Open Access Article
Open Access ArticleIdentification of absorbed compounds of Xiao Yao San Jia Wei and pharmacokinetic study in depressed rats by force swimming stress†
Chenxiao Shanab,
Jia Lia,
Bo Suna,
Runze Zhoua,
Min Xua,
Qiulong Zhaoa,
Ping Rena,
Hongmei Wenb and
Xi Huang*a
aInstitute of TCM-related Comorbid Depression, Nanjing University of Chinese Medicine, 138 Xianlin Road, Nanjing 210023, Jiangsu, China. E-mail: 290606@njucm.edu.cn; Tel: +86 13951761464
bSchool of Pharmacy, Nanjing University of Chinese Medicine, Nanjing 210023, Jiangsu, China
First published on 4th February 2022
Abstract
Xiao-Yao-San-Jia-Wei (XYSJW) is a commonly prescribed formulation for depression and anorexia in the Jiang Su Province Hospital of Chinese Medicine. Unfortunately, the proper dosage of this formulation is still unclear due to its limited chemical and pharmacokinetic profiles. Thus, in the present study, a sensitive, precise, and rapid procedure for the identification of absorbed compounds (Cs) in the plasma of depressed rats together with a pharmacokinetic analysis was established with the help of ultra-flow liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UFLC-Q-TOF MS/MS) and ultra-flow liquid chromatography coupled with electrospray ionization triple quadrupole tandem mass spectrometry (UFLC-QQQ MS/MS). Based on the characteristic fragmentation, neutral loss, mass defect filter, relevant literature and reference standards, 225 Cs in the XYSJW extract and 20 Cs in the plasma of the depressed rats were tentatively recognized via UFLC-Q-TOF MS/MS and UFLC-QQQ MS/MS. Then, the 12 major absorbed Cs in the depressed rats after oral XYSJW administration were chosen to further investigate its pharmacokinetic profile by UFLC-QQQ MS/MS. This study provides a systematic approach for the rapid and qualitative analysis of absorbed Cs in depressed rats and investigating the pharmacokinetics of XYSJW. More importantly, our work provides key information on the chemical and pharmacokinetic profiles of XYSJW in vitro and in vivo, which may benefit its therapeutic efficacy and further pharmacological studies involving this formulation.
1. Introduction
The Global Burden of Disease Study 2017 identified major depressive disorder (MDD) as the second largest worldwide producer of disability.1 MDD is characterized by poor mood, disinterest, and displeasure, together with somatic and psychiatric symptomology such as nausea, headache, insomnia, and agitation.2 In the majority of cases, MDD therapy involves the use of antidepressants, namely selective serotonin reuptake inhibitors (SSRIs).3 However, although positive effects have been observed with SSRIs, the presence of multiple adverse reactions associated with this type of drug warrants caution.4Traditional Chinese medicine (TCM) frequently provides therapy for poor mental health in China.5 Xiao-Yao-San is a Chinese herbal formulation that was first introduced in the Taiping Huimin Heji Jufang, the initial global medical official documentation during the Song Dynasty (960–1127 AD). Growing evidence suggests that Xiao-Yao-San is beneficial for the correction of liver qi stagnation and spleen deficiency, a condition that induces both states of depression and loss of appetite.6 In prior investigations, we demonstrated that Xiao-Yao-San and Xiao-Yao-San-Jia-Wei (modified Xiao-Yao-San, XYSJW) exert an anti-depressive effect via modulation of the hippocampus,7,8 amygdala,9 locus coeruleus,10 and hypothalamus.11 Additionally, XYSJW can relieve depressive symptoms via regulation of gut microbes.12 Recently, XYSJW has become the first choice for treating depression, sleep disturbances, and anxiety disorder due to its good clinical manifestations and low side effects in China.13
As is known, most TCM are orally administrated, and thus only the ingredients absorbed into the blood can exert their bioactivities.14 Therefore, tracing the compounds in a TCM prescription in vivo and evaluation their pharmacokinetics provide more in-depth insight into the main active components and therapeutic mechanisms of TCM formulations. Compared to the clinical and pharmacological studies on XYSJW, the chemical and pharmacokinetic profile of this formulation is limited, and to date, pharmacokinetic studies in depressed rats are also rare. This study utilized ultra-flow liquid chromatography coupled with mass spectrometry (UFLC-MS/MS), a robust detecting system, with high resolution and sensitivity,15 to detect hundreds of active compounds (Cs) in XYSJW. Based on our research, 225 Cs and 20 absorbed Cs were tentatively identified in the XYSJW extract and depressed rat plasma after 30 kg g−1 oral XYSJW administration, respectively. Then, 12 absorbed Cs were detected by UFLC-QQQ-MS/MS and their pharmacokinetic parameters were determined. The chemical and pharmacokinetic data from this study will provide meaningful information in the fields of pharmacological research and rational use of this formulation in clinics.
2. Materials and method
2.1. Materials and reagent
Prepared slices of the crude drugs, namely, Paeoniae Radix Alba, Angelicae sinensis Radix, Aurantii Fructus, Radix Puerariae, Glycyrrhiza uralensis Fisch, Atractylodes macrocephala Koidz., Jujubae Fructus, Zingiberis Rhizoma, Cortex Moutan, Poria cocos (Schw.) Wolf, Gardeniae Fructus, Menthae Herba, Bupleuri Radix, and Magnolia officinalis Rehd. Et Wils were obtained from Laobaixing Pharmaceutical Co., Ltd. All drugs were critically examined and validated by Prof. Jian-wei Chen (Nanjing University of Chinese Medicine). HPLC-grade acetonitrile, formic acid, and methanol were purchased from Merck Co. (Darmstadt, Germany). Ultra-pure water was obtained from a Milli-Q SP Reagent system (Bedford, MA, USA). Meranzin hydrate, atractylenolide III, hesperetin, daidzein, liquirtin, magnolol, puerarin, paeoniflorin, naringin, hesperidin, glycyrrhizic acid, ferulic acid, geniposide, and paracetamol with purity ≥98%, were obtained from Nanjing Jin Yibai Biological Technology Co. Ltd. Their chemical structures are illustrated in Fig. 1.2.2. UFLC-MS/MS conditions
A UFLC (Shimadzu Corporation UFLC XR; Kyoto, Japan) attached to a Triple TOF MS (Triple TOF MS 5600 system, AB SCIEX, Los Angeles, CA, USA), using an electrospray ionization (ESI) source, was employed for qualitative analysis. For chromatographic separation (CGS), Shimadzu Shim-pack XR-ODS C18 (100 × 2.0 mm, 2.2 µm Kyoto, Japan) was used. A binary solvent gradient was prepared using water with 0.1% formic acid and acetonitrile with 0.1% formic acid. The flow rate was adjusted to 0.4 mL min−1 and the complete run time was 60 min. The gradient was as follows: 0–5 min 2–8% B; 5–12 min 8–10% B; 12–16 min 10–15% B; 16–38 min 15–35% B; 38–45 min 35–50% B; 45–52 min 50–95% B; 52–55 min 95% B; 55–57 min 95–2% B; and 57–60 min 2% B.A UFLC (Shimadzu Corporation UFLC XR; Kyoto, Japan) attached to a Q-Trap-MS (Q-Trap 5500 system, AB SCIEX, Los Angeles, CA, USA), using an electrospray ionization (ESI) source, was employed for quantitative analysis. For the CGS, a Waters BEH C18 column was used (2.1 mm × 50 mm, 1.7 µm) (Waters Corporation, Milford, MA, USA). A binary solvent gradient was prepared using water with 0.1% formic acid and acetonitrile with 0.1% formic acid. The flow rate was adjusted to 0.35 mL min−1 and the complete run time was 10 min. The gradient was as follows: 0–5 min 8–95% B; 5–7 min 95% B; 7–8 min 95–8% B; and 8–10 min 8% B. Lastly, the column temperature and injection volume were 40 °C and 2 µL, respectively.
Samples were subjected to full scans and multiple reaction surveillance scans using positive (+) and negative (−) ion modes. ESI was used to maintain the temperature at 550 °C. Nitrogen gas and ions were utilized to form the collision gas. Ion source gases 1 and 2, 55 psi each; entrance potential, ±10 V; curtain gas, 45 psi and ion spray voltage floating, 5500 V and −4500 V, under + and − ion modes respectively.
2.3 Formulation preparation
XYSJW was prepared using routine protocols in the Formularies of the Bureau of People's Welfare Pharmacies. The crude drug form of Paeoniae Radix Alba (25 g), Angelicae sinensis Radix (25 g), Aurantii Fructus (30 g), Radix Puerariae (40 g), Glycyrrhiza uralensis Fisch (15 g), Atractylodes macrocephala Koidz. (25 g), Jujubae Fructus (15 g), Zingiberis Rhizoma (25 g), Cortex Moutan, Poria Cocos (Schw.) Wolf (15 g), Gardeniae Fructus (15 g), Menthae Herba (15 g), Bupleuri Radix (25 g), and Magnolia officinalis Rehd Et Wils (30 g) were precisely weighed and boiled twice in 3.150 L and 2.52 L of water, respectively. The samples were then filtered and lyophilized. Subsequently, 2 mL methanol: water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) was combined with 5 mg lyophilized powder, vortexed for 3.0 min, and centrifuged for 10 min at 12
50 v/v) was combined with 5 mg lyophilized powder, vortexed for 3.0 min, and centrifuged for 10 min at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. 2 µL of the supernatant (S) was then injected into the UFLC-Q-TOF MS/MS.
000 rpm. 2 µL of the supernatant (S) was then injected into the UFLC-Q-TOF MS/MS.
2.4. Sample processing
5 µL IS (4 µg mL−1) and 400 µL methanol were combined with 50 µL of plasma sample to allow precipitation of protein and vortexed for 3.0 min, then centrifuged for 10 min at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 400 µL of S was retrieved and dried at 40 °C under an N2 flow, and then reconstituted in 50 µL of acetonitrile: water (5
000 rpm, 400 µL of S was retrieved and dried at 40 °C under an N2 flow, and then reconstituted in 50 µL of acetonitrile: water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v). Subsequently, after vortexing for another 3 min and centrifugation at 12
5, v/v). Subsequently, after vortexing for another 3 min and centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, 5 µL of S was injected into the UFLC-Q-TOF MS/MS for qualitative examination and 2 µL of S was injected into the UFLC-QQQ MS/MS for quantitative examination.
000 rpm for 10 min, 5 µL of S was injected into the UFLC-Q-TOF MS/MS for qualitative examination and 2 µL of S was injected into the UFLC-QQQ MS/MS for quantitative examination.
2.5. Verifying the UFLC-MS/MS technique
We confirmed our UFLC-MS/MS results based on the US FDA principles for the verification of bioanalytical methods, which include selectivity, carry-over effect calibration curves, lower limit of quantification (LLOQ), precision, accuracy, recovery, and matrix effect.To assess the carry-over effect, the highest calibration standard and IS were injected five times before the injection of the blank sample. Carry-over in the blank plasma was required to be <20% for the LLOQ of each sample and <5% for IS.
2.6 Animal experiments
Six Sprague Dawley rats (weighing 200 ± 20 g) were obtained from the Laboratory Animal Center of Nanjing University of Chinese Medicine and maintained in a controlled environment at 22 °C ± 2 °C. The forced swimming stress rat model was established according to Yoshimi Kitsda's protocol16 and involved a swimming test and open field test (ESI Table 1†). For pharmacokinetic examination, 30 g kg−1 XYSJW was administered to the rats and 0.2 mL blood samples were extracted at different time points, namely, 0.0833, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 h. The blood samples were from the jugular veins and stored in heparinized tubes. Subsequently, the samples were centrifuged at 2000 g for 10 min and Ss were maintained at −20 °C until further examination. Meanwhile, 2 mL of sterile isotonic saline was injected into the rats to eliminate any adverse effects from blood loss. Plasma was isolated at various times after oral formulation ingestion, namely at 1 h, 2 h, and 4 h, and used for qualitative analysis. All protocols involving care and treatment were approved by the Animal Ethics Committee of NJUCM (no. ACU-11(20201120)).2.7 Data processing
UFLC-QQQ- MS/MS and UFLC-Q-TOF- MS/MS data were generated using Analyst® version 1.6 and analyzed using PeakView® version 1.2 and MultiQuant™ version 3.2. All the above-mentioned software were obtained from AB SCIEX, Los Angeles, CA, USA. The main pharmacokinetic parameters were measured using Phoenix®WinNonlin® 8.1 (Pharsight Corporation, Mountain View, CA, USA), utilizing the non-compartmental method.3. Results and discussion
3.1 MS and chromatographic condition developments
To achieve appropriate chromatographic parameters, several analytical columns were investigated, including Shimadzu Shim-pack XR-ODS C18 (100 × 2.0 mm, 2.2 µm Kyoto, Japan), Waters XBridge Amide column (4.6 × 100 mm, 3.5 µm Milford, USA), and Waters BEH C18 (2.1 × 50 mm, 1.7 µm Milford, USA) column. The results revealed that the Shimadzu Shim-pack XR-ODS C18 exhibited the greatest separation in the qualitative analysis and Waters BEH C18 column exhibited the greatest resolution and sensitivity in the quantitative analysis. Furthermore, several mobile phases, flow rates, and gradient elution were investigated. The acetonitrile–water system was chosen as the mobile phase due to its larger signal outcomes compared to the methanol–water system. To achieve higher ionization efficiency and shape peak, four mobile phase systems were investigated in our research including pure water, 0.1% formic acid, 3 mM ammonium acetate, and 0.1% formic acid plus 3 mM ammonium acetate. Finally, 0.1% formic acid was added to the mobile phase. To increase the selectivity of our identification, 30 standard Cs and several gradients were tested, where all the standard Cs were baseline separated at 60 min including some isomers in the current condition. To achieve a selective and sensitive MRM mode, the corresponding standard solutions of the target Cs were separately injected into the MS, with the spectra recorded between m/z 50 and 1000. The result demonstrated that some Cs responded well in the negative mode, whereas others were better in the positive mode. The optimized results, including ion transitions (ESI Fig. 1†) and C-independent mass parameters, are presented in Table 1. In addition, another 18 Cs in XYSJW were optimized and validated, including albiflorin, rutin, narirutin, rosmarinic acid, neohesperidin, naringenin, isoliquiritigenin, 6-gingerol, saikosaponin (SS) A, genipin, honokiol, atractylenolide I, glycyrrhetic acid, atractylenolide I, poricoic acid A, gardenoside, zizyvoside I, and ononin. However, they were not detected in the plasma samples of the depressed rats, which is likely due to the excessive herb content in this formula. Moreover, each dosage of these undetectable Cs was 0.35–2.71 mg kg−1 after oral administration of the XYSJW extract and 0.5–1 ng mL−1 in real plasma (their concentrations in the plasma were lower than the LLOQ). In addition, their bioavailabilities were very low. Although the real method validation included 30 Cs between 2 ng mL−1 and 2 µg mL−1, only 12 Cs were quantified and their pharmacokinetic parameters profiled.| Analyte | Precursor ion (Da) | Product ion (Da) | Collision energy (eV) | Cell exit potential (V) | Declustering potential (V) | Retention time (min) |
|---|---|---|---|---|---|---|
| Meranzin hydrate | 279.0 | 261.1 | +7 | +10 | +60 | 2.66 |
| Puerarin | 417.0 | 296.9 | +35.0 | +14 | +80 | 1.69 |
| Paracetamol (IS) | 152.0 | 110.0 | +21 | +14 | +80 | 0.75 |
| Atractylenolide III | 247.0 | 203.0 | −20 | −17 | −110 | 4.02 |
| Daidzein | 253.0 | 224.0 | −36 | −17 | −185 | 2.7 |
| Ferulic acid | 193.0 | 134.0 | −22 | −13 | −85 | 2.18 |
| Geniposide | 433.1 | 224.9 | −18 | −13 | −40 | 1.77 |
| Glycyrrhizic acid | 821.3 | 351.0 | −52 | −13 | −150 | 3.46 |
| Hesperidin | 609.1 | 301.0 | −36 | −25 | −150 | 2.41 |
| Liquiritin | 417.0 | 255.0 | −26 | −19 | −120 | 2.16 |
| Magnolol | 265.0 | 247.0 | −28 | −13 | −155 | 4.66 |
| Naringin | 579.0 | 270.9 | −42 | −19 | −180 | 2.32 |
| Paeoniflorin | 525.0 | 449.0 | −20 | −43 | −75 | 1.99 |
| Paracetamol (IS) | 150.3 | 106.9 | −22 | −13 | −100 | 0.75 |
To obtain an appropriate internal standard that responds well under both + and − modes, several synthetic Cs were investigated, including paracetamol, glimepiride sulfonamide, and penicillin. High extract recovery in the rat plasma and great stability are the basic requirements for selecting the internal standard. Based on our result, paracetamol achieved 89.5 ± 3.91% recovery, ±5.5% stability and 91.34 ± 4.71% matrix effect, and thus was chosen for further investigation in our study.
3.2 UFLC-Q-TOF MS analysis of absorbed Cs in depressed rat plasma
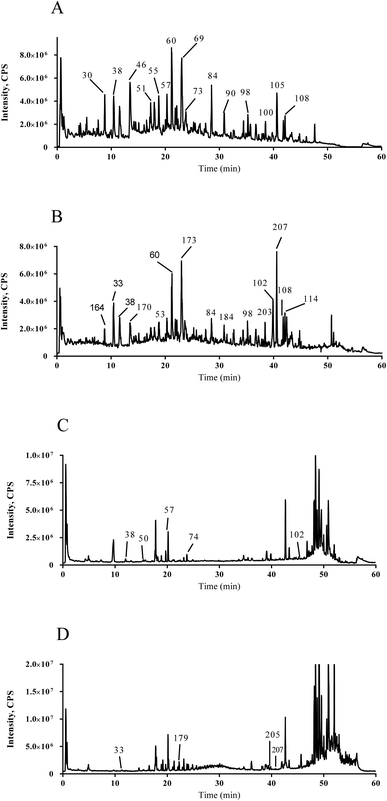
The total ion chromatogram profiles of the XYSJW extract and RRP after oral administration were expressed in positive and negative modes, as depicted in Fig. 2. The components in XYSJW and RRP were sufficiently separated by the UFLC/Q-TOF–MS/MS technique. Specifically, there were 225 Cs in the XYSJW extract (ESI Table 2† and Table 3) according to the characteristic fragmentation, neutral loss, mass defect filter, relevant literature and reference standards (CF). Furthermore, 16 Cs in RRP were tentatively identified by UFLC-QTOF-MS/MS. Their retention times (RTs) and the MS/MS information of the absorbed compounds are listed in Table 2 and Table 3.| No. | TR (min) | Identification | Molecular formula | Ion species | Theoretical mass (Da) | Measured mass (Da) | Mass accuracy (ppm) | MS/MS fragments ions | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.33 | Shanzhiside | C16H24O11 | [M − H]− | 391.12459 | 391.12389 | −1.8 | 229.0723, 59.0170 | 17 |
| 2 | 4.53 | Geniposidic acid | C16H22O10 | [M − H]− | 373.11402 | 373.11419 | 0.5 | 211.0602, 167.0701, 149.0601, 123.0447 | 17 |
| 3 | 10.57 | Genipin | C11H14O5 | [M − H]− | 225.07685 | 225.07742 | 2.5 | 210.0494, 180.0431, 165.0181, 137.0228 | 17 |
| 4 | 11.52 | Puerarin | C21H20O9 | [M − H]− | 415.10346 | 415.10326 | −0.5 | 295.0594, 277.0594, 267.0647 | 18 |
| 5 | 17.31 | Liquiritin | C21H22O9 | [M − H]− | 417.11911 | 417.11819 | −2.2 | 255.0683, 135.0076, 119.0496 | 19 and 20 |
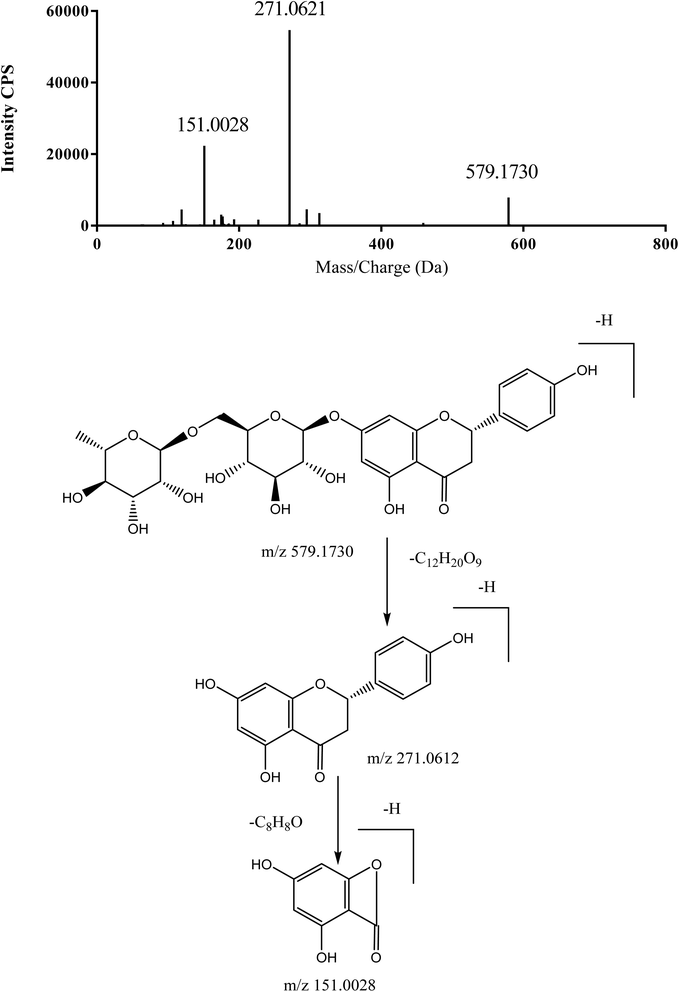
| 6 | 20.25 | Naringin | C27H32O14 | [M − H]− | 579.17193 | 579.1704 | −2.6 | 271.0596, 151.0028 | 21 and 22 |
| 7 | 22.11 | Hesperidin | C28H34O15 | [M − H]− | 609.18249 | 609.18019 | −3.8 | 609.1521, 301.0692 | 21 and 22 |
| 8 | 23.5 | Neohesperidin | C28H34O15 | [M − H]− | 609.18249 | 609.18019 | −3.8 | 489.1402, 343.0812, 301.0691 | 21 and 22 |
| 9 | 24.21 | Daidzein | C15H10O4 | [M − H]− | 253.05063 | 253.05107 | 1.7 | 233.0388, 208.0522 | 23 |
| 10 | 42.231 | 6-Gingerol | C17H26O4 | [M − H]− | 293.17583 | 293.17611 | 0.9 | 99.0800, 57.0366 | 24 |
| 11 | 47.909 | Magnolol | C18H18O2 | [M − H]− | 265.1234 | 265.12415 | 2.8 | 247.1101, 245.0952, 243.0803, 223.0747 | 25 |
| No. | TR (min) | Identification | Molecular formula | Ion species | Theoretical mass (Da) | Measured mass (Da) | Mass accuracy (ppm) | MS/MS fragments ions | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10.49 | Genipin | C11H14O5 | [M + H]+ | 227.0914 | 227.09161 | 0.9 | 177.0549, 149.0600, 121.0663, 91.0567, 77.0422 | 17 |
| 2 | 23.56 | 4′,7-Dihydroxyflavone | C15H10O4 | [M + H]+ | 255.06519 | 255.06527 | 0.3 | 237.0552, 227.0716, 199.0761, 181.0654, 137.0243, 91.0570 | 17 |
| 3 | 23.64 | Meranzin hydrate | C15H18O5 | [M + H]+ | 279.1227 | 279.1227 | 0.1 | 243.1031, 189.0551, 131.0504 | 21 and 22 |
| 4 | 39.93 | Nobiletin | C21H22O8 | [M + H]+ | 403.13874 | 403.13909 | 0.9 | 388.1173, 373.0929 | 21 and 22 |
| 5 | 40.98 | Glycyrrhizic acid | C42H62O16 | [M + H]+ | 823.41106 | 823.41152 | 0.6 | 647.3800, 417.3479, 453.3360, 435.3269 | 19 and 20 |
| 6 | 41.06 | Atractylenolide III | C15H20O3 | [M + H]+ | 249.14852 | 249.14865 | 0.5 | 231.1389, 163.0756, 105.0708 | 26 |
Using narirutin (C 57) as the authentic standard in negative mode, we found a neutral loss (NL) of 308 Da (Fig. 4), and mass defect at 170 ± 80 mDa, which are important characteristics in the identification of flavonoids. Based on the NL of 308 Da and the reference standards, C 51, C 60, C 63, C 69, and C 90 were unambiguously identified as rutin, naringin, hesperidin, neohesperidin, and hesperetin, respectively.
3.3. Procedural verification
After injection of the highest standards, the analyte peaks were <5% of the LLOQ in the BRP, indicating the absence of a marked carry-over effect. The typical MRM chromatograms are presented in ESI Fig. 2.†
| Analyte | Regression equation | R | Linear range (ng mL−1) | LLOQ (ng mL−1) |
|---|---|---|---|---|
| Atractylenolide III | Y = 0.000173X − 0.000519 | 0.9961 | 2.0–2000 | 2.0 |
| Daidzein | Y = 0.00485X + 0.00497 | 0.9990 | 2.0–2000 | 2.0 |
| Ferulic acid | Y = 0.00411X − 0.00433 | 0.9981 | 2.0–2000 | 2.0 |
| Geniposide | Y = 0.00892X + 0.00724 | 1.0000 | 2.0–2000 | 2.0 |
| Glycyrrhizic acid | Y = 0.00164X + 0.00329 | 0.9994 | 2.0–2000 | 2.0 |
| Hesperidin | Y = 0.00456X + 0.00862 | 0.9983 | 2.0–2000 | 2.0 |
| Liquiritin | Y = 0.0137X + 0.0185 | 1.0000 | 2.0–2000 | 2.0 |
| Magnolol | Y = 0.0232X + 0.0197 | 0.9971 | 2.0–2000 | 2.0 |
| Meranzin hydrate | Y = 0.0000463X + 0.0000521 | 0.9996 | 2.0–2000 | 2.0 |
| Naringin | Y = 0.0337X + 0.0415 | 0.9994 | 2.0–2000 | 2.0 |
| Paeoniflorin | Y = 0.00456X + 0.00862 | 0.9975 | 2.0–2000 | 2.0 |
| Puerarin | Y = 0.000114X + 0.000114 | 1.0000 | 2.0–2000 | 2.0 |
3.4 Pharmacokinetic study
Our established technique was effectively used toward the pharmacokinetic investigation of 30 g kg−1 XYSJW-administered depressed rats. The profiles of the average plasma concentration versus duration of each analyte are depicted in Fig. 8. The significant pharmacokinetic profiles are listed in Tables 5–7. | ||
| Fig. 8 Mean (±SD, n = 6) plasma concentration–time profiles of twelve compounds in depression rats induced by force swimming stress. 8. | ||
| Parameters | Atractylenolide III | Daidzein | Ferulic acid | Geniposide | |
|---|---|---|---|---|---|
| Dose | (mg kg−1) | 0.80 ± 0.00 | 0.73 ± 0.00 | 0.08 ± 0.00 | 1.15 ± 0.00 |
| Tmax | (h) | 4.51 ± 3.76 | 0.29 ± 0.10 | 1,67 ± 0.41 | 1.67 ± 1.13 |
| Cmax | (ng mL−1) | 291.00 ± 123.74 | 31.62 ± 6.58 | 35.23 ± 13.07 | 724.33 ± 330.02 |
| T1/2 | (h) | 4.50 ± 1.19 | 3.91 ± 1.97 | 9.54 ± 3.83 | 8.00 ± 6.02 |
| AUC0-t | (h ng mL−1) | 2270.12 ± 622.78 | 93.41 ± 13.07 | 128.52 ± 35.04 | 6041.50 ± 1723.20 |
| AUC0–∞ | (h ng mL−1) | 2523.37 ± 634.62 | 106.88 ± 9.37 | 173.84 ± 47.70 | 6912.69 ± 1312.93 |
| Clz/F | (mL h−1 kg−1) | 368.10 ± 110.36 | 6886.78 ± 598.63 | 467.24 ± 162.62 | 242.98 ± 124.36 |
| MRT0-t | (h) | 9.90 ± 3.12 | 4.24 ± 0.74 | 6.59 ± 2.14 | 6.99 ± 1.29 |
| MRT0–∞ | (h) | 16.58 ± 7.31 | 6.35 ± 2.25 | 14.03 ± 5.14 | 11.08 ± 5.72 |
| Parameter | Glycyrrhizic acid | Hesperidin | Liquiritin | Magnolol | |
|---|---|---|---|---|---|
| Dose | (mg kg−1) | 8.43 ± 0.00 | 61.13 ± 0.00 | 8.45 ± 0.00 | 3.55 ± 0.00 |
| Tmax | (h) | 2.25 ± 2.82 | 0.33 ± 0.13 | 1.21 ± 1.40 | 0.33 ± 0.13 |
| Cmax | (ng mL−1) | 16.68 ± 7.78 | 233.25 ± 119.82 | 58.70 ± 14.74 | 18.78 ± 14.80 |
| T1/2 | (h) | 14.54 ± 3.32 | 13.27 ± 6.42 | 11.26 ± 6.79 | 12.94 ± 7.72 |
| AUC0-t | (h ng mL−1) | 108.75 ± 58.44 | 499.61 ± 151.29 | 316.16 ± 111.56 | 33.42 ± 9.27 |
| AUC0–∞ | (h ng mL−1) | 149.27 ± 60.85 | 574.94 ± 182.33 | 319.01 ± 189.99 | 41.52 ± 12.84 |
| Clz/F | (mL h−1 kg−1) | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 421.79 ± 19 421.79 ± 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 547.67 547.67 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 463.59 ± 13 463.59 ± 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 912.24 912.24 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 021.80 ± 6734.19 021.80 ± 6734.19 |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 217.80 ± 712.78 217.80 ± 712.78 |
| MRT0-t | (h) | 8.26 ± 2.66 | 7.79 ± 1.04 | 9.26 ± 2.06 | 3.91 ± 1.54 |
| MRT0–∞ | (h) | 30.88 ± 20.21 | 19.19 ± 6.19 | 30.31 ± 20.96 | 17.73 ± 1.95 |
| Parameter | Meranzin hydrate | Naringin | Paeoniflorin | Puerarin | |
|---|---|---|---|---|---|
| Dose | (mg kg−1) | 22.41 ± 0.00 | 14.73 ± 0.00 | 37.10 ± 0.00 | 115.44 ± 0.00 |
| Tmax | (h) | 0.96 ± 0.51 | 0.33 ± 0.13 | 2.50 ± 1.18 | 0.67 ± 0.49 |
| Cmax | (ng mL−1) | 999.67 ± 131.67 | 43.78 ± 22.39 | 460.17 ± 280.95 | 13.37 ± 6.11 |
| T1/2 | (h) | 9.91 ± 4.75 | 14.62 ± 6.18 | 9.73 ± 4.15 | 11.16 ± 9.34 |
| AUC0-t | (h ng mL−1) | 4098.00 ± 396.59 | 59.08 ± 17.94 | 2011.61 ± 854.80 | 46.73 ± 16.08 |
| AUC0–∞ | (h ng mL−1) | 5142.68 ± 1067.26 | 74.2 ± 35.32 | 2512.79 ± 1240.32 | 61.38 ± 30.21 |
| Clz/F | (mL h−1 kg−1) | 4490.97 ± 276.78 | 127![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960.57 ± 8100.51 960.57 ± 8100.51 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 676.94 ± 2273.36 676.94 ± 2273.36 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 091 091![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 817.62 ± 63 817.62 ± 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106.15 106.15 |
| MRT0-t | (h) | 6.27 ± 1.82 | 4.79 ± 4.84 | 7.60 ± 0.58 | 6.78 ± 3.68 |
| MRT0–∞ | (h) | 12.59 ± 6.77 | 10.88 ± 7.58 | 13.29 ± 4.67 | 18.65 ± 15.66 |
3.5 Discussion
Although 225 Cs were tentatively identified in the XYSJW extract, only 20 absorbed Cs were identified in the depressed rats after oral 30 g kg−1 XYSJW extract administration using the current Q-TOF MS/MS (16 Cs) and QQQ-MS/MS (12 Cs) methods. According to the relevant literature,27 the absorption in the depressed rats was lower than that in the normal rats. In addition, the oral bioavailability of over 30% Cs in the XYSJW extract was 12.7% by screening the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform. Therefore, only 8.5% of the 225 Cs was identified in the depressed rats. In our future investigation, the metabolites of the 225 Cs in the depressed rats will be tentatively identified and their potential pharmacological effects will be examined.According to the area under the curve and the dosage (0.08–1.15 mg kg−1), atractylenolide III, daidzein, ferulic acid, and geniposide exhibited good oral absorption in the depressed rats in our current study. In contrast, based on our previous study,28 puerarin displayed poor oral absorption in the depressed rats with a relatively low maximum plasma drug concentration compared to the orally administered 115.44 mg kg−1 puerarin in the XYSJW extract. Ironically, although the concentration of glycyrrhetic acid was much higher than glycyrrhizic acid in the extract, glycyrrhetic acid was hardly detected in the depressed rats, which indicated that the UDP-glucuronosyl transferases were highly activated in the depressed rats after oral administration of the XYSJW extract.29 According to our current study, the CL/Fz data of atractylenolide III, ferulic acid and geniposide were not more than 500 mL h−1 kg−1, while the CL/Fz data of hesperidin, naringin and puerarin were more than 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL h−1 kg−1. Compared with the literature,28,30–34 the results indicate that some metabolic enzymes in the depressed rats were activated by acute stress, while some were inhibited, highlighting the value of studying the metabolism of depression in vivo and in vitro. Thus, our future research will focus on the metabolism of XYSJW and compatibility mechanism using depressed rats and rat liver microsomes.
000 mL h−1 kg−1. Compared with the literature,28,30–34 the results indicate that some metabolic enzymes in the depressed rats were activated by acute stress, while some were inhibited, highlighting the value of studying the metabolism of depression in vivo and in vitro. Thus, our future research will focus on the metabolism of XYSJW and compatibility mechanism using depressed rats and rat liver microsomes.
Moreover, the concentration of each C in the depressed rat plasma provides evidence for in vitro experiments. In our current DPPH research, the 12 absorbed Cs represented 95.7% antioxidant effect of the XYSJW extract. The antidepressant effect of the 12 absorbed Cs in this formulation will be studied in cell research and the contribution rate of each C will be published in the future.
4 Conclusions
Firstly, we developed an UFLC-Q- TOF-MS/MS method for the tentative identification of 225Cs in XYSJW extract and 20 absorbed Cs of XYSW in depressed rats. Subsequently, we established and verified a quick, selective, sensitive, and reliable UFLC-QQQ-MS/MS procedure for the simultaneous quantification of the 12 main absorbed Cs in depressed rats and their pharmacokinetics characteristics were studied further. To the best of our knowledge, this research is the first study on the chemical and pharmacokinetic profiles of XYSJW, especially in depressed rats. Our findings will be of high interest in clinical applications and in the development of anti-depressive therapy. However, this method has some limitations. Thus, to comprehend the true pharmacokinetic profiles of Cs in the XYSJW extract, we will do our best to improve our method and determine more Cs and their potential pharmacokinetic effects in depressed rats.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This project was funded by the Graduate Research and Innovation Projects of Jiangsu Province (Grant KYXC20_1512) and the National Natural Science foundation of China (grant numbers: 81973589 and 81373855).References
- Q. Liu, H. He, J. Yang, X. Feng, F. Zhao and J. Lyu, J. Psychiatr. Res., 2020, 126, 134–140 CrossRef PubMed.
- J. C. Nelson, E. Weiller, P. Zhang, C. Weiss and M. Hobart, J. Affective Disord., 2018, 227, 103–108 CrossRef CAS PubMed.
- J. Marjoribanks, J. Brown, P. M. S. O’Brien and K. Wyatt, Cochrane Database Syst. Rev., 2013,(6), CD001396 Search PubMed.
- M. Xu, Y. Liu, Y. Guo, C. Liu, Y. Liu, Z. Yan, Y. Hou, X. Li, Q. Ma and X. Zhou, Medicine, 2020, 99, e19425 CrossRef PubMed.
- W. Dai, K. Feng, X. Sun, L. Xu, S. Wu, K. Rahmand, D. Jia and T. Han, J. Ethnopharmacol., 2021, 114692, DOI:10.1016/j.jep.2021.114692.
- Q. Ma, X. Li, Z. Yan, H. Jiao, T. Wang, Y. Hou, Y. Jiang, Y. Liu and J. Chen, Front. Psychiatry, 2019, 10, 910 CrossRef PubMed.
- L. Gao, P. Huang, Z. Dong, T. Gao, S. Huang, C. Zhou, Y. Lai, G. Deng, B. Liu and G. Wen, Front. Pharmacol., 2018, 9, 1098 CrossRef CAS PubMed.
- H. Jiao, Z. Yan, Q. Ma, X. Li, Y. Jiang, Y. Liu and J. Chen, Neuropsychiatr. Dis. Treat., 2019, 15, 21 CrossRef CAS PubMed.
- X. Guo, W. Qiu, Y. Liu, Y. Zhang, H. Zhao and J. Chen, Molecules, 2017, 22, 1386 CrossRef PubMed.
- X. Zhu, O. Xia, W. Han, M. Shao, L. Jing, Q. Fan, Y. Liu, J. Diao, Z. Lv and X. Sun, Evid.-based Complement. Altern. Med., 2014, 2014, 902516 Search PubMed.
- Z. Yan, H. Jiao, X. Ding, Q. Ma, X. Li, Q. Pan, T. Wang, Y. Hou, Y. Jiang and Y. Liu, Molecules, 2018, 23, 1073 CrossRef PubMed.
- H.-Z. Zhu, Y.-D. Liang, Q.-Y. Ma, W.-Z. Hao, X.-J. Li, M.-S. Wu, L.-J. Deng, Y.-M. Li and J.-X. Chen, Biomed. Pharmacother., 2019, 112, 108621 CrossRef CAS PubMed.
- D. N. H. Tran, I. H. Hwang, F. J. Chen, Y. P. Tseng, C. M. Chang, S. J. Tsai, J. L. Yang, T. P. Wu, C. H. Hsu, F. P. Chen and Y. Y. Kung, Integr. Med. Res., 2021, 10, 100707 CrossRef PubMed.
- Y. Tao, H. Y. Xu, S. S. Wang, B. Wang, Y. C. Zhang, W. H. Wang, B. Huang, H. W. Wu, D. F. Li, Y. Zhang, X. F. Xiao, Y. B. Li, H. J. Yang and L. Q. Huang, J. Chromatogr. B, 2013, 935, 1–9 CrossRef CAS PubMed.
- L. Shan, N. Yang, Y. Zhao, X. Sheng, S. Yang and Y. Li, J. Sep. Sci., 2018, 41, 3791–3805 CrossRef CAS PubMed.
- Y. Kitada, T. Miyauchi, A. Satoh and S. Satoh, Eur. J. Pharmacol., 1981, 72, 145–152 CrossRef CAS PubMed.
- Z. Fu, Y. Ling, Z. Li, M. Chen, Z. Sun and C. Huang, Biomed. Chromatogr., 2014, 28, 475–485 CrossRef CAS PubMed.
- T. Wu, C. Liu, Y. Huang, S. Li and Y. Wang, J. Sep. Sci., 2018, 41, 4458–4468 CrossRef CAS PubMed.
- Q. Wang, Y. Qian, Q. Wang, Y. F. Yang, S. Ji, W. Song, X. Qiao, D. A. Guo, H. Liang and M. Ye, J. Pharm. Biomed. Anal., 2015, 115, 515–522 CrossRef CAS PubMed.
- Y. F. Wong, F. Cacciola, S. Fermas, S. Riga, D. James, V. Manzin, B. Bonnet, P. J. Marriott, P. Dugo and L. Mondello, Electrophoresis, 2018, 39, 1993–2000 CrossRef CAS PubMed.
- Y. Bai, Y. Zheng, W. Pang, W. Peng, H. Wu, H. Yao, P. Li, W. Deng, J. Cheng and W. Su, Molecules, 2018, 23, 803 CrossRef PubMed.
- Y. He, Z. Li, W. Wang, S. R. Sooranna, Y. Shi, Y. Chen, C. Wu, J. Zeng, Q. Tang and H. Xie, Molecules, 2018, 23, 2189 CrossRef PubMed.
- Y. Yan, C.-Z. Chai, D.-W. Wang, X.-Y. Yue, D.-N. Zhu and B.-Y. Yu, J. Pharm. Biomed. Anal., 2013, 80, 192–202 CrossRef CAS PubMed.
- X. Nöst, E.-M. Pferschy-Wenzig, S. Nikles, X. He, D. Fan, A. Lu, J. Yuk, K. Yu, G. Isaac and R. Bauer, Molecules, 2019, 24, 3116 CrossRef PubMed.
- Z. Xue, C. Lai, L. Kang, A. Kotani, H. Hakamata, Z. Jing, H. Li, W. Wang and B. Yang, J. Chromatogr. A, 2020, 1611, 460583 CrossRef CAS PubMed.
- X. Sun, X.-b. Cui, H.-m. Wen, C.-x. Shan, X.-z. Wang, A. Kang, C. Chai and W. Li, J. Pharm. Biomed. Anal., 2017, 141, 19–31 CrossRef CAS PubMed.
- X. J. Li, W. Q. Qiu, X. L. Da, Y. J. Hou, Q. Y. Ma, T. Y. Wang, X. M. Zhou, M. Song, Q. L. Bian and J. X. Chen, Anat. Rec., 2020, 303, 2154–2167 CrossRef CAS PubMed.
- C. Shan, Q. Yuan, X. Cui, C. Chai, S. Yu, H. Wen and X. Huang, Biomed. Chromatogr., 2020, 34, e4818 CAS.
- F. Li, S. Wang, D. Lu, Y. Wang, D. Dong and B. Wu, Xenobiotica, 2017, 47, 369–375 CrossRef CAS PubMed.
- W. Li, J. Guo, Y. Tang, H. Wang, M. Huang, D. Qian and J. A. Duan, Int. J. Mol. Sci., 2012, 13, 3583–3597 CrossRef CAS PubMed.
- T. P. Hsueh and T. H. Tsai, Molecules, 2018, 23(10), 2716 CrossRef PubMed.
- J. A. Araujo-Leon, R. Ortiz-Andrade, R. A. Vera-Sanchez, J. E. Oney-Montalvo, T. I. Coral-Martinez and Z. Cantillo-Ciau, Molecules, 2020, 25(18), 4241 CrossRef CAS PubMed.
- Y. Bai, W. Peng, C. Yang, W. Zou, M. Liu, H. Wu, L. Fan, P. Li, X. Zeng and W. Su, Front. Pharmacol., 2020, 11, 364 CrossRef CAS PubMed.
- S. H. Jeong, J. H. Jang, G. Y. Lee, S. J. Yang, H. Y. Cho and Y. B. Lee, J. Pharm. Anal., 2021, 11, 444–457 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08778a |
| This journal is © The Royal Society of Chemistry 2022 |