Open Access Article
Open Access ArticleIn situ integration of cobalt diselenide nanoparticles on CNTs realizing durable hydrogen evolution†
Hongfeng Ye,
Xuejiao Zhou,
Zhitao Shao,
Jing Yao,
Wenjie Ma,
Lili Wu * and
Xinzhi Ma*
* and
Xinzhi Ma*
Key Laboratory for Photonic and Electronic Bandgap Materials, Ministry of Education, School of Physics and Electronic Engineering, Harbin Normal University, Harbin, 150025, People's Republic of China. E-mail: wll790107@hotmail.com; maxz@hrbnu.edu.cn
First published on 3rd February 2022
Abstract
Cobalt diselenide (CoSe2) is considered to be a promising economical and efficient electrocatalyst for the hydrogen evolution reaction (HER). Here carbon nanotubes (CNTs) were employed as a conductive skeleton to optimize the electrocatalytic performance of CoSe2 through a simple one-step hydrothermal method. Beyond the expected, the introduction of CNTs not only accelerates electron transportation and ion diffusion, but also improves the reaction kinetics for HER by forming a CoSe2/CNT heterointerface. Consequently, the CoSe2/CNTs composite exhibits an optimal overpotential of 153 mV with a weight ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and sustains a long period of 48 hours with an negligible overpotential deterioration. In addition, a Faraday efficiency of 97.67% is achieved with a H2/O2 molar ratio of 2
1, and sustains a long period of 48 hours with an negligible overpotential deterioration. In addition, a Faraday efficiency of 97.67% is achieved with a H2/O2 molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Therefore, these results open up further opportunities for yielding efficient and durable hydrogen evolving electrocatalysts from low-cost transition metal compounds.
1. Therefore, these results open up further opportunities for yielding efficient and durable hydrogen evolving electrocatalysts from low-cost transition metal compounds.
1 Introduction
With the serious consumption of fossil energy and concomitant environmental pollution, renewable energy is receiving increasing attention. Hydrogen is considered to be an effective solution for global energy shortage due to its advantages: high energy density, cleanliness, and renewability.1–3 The electrolysis of water is an efficient way to produce hydrogen, and the most efficient cathode electrocatalysts toward the hydrogen evolution reaction (HER) are platinum-based catalysts, which have low overpotential and fast catalytic reaction kinetics. However, the high cost of platinum-based cathode electrocatalysts severely limits their large-scale commercial application.4,5For saving costs, Pt or other noble metal-based electrocatalysts on atomic-level, like the noble-metal single-atom catalysts supported by non-noble substrate, have been extensively investigated.6,7 Alternatively, non-noble-metal-based nanocatalysts including alloys, chalcogenides, phosphides, carbides and nitrides are closely pursued to maximize economic benefit.5,8–10 Among these non-noble-metal-based nanocatalysts, transition metal selenides stand out from the crowd due to their more superior free energy than pristine metals for proton adsorption and relatively lower electrical conductivity than the counterparts of sulfides.11 Especially, CoSe2 has received much attention due to its good electrical conductivity, good catalytic activity, huge storage on earth, and indispensability for human being.12–15 Unfortunately, during the growth process, CoSe2 commonly possesses a large individual size or tends to aggregates together, limiting the exposure of HER active center. In this regard, researchers devoted a lot of attentions aiming to enlarge the number of active centers of CoSe2. For example, Kim et al. integrated CoSe2 with CNTs through a spray pyrolysis followed with a selenization.16 The obtained CoSe2–CNTs composite has a porous structure, which increases the number of the exposed active centers besides guaranteeing fast ions' transmission in electrolyte. These advantages contributed CoSe2–CNTs an improved overpotential of 174 mV and a good long-term stability for 1000 cycles. Besides that, Yang et al. synthesized hollow mesoporous carbon (HC) nanospheres, wherein the carbon walls were grafted with CoSe2 by successive infiltration and selenization processes.13 The classical structure of HC not only facilitates the penetration of electrolyte and the exposure of active centers, but also stabilizes the structure by hindering electrolyte etching. As a result, the CoSe2@HC composites harvest an optimal overpotential of 171.7 mV at 10 mA cm−2 and a stable HER for 12 h.
Although these exciting breakthroughs in exposing more active centers of CoSe2 have been achieved, there's plenty room for stability improvement. Generally, HER stability is tested by rapid CV cycling for one to several thousand cycles, or under static (potential/galvanic) mode for several hours to a few days. At the end of these endurance tests, the change in overpotential or the percentage degradation in activity is frequently reported as the stability marker, and an increase in overpotential by ∼30 mV or activity degradation no more than 5% is accepted as a good catalyst.10 Furthermore, faradaic efficiency is an importantly index reflecting the selectivity of an electrocatalyst, scilicet the utilization efficiency of applied energy. It is usually less than 100%. A low faradaic efficiency should ascribe to the presence of side reactions.
Herein, we in situ grew CoSe2 nanoparticles on CNTs via an artful one-pot hydrothermal method. The employment of CNTs guarantees fast ion and electronic transfer. In addition, it significantly restricts the growth and inhibits the agglomeration of CoSe2 crystals. These advantages endow the CoSe2/CNTs composites obviously enlarged number of active centers, which is seriously conducive to HER activity. As a result, the CoSe2/CNTs composites output a current density of 10 mA cm−2 with an optimal overpotential of 153 mV. More importantly, the CoSe2/CNTs composites exhibit an excellent stability in the long-term cycle test for 48 h, which is much superior to the commercial platinum carbon (Pt/C) composite electrode. The faradaic efficiency yielded during HER process is 97.67%.
2 Experimental sections
2.1 Materials
Carbon nanotube dispersion (TNWDM-M2) was purchased from Chinese Academy of Science, Chengdu Organic Chemistry Co. Ltd, NaBH4 (CAS No: 16940-66-2, 98%), CoCl2·6H2O (CAS No: 7791-13-1, 98%), and Se (CAS No: 7782-49-2, 99.9%) powders were purchased from Aladdin Co. Ltd. All the chemicals are analytical grade without further purification.2.2 Synthesis of CoSe2/CNTs composites
Firstly, 671 μL carbon nanotube solution (41.2 mg mL−1) was added into 20 mL deionized (DI) water, and then ultrasound for one hour to make it dispersed evenly. Secondly, 215 mg Se powder and 128.6 mg NaBH4 were dissolved in 10 mL DI, and named it as solution A after being dispersed evenly. Thirdly, the dispersed carbon nanotube solution and 323.18 mg CoCl2·6H2O were added into solution A and then dispersed evenly. Finally, the prepared solution was transferred to a 50 mL Teflon-lined autoclave, and then it was heated at 200 °C for 20 hours. The product obtained after the hydrothermal treatment was washed with DI, and then vacuum dried at 60 °C. Except for adding CNTs, the synthesis method of pure CoSe2 is the same as that of CoSe2/CNTs.2.3 Preparation of CoSe2/CNTs electrodes
Weigh 20 mg CoSe2/CNTs and grind it in a mortar for 1–2 h. CoSe2/CNTs and 5 wt% polyvinylidene fluoride (PVDF) (mass ratio: CoSe2/CNTs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PVDF is 9
PVDF is 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were added to NMP, ultrasonic treatment for 3 hours to obtain uniformly dispersed ink. The 1 mg/10 μL ink was evenly coated on hydrophilic carbon paper (1 cm × 1 cm, load ≈ 2 mg cm−2) by drip coating, and dried under vacuum at 60 °C for 12 hours.
1) were added to NMP, ultrasonic treatment for 3 hours to obtain uniformly dispersed ink. The 1 mg/10 μL ink was evenly coated on hydrophilic carbon paper (1 cm × 1 cm, load ≈ 2 mg cm−2) by drip coating, and dried under vacuum at 60 °C for 12 hours.
For comparison, pure CoSe2 nanoparticles without CNTs and the mixture of CoSe2 nanoparticles with CNTs (denoted as CoSe2–CNTs) were also coated onto hydrophilic carbon paper to prepare pure CoSe2 and CoSe2–CNTs electrodes using the same method.
2.4 Characterization
Scanning electron microscope (SEM, SU70, Hitachi, Japan) analyzes the morphology of the composite material. The microstructure of the material was further characterized by a transmission electron microscope (TEM, FEI, Tecnai TF20) with an energy dispersive X-ray spectrometer (EDX). Use X-ray diffraction (XRD, D/max 2600, Rigaku, Japan) with Cu Kα radiation (λ = 1.5418 Å) to characterize the crystal structure of the material. The Raman spectrum was measured with a miniature Raman spectrometer (JY; HR800, France) at an excitation wavelength of 488 nm. The chemical composition of the material was characterized by X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi).2.5 Electrochemical measurements
The composite material's electrochemical catalytic ability, stability and other electrochemical properties were tested by the VMP3 electrochemical workstation (France Bio-logic) in three-electrode configuration. The pre-prepared electrode was used as the working electrode, the saturated calomel electrode (SCE) was used as the reference electrode, and the carbon rod was used as the counter electrode.The polarization curve was obtained by linear sweep voltammetry (LSV) at 5 mV s−1. Electrochemical impedance spectroscopy (EIS) was tested under an overpotential of −0.25 V (vs. RHE), and the frequency parameter was 100 mHz to 100 kHz. Cyclic voltammetry (CV) was used to measure the electric double-layer capacitance (Cdl) at 0.45–0.55 V (vs. RHE) and the scan rate range was 20–200 mV. Turnover frequency (TOF) was obtained from the CV test in a pH = 7 phosphate buffer (PBS), within the voltage range of −0.2–0.6 V (vs. RHE), the scan rate was 50 mv s−1. The scanning rate was 100 mV s−1 for 3000 CV tests, and the chronopotentiometry was used for a 48 h long cycle test at 30 mA cm−2 to investigate the catalytic stability of the catalyst. All overpotentials were corrected by 85% ohm potential drop (iR) compensation.
3 Results and discussion
3.1 Morphologies and structures
Fig. 1a illustrates the facile preparation process of CoSe2/CNTs composites. First, Se and NaBH4 powders were added into deionized water and stirred continuously. When the aqueous solutions were homogeneous, the pre-dispersed CNTs suspension with a certain amount of CoCl2·6H2O dissolved in it was added. The CNTs here used are multiwall CNTs with a uniform diameter of ∼10 nm (Fig. S1†). After the hydrothermal treatment at 200 °C for 20 hours, final products were obtained. In order to analyze their crystalline structure, XRD test was first conducted. As shown in Fig. 1b, the wide peak located at ∼26° belongs to CNTs, and other diffraction peaks all derive from the orthorhombic structure of CoSe2 (JCPDS #00-053-0449).17 It strongly confirmed that orthorhombic CoSe2 nanocrystallines have been grown in situ on the surface of CNTs (denoted as CoSe2/CNTs) with no impurities observed.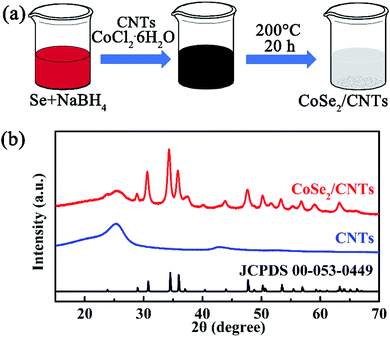 | ||
| Fig. 1 (a) Preparation process of CoSe2/CNTs composite, and (b) XRD patterns of CoSe2/CNTs composite and CNTs. | ||
The morphology of the CoSe2/CNTs composite is characterized by SEM. In Fig. 2a, numerous nanoparticles can be clearly watched snatchily decorating the surface of CNTs without severely agglomeration. The size of these nanoparticles mainly ranges from 10 nm to 30 nm, and the average diameter of ∼100 nanoparticles is 22.5 nm (the inset in Fig. 2a). It is remarkably smaller than that of the bare CoSe2 nanoparticles (37.5 nm) preparing under the same conditions (Fig. S2 and S3†). Meanwhile, the smaller nanoparticles in the composite exhibit better distribution than bare CoSe2 nanoparticles, which is desirable for high-performance HER electrocatalysis. In Fig. 2b, EDX analysis reveals that the composite consists of Se, Co, C, B, and O elements. The stoichiometric ratio of Co and Se is nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, indicating the small nanoparticles in the composite are CoSe2 nanoparticles. B element should be inherited from sodium borohydride precursor and conducive for HER. O element might be due to the partial oxidation of CoSe2 (vide infra).
2, indicating the small nanoparticles in the composite are CoSe2 nanoparticles. B element should be inherited from sodium borohydride precursor and conducive for HER. O element might be due to the partial oxidation of CoSe2 (vide infra).
The weight ratio of CoSe2 and CNTs was subsequently investigated by thermogravimetric analysis (TGA). As shown in Fig. 2c, the slight weight loss before 200 °C is derived from the evaporation of adsorbed water in the sample, and the slight increase in weight between 200–350 °C is related to the oxidation of CoSe2. The weight loss between 350–800 °C is due to the further conversion from CoSe2O5 to Co3O4 along with the combustion of CNTs.18 Finally, according to the Co3O4 content displayed by the TGA curve, the CoSe2 content in CoSe2/CNTs is calculated to be 90.89 wt%. That is, the CoSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CNTs weight ratio approaches to 10
CNTs weight ratio approaches to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Fig. 2d is a typical TEM image of the CoSe2/CNTs composite, in which it shows the irregular morphology and nonuniform diameters of the CoSe2 nanoparticles in composite. In Fig. 2e (corresponding to the yellow dashed area in Fig. 2d), well-resolved lattice fringes of 0.29 nm are corresponding to the (101) crystal plane of CoSe2, and the ones of 0.36 nm belong to the (002) plane of CNTs. The wall number of CNTs is 5–10, and the inner diameter is ∼5 nm. Fig. 2f is the SAED pattern of CoSe2/CNTs composite. It mainly exhibits four concentric rings. The three yellow lines marked ones are indexed to the (101), (111), and (211) of orthorhombic CoSe2, and the white one corresponds to the (002) crystal plane of CNTs,19 confirming the successful construction of CoSe2/CNTs composite with both polycrystalline nature of CoSe2 nanoparticles and CNTs.20
1. Fig. 2d is a typical TEM image of the CoSe2/CNTs composite, in which it shows the irregular morphology and nonuniform diameters of the CoSe2 nanoparticles in composite. In Fig. 2e (corresponding to the yellow dashed area in Fig. 2d), well-resolved lattice fringes of 0.29 nm are corresponding to the (101) crystal plane of CoSe2, and the ones of 0.36 nm belong to the (002) plane of CNTs. The wall number of CNTs is 5–10, and the inner diameter is ∼5 nm. Fig. 2f is the SAED pattern of CoSe2/CNTs composite. It mainly exhibits four concentric rings. The three yellow lines marked ones are indexed to the (101), (111), and (211) of orthorhombic CoSe2, and the white one corresponds to the (002) crystal plane of CNTs,19 confirming the successful construction of CoSe2/CNTs composite with both polycrystalline nature of CoSe2 nanoparticles and CNTs.20
More structural features of the CoSe2/CNTs composite were further revealed by the Raman spectrum. In Fig. 3a, the two sharp peaks located at 173.5 and 674 cm−1 are the fingerprint characters of orthorhombic CoSe2 and correspond to the Ag and A1g modes, and other two tiny ones at 469, 512 cm−1 correspond to the Eg, and F12g modes of orthorhombic CoSe2. Besides, the peaks appearing at 1354 and 1584 cm−1 are corresponded to the sp3 (D band) and sp2 (G band) hybridizations of carbonaceous materials, respectively.21 The intensity ratio (ID/IG) is often used as a diagnostic tool for evaluating the disordered degree of carbonaceous materials. Here it is calculated to be 1.02, implying the existence of affluent carbon defects. The local electronic structures of the CoSe2/CNTs composite were elucidated by XPS measurement. The XPS survey spectrum confirms Co, Se, C, B, and O elements in the composite (Fig. 3b). The Co 2p spectrum in Fig. 3c presents two broad peaks of Co 2p3/2 (781.2 eV) and Co 2p1/2 (796.9 eV) consisting of three doublets: Co2+ (Co–Se), Co3+ (Co–O/B, arising from the partial surface oxidation of CoSe2),22 and satellite peaks. In Fig. 3d, the Se 3d spectrum consists of pronounced Se 3d3/2, Se 3d5/2 and SeOx (the partial oxidation of CoSe2).23 The C 1s signal (Fig. 3e) mainly contains C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–C, and C–O/B bonds.16 Fig. 3f shows the XPS high-resolution spectrum of the B 1s region. The B 1s signal is located at 186.5 eV, which belongs to the three-fold coordination of B element.24,25 It is reasonable to believe that the presence of three fold coordinated B element would increase defect density and improve the HER performance of CoSe2/CNTs composite.
C, C–C, and C–O/B bonds.16 Fig. 3f shows the XPS high-resolution spectrum of the B 1s region. The B 1s signal is located at 186.5 eV, which belongs to the three-fold coordination of B element.24,25 It is reasonable to believe that the presence of three fold coordinated B element would increase defect density and improve the HER performance of CoSe2/CNTs composite.
 | ||
| Fig. 3 (a) Raman image, (b) XPS survey spectrum, (c) Co 2p, (d) Se 3d and (e) C 1s and (f) B 1s XPS spectra of CoSe2/CNT composite. | ||
3.2 Electrochemical performances
The CoSe2/CNTs composite was measured electrochemical performances in 0.5 M H2SO4. For comparison, commercial Pt/C (20%), pure CoSe2 and CNTs were also checked. In Fig. 4a, the CoSe2/CNTs composite only needs 153 mV overpotential to achieve 10 mA cm−2. Based on the phenomenon that CNTs has almost no HER performance, the catalytic performance of CoSe2/CNTs composite could be completely attributed to CoSe2 nanoparticles. While compared with the overpotential of 189 mV for bare CoSe2 nanoparticles, the overpotential differential of 36 mV should profit from the introduction of CNTs supporting. Furthermore, the compact connections of CNTs with CoSe2 nanoparticles derived from the in situ growth also plays a vital role in boosting the catalytic activity of CoSe2 for HER, which can be validated by the further comparison with the mixture of bare CoSe2 nanoparticle and CNTs (denoted as CoSe2–CNTs). The CoSe2–CNTs needs 177 mV overpotential to achieve 10 mA cm−2, which is still 24 mV higher than CoSe2/CNTs composite. It should be noted that the CNTs content in CoSe2/CNTs composite has a significant impact on the electrocatalytic property of resultant composite (Fig. S4†). As CNTs content increases, the overpotential (10 mA cm−2) of CoSe2/CNTs composites firstly ameliorates from 189 mV and then deteriorates to 173 mV. The optimal overpotential of 153 mV is obtained from the CoSe2/CNTs composite with a weight ratio of CoSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CNTs = 10
CNTs = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. To directly illustrate the superiority of CoSe2/CNTs composite to the bare CoSe2 nanoparticles and CoSe2–CNTs in catalytic activity, the current density at the fixed overpotential of 0.153 V (vs. RHE) are recorded (Fig. 4b). The current density of 10 mA cm−2 for the CoSe2/CNTs composite is 3.35 and 4.23 times of pure CoSe2 (2.98 mA cm−2) and times of CoSe2–CNTs (2.36 mA cm−2), respectively. The Tafel slope values of these catalysts can be obtained from Fig. 4c. Obviously, CoSe2/CNTs composite has the smallest Tafel slope value of 53.6 mV dec−1, which is smaller than both of bare CoSe2 (69.8 mV dec−1) and CoSe2–CNTs (59.2 mV dec−1), indicating the introduction of conductive CNTs scaffold and the formation of CoSe2/CNT heterointerface have obviously accelerated the reaction kinetics. According to the Tafel equation,26,27 the charge transfer coefficient of the CoSe2/CNTs composite is 1. The rate-determining step (RDS) involves zero electron transfer, and the number of electron transfer before RDS is 1. It means the RDS of the HER process of the CoSe2/CNTs composite is the Tafel reaction. Based on the above analysis, the reaction mechanism of the CoSe2/CNTs composite is deduced as shown in Fig. 4d. Firstly, the active site on the surface of CoSe2/CNTs composite capture a hydrogen ion (H+) and an electron to form an adsorbed hydrogen atom (H*). Then, the subsequently generated H* recombined with the adjacent H* to evolve H2. Thus, the obviously accelerated reaction kinetics of CoSe2/CNTs composite than bare CoSe2 and CoSe2–CNTs should attribute to the speed up generation of H*.
1. To directly illustrate the superiority of CoSe2/CNTs composite to the bare CoSe2 nanoparticles and CoSe2–CNTs in catalytic activity, the current density at the fixed overpotential of 0.153 V (vs. RHE) are recorded (Fig. 4b). The current density of 10 mA cm−2 for the CoSe2/CNTs composite is 3.35 and 4.23 times of pure CoSe2 (2.98 mA cm−2) and times of CoSe2–CNTs (2.36 mA cm−2), respectively. The Tafel slope values of these catalysts can be obtained from Fig. 4c. Obviously, CoSe2/CNTs composite has the smallest Tafel slope value of 53.6 mV dec−1, which is smaller than both of bare CoSe2 (69.8 mV dec−1) and CoSe2–CNTs (59.2 mV dec−1), indicating the introduction of conductive CNTs scaffold and the formation of CoSe2/CNT heterointerface have obviously accelerated the reaction kinetics. According to the Tafel equation,26,27 the charge transfer coefficient of the CoSe2/CNTs composite is 1. The rate-determining step (RDS) involves zero electron transfer, and the number of electron transfer before RDS is 1. It means the RDS of the HER process of the CoSe2/CNTs composite is the Tafel reaction. Based on the above analysis, the reaction mechanism of the CoSe2/CNTs composite is deduced as shown in Fig. 4d. Firstly, the active site on the surface of CoSe2/CNTs composite capture a hydrogen ion (H+) and an electron to form an adsorbed hydrogen atom (H*). Then, the subsequently generated H* recombined with the adjacent H* to evolve H2. Thus, the obviously accelerated reaction kinetics of CoSe2/CNTs composite than bare CoSe2 and CoSe2–CNTs should attribute to the speed up generation of H*.
 | ||
| Fig. 4 (a) Polarization curve, (b) cathode current density at the same voltage (c) Tafel slope, and (d) reaction mechanism diagram. | ||
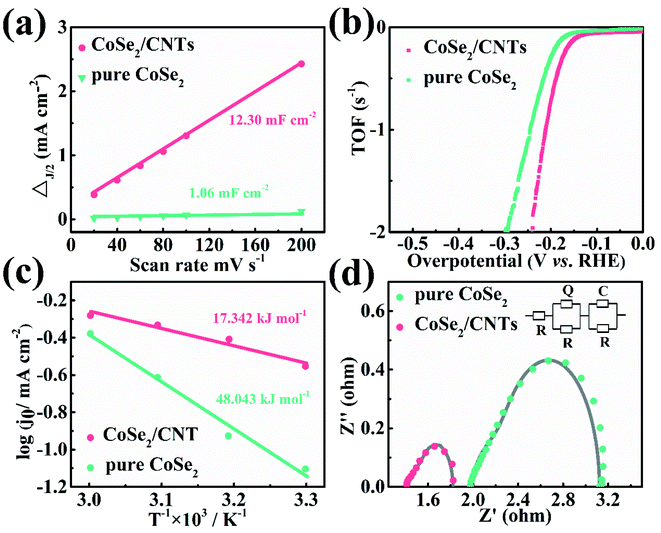
The Cdl values of CoSe2/CNTs composite and bare CoSe2 were estimated from the CV curves in Fig. S5.† The rectangular shape implies the favorable ion and electron transport of CoSe2/CNTs composite. In Fig. 5a, the linear slope, that is Cdl value, is 12.30 mF cm−2 for the CoSe2/CNTs composite. It is significantly larger than that of bare CoSe2 nanoparticles (1.06 mF cm−2), which should give the credit to the smaller size and better distribution of the CoSe2 nanoparticles in the composite than bare CoSe2 ones (Fig. 2a and S2†) besides the hollow structure of CNTs. The TOF value of CoSe2/CNTs composite can be extracted from the CV curve in Fig. S6.† According to the eqn (1):28
| TOF (s−1) = (j × A)/(2 × n × F) | (1) |
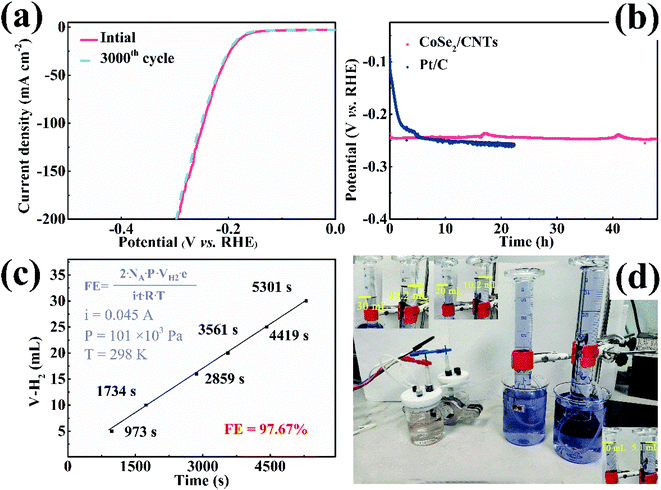
Durability is a significant indicator for the practical application of an electrocatalyst. In Fig. 6a, the LSV curve of CoSe2/CNTs composite after 3000 CV cycles superimposes over the initial curve before cycling with an ignorable increase of 6 mV in overpotential, indicating the excellent stability of CoSe2/CNTs composite. The chronopotentiometric curve of the CoSe2/CNTs composite was captured at 30 mA cm−2 (Fig. 6b). It shows the deterioration of 5% in overpotential after a long period of 48 hours, which is obviously superior to the unstable catalytic performance of Pt/C. The calculated Faraday efficiency at a constant current of 45 mA of CoSe2/CNTs electrode is 97.67% with a H2/O2 molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 6c and d). The CoSe2/CNTs composite after cycling was characterized by SEM, XRD, XPS, and TEM (Fig. 7 and S8†). In short, there is almost no change in morphology and microstructure after cycling. Especially, the well resolved lattice fringes without malposition in Fig. 7e and the sharp diffraction pattern in Fig. 7f provide reasonable assurance regarding the excellent stability of CoSe2/CNTs composite, which is remarkable compared with the reported CoSe2-based composite electrocatalysts (Table S1†). The high stability of CoSe2/CNTs composite should be attributed to the compact connections of CNTs with CoSe2 nanoparticles derived from the in situ growth.
1 (Fig. 6c and d). The CoSe2/CNTs composite after cycling was characterized by SEM, XRD, XPS, and TEM (Fig. 7 and S8†). In short, there is almost no change in morphology and microstructure after cycling. Especially, the well resolved lattice fringes without malposition in Fig. 7e and the sharp diffraction pattern in Fig. 7f provide reasonable assurance regarding the excellent stability of CoSe2/CNTs composite, which is remarkable compared with the reported CoSe2-based composite electrocatalysts (Table S1†). The high stability of CoSe2/CNTs composite should be attributed to the compact connections of CNTs with CoSe2 nanoparticles derived from the in situ growth.
4 Conclusions
In summary, artful CoSe2/CNTs composites were synthesized by a simple one-step hydrothermal method. The optimal overpotential of CoSe2/CNTs composites is 153 mV. Furthermore, the CoSe2/CNTs composite sustains a superior durability to commercial Pt/C electrode with Faraday efficiency nearly 100%. These desirable performances are attributed to the conductive CNTs scaffold, the smaller size and better distribution of the CoSe2 nanoparticles, as well as the compact connections of CoSe2 nanoparticles with CNTs. It is reasonable to believe these results open up further opportunities for yielding efficient and durable hydrogen evolving electrocatalysts from low-cost transition metal compounds.Author contributions
Hongfeng Ye, conceptualization, data curation, writing – original draft, Xuejiao Zhou, data curation, Zhitao Shao, data curation, Jing Yao, data curation, Wenjie Ma, data curation, Lili Wu, writing – review & editing and Xinzhi Ma writing – review & editing.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (No. 11504097, 51772069, 52072099), Team program of the Natural Science Foundation of Heilongjiang Province, China (No. TD2021E005).References
- Z. P. Lin, B. B. XiaO, Z. P. Wang, W. Y. Tao, S. J. Shen, L. G. Huang, J. T. Zhang, F. Q. Meng, Q. H. Zhang, L. Gu and W. W. Zhong, Adv. Funct. Mater., 2021, 31, 2102321 CrossRef CAS.
- T. Egeland-Eriksen, A. Hajizadeh and S. Sartori, Int. J. Hydrogen Energy, 2021, 46, 31963–31983 CrossRef CAS.
- M. Yue, H. Lambert, E. Pahon, R. Roche, S. Jemei and D. Hissel, Renewable Sustainable Energy Rev., 2021, 146, 111180 CrossRef.
- N. Dubouis and A. Grimaud, Chem. Sci., 2019, 10, 9165–9181 RSC.
- F. Zhou, Y. Zhou, G. G. Liu, C. T. Wang and J. Wang, Rare Met., 2021, 40, 3375–3405 CrossRef CAS.
- X. H. He, Y. C. Deng, Y. Zhang, Q. He, D. Q. Xiao, M. Peng, Y. Zhao, H. Zhang, R. C. Luo, T. Gan, H. B. Ji and D. Ma, Cell Rep. Phys. Sci., 2020, 1, 100004 CrossRef.
- D. W. Ma, T. X. Li, Q. G. Wang, G. Yang, C. Z. He, B. Y. Ma and Z. S. Lu, Carbon, 2015, 95, 756–765 CrossRef CAS.
- P. Yu, F. Wang, T. A. Shifa, X. Zhan, X. Lou, F. Xia and J. He, Nano Energy, 2019, 58, 244–276 CrossRef CAS.
- D. Zhang, X. Hui, C. Y. Wu and Y. B. Zhu, ChemCatChem, 2021, 13, 3370–3380 CrossRef CAS.
- J. Pan, S. W. Yu, Z. W. Jing, Q. T. Zhou, Y. F. Dong, X. D. Lou and F. Xia, Small Struct., 2021, 2, 2100076 CrossRef.
- S. Anantharaj, S. Kundu and S. Noda, J. Mater. Chem. A, 2020, 8, 4174–4192 RSC.
- C. L. Mccarthy, C. A. Downes, E. C. Schueller, K. Abuyen and R. L. Brutchey, ACS Energy Lett., 2016, 1, 607–611 CrossRef CAS.
- S. H. Yang, G. D. Park, J. K. Kim and Y. C. Kang, Chem. Eng. J., 2021, 424, 130341 CrossRef CAS.
- C. P. Lee, W. F. Chen, T. Billo, Y. G. Lin, F. Y. Fu, S. Samireddi, C. H. Lee, J. S. Hwang, K. H. Chen and L. C. Chen, J. Mater. Chem. A, 2016, 4, 4553–4561 RSC.
- K. Wang, D. Xi, C. J. Zhou, Z. Q. Shi, H. Y. Xia, G. W. Liu and G. J. Qiao, J. Mater. Chem. A, 2015, 3, 9415–9420 RSC.
- J. K. Kim, G. D. Park, J. H. Kim, S. K. Park and Y. C. Kang, Small, 2017, 13, 1700068 CrossRef PubMed.
- H. H. Yue, B. Yu, F. Qi, J. H. Zhou, X. Q. Wang, B. J. Zheng, W. L. Zhang, Y. R. Li and Y. F. Chen, Electrochim. Acta, 2017, 253, 200–207 CrossRef CAS.
- J. Yang, H. C. Gao, S. Men, Z. Q. Shi, Z. Lin, X. W. Kang and S. W. Chen, Adv. Sci., 2018, 5, 1800763 CrossRef PubMed.
- Z. T. Shao, L. L. Wu, Y. Yang, X. Z. Ma, L. Li, H. F. Ye and X. T. Zhang, New Carbon Mater., 2021, 36, 219–226 CrossRef.
- H. X. Zhang, B. Yang, X. L. Wu, Z. J. Li, L. C. Lei and X. W. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 1772–1779 CrossRef CAS PubMed.
- B. Yu, F. Qi, B. J. Zheng, W. Q. Hou, W. L. Zhang, Y. R. Li and Y. F. Chen, J. Mater. Chem. A, 2018, 6, 1655–1662 RSC.
- S. K. Park, J. K. Kim and Y. C. Kang, Chem. Eng. J., 2017, 328, 546–555 CrossRef CAS.
- X. Q. Wang, J. R. He, B. Yu, B. C. Sun, D. X. Yang, X. J. Zhang, Q. H. Zhang, W. L. Zhang, L. Gu and Y. F. Chen, Appl. Catal., B, 2019, 258, 117996 CrossRef CAS.
- S. Lee, S. Park, J. Park, Y. Kim, K. Yoon, C. Shin, S. Baek, J. Kim, Y.-J. Lee and J. Yi, Jpn. J. Appl. Phys., 2011, 50, 095801 CrossRef.
- K. Ohmori, N. Esashi, E. K. Atoro, D. K. Sato, H. K. Kawanishi, Y. Higashiguchi and Y. Hayafuji, Jpn. J. Appl. Phys., 2007, 46, 14–20 CrossRef CAS.
- Y. Hui Fang and Z. P. Liu, ACS Catal., 2014, 4, 4364–4376 CrossRef.
- Y. M. Shi and B. Zhang, Chem. Soc. Rev., 2016, 45, 1529–1541 RSC.
- D. H. Kweon, M. T. S. Okyay, S. J. Kim, J. P. Jeon, H. J. Noh, N. Park, J. Mahmood and B. J. Beom, Nat. Commun., 2020, 11, 1278 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07301j |
| This journal is © The Royal Society of Chemistry 2022 |