 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Intriguing electronic, optical and photocatalytic performance of BSe, M2CO2 monolayers and BSe–M2CO2 (M = Ti, Zr, Hf) van der Waals heterostructures
M. Munawara,
M. Idreesa,
Iftikhar Ahmadbc,
H. U. Dind and
B. Amin *a
*a
aDepartment of Physics, Abbottabad University of Science & Technology, Abbottabad 22010, Pakistan. E-mail: binukhn@gmail.com
bCenter for Computational Materials Science, University of Malakand, Chakdara 18800, Pakistan
cDepartment of Physics, Gomal University, DI Khan, Pakistan
dDepartment of Physics, Bacha Khan University, Charsadda, Pakistan
First published on 21st December 2021
Abstract
Using density functional (DFT) theory calculations, we have investigated the electronic band structure, optical and photocatalytic response of BSe, M2CO2 (M = Ti, Zr, Hf) monolayers and their corresponding BSe–M2CO2 (M = Ti, Zr, Hf) van der Waals (vdW) heterostructures. Optimized lattice constant, bond length, band structure and bandgap values, effective mass of electrons and holes, work function and conduction and valence band edge potentials of BSe and M2CO2 (M = Ti, Zr, Hf) monolayers are in agreement with previously available data. Binding energies, interlayer distance and Ab initio molecular dynamic simulations (AIMD) calculations show that BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures are stable with specific stacking and demonstrate that these heterostructures might be synthesized in the laboratory. The electronic band structure shows that all the studied vdW heterostructures have indirect bandgap nature – with the CBM and VBM at the Γ–K and Γ-point of BZ for BSe–Ti2CO2, respectively; while for BSe–Zr2CO2 and BSe–Hf2CO2 vdW heterostructures the CBM and VBM lie at the K-point and Γ-point of BZ, respectively. Type-II band alignment in BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures prevent the recombination of electron–hole pairs, and hence are crucial for light harvesting and detection. Absorption spectra are investigated to understand the optical behavior of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures, where the lowest energy transitions are dominated by excitons. Furthermore, BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures are found to be potential photocatalysts for water splitting at pH = 0, and exhibit enhanced optical properties in the visible light zones.
1. Introduction
After the successful synthesis of graphene,1–4 great attention has been paid to other 2D materials, such as hexagonal boron nitrides (h-BN),5 blue and black phosphorene,6 transition metal dichalcogenides (TMDCs),7 silicene,8 germanene,9 MXenes,10 and Janus transition metal dichalcogenides (JTMDCs).11 Among these materials, MXenes (Mn+1Xn), synthesized by eliminating the A-layer from their bulk counterpart the MAX phase (Mn+1AXn, M refers to early transition metals, “A” represents the group of sp elements, “X” represents C or N atoms, and n is 1, 2, 3), has received wide research attention12 due to a wide range of applications in Li-ion batteries,13 catalysis,14 electrochemical capacitors15 and also in fuel cells.16 The M–X bond in the MAX crystals is stronger than the M–A bond, making it possible to etch “A” atoms between the Mn+1Xn layer.17 All the MXenes are metals, while appropriate surface termination (Mn+1XnTx, Tx denotes surface terminations, i.e. O, F, OH) makes them semiconductors.18Tuning the properties of 2D materials has led to a new field that assembles 2D materials (isolated) into hybrid heterostructures in a precisely controlled sequence of layer by layer stacking, called vdW heterostructures.19 It provides a versatile platform for exploring new phenomena and designing novel nanoelectronic devices.20,21 To date, a great deal of vdW heterostructures have been studied theoretically22–27 and perceived experimentally.28–31 These vdW heterostructures are also utilized to create electronic and optoelectronic devices with novel physical properties and applications.32–37
MXenes-based vdW heterostructures, such as MXenes–MXenes,38 MXene and nitrogen-doped graphene,39 MXenes–TMDCs,40 MXene–blue phosphorene,41 MXenes and B-doped graphene,42 have already been fabricated and investigated in detail. BSe, another 2D material, has been proposed and predicted to be thermally stable with indirect bandgap nature.43,44
Motivated by the fascinating optoelectronic and photocatalytic performance of MXenes with other monolayers in the form of vdW heterostructures, we have fabricated BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures. Indeed small lattice mismatch and the same hexagonal symmetry of the BSe and M2CO2 (M = Ti, Zr, Hf) monolayer allow the creation of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures. It is also surprising that there is no previous work on the BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures. We have investigated the structural and electronic properties, band alignments, average and planar electrostatic potentials, Bader charge analysis, optical and photocatalytic response of BSe, M2CO2 (M = Ti, Zr and Hf) monolayers and their vdW heterostructure. Our results show that BSe–M2CO2 (M = Ti, Zr) vdW heterostructures are a promising novel material for visible light photocatalysis, electronic and optoelectronic devices.
2. Computational details
We used DFT45 with empirical dispersion correction of Grimme46 and Perdew–Burke–Ernzerhof (PBE)47 functional in Vienna ab initio simulation package (VASP).48,49 In the first Brillouin zone, a Γ-point centered 6 × 6 × 1 Monkhorst–Pack k-point grid and 500 eV cutoff energy were used. A vacuum layer thickness of 25 Å is established to avoid the interaction of the adjacent layers of atoms. The geometric relaxations are carried out until we achieve the convergence criterion of 10−4 eV Å−1 (10−5 eV) for forces (energy). Commonly, the PBE functional underestimates the band gap values of semiconductors, therefore, we have also performed a computationally expensive HSE06 (Heyd–Scuseria–Ernzerhof)50 functional for the precise calculation of the electronic structure and band gap values.Ab initio molecular dynamic simulations (AIMD)51 are used to investigate the thermal stabilities of BSe–M2CO2 (M = Ti, Zr) vdW heterostructures. AIMD simulations are performed through the Nose thermostat algorithm at a temperature of 300 K for a total of 6 ps with a time interval of 1 fs.
Furthermore, we have solved the Bethe–Salpeter equation (BSE) in GW calculations using the Quantum-Espresso program package,52 to explore the optical spectra estimated by the imaginary part of the dielectric function (ε2(ω)) of the BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures.53–55
3. Results and discussion
Optimized lattice constant, bond length, bandgap values, effective mass of electrons and holes, work function and conduction and valence band edge potentials (ECB and EVB) of BSe and M2CO2 (M = Ti, Zr, Hf) monolayers in Table 1, are in agreement with ref. 56–58. Optimized geometry (top view) and electronic band structure (using PBE and HSE06 functional) are presented in Fig. 1, and show that both BSe and M2CO2 (M = Ti, Zr, Hf) monolayers are indirect bandgap semiconductors with CBM(VBM) at the M(Γ)-point of BZ. The calculated effective mass for both holes and electrons in Table 1, show that BSe and Hf2CO2 monolayers would have high carrier mobility.59 Difference in the work functions in Table 1, show that in the case of the interface of these materials, electrons will spontaneously flow from M2CO2 to the BSe monolayer, which is further explained in detail later in the vdW heterostructure of BSe and M2CO2 (M = Ti, Zr, Hf) monolayers.60 Furthermore, the imaginary part of the dielectric function in Fig. 1, shows that the first excitonic peak at 3.851 for BSe, 0.286 for Ti2CO2, 1.79 for Zr2CO2, and 2.416 eV for the Hf2CO2 monolayer, lies in the visible range of the spectrum, consistent with ref. 61–63. In the case of the photocatalytic response at pH = 0, BSe and Hf2CO2 cross both the conduction and valence band edge potentials, while Ti2CO2 and Zr2CO2 cross the valence band edge potential only and fail to cross the conduction band edge, in agreement with ref. 56, 59 and 64, hence showing the potential of these systems in electronic, optoelectronic and photocatalytic applications. The above discussed consistencies for BSe and M2CO2 (M = Ti, Zr, Hf) monolayers, show the authenticity of the present approach for the calculation of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures. and
and  ), work function (ϕ in eV) and conduction and valence band edge potentials (ECB and EVB in eV) for BSe monolayer and M2CO2 (M = Ti, Zr, Hf) MXenes
), work function (ϕ in eV) and conduction and valence band edge potentials (ECB and EVB in eV) for BSe monolayer and M2CO2 (M = Ti, Zr, Hf) MXenes
| Monolayers | BSe | Ti2CO2 | Zr2CO2 | Hf2CO2 |
| a | 3.26 | 3.01 | 3.31 | 3.27 |
| B–Se | 2.10 | — | — | — |
| M–O | — | 1.970 | 2.119 | 2.091 |
| M–C | — | 2.210 | 2.359 | 2.332 |
| Eg-PBE | 2.635 | 0.300 | 0.865 | 0.99 |
| Eg-HSE06 | 3.56 | 0.920 | 1.590 | 1.70 |
| d1ra07569a-t3 | 0.42 | 0.87 | 0.69 | 0.61 |
| d1ra07569a-t3 | 0.93 | 1.32 | 1.05 | 1.27 |
| Φ | 3.953 | 5.536 | 4.835 | 4.450 |
| ECB | −1.255 | 0.354 | 0.069 | −0.005 |
| EVB | 2.304 | 1.248 | 1.659 | 1.695 |
Lattice mismatch of BSe, with Ti2CO2 of 4.9%, with Zr2CO2 of 1.2% and with Hf2CO2 of 0.03%, are experimentally achievable65 and the same hexagonal symmetry realizes the fabrication of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures. The electronic band structure is very sensitive to layer stacking,66 therefore we have chosen five possible stacking configurations of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures, see Fig. 2. In stacking (a) the M(O) atom of M2CO2 is placed on top of the Se(B) atom of the BSe monolayer; in stacking (b) the M(C) atom of M2CO2 is placed on top of the B(Se) atom of the BSe monolayer; in stacking (c) the O(C) atom of M2CO2 is placed on top of the Se(B) atom of the BSe monolayer; in stacking (d) the O(M) atom of M2CO2 is placed on top of the Se(B) atom of the BSe monolayer; and in stacking (e) the O(M) atom of M2CO2 is placed on top of the (B) atom of the BSe monolayer, while the C is on a hexagonal site.
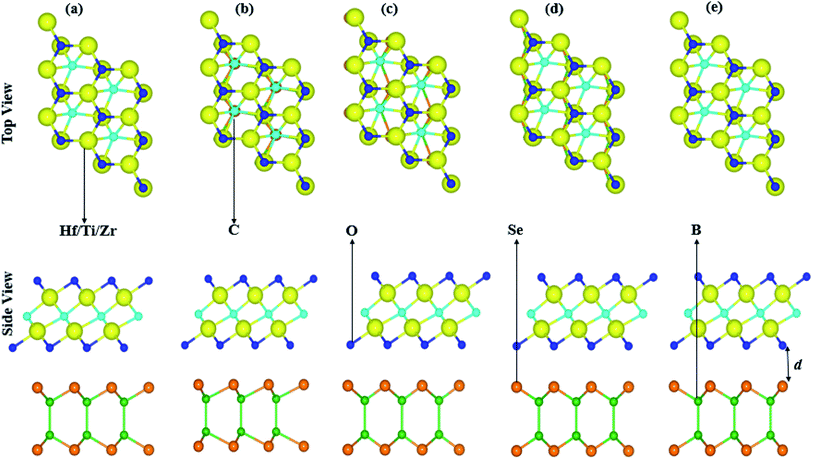 | ||
| Fig. 2 Possible stacking configurations of the BSe–M2CO2 (M = Ti, Zr, Hf) van der Waal heterostructures. | ||
Binding energy; Eb = EBSe–M2CO2 − EM2CO2 − EBSe, where EBSe–M2CO2 is the total energy of the BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructure, EM2CO2 is the total energy of the isolated M2CO2 (M = Ti, Zr, Hf) MXene, and EBSe is the total energy of the isolated BSe monolayer along with interlayer distance of the stacking as presented in Table 2. Smaller interlayer distance and binding energies represent the most stable stacking configuration, therefore, stacking (a) of the BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures is the most stable configuration. Obviously, negative binding energies show that the formation of all heterostructures are exothermic, see Table 2. These values are in the range of binding energies for other vdW heterostructures,67,68 hence suggest the possible experimental fabrication of BSe–M2CO2 vdW heterostructures. The calculated interlayer distance (see Table 2) also confirms weak vdW interactions in the stacked layers of these heterostructures. Optimized lattice constants of the most stable stacking configurations are presented in Table 3.
| Stacking | BSe–Ti2CO2 | BSe–Zr2CO2 | BSe–Hf2CO2 |
| Eb (a) | −0.429 | −0.395 | −0.297 |
| d | 3.33 | 3.32 | 3.33 |
| Eb (b) | −0.326 | −0.316 | −0.268 |
| d | 3.42 | 3.41 | 3.39 |
| Eb (c) | −0.331 | −0.337 | −0.284 |
| d | 3.39 | 3.38 | 3.35 |
| Eb (d) | −0.409 | −0.305 | −0.277 |
| d | 3.37 | 3.41 | 3.39 |
| Eb (e) | −0.398 | −0.327 | −0.281 |
| d | 3.46 | 3.39 | 3.36 |
 and
and  ), work function (ϕ in eV), potential difference (ΔV) conduction and valence band edges (EVB and ECB in eV) of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures
), work function (ϕ in eV), potential difference (ΔV) conduction and valence band edges (EVB and ECB in eV) of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures
To further verify the thermal stability of the stacking of (a) BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures, we have used the AIMD simulation. We have chosen a 3 × 3 supercell with top view, see Fig. 3. It is clear from the figure that after heating for 5 ps at 1 fs time steps at 300 K, the BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures show no broken bonds (remain stable), while the free energy oscillates slightly (see Fig. 3, middle row), which confirms the thermal stability of these systems at 300 K. Therefore, the stacking of the (a) BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures is the most stable structure configuration and will be further examined in detail.
Using both PBE and HSE06 functionals, we have calculated the electronic band structures of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures, see Fig. 4, while the calculated bandgap values are presented in Table 3. The electronic band structure shows that all the studied vdW heterostructures have an indirect band nature with the CBM and VBM at the Γ–K and Γ-point of BZ for BSe–Ti2CO2, (see Fig. 4(a)), while both BSe–Zr2CO2 and BSe–Hf2CO2 vdW heterostructures are indirect bandgap semiconductors with CBM at the K-point and VBM at the Γ-point of the first BZ (see Fig. 4(b) and (c)). In the case of the BSe–Ti2CO2 vdW heterostructure direct recombination of photogenerated electrons and holes hence play a crucial rule in optoelectronic devices.69 In the case of the BSe–Zr2CO2 and BSe–Hf2CO2 vdW heterostructures, the recombination of photogenerated electrons and holes is slow because firstly the CBM and VBM momenta align themselves and then recombination occurs, which is useful for laser applications.70–72 The variation in bandgap values (given in Table 3) and the band structures of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures from their parent monolayers, reveals the bandgap engineering making the vdW heterostructures.73 The contribution of the different atomic states to the Fermi level is further explored by investigating the partial density of states (PDOS), see Fig. 4 (b), (d) and (f). One can see that the CBM is mainly due to the d state of Ti/Zr/Hf atoms of the M2CO2 layer, while the VBM is due to the p state of the Se atom of BSe layer.
To verify the contribution of different atomic states in the VBM and CBM, and nature of the band structure for type-I and type-II, we have calculated the weighted band structure of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures, plotted in Fig. 5. One can clearly see that in the case of BSe–Ti2CO2 vdW heterostructures (Fig. 5(a)) at the Γ-point of BZ, the main contribution in the CBM is due to the Ti-dxy atom of Ti2CO2 monolayers while the VBM is due to the Se-pxy state of BSe monolayers, hence confirming type-II band alignment.74,75 In the case of the BSe–Zr2CO2 and BSe–Hf2CO2 vdW heterostructures (see Fig. 5(b) and (c), respectively) the main contribution in the CBM(VBM) is due to the Zr/Hf-dxy (Se-pxy) states of the Zr2CO2, Hf2CO2 (BSe) monolayers at the K(Γ)-point of BZ, which also shows type-II band alignment. The localization of the VBM and CBM from different layers are obtained without any external electric field, as the intrinsic electric field induces bond bending in making the vdW heterostructures.76,77 This induced field drive photogenerated electrons and holes in different directions. Type-II band alignment is an effective tool to enhance electron–holes pairs which reduce the recombination time, applicable for light harvesting and detection.74,75 The spontaneous apprehension about the charge transfer is obtained from the deportation charge density (DCD) isosurface, presented in Fig. 5(d–f) for BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures. In Fig. 5(d–f) the cyan(yellow) color shows the charge electrons depletion(accumulation), hence confirming that charge is transferred from M2CO2 (M = Ti, Zr, Hf) to BSe monolayers at the interface of the BSe–M2CO2 vdW heterostructures, which leads to p-doping in Ti2CO2, Zr2CO2 and Hf2CO2, and n-doping in the BSe monolayer. For further verification and quantification of charge transfer we have investigated the Bader charge analysis, which shows that the charge of about 0.17, 0.09 and 0.11 e/unitcell is transferred from the Ti2CO2, Zr2CO2 and Hf2CO2 to the BSe monolayer, respectively.78 This transfer of charge confirms that due to long range vdW forces, the interlayer bonding of Ti2CO2, Zr2CO2, Hf2CO2 and BSe monolayers can be weak and diminishes with increasing bond length.
Furthermore, we have verified the transfer of charge and potential difference by calculating the average and planar electrostatic potential difference along the z-axis, see Fig. 6. One can easily see that the BSe monolayer has a deeper potential then Ti2CO2, Zr2CO2 and Hf2CO2 monolayers in BSe–M2CO2 vdW heterostructures (see Fig. 6), confirming the transfer of charge from Ti2CO2, Zr2CO2 and Hf2CO2 to the BSe layer. Also, the potential drop (DV) across the vdW heterostructures, given in Table 3, facilitates the separation of electrons and holes at the interface. Making vdW heterostructures may effect the work function, which leads to enhanced electronic properties of the vdW heterostructures. Therefore, we have calculated the work function of monolayers and their vdW heterostructures, as presented in Table 1 and 3. One can easily see that the work function of vdW heterostructures is almost the average of the corresponding monolayers, efficient for charge transfer.
 | ||
| Fig. 6 Average and planar electrostatic potential of (a and b) BAse–Ti2CO2, (c and d) BSe–Zr2CO2 and (e and f) BSe–Hf2CO2. The work function (ϕ) and potential drop (ΔV) are highlighted. | ||
Furthermore, we have calculated the effective mass of electrons and holes in the BSe–M2CO2 vdW heterostructures. Smaller effective mass leads to higher carrier mobility which is useful for high performance nanoelectronic devices.79 We used parabolic fitting for the VBM and CBM and investigated the effective mass of electrons and holes of the BSe–M2CO2 vdW heterostructures. The value for effective mass of the holes and electrons are given in Table 3. One can see that the effective mass of vdW heterostructures (for holes and electrons) is smaller than that of the corresponding monolayers in Table 1, hence are suitable for application in high-performance nanoelectronic devices.
We have also calculated the optical performance in terms of imaginary parts of the dielectric function (ε2(ω)) of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures as a function of photon energy, given in Fig. 7. One can see that optical transitions are dominated by excitons at 2.59 eV for Ti2CO2, at 2.27 eV for Zr2CO2 and at 2.43 eV for Hf2CO2. The calculated exciton binding energies are 0.77, 0.048 and 0.143, respectively (see Fig. 7). All these BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures show substantial absorption in visible and UV regions of the spectrum. This can be attributed to the fact that the charge transfer and interlayer coupling, which can result in the overlap of electronic states in the valence bands of the heterostructure, and which enhances the optical absorption (see Fig. 1 and 7).80–82
We have also investigated the photocatalytic83–86 properties of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures using the Mulliken electronegativity.87,88 Appropriate bandgap size, valence and conduction band edges must straddle the redox potentials of water, as reported in our previous work89 for use in the water splitting reaction. The standard water redox potentials are −4.50 eV for the reduction (H+/H2) and −5.73 eV for the oxidation (H2O/O2).90 The calculated band edge potentials EVBM and ECBM of the heterostructures by the HSE06 functional are summarized in Table 3 and presented in Fig. 8. Valence band edge potential and conduction band edge potential, (EVBM and ECBM) for BSe–Hf2CO2 and BSe–Zr2CO2 vdW heterostructures are higher than that of H+/H2 and H2O/O2. These results signify that, BSe–Hf2CO2 and BSe–Zr2CO2 vdW heterostructures can oxidize H2O/O2 and reduce H+/H2,90 which is suitable for the production of clean and renewable energy equipment applications.91 Although, the Zr2CO2 monolayer fails to oxidize water (see Fig. 1 and Table 1), the BSe–Zr2CO2 vdW heterostructure shows a good response to water redox potential, hence making the vdW heterostructure suitable for the production of clean and renewable energy device applications.91 Similar to the corresponding monolayer, in the case of BSe–Ti2CO2, the EVB(ECB) cross(fail to cross) the reduction level. All these findings demonstrate that the BSe–M2CO2 heterostructures can be considered as potential photocatalysts for water splitting and provide theoretical guidance for designing high-performance nano-electronic and optoelectronic devices based on the BSe–M2CO2 heterostructures.92–94
4. Conclusion
In summery, using first principles DFT calculations, we have investigated the electronic band structure, optical and photocatalytic response of BSe, M2CO2 (M = Ti, Zr, Hf) monolayers and their corresponding BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures. The calculated lattice parameters, electronic band structure, bandgap values and valence and conduction band edge potentials of BSe and M2CO2 (M = Ti, Zr, Hf) monolayers are in good agreement with previous available data, showing the authenticity of the present approach for the calculations of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures. Based on the binding energy and interlayer distance calculations, stacking (a) of the five different stacking of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures is the most stable stacking configuration. Furthermore, AIMD simulations also show that stacking (a) for all studied systems, is thermally stable at 300 K. Surprisingly, in contrast to the parent monolayers, BSe–Ti2CO2 (BSe–Zr2CO2 and BSe–Hf2CO2) vdW heterostructures are direct (indirect) band gap semiconductor(s). All studied vdW heterostructures have type-II band alignment, hence play a major role in light harvesting and detection. Bader charge analysis shows transfer of charge from M2CO2 (M = Ti, Zr, Hf) to the BSe layer, hence N(P)-type doping is achieved in the M2CO2(BSe) monolayer at the interface of BSe–M2CO2 vdW heterostructures. The imaginary part of the dielectric function is also investigated to understand the optical absorption of BSe–M2CO2 (M = Ti, Zr, Hf) vdW heterostructures, where the lowest energy transitions are dominated by excitons. The calculated photocatalytic response signifying that BSe–Zr2CO2 and BSe–Hf2CO2 vdW heterostructures can oxidized H2O/O2 and reduce H+/H2, while the Zr2CO2 monolayer fails to oxidize water, hence making BSe–M2CO2 vdW heterostructures viable for the production of clean and renewable energy device applications. Similar to the corresponding monolayer, in the case of BSe–Ti2CO2, the EVBM(ECBM) cross(fail to cross) the reduction level.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors thank the Center for Computational Materials Science, the University of Malakand Chakdara, Pakistan, for their computing support.References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS PubMed.
- K. S. Novoselov, V. I. Faľko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, Nature, 2012, 490, 192 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451 CrossRef CAS PubMed.
- Y. F. Li, Z. Zhou, S. B. Zhang and Z. F. Chen, J. Am. Chem. Soc., 2008, 130, 16739 CrossRef CAS PubMed.
- J. N. Coleman, M. Lotya, A. O. Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H.-Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568 CrossRef CAS PubMed.
- P. Vogt, P. D. Padova, C. Quaresima, J. Avila, F. Frantzeskakis, M. C. Asensio, A. Resta, B. Ealet and G. L. Lay, Phys. Rev. Lett., 2012, 108, 155501 CrossRef PubMed.
- Z. Ni, Q. Liu, K. Tang, J. Zheng, J. Zhou, R. Qin, Z. Gao, D. Yu and J. Lu, Nano Lett., 2012, 12, 113 CrossRef CAS PubMed.
- I. R. Shein and A. L. Ivanovskii, Micro Nano Lett., 2013, 8, 59 CrossRef CAS.
- J. Zhang, S. Jia, I. Kholmanov, L. Dong, D. Er, W. Chen, H. Guo, Z. Jin, V. B. Shenoy, L. Shi and J. Lou, ACS Nano, 2017, 11, 8192 CrossRef CAS PubMed.
- A. L. Ivanovskii and A. N. Enyashin, Russ. Chem. Rev., 2013, 82, 735 CrossRef.
- O. Mashtalir, M. Naguib, V. N. Mochalin, Y. D. Agnese, M. Heon, M. W. Barsoum and Y. Gogotsi, Nat. Commun., 2013, 4, 1716 CrossRef PubMed.
- X. Xie, S. Chen, W. Ding, Y. Nie and Z. Wei, Chem. Commun., 2013, 49, 10112 RSC.
- M. R. Lukatskaya, O. Mashtalir, C. E Ren, Y. D. Agnese, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Science, 2013, 341, 1502 CrossRef CAS PubMed.
- N.H. A. Junaidi, W. Y. Wong, K. S. Loh, S. Rahman and W. R. W. Daud, Int. J. Energy Res., 2021, 45, 15760 CrossRef.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992 CrossRef CAS PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248 CrossRef CAS PubMed.
- L. A. Ponomarenko, A. K. Geim, A. A. Zhukov, R. Jalil, S. V. Morozov, K. S. Novoselov, I. V. Grigorieva, E. H. Hill, V. V. Cheianov, V. I. Fal’ko, K. Watanabe, T. Taniguchi and R. V. Gorbachev, Nat. Phys., 2011, 7, 958 Search PubMed.
- A. K. Geim and I. V. Grigorieva, Nature, 2013, 499, 419 CrossRef CAS PubMed.
- Y. Liu, N. O. Weiss, X. Duan, H.-C. Cheng, Y. Huang and X. Duan, Nat. Rev. Mater., 2016, 1, 16042 CrossRef CAS.
- B. Amin, N. Singh and U. Schwingenschlgl, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 075439 CrossRef.
- M. Sun, J.-P. Chou, J. Yu and W. Tang, Phys. Chem. Chem. Phys., 2017, 19, 17324 RSC.
- D. D. Vo, T. V. Vu, N. V. Hieu, N. N. Hieu, H. V. Phuc, N. T. T. Binh, L. T. T. Phuong, M. Idrees, B. Amin and C. V. Nguyen, Phys. Chem. Chem. Phys., 2019, 21, 25849 RSC.
- K. D. Pham, L. G. Bach, B. Amin, M. Idrees, N. N. Hieu, H. V. Phuc, H. D. Bui and C. V. Nguyen, J. Appl. Phys., 2019, 125, 225304 CrossRef.
- T. V. Vu, N. V. Hieu, H. V. Phuc, N. N. Hieu, H. D. Bui, M. Idrees, B. Amin and C. V. Nguyen, Appl. Surf. Sci., 2020, 507, 145036 CrossRef CAS.
- H. T. T. Nguyen, M. M. Obeid, A. Bafekry, M. Idrees, T. V. Vu, H. V. Phuc, N. N. Hieu, L. T. Hoa, B. Amin and C. V. Nguyen, Phys. Rev. B, 2020, 102, 075414 CrossRef CAS.
- X. Liu and M. C. Hersam, Adv. Mater., 2018, 30, 1801586 CrossRef PubMed.
- J. Wang, Z. Li, H. Chen, G. Deng and X. Niu, Nano-Micro Lett., 2019, 11, 48 CrossRef PubMed.
- D. Pashnev, V. V. Korotyeyev, J. Jorudas, T. Kaplas, V. Janonis, A. Urbanowicz and I. Kašalynasa, Appl. Phys. Lett., 2020, 117, 162101 CrossRef CAS.
- J. Yu, E. Han, M. A. Hossain, K. Watanabe, T. Taniguchi, E. Ertekin, A. Zande and P. Y. Huang, Adv. Mater., 2021, 33, 2007269 CrossRef CAS PubMed.
- Z. Wu, Y. Zheng, S. H. Zheng, S. Wang, C. L. Sun, K. Parvez, T. Ikeda, X. Bao, K. Müllen and X. Feng, Adv. Mater., 2016, 29, 1602960 CrossRef PubMed.
- M. S. Long, E. F. Liu, P. Wang, A. Y. Gao, H. Xia, W. Luo, B. G. Wang, J. W. Zeng, Y. J. Fu, K. Xu, W. Zhou, Y. Y. Lv, S. H. Yao, M. H. Lu, Y. F. Chen, Z. H. Ni, Y. M. You, X. A. Zhang, S. Q. Qin, Y. Shi, W. D. Hu, D. Y. Xing and F. Miao, Nano Lett., 2016, 15, 2254 CrossRef PubMed.
- D. Li, X. J. Wang, Q. C. Zhang, L. P. Zou, X. F. Xu and Z. X. Zhang, Adv. Funct. Mater., 2015, 25, 7362 Search PubMed.
- X. H. Li, B. J. Wang, X. L. Cai, L. W. Zhang, G. D. Wang and S. H. Ke, RSC Adv., 2017, 7, 28393 RSC.
- X. H. Li, B. J. Wang, X. L. Cai, W. Y. Yu, L. W. Zhang, G. D. Wang and S. H. Ke, RSC Adv., 2017, 7, 44394 RSC.
- Q. Zhang, X. Xiao, R. Zhao, D. Lv, G. Xu, Z. Lu, L. Sun, S. Lin, X. Gao, J. Zhou, C. Jin, F. Ding and L. Jiao, Angew. Chem., Int. Ed., 2015, 54, 8957 CrossRef CAS PubMed.
- J. Cao, Z. Sun, J. Li, Y. Zhu, Z. Yuan, Y. Zhang, D. Li, L. Wang and W. Han, ACS Nano, 2021, 15, 3423 CrossRef CAS PubMed.
- B. Shen, H. Huang, H. Liu, Q. Jiang and H. He, Int. J. Hydrogen Energy, 2021, 46, 29984 CrossRef CAS.
- B. Zhu, F. Zhang, J. Qiu, X. Chen, K. Zheng, H. Guo, G. Yu and J. Bao, Mater. Sci. Semicond. Process., 2021, 133, 105947 CrossRef CAS.
- Z. Guo, N. Miao, J. Zhou, B. Sa and Z. Sun, J. Mater. Chem. C, 2017, 5, 978 RSC.
- P. Zhao, X. Qin, H. Li, K. Qu and R. Li, J. Solid State Chem., 2021, 302, 122418 CrossRef CAS.
- S. Demirci, N. Avazli, E. Durgun and S. Cahangirov, Phys. Rev. B, 2017, 95, 115409 CrossRef.
- B. Mortazavi and T. Rabczuk, Energies, 2018, 11, 1573 CrossRef.
- W. Kohn and L. J. Sham, Phys. Rev., 1965, 140, A1133 CrossRef.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558 CrossRef CAS PubMed.
- P. E. Blochl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef PubMed.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2006, 124, 219906 CrossRef.
- R. Yuan, J. A. Napoli, C. Yan, O. Marsalek, T. E. Markland and M. D. Fayer, ACS Cent. Sci., 2019, 5, 1269 CrossRef CAS PubMed.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. D. Corso, S. D. Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. M. Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed.
- M. Shishkin and G. Kresse, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 035101 CrossRef.
- M. Rohlfing and S. G. Louie, Phys. Rev. Lett., 1998, 81, 2312 CrossRef CAS.
- E. Mosconi, P. Umari and F. D. Angelis, Phys. Chem. Chem. Phys., 2016, 8, 27158–27164 RSC.
- S. A. Khan, B. Amin, Li-Y. Gan and I. Ahmad, Phys. Chem. Chem. Phys., 2017, 19, 14738 RSC.
- P. Mishra, D. Singh, Y. Sonvane and R. Ahuja, Sustainable Energy Fuels, 2020, 4, 2363 RSC.
- Y. Zhang, R. Xiong, B. Sa, J. Zhou and Z. Sun, Sustainable Energy Fuels, 2021, 5, 135 RSC.
- Z. Guo, J. Zhou, L. Zhu and Z. Sun, J. Mater. Chem. A, 2016, 4, 11446 RSC.
- C. V. Nguyen, M. Idrees, H. V. Phuc, N. N. Hieu, N. T. T. Binh, B. Amin and T. V. Vu, Phys. Rev. B, 2020, 101, 235419 CrossRef CAS.
- S. S. Li, X.-H. Li, R.-Z. Zhang and H.-L. Cui, Int. J. Quantum Chem., 2020, 120, e26365 CAS.
- A. Mostafaei, E. Faizabadi and E. Semiromi, Phys. E, 2019, 114, 113559 CrossRef CAS.
- K. Pham, N. Hieu, L. Bui, I. Ershov, N. Hieu, H. Phuc, B. Hoi, L. Phuong, L. Duc, M. Idrees, B. Amin and C. Nguyen, Mater. Res. Express, 2019, 6, 065910 CrossRef CAS.
- X. Zhang, Z. Zhang, J. Li, X. Zhao, D. Wu and Z. Zhou, J. Mater. Chem. A, 2017, 5, 12899 RSC.
- M. Liao, P. Nicolini, L. Du, J. Yuan, S. Wang, H. Yu, J. Tang, P. Cheng, K. Watanabe, T. Taniguchi, L. Gu, V. Claerbout, A. Silva, D. Kramer, T. Polcar, R. Yang, D. Shi and G. Zhang, Nat. Mater., 2021 DOI:10.1038/s41563-021-01058-4.
- N. Kharche, Y. Zhou, K. P. OBrien, S. Kar and S. K. Nayak, ACS Nano, 2011, 5, 6096 CrossRef CAS PubMed.
- H. U. Din, M. Idrees, A. Albar, M. Shafiq, I. Ahmad, C. V. Nguyen and B. Amin, Phys. Rev. B, 2019, 100, 165425 CrossRef CAS.
- M. Idrees, H. U. Din, R. Ali, G. Rehman, T. Hussain, C. V Nguyen, I. Ahmad and B. Amin, Phys. Chem. Chem. Phys., 2019, 21, 18612 RSC.
- H. Terrones, F. Lopez-Urias and M. Terrones, Sci. Rep., 2013, 3, 1549 CrossRef PubMed.
- V. D. Ganesan, J. Linghu, C. Zhang, Y. P. Feng and L. Shen, Appl. Phys. Lett., 2016, 108, 122105 CrossRef.
- M. Tangi, P. Mishra, M.-Y. Li, M. K. Shakfa, D. H. Anjum, M. N. Hedhili, T. K. Ng, L.-J. Li and B. S. Ooi, Appl. Phys. Lett., 2017, 111, 092104 CrossRef.
- M. Z. Bellus, M. Li, S. D. Lane, F. Ceballos, Q. Cui, X. C. Zengand and H. Zhao, Nanoscale Horiz., 2017, 2, 31 RSC.
- B. J. Wang, X.-H. Li, R. Zhao, X. Cai, W.-Y. Yu, W.-B. Li, Z.-S. Liu, L.-W. Zhanga and S.-H. Ke, J. Mater. Chem. A, 2018, 6, 8923 RSC.
- J. Kang, S. Tongay, J. Zhou, J. Li and J. Wu, Appl. Phys. Lett., 2013, 102, 012111 CrossRef.
- X. L. Wei, H. Zhang, G. C. Guo, X. B. Li, W. M. Lau and L. M. Liu, J. Mater. Chem. A, 2014, 2, 2101 RSC.
- T. P. Kaloni, G. Schreckenbach and M. S. Freund, J. Phys. Chem. C, 2014, 118, 23361 CrossRef CAS.
- T. P. Kaloni, G. Schreckenbach and M. S. Freund, J. Phys. Chem. C, 2015, 119, 3979 CrossRef CAS.
- Y. Q. Cai, G. Zhang and Y. W. Zhang, J. Phys. Chem. C, 2008, 119, 13929 CrossRef.
- Y. Liu, X. Duan, Y. Huang and X. Duan, Chem. Soc. Rev., 2018, 47, 6388 RSC.
- X. H. Niu, Y. H. Li, H. B. Shu, X. J. Yao and J. L. Wang, J. Phys. Chem. C, 2017, 121, 3648 CrossRef CAS.
- J. M. Liao, B. S. Sa, J. Zhou, R. Ahuja and Z. M. Sun, J. Phys. Chem. C, 2014, 118, 17594 CrossRef CAS.
- F. Wu, Y. Liu, G. Yu, D. Shen, Y. Wang and E. Kan, J. Phys. Chem. Lett., 2012, 3, 3330 CrossRef CAS.
- Y. H. Chiu, T. H. Lai, M. Y. Kuo, P. Y. Hsieh and Y. J. Hsu, APL Mater., 2019, 7(8), 080901 CrossRef.
- Y. H. Chiu, T. F. M. Chang, C. Y. Chen, M. Sone and J. Hsu, Catalysts, 2019, 9(5), 430 CrossRef CAS.
- P. Y. Hsieh, J. Y. Wu, T. F. Chang, C. Y. Chen, M. Sone and Y. J. Hsu, Arabian J. Chem., 2020, 13(11), 8372–8387 CrossRef CAS.
- M. J. Fang, C. W. Tsao and Y. J. Hsu, J. Phys. D: Appl. Phys., 2020, 53(14), 143001 CrossRef CAS.
- J. J. Liu, X. L. Fu, S. F. Chen and Y. F. Zhu, Appl. Phys. Lett., 2011, 99, 191903 CrossRef.
- H. L. Zhuang and R. G. Hennig, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 115314 CrossRef.
- M. Idrees, C. Nguyen, H. Bui, I. Ahmad and B. Amin, Phys. Chem. Chem. Phys., 2020, 22, 20704 RSC.
- X. Hong, J. Kim, S. F. Shi, Y. Zhang, C. Jin, Y. Sun, S. Tongay, J. Wu, Y. Zhang and F. Wang, Nat. Nanotechnol., 2014, 9, 682 CrossRef CAS PubMed.
- P. Rivera, J. R. Schaibley, A. M. Jones, J. S. Ross, S. Wu, G. Aivazian, P. Klement, N. J. Ghimire, J. Yan, D. G. Mandrus, W. Yao and X. Xu, Nat. Commun., 2015, 6, 6242 CrossRef CAS PubMed.
- Y. A. Chen, Y. T. Wang, H. S. Moon, K. Yong and J. Hsu, RSC Adv., 2021, 11(20), 12288–12305 RSC.
- H. Lai, K. I. Katsumata and Y. J. Hsu, Nanophotonics, 2021, 10(2), 777–795 CrossRef.
- C. W. Tsao, M. J. Fang and J. Hsu, Coord. Chem. Rev., 2021, 438, 213876 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |








