Open Access Article
Open Access ArticleA thiadiazolopyridine-functionalized Zr(IV)-based metal–organic framework for enhanced photocatalytic synthesis of tetrahydroquinolines under visible light†
Changyun Li‡
a,
He Zhang‡a,
Xuefei Wanga,
Qiu-Yan Li *a,
Xinsheng Zhaob and
Xiao-Jun Wang
*a,
Xinsheng Zhaob and
Xiao-Jun Wang *a
*a
aJiangsu Key Laboratory of Green Synthetic Chemistry for Functional Materials, School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou 221116, P. R. China
bSchool of Physics and Electronic Engineering, Jiangsu Normal University, Xuzhou 221116, P. R. China. E-mail: qyli@jsnu.edu.cn; xjwang@jsnu.edu.cn
First published on 10th January 2022
Abstract
Metal–organic framework (MOF) materials provide a versatile and promising platform for constructing heterogeneous photocatalysts with applications in organic transformations. One of the methods for enhancing MOFs' performance in photocatalysis relies on the elaborate design and functionalization of organic linkers. Here we reported a photoactive thiadiazolopyridine (TDP) moiety functionalized UiO-68 isoreticular Zr(IV)-based MOF (denoted as UiO-68-TDP) that was synthesized by the de novo approach of mixed dicarboxylate struts. Under blue LED irradiation and in an open air atmosphere, MOF UiO-68-TDP exhibited a largely higher photocatalytic activity for the synthesis of tetrahydroquinolines by oxidative annulation reaction between N,N-dimethylanilines and maleimides, in comparison to the benzothiadiazole decorated analogue MOF. Besides, UiO-68-TDP can be reused at least three times without significant loss of its photocatalytic activity and its framework was well maintained after these cycles. Furthermore, the related mechanism involving reactive oxygen species was properly proposed.
Introduction
Visible light is regarded as an abundant, inexpensive, readily available and renewable source of clean energy. Its application in synthesis of value-added organic compounds has received great attention from chemists over the past years.1–5 However, since a large proportion of simple organic molecules cannot absorb visible light, photocatalysts such as metal complexes and organic dyes are typically used to efficiently promote the photochemical transformation.6–9The tetrahydroquinoline (THQ) motif is an important structural scaffold of many natural products and medicinal agents that have demonstrated a variety of biological activities.10,11 Due to their widespread existence in natural products and pharmaceutical agents, a number of methods have been developed for the construction of THQ skeleton.12–14 Among them, the photochemical approach mediated by visible light offered a more sustainable methodology in this process.15–18 However, most of these photochemical reactions were conducted in homogenous systems. Thus, it is problematic to recycle these photocatalysts for reuse, especially considering the high cost of precious metal complexes. The development of heterogeneous photocatalysts that are free of noble metals as well as possessing outstanding catalytic activity is highly desirable. In this regard, there are only a handful of reported examples to date.19–21 For example, Zhang et al. developed a series of benzobisthiadiazole conjugated nanoporous polymers for the synthesis of THQ derivatives under visible light.19 Modified TiO2 semiconductors were also employed as efficient photocatalysts in this photochemical reaction.20,21 However, the reported heterogeneous photocatalyst based materials are still limited and suffered from some drawbacks, such as tedious preparative procedure and low surface area. It is highly demanded to exploit more different materials toward this end.
Metal–organic frameworks (MOFs), that are assembled by the coordination of metal centers with organic linkers, have emerged as a newly promising type of photoactive materials for photocatalyzing organic reactions on account of their unique structural characteristics.22–25 In sharp contrast to metal oxide semiconductive photocatalysts, the hybrid and modular inherent nature of MOF materials allows their properties to be readily tailored by the metal nodes, organic linkers as well as the cavity in MOFs, thus resulting in the remarkable enhancement of photocatalytic activities. Our research group has recently devoted many efforts into the modulation of MOFs photocatalytic performance starting from the design and synthesis of photoactive organic linkers; subsequently, a series of functional and robust Zr(IV) MOFs with UiO-isoreticular topology were constructed by using these linkers and their applications in light-induced organic reactions were further explored.26–30 To the best of our knowledge, there is no report that MOFs photocatalyzed THQ synthesis until now.
Along our research line in functionalization of organic linkers, here we designed and synthesized a photoactive thiadiazolopyridine (TDP) moiety31–33 conjugated terphenyldicarboxylate linker (denoted as H2-TDP), which was integrated into the porous and robust UiO-68 isoreticular Zr-MOF (denoted as UiO-68-TDP, Scheme 1) by a mix-and-match approach of mixed dicarboxylate struts. In the open air atmosphere and under blue LEDs irradiation, UiO-68-TDP can efficiently photocatalyze the oxidative cyclization synthesis of THQs from N,N-dimethylanilines and maleimides. And it demonstrated a remarkably higher photocatalytic activity for this reaction than its analogue MOF UiO-68-BTD containing the benzothiadiazole (BTD) unit.
Results and discussion
As described in Scheme 2, the TDP-conjugated terphenyldicarboxylate linker (H2-TDP) can be readily synthesized by two step reactions. The well-known Suzuki coupling reaction of 4,7-dibromo-[1,2,5]thiadiazolo[3,4-c]pyridine (1) and methyl 4-boronobenzoate catalyzed by Pd(dppf)Cl2 and Pd(PPh3)4 in the presence of Cs2CO3 and CsF gave rise to methyl ester precursor compound 2, which further hydrolyzed by KOH to give the target linker H2-TDP in a high yield. Due to limited solubility of H2-TDP, the mix-and-match synthetic strategy was used to incorporate it into the porous and robust UiO-68 framework. Specifically, a combination of organic linkers H2-TDP and H2-DMT (in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio) with the same ligand length was reacted with ZrCl4 under the assistance of HAc as a modulator in N,N-dimethylformamide (DMF) at 90 °C for 48 h to generate MOF UiO-68-TDP (Scheme 1).
3 molar ratio) with the same ligand length was reacted with ZrCl4 under the assistance of HAc as a modulator in N,N-dimethylformamide (DMF) at 90 °C for 48 h to generate MOF UiO-68-TDP (Scheme 1).
As expected, powder X-ray diffraction (XRD) of UiO-68-TDP was investigated to reveal its isostructural framework as the same with the parent MOF UiO-68 and its highly crystalline nature (Fig. 1a). Additionally, the porosity of UiO-68-TDP was examined by nitrogen sorption measurement at 77 K (Fig. 1b), indicating a classic type I reversible isotherm with BET surface area of 3060 m2 g−1. The pore size distribution was calculated as ∼1.6 nm by using nonlocal density functional theory (NLDFT). Therefore, the high porosity and big pore width of UiO-68-TDP can facilitate adsorption and diffusion of substrates in the framework for achieving an efficient heterogeneous catalysis.
 | ||
| Fig. 1 Powder XRD patterns (a) and nitrogen sorption isotherm (b) for UiO-68-TDP (inset: the pore size distribution calculated from NLDFT). | ||
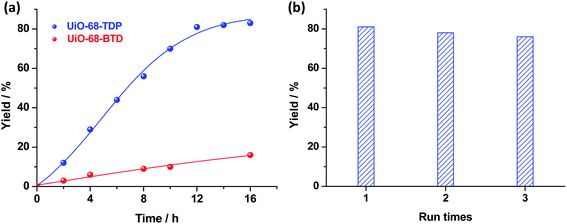
In order to evaluate the photocatalytic activity of UiO-68-TDP, the oxidative annulation reaction of N,N-dimethylaniline (1a, 0.2 mmol) and N-(4-methylphenyl)maleimide (2a, 0.1 mmol) was employed as a model reaction. As shown in Table 1, the reaction mixture of 1a and 2a containing a catalytic amount of UiO-68-TDP in CH3CN was irradiated by blue LEDs in an open air atmosphere at room temperature. The desired annulation product of THQ derivative 3a can be optimized and obtained in good-to-excellent yield after 12 h irradiation (entries 1–3). The very low yield was achieved in the absence of UiO-68-TDP (entry 4), confirming its photocatalytically active role in this reaction. On the other hand, when the photocatalyst was changed from UiO-68-TDP into its analogue MOF UiO-68-BTD containing benzothiadiazole moiety (Fig. S4 in ESI†), the product yield of 3a was largely decreased (entry 5). Furthermore, we monitored and compared this photocatalytic progress by using 1H NMR analysis. As shown in Fig. 2a, it is clear that UiO-68-TDP exhibited a largely higher photocatalytic activity for this light-induced oxidative annulation reaction in contrast to UiO-68-BTD. This result validates the critical role of photoactive organic linker in determining MOF's photocatalytic performance. Besides, the reusability of UiO-68-TDP as a heterogeneous photocatalyst was investigated. The MOF photocatalyst can be readily recovered from the reaction mixture by centrifugation and reused at least three times without obvious loss of activity (Fig. 2b). The powder XRD pattern of UiO-68-TDP after three photocatalyzed cycles revealed the good maintenance of its crystalline structure and framework (Fig. 1a), indicative of the high stability of Zr-based UiO frameworks.
| Entry | Conditions | Light | Additive | Yieldb |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.1 mmol) in CH3CN (1 mL) under an air atmosphere at room temperature for 12 h, blue LEDs (λmax = 450 nm, 3 W).b Yield was determined by 1H NMR analysis with 1-methyl-2,4-dinitrobenzene as an internal standard.c The reaction time was 16 h.d No light.e No oxygen.f p-Benzoquinone (BQ) as the superoxide scavenger.g NaN3 as the single oxygen 1O2 scavenger. | ||||
| 1 | UiO-68-TDP, 3 mg | + | − | 62% |
| 2 | UiO-68-TDP, 4 mg | + | − | 82% |
| 3 | UiO-68-TDP, 5 mg | + | − | 82% |
| 4 | No photocatalyst | + | − | 12% |
| 5c | UiO-68-BTD, 4 mg | + | − | 16% |
| 6d | UiO-68-TDP, 4 mg | − | − | Trace |
| 7e | UiO-68-TDP, 4 mg | + | − | Trace |
| 8f | UiO-68-TDP, 4 mg | + | BQ | 8% |
| 9g | UiO-68-TDP, 4 mg | + | NaN3 | 16% |
To gain insights into the photochemical reaction mechanism, several control experiments were conducted as shown in Table 1 (entries 6–9). These results indicated that each component, including photocatalyst UiO-68-TDP, light, and oxygen, is essential for the effective progress in the photo-induced reaction. Additionally, p-benzoquinone (BQ) and NaN3, as a superoxide radical anion O2˙− scavenger and a singlet oxygen 1O2 scavenger, were added into the reaction mixture, respectively. The product yield was greatly decreased (entries 8 and 9), suggesting that both of active oxygen species (O2˙−and 1O2) were involved in this photo-oxidative annulation reaction. Furthermore, electron paramagnetic resonance (EPR) measurements were employed to validate these oxygen active species by using DMPO and TEMP as spin-trapping reagents for O2˙−and 1O2 after blue LEDs light irradiation. As shown in Fig. 3, no signal was observed for the mixture of UiO-68-TDP with TEMP or DMPO in the dark condition. While the typically characteristic of 1O2 and O2˙− corresponding TEMPO and DMPO–OOH were clearly detected in EPR spectra (Fig. 3a and c), these results suggest the effective energy transfer and/or electron transfer between excited UiO-68-TDP species to molecular oxygen during the photocatalytic process.
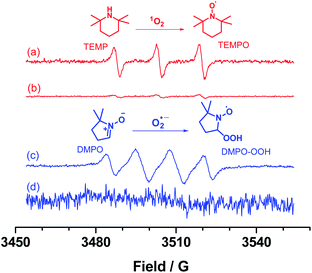 | ||
| Fig. 3 EPR spectra of a mixture of UiO-68-TDP in CH3CN with TEMP upon light irradiation (a) and in the dark (b) as well as DMPO upon light irradiation (c) and in the dark (d). | ||
Based on these observations in the above control experiments and reported literatures, we proposed a plausible reaction mechanism for the formation of THQ derivative 3a, as illustrated in Scheme 3. Upon light illumination, MOF UiO-68-TDP as photocatalyst (PC) was excited from the ground state to its excited state (PC*), which can activate molecular oxygen O2 through energy transfer or single electron transfer (SET) process, generating two reactive oxygen species of 1O2 and O2˙−. Simultaneously, the radical cation 1a˙+ was produced in the oxidation of 1a by PC* and 1O2 through a SET process, which was deprotonated by the superoxide anion radical to give α-aminoalkyl radical 4. Then, the radical addition of 4 to maleimide 2a formed radical 5, along with the subsequent intramolecular cyclization leading to intermediate 6. At last, the electron and proton separation from 6 mediated by active oxygen species gave birth to the desired product 3a.
 | ||
| Scheme 3 Proposed mechanism for the photocatalytic oxidative annulation reaction by MOF UiO-68-TDP (photocatalyst, PC). | ||
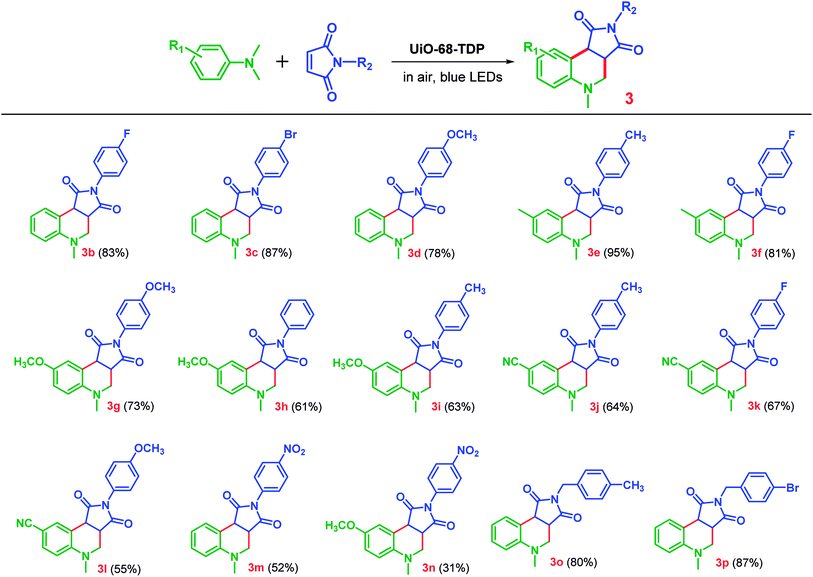
Furthermore, the scope of this photocatalytic reaction was investigated to explore its broad applications by varying substrates with both electron-donating and electron-withdrawing groups under the same reaction conditions using UiO-68-TDP as photocatalyst. As shown in Table 2, most of reactions can afford modest to good yields. A range of N,N-dimethylanilines with methyl, methoxy and cyano groups on the phenyl ring can react with various maleimides to afford the corresponding THQ derivatives in good to excellent yields (3b–3l, 55–95%), while maleimides with a nitro group gave a relative poor yield (3m–3n, 31–52%). Besides, a very good yield can be obtained by using benzyl substituted maleimides (3o–3p, 80–87%).
| a Reaction conditions: N,N-dimethylanilines (0.2 mmol), maleimides (0.1 mmol) and MOF UiO-68-TDP (4 mg) in CH3CN (1 mL) under an air atmosphere at room temperature for 12 h, blue LEDs (λmax = 450 nm, 3 W). Yield was determined by 1H-NMR analysis with 1-methyl-2,4-dinitrobenzene as an internal standard. |
|---|
 |
Conclusions
In summary, we have conjugated a photoactive thiadiazolopyridine moiety into the terphenyldicarboxylate linker, which was subsequently integrated into the porous and robust UiO-type isoreticular Zr-MOF UiO-68-TDP by a mix-and-match approach using mixed dicarboxylate struts. In the open air atmosphere, UiO-68-TDP can efficiently photocatalyze the oxidative cyclization synthesis of tetrahydroquinolines from N,N-dimethylanilines and maleimides under blue-LED irradiation. Moderate to high yields were achieved from a wide range of substrates by using UiO-68-TDP as heterogeneous photocatalyst under this reaction conditions. In comparison to its analogue UiO-68-BTD containing benzothiadiazole unit, UiO-68-TDP demonstrated a remarkably higher photocatalytic activity for this reaction. This work demonstrates the great potential of MOF based photocatalysts as well as the critical role of organic linker design in determining MOF's photocatalytic performance.Experimental section
Synthesis and characterizations
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was financially supported from Natural Science Foundation of Jiangsu Province (BK20181001).References
- D. M. Schultz and T. P. Yoon, Solar Synthesis: Prospects in Visible Light Photocatalysis, Science, 2014, 343, 1239176 CrossRef PubMed.
- N. A. Romero and D. A. Nicewicz, Organic Photoredox Catalysis, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed.
- J. Xie, H. Jin and A. S. K. Hashmi, The Recent Achievements of Redox-Neutral Radical C-C Cross-Coupling Enabled by Visible-Light, Chem. Soc. Rev., 2017, 46, 5193–5203 RSC.
- B. Chen, L.-Z. Wu and C.-H. Tung, Photocatalytic Activation of Less Reactive Bonds and Their Functionalization Via Hydrogen-Evolution Cross-Couplings, Acc. Chem. Res., 2018, 51, 2512–2523 CrossRef CAS PubMed.
- X.-Y. Yu, J.-R. Chen and W.-J. Xiao, Visible Light-Driven Radical-Mediated C–C Bond Cleavage/Functionalization in Organic Synthesis, Chem. Rev., 2021, 121, 506–561 CrossRef CAS PubMed.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed.
- X. Huang and E. Meggers, Asymmetric Photocatalysis with Bis-Cyclometalated Rhodium Complexes, Acc. Chem. Res., 2019, 52, 833–847 CrossRef CAS PubMed.
- A. Tlili and S. Lakhdar, Acridinium Salts and Cyanoarenes as Powerful Photocatalysts: Opportunities in Organic Synthesis, Angew. Chem., Int. Ed., 2021, 60, 19526–19549 CrossRef CAS PubMed.
- J. D. Bell and J. A. Murphy, Recent Advances in Visible Light-Activated Radical Coupling Reactions Triggered by (i) Ruthenium, (ii) Iridium and (Iii) Organic Photoredox Agents, Chem. Soc. Rev., 2021, 50, 9540–9685 RSC.
- N. Goli, P. S. Mainkar, S. S. Kotapalli, T. K, R. Ummanni and S. Chandrasekhar, Expanding the Tetrahydroquinoline Pharmacophore, Bioorg. Med. Chem. Lett., 2017, 27, 1714–1720 CrossRef CAS PubMed.
- J. Liu, G. S. Cremosnik, F. Otte, A. Pahl, S. Sievers, C. Strohmann and H. Waldmann, Design, Synthesis, and Biological Evaluation of Chemically and Biologically Diverse Pyrroquinoline Pseudo Natural Products, Angew. Chem., Int. Ed., 2021, 60, 4648–4656 CrossRef CAS PubMed.
- V. Sridharan, P. A. Suryavanshi and J. C. Menéndez, Advances in the Chemistry of Tetrahydroquinolines, Chem. Rev., 2011, 111, 7157–7259 CrossRef CAS PubMed.
- I. Muthukrishnan, V. Sridharan and J. Carlos Menendez, Progress in the Chemistry of Tetrahydroquinolines, Chem. Rev., 2019, 119, 5057–5191 CrossRef CAS PubMed.
- N. Hofmann, L. Homberg and K. C. Hultzsch, Synthesis of Tetrahydroquinolines Via Borrowing Hydrogen Methodology Using a Manganese Pn3 Pincer Catalyst, Org. Lett., 2020, 22, 7964–7970 CrossRef CAS PubMed.
- T. P. Nicholls, G. E. Constable, J. C. Robertson, M. G. Gardiner and A. C. Bissember, Bronsted Acid Cocatalysis in Copper(I)-Photocatalyzed Alpha-Amino C–H Bond Functionalization, ACS Catal., 2016, 6, 451–457 CrossRef CAS.
- J.-T. Guo, D.-C. Yang, Z. Guan and Y.-H. He, Chlorophyll-Catalyzed Visible-Light-Mediated Synthesis of Tetrahydroquinolines from N,N-Dimethylanilines and Maleimides, J. Org. Chem., 2017, 82, 1888–1894 CrossRef CAS PubMed.
- X.-L. Yang, J.-D. Guo, T. Lei, B. Chen, C.-H. Tung and L.-Z. Wu, Oxidative Cyclization Synthesis of Tetrahydroquinolines and Reductive Hydrogenation of Maleimides under Redox-Neutral Conditions, Org. Lett., 2018, 20, 2916–2920 CrossRef CAS PubMed.
- C.-W. Hsu and H. Sunden, Alpha-Aminoalkyl Radical Addition to Maleimides Via Electron Donor-Acceptor Complexes, Org. Lett., 2018, 20, 2051–2054 CrossRef CAS PubMed.
- Z. J. Wang, S. Ghasimi, K. Landfester and K. A. I. Zhang, Bandgap Engineering of Conjugated Nanoporous Poly-Benzobisthiadiazoles Via Copolymerization for Enhanced Photocatalytic 1,2,3,4-Tetrahydroquinoline Synthesis under Visible Light, Adv. Synth. Catal., 2016, 358, 2576–2582 CrossRef CAS.
- J. Tang, G. Grampp, Y. Liu, B.-X. Wang, F.-F. Tao, L.-J. Wang, X.-Z. Liang, H.-Q. Xiao and Y.-M. Shen, Visible Light Mediated Cyclization of Tertiary Anilines with Maleimides Using Nickel(Ii) Oxide Surface-Modified Titanium Dioxide Catalyst, J. Org. Chem., 2015, 80, 2724–2732 CrossRef CAS PubMed.
- M. Hosseini-Sarvari, M. Koohgard, S. Firoozi, A. Mohajeri and H. Tavakolian, Alizarin Red S–TiO2-Catalyzed Cascade C(sp3)–H to C(sp2)–H Bond Formation/Cyclization Reactions toward Tetrahydroquinoline Derivatives under Visible Light Irradiation, New J. Chem., 2018, 42, 6880–6888 RSC.
- T. Zhang and W. Lin, Metal–Organic Frameworks for Artificial Photosynthesis and Photocatalysis, Chem. Soc. Rev., 2014, 43, 5982–5993 RSC.
- X. Deng, Z. Li and H. Garcia, Visible Light Induced Organic Transformations Using Metal–Organic-Frameworks (Mofs), Chem.–Eur. J., 2017, 23, 11189–11209 CrossRef CAS PubMed.
- Y. Fang, Y. Ma, M. Zheng, P. Yang, A. M. Asiri and X. Wang, Metal–Organic Frameworks for Solar Energy Conversion by Photoredox Catalysis, Coord. Chem. Rev., 2018, 373, 83–115 CrossRef CAS.
- T. Zhang, Y. Jin, Y. Shi, M. Li, J. Li and C. Duan, Modulating Photoelectronic Performance of Metal–Organic Frameworks for Premium Photocatalysis, Coord. Chem. Rev., 2019, 380, 201–229 CrossRef CAS.
- Q.-Y. Li, Z. Ma, W.-Q. Zhang, J.-L. Xu, W. Wei, H. Lu, X. Zhao and X.-J. Wang, Aie-Active Tetraphenylethene Functionalized Metal–Organic Framework for Selective Detection of Nitroaromatic Explosives and Organic Photocatalysis, Chem. Commun., 2016, 52, 11284–11287 RSC.
- W.-Q. Zhang, Q.-Y. Li, Q. Zhang, Y. Lu, H. Lu, W. Wang, X. Zhao and X.-J. Wang, Robust Metal–Organic Framework Containing Benzoselenadiazole for Highly Efficient Aerobic Cross-Dehydrogenative Coupling Reactions under Visible Light, Inorg. Chem., 2016, 55, 1005–1007 CrossRef CAS PubMed.
- W.-Q. Zhang, Q.-Y. Li, X. Yang, Z. Ma, H. Wang and X.-J. Wang, Benzothiadiazole Conjugated Metal–Organic Framework for Organic Aerobic Oxidation Reactions under Visible Light, Acta Chim. Sinica, 2017, 75, 80–85 CrossRef CAS.
- W.-Q. Zhang, Q.-Y. Li, J.-Y. Cheng, K. Cheng, X. Yang, Y. Li, X. Zhao and X.-J. Wang, Ratiometric Luminescent Detection of Organic Amines Due to the Induced Lactam–Lactim Tautomerization of Organic Linker in a Metal–Organic Framework, ACS Appl. Mater. Interfaces, 2017, 9, 31352–31356 CrossRef CAS PubMed.
- W.-Q. Zhang, K. Cheng, H. Zhang, Q.-Y. Li, Z. Ma, Z. Wang, J. Sheng, Y. Li, X. Zhao and X.-J. Wang, Highly Efficient and Selective Photooxidation of Sulfur Mustard Simulant by a Triazolobenzothiadiazole-Moiety-Functionalized Metal–Organic Framework in Air, Inorg. Chem., 2018, 57, 4230–4233 CrossRef CAS PubMed.
- Y. Ji, B. He, H. Lu, J. Xu, R. Wang, Y. Jin, C. Zhong, Y. Shan, F. Wu and L. Zhu, Core Structure Engineering in Hole-Transport Materials to Achieve Highly Efficient Perovskite Solar Cells, Chemsuschem, 2019, 12, 1374–1380 CrossRef CAS PubMed.
- B. Wang, M. Wang, A. Mikhailovsky, S. Wang and G. C. Bazan, A Membrane-Intercalating Conjugated Oligoelectrolyte with High-Efficiency Photodynamic Antimicrobial Activity, Angew. Chem., Int. Ed., 2017, 56, 5031–5034 CrossRef CAS PubMed.
- T. Ishi-i, H. Tanaka, H. Koga, Y. Tanaka and T. Matsumoto, Near-Infrared Fluorescent Organic Porous Crystal That Responds to Solvent Vapors, J. Mater. Chem. C, 2020, 8, 12437–12444 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07363j |
| ‡ These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |