10, 7–13 CrossRef CAS.
- Y. Zhang, B. Cetin, W. J. Likos and T. B. Edil, Fuel, 2016, 184, 815–825 CrossRef CAS.
- H. S. Shi and L. L. Kan, J. Hazard. Mater., 2009, 164, 750–754 CrossRef CAS PubMed.
- Y. Zhao and Y. T. Zhu, Water, 2019, 11, 2186–2202 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |