Open Access Article
Open Access ArticleCarbazole-based bis-imidazole ligand-involved synthesis of inorganic–organic hybrid polyoxometalates as electrochemical sensors for detecting bromate and efficient catalysts for selective oxidation of thioether†
Xiang Wang *,
Jiafeng Lin,
Huan Li,
Chenying Wang and
Xiuli Wang
*,
Jiafeng Lin,
Huan Li,
Chenying Wang and
Xiuli Wang *
*
Liaoning Professional Technology Innovation Center of Liaoning Province for Conversion Materials of Solar Cell, College of Chemistry and Materials Engineering, Bohai University, Jinzhou, 121000, P. R. China. E-mail: xwang@bhu.edu.cn; wangxiuli@bhu.edu.cn
First published on 3rd February 2022
Abstract
Considering the potential application on preparing electrode and catalyst materials of inorganic–organic hybrid polyoxometalates, a bis-imidazole ligand with carbazole as a connector, 3,6-di(1H-imidazol-1-yl)-9H-carbazole (L), was used for preparing inorganic–organic hybrid polyoxometalates. As a result, three complexes formulated by [NiL2(Mo2O7)] (1), [Cu(H2O)2(HL)2 (β-Mo8O26)]·H2O (2) and [Ni2(H2O)4L2 (CrMo6(OH)5O19)]·6H2O (3) were obtained successfully. Structural analysis indicated that the different polyoxoanions and metal ions showed important influences on the formation of structures. In the presence of Ni2+ ions and heptamolybdate, a 2D network constructed from Ni2+ ions and L ligands was formed in complex 1, in which the [Mo4O14]4− polyoxoanions were encapsulated. But the use of Cu2+ ions led to a 1D chain of complex 2, which was composed of [β-Mo8O26]4− polyoxoanions and mononuclear {CuL2} units. By utilizing [CrMo6(OH)5O19]4− as the inorganic building block, complex 3 showed a 2D (4, 4)-connected layer. Complexes 1–3 could be employed as electrode materials for sensing bromate with the limits of detection of 0.315 μM for 1, 0.098 μM for 2 and 0.551 μM for 3. Moreover, these complexes showed efficient catalytic activity for the selective oxidation of thioethers.
Introduction
Nowadays, polyoxometalates (POMs) as anionic metal oxide clusters have attracted more and more attention because of their satisfactory properties and potential applications in many fields due to excellent redox properties, such as electrochemistry,1 catalysis,2,3 energy storage and battery,4–6 and so on, and also their diverse architectures,7,8 including classical Keggin, Wells–Dawson, Anderson, Waugh and Lindquist types. However, their disadvantages, such as aggregation and instability in most solvents, often hinder their promising application. Many strategies to overcome these disadvantages have been exploited. For example, the use of a host material is usually considered as a helpful approach, such as carbon nanotubes,9 or polypyrroles,10 because they can strengthen the stability of the structure, as well as conductivity and energy density of materials.Another method is to combine a POM and metal–organic framework (MOF), because MOFs own usually not only high crystallinity and satisfying stability, especially these obtained from hydrothermal condition, but also porous structures featuring 2D or 3D networks resulting from organic ligands as linkers and metal ions, as well as secondary building units, in which the POMs acting as guest molecules or inorganic linkages are involved.11,12 For instance, the Keggin type POMs as guest molecules were successfully embraced in the porous Cu2+/1,3,5-tricarboxybenzene MOF.13,14 The N-donor ligands are also popular candidates, because their abundant configurations and linking modes with metal centers usually result in the stable frameworks to accommodate POMs. To this day, a plenty of N-donor ligands have been employed to react with metal ions and POMs under hydrothermal condition, resulting in POM-based complexes materials with excellent properties and structures, where the POMs play different roles in the final structures. For example, the use of metalloporphyrin ligand generated stable POM-based MOFs with remarkable performance for scavenging of dyes and for heterogeneous selective oxidation of alkylbenzenes, or highly selective electroreduction of CO2.15–18 The carbazole-contained ligands, 4-{[4-(9H-carbazol-9-yl)phenyl]ethynyl}aniline and 2-(9H-carbazol-9-yl)-N-(1,3-dihydroxy-2-(hydroxymethyl) propan-2-yl)acetamide have been also designed and used to prepare the complexes based on Lindqvist and Anderson POMs, the non-linear optical property and genotoxicity of which were evaluated.19,20
Based on their potential feasibilities of the analysis of the electrocatalytic reaction,21 the electrochemical techniques were regarded as considerable means for detecting bromate because of their high sensitivity, uncomplicated and fast procedures,22 compared with other ways such as ion chromatography,23 liquid chromatography-mass spectrometry,24 spectrofluorometry,25 and so on. The POM-based complexes may be satisfactory candidates as electrode materials to prepare the electrochemical sensor for detecting bromate due to their stable structures and satisfactory redox abilities. For example, the complexes based on Keggin and Wells–Dawson POMs by using 1,4-bis(1-imidazol-yl)-2,5-dimethyl benzene, 1,2,4-triazole and terphenyl-based tricarboxylate as organic ligands, could be chosen as electrode materials to fabricate electrochemical sensors for the detection of bromate.26–28 The complexes based on octamolybdate and Anderson POMs originated from bis-amide ligands exhibited promising applications on the preparation of electrochemical sensors of bromate.29,30 Further, such complexes could also be utilized to prepare composite materials or films as the electrode materials of electrochemical sensors of bromate.31–33 The above investigations manifest that the synthesis of polyoxometalate-based complexes composed of metal ions and organic ligands is of great significance for the development of catalysts and electrode materials.
In this work, a bi-imidazole ligand bridged each other by a carbazole connector, 3,6-di(1H-imidazol-1-yl)-9H-carbazole(L), was used in order to synthesize the POM-based complex materials. By adjusting the polyoxoanions and metal ions, three POM-based complexes, [NiL2(Mo2O7)] (1), [Cu(H2O)2(HL)2 (β-Mo8O26)]·H2O (2) and [Ni2(H2O)4L2 (CrMo6(OH)5O19)]·6H2O (3), have been prepared under hydrothermal condition. Complex 1 is a 2D structure, and the [Mo4O14]4− inorganic units are encapsulated in a network constructed from Ni2+ ions and L ligands. Complex 2 shows a chilopod-like chain composed of mononuclear {CuL2} units and [β-Mo8O26]4− polyoxoanions. In 3, the L ligands link the 1D [CrMo6(OH)5O19]4− POM-based inorganic chains into a 2D (4,4)-connected network. The metal centers and polyoxoanions play important roles in tuning the diverse architectures. Moreover, the complexes display satisfying electrochemical sensing behaviors for BrO3− and catalytic performance for oxidation of thioether to sulfoxide.
Experimental
Reagent and apparatus
The L ligand and other reagents during experimental operation were purchased from commercial sources and used directly. FT-IR were measured on a Varian 640 FT-IR spectrometer (KBr pellets). The morphological characterization was analysed by using a Hitachi S-4800 scanning electron microscope (SEM). The powder X-ray diffraction (PXRD) data was collected by using a Rigaku Ultima IV diffractometer. The catalytic reaction of oxidation of thioether was monitored by utilizing a Shimadzu Techcomp GC-7900.Preparation of complexes 1–3
Electrochemical measurements
All electrochemical studies were achieved on a CHI 660 electrochemical workstation. The cyclic voltammogram and amperometric measurements were performed to study the response current and sensing behavior toward bromate of the electrode materials prepared from three complexes. Electrochemical measurements were performed in 0.1 M H2SO4 + 0.5 M Na2SO4 aqueous solution containing a certain number of bromate. The carbon paste electrodes (CPEs) modified by complexes 1–3 (1–3-CPEs),34 Pt wire and saturated calomel electrode were chosen as the working, counter and reference electrodes.General procedure for the oxidation of sulfides
Sulfides (0.5 mmol), tert-butyl hydroperoxide (TBHP) (0.75 mmol), catalyst (3 μmol), and methanol (2 mL) were added into a 25 mL round-bottom tube. The mixture was reacted at the set temperature. The conversion and selectivity of the resulting products were analyzed by GC with naphthalene as an internal standard.X-ray structure determination
The crystal data of complexes 1–3 were collected on a Bruker SMART APEX II with Mo Kα (λ = 0.71073 Å) at 293 K by ω and θ scan mode. All structures of 1–3 were solved and refined by employing SHELXTL-2014 packages and Olex2 software and using direct methods.35,36 The primary crystal data and structure refinements of 1–3 are summarized in Table 1. The crystallographic data of 1–3 have been deposited in the Cambridge Crystallographic Data Center with CCDC No. 2123433–2123435. Table S1† includes the selected bond lengths and angles.| Complex | 1 | 2 | 3 |
|---|---|---|---|
| a R1 = ∑∥Fo| − |Fc∥/∑|Fo|.b wR2 = [∑w(Fo2 − Fc2)2]/∑[w(Fo2)2]1/2. | |||
| Formula | C36H26Mo2N10NiO7 | C36H34CuMo8N10O29 | C36H51CrMo6N10Ni2O34 |
| Fw | 961.24 | 1901.77 | 1912.86 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | P21/n | C2/c | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a Å−1 | 12.1311 (14) | 8.4459 (4) | 7.8269 (4) |
| b Å−1 | 19.995 (2) | 24.3390 (11) | 10.4537 (5) |
| c Å−1 | 14.5390 (16) | 24.7639 (11) | 17.1966 (8) |
| α °−1 | 90.000 (5) | 90 | 95.1090 (10) |
| β °−1 | 93.938 (2) | 95.4410 (10) | 91.5670 (10) |
| γ °−1 | 90.000 (5) | 90 | 97.2660 (10) |
| V Å−3 | 3518.2 (7) | 5067.6 (4) | 1389.12 (12) |
| Z | 4 | 4 | 1 |
| Dc (g cm−3) | 1.815 | 2.487 | 2.261 |
| μ mm−1 | 1.299 | 2.427 | 2.264 |
| F (000) | 1920.0 | 3652.0 | 920.0 |
| Final R1,a wR2b [I >2σ(I)] | 0.0490, 0.1303 | 0.0355, 0.0721 | 0.0383, 0.0908 |
| Final R1,a wR2b (all data) | 0.0813, 0.1552 | 0.0623,0.0810 | 0.0524, 0.0974 |
| Goodness on F2 | 0.918 | 1.017 | 1.042 |
Results and discussion
Crystal structures
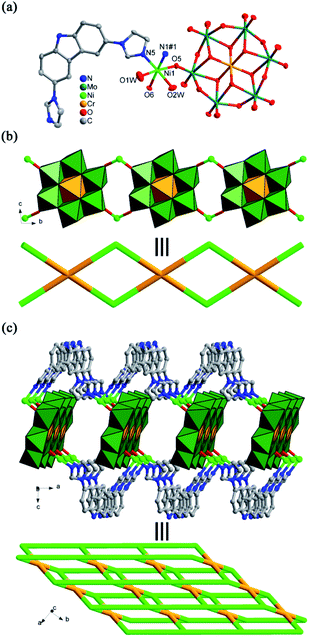
Complex 1 consists of one Ni2+ ion, two L ligands and one [Mo2O7]2− anion. Bond valence sum calculation indicates the Ni and Mo ions are in +II and +VI oxidation states,37 respectively. The Ni atom shows a six-coordinated mode with an octahedral configuration (Fig. 1a), surrounded by two terminal oxygen atoms belonging to [Mo2O7]2− anion and four nitrogen atoms from two L ligands. The bond length of Ni–O and Ni–N are 2.056–2.192 Å. All two Mo atoms are five-coordinated modes. Both {MoO2} and {MoO3} clusters are aggregated through one bridged oxygen atom μ2-O7 to form a {Mo2O6} cluster (Fig. 1b). Two μ3-O6 oxygen atoms connect two {Mo2O6} clusters into a {Mo4O14} cluster (Fig. 1c).Complex 1 is a 2D structure. Firstly, a binuclear metal–organic ring {Ni2L2} with a size of 11.58 Å × 9.90 Å is constructed from two Ni atoms and two L ligands depending on the coordination bonds between imidazole group and Ni atom (Fig. 2a). The size of the {Ni2L2} ring is enough to accommodate one {Mo4O14} cluster, in which one {Mo4O14} cluster can be perfectly encapsulated, resulting in a POM-based ring. Moreover, the {Mo4O14} cluster provides two pairs of oxygen atoms including two terminal O5 and two μ2-O7 atoms to coordinate together with the Ni atoms from the {Ni2L2} ring (Fig. 2b), respectively. Further, the remaining L ligand links the POM-based ring into a 2D layer (Fig. 2c), which offers two N atoms of imidazole group to coordinated with the Ni atom from adjacent POM-based ring. In fact, each of Ni atoms of the POM-based ring is surrounded by four L ligand. Or to say, the {Ni2L2} rings are extended via four L ligands into a 2D network and the {Mo4O14} cluster are embedded into these {Ni2L2} rings. Considering the POM-based rings as four connected modes, the 2D layer can be simplified as a (4, 4)-connected network (Fig. 2d).
 | ||
| Fig. 2 (a) View of the metal–organic ring {Ni2L2}. (b) The {Mo4O14} cluster-encapsulated ring. (c) View of the 2D layer of complex 1. (d) View of the (4, 4)-connected network of 1. | ||
Complex 2 is compose of one Cu2+ ion, one [β-Mo8O26]4− (abbreviated to Mo8) polyoxoanion, two L ligands, two coordinated water molecules and one crystal water molecule. One of two imidazole groups of L ligand is protonated. Bond valence sum calculation indicates the Cu and Mo ions are in +II and +VI oxidation states,37 respectively. The Cu atom has a six-coordinated mode (Fig. 3a), defined by two nitrogen atoms from two L ligands, two oxygen atoms of the Mo8 anion and two water molecules. The bond length of Cu–O and Cu–N are 1.951–2.303 Å.
In the structure of complex 2, the combination of two L ligands and one Cu atom via Cu–N coordination bond to generate a mononuclear unit {CuL2} (Fig. 3b). Each of Mo8 polyoxoanions acting as bidentate inorganic building block utilizes two symmetric terminal oxygen atoms to coordinate with the Cu atom from the mononuclear unit {CuL2}, forming a 1D chilopod-like chain of complex 2 (Fig. 3c). Meanwhile, two water molecules compensate the coordination numbers of the Cu center.
Complex 3 consists of one Ni2+ ion, two L ligands, one Anderson-type polyoxoanion [CrMo6(OH)5O19]4− (abbreviated to CrMo6), three coordinated water molecules and six crystal water molecules. Bond valence sum calculation indicates the Ni and Mo ions are in +II and +VI oxidation states,37 respectively.
The six-coordinated Ni atom is surrounded by two water molecules, two nitrogen atoms from two imidazole groups of L ligand, two oxygen atoms of the CrMo6 anion (Fig. 4a), showing an octahedral configuration. The CrMo6 anion acts as a tetra-dentate inorganic ligand to provide four oxygen atoms to coordinate with four Ni atoms.
Complex 3 has a 2D structure. The Ni atoms join the CrMo6 anions in pairs into a 1D POM-based inorganic chain (Fig. 4b). Then the L ligand plays a role of bi-dentate linker, utilizing two nitrogen atoms of imidazole group to coordinate with two Ni atoms from neighboring POM-based inorganic chains. Such the coordination pattern induces a 2D structure of complex 3. If both Ni atoms and CrMo6 anions are considered as four-connected nodes, the 2D layer can be simplified as a (4,4)-connected network (Fig. 4c).
Morphology, PXRD and FT-IR spectra
The SEM of the crystalline complexes was carried out, as shown in Fig. S1.† It can be observed that the lumpy crystals of complexes 1–3 were synthesized during their preparation process. The phase purity of complexes 1–3 was verified by PXRD. The experimental diffraction peaks are consistent with that simulated by crystal data, as shown in Fig. S2,† revealing that two complexes have good phase purities. Additionally, the IR spectra of 1–3 and L were recorded in the range of 4000–400 cm−1 (Fig. S3†). The absorption bands in the region of 1582 to 1062 cm−1 indicate the presence of organic ligand L. The absorption bands at 929, 908, 880, 807 cm−1 for 1, 955, 904,836, 817 cm−1 for 2, 938, 918, 885, 634, 568 cm−1 for 3, should be ascribed to the characteristic peaks of polyoxoanions.30,38 These results confirm further the composition of all three complexes.Electrochemical properties
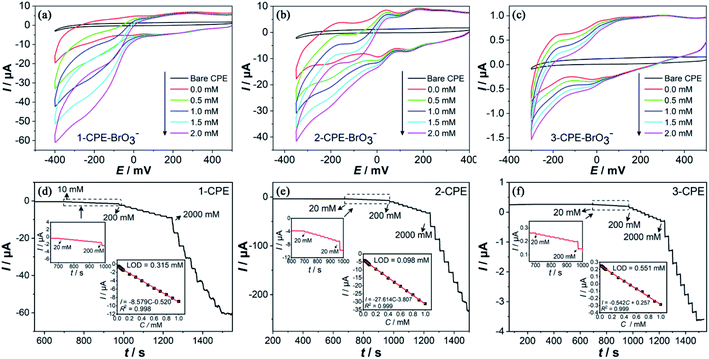
The electrochemical properties of complexes 1–3, including the cyclic voltammogram, electrocatalytic and amperometric sensing behaviors, were investigated. As shown in Fig. 5a–c, three complexes show three pairs of redox peaks with the average peak potentials calculated based on E1/2 = (Epa + Epc)/2 of +284 mV (I–I′), +30 mV (II–II′), −160 mV (III–III′) for 1-CPE, +208 mV (I–I′), +65 mV (II–II′), −146 mV (III–III′) for 2-CPE, +186 mV (I–I′), +41 mV (II–II′), −99 mV (III–III′) for 3-CPE, respectively, which should be attributed to three consecutive two-electron redox processes of Mo centers.39 The linear relationship between peak current and scan rate (II–II′) indicates that the redox reaction belongs to be surface-controlled (Fig. 5d). The excellent redox abilities of complexes 1–3 indicate their potential applications as electrode materials in the electrochemical fields.Based on the excellent redox abilities of complexes 1–3, the electrocatalytic activities of 1-, 2- and 3-CPEs toward the reduction of BrO3− were inspected in the 0.1 M H2SO4 + 0.5 M Na2SO4 aqueous solution. It can be observed that the gradual addition of BrO3− causes the obvious increase of reduction peak currents of 1–3-CPEs (Fig. 6a–c), especially the third reduction peak, while the corresponding oxidation peak currents decrease, indicating that complexes 1–3 have good electrocatalytic activities toward the reduction of BrO3−, which suggests their potential applications for preparing electrochemical sensors depending on the current responses with the change of concentration.
Further, the electrochemical sensing activities toward BrO3− of 1–3-CPEs as amperometric sensors were evaluated. Fig. 6d–f illustrates that 1–3-CPEs exhibit quick current responses when the BrO3− is added continuously at 30 s intervals at optimal voltage, respectively. At the same time, there is a remarkable linear relationship between response current and substrate concentration in the range of 10 to 1000 μM. Based on the linear regression equation of I = −8.579C − 0.520 (R2 = 0.998) for 1-CPE, I = −27.614C − 3.807 (R2 = 0.999) for 2-CPE and I = −0.542C + 0.257 (R2 = 0.999) for 3-CPE, the sensitivity and limit of detection (LOD) of 1–3-CPEs are calculated as 8.58 μA mM−1 and 0.315 μM for 1-CPE, 27.61 μA mM−1 and 0.098 μM for 2-CPE, 0.54 μA mM−1 and 0.551 μM for 3-CPE. The LOD of 2-CPE is lowest in comparison with that 1-CPE and 3-CPE, which are comparable to the reported electrochemical sensors (Table S2†). To understand the differences of electrocatalytic activity of the CPEs modified by three complexes, the electrochemical impedance spectroscopy (EIS) could be carried out to determine the electroconductivity of 1–3-CPEs (Fig. S4†).40 The Nyquist plot of 2-CPE possesses smallest charge-transfer impedance than that of 1- and 3-CPEs, which may be resulted by their different structures, that may be beneficial to the electron transfer during the electrocatalytic reduction of BrO3−,40 suggesting the high electrochemical performance of 2-CPE. The mechanism may be that the BrO3− could be reduced by the multi-electron-reduced products of complexes. Three pairs of redox peaks (I–I′, II–II′, III–III′) of 1–3-CPEs should belong to three consecutive two-electron redox processes of Mo centers, and the electrochemical reaction can be shown as follows.41,42
| POM − complex + 2H+ + 2e− → H2POM − complex |
| H2POM − complex + 2H+ + 2e− → H4POM − complex |
| H4POM − complex + 2H+ + 2e− → H6POM − complex |
Considering that the addition of BrO3− can lead to the obvious increase of reduction peak currents (II–II′, III–III′), especially the third reduction peak, so the catalytic behavior toward BrO3− may involve the four-electron-reduced and six-electron-reduced products, producing bromide as the product.26,43 The catalytic chemical steps can be explained by the following reactions.
| 3H4POM − complex + 2BrO3− → 3POM − complex + 2Br− + 6H2O |
| H6POM − complex + BrO3− → POM − complex + Br− + 3H2O |
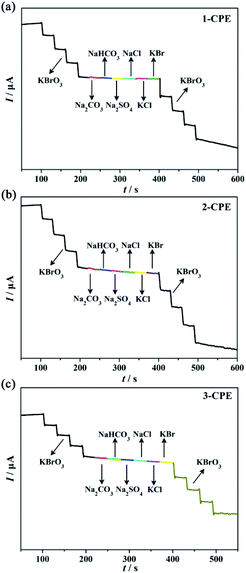
The anti-interference ability is also usually considered as the key to evaluate the performance of the electrochemical sensors. Here, the CO32−, HCO32, SO42−, Cl− and Br− as potential interference substances are introduced to investigate the anti-interference abilities of 1–3-CPEs as electrochemical sensors in the process of detecting BrO3−. As shown in Fig. 7, the addition of BrO3− can lead to obvious current responses of the corresponding CPEs, but the response remained unchanged when the selected potential interference substances are added, indicating that 1–3-CPEs as electrochemical sensors of BrO3− can exhibit excellent selectivity and anti-interference ability.
Catalytic oxidation of thioether
Generally, the POM-based complexes could usually be recognized as good catalysts for oxidation of thioether,44,45 so the catalytic performances of complexes 1–3 for the oxidation of thioether were also studied here. The reaction of oxidation of methyl phenyl thioether (MPS) with 1 as catalyst and TBHP as oxidant was used to investigate the influences on the catalytic efficiencies of dosage of catalyst, reaction time, oxidant and temperature (Table 2). Firstly, the influence of reaction temperature is evaluated under the reaction condition containing 0.5 mmol substrate, 3 μmol catalysts and 0.75 mmol TBHP. Increasing the reaction temperature from room temperature to 50 °C, the conversion and selectivity reach to 99.5% and 98.6% within 40 minutes (Entries 1–3). When the temperature is raised to 60 °C, the conversion and selectivity are reduced to 99.0% and 96.3% (Entry 4), which reveals that the optimum reaction temperature is 50 °C.| Entry | Catalyst (μmoL) | TBHP (mmoL) | Time (min) | Temp. (°C) | Conv. (%)b | Sel. (%)c |
|---|---|---|---|---|---|---|
| a Reaction condition: substrate (0.5 mmoL), methanol (2 mL), 1 (3 μmoL, 0.6 mol%).b Conversion and selectivity were analyzed by GC.c Conversion and selectivity were analyzed by GC.d H2O2.e Ethanol (2 mL).f CHCl3 (2 mL).g Acetonitrile (2 mL). | ||||||
| 1 | 3 | 0.75 | 40 | rt | 81.5 | 91.1 |
| 2 | 3 | 0.75 | 40 | 40 | 99.1 | 97.0 |
| 3 | 3 | 0.75 | 40 | 50 | 99.5 | 98.6 |
| 4 | 3 | 0.75 | 40 | 60 | 99.0 | 96.3 |
| 5 | 3 | 0.75 | 30 | 50 | 78.5 | 99.5 |
| 6 | 3 | 0.5 | 40 | 50 | 91.0 | 99.2 |
| 7 | 3 | None | 40 | 50 | <10 | 96.5 |
| 8 | 6 | 0.75 | 40 | 50 | 98.9 | 96.6 |
| 9 | None | 0.75 | 40 | 50 | 33.2 | 98.9 |
| 10 | 3d | 0.75 | 40 | 50 | 98.0 | 84.0 |
| 11 | 3e | 0.75 | 40 | 50 | 93.1 | 98.5 |
| 12 | 3f | 0.75 | 40 | 50 | 87.8 | 97.4 |
| 13 | 3g | 0.75 | 40 | 50 | 78.0 | 98.7 |
Further, the changes of reaction time, dosage of catalyst and oxidants under the optimum reaction temperature lead to the decreases of conversion and selectivity (Entries 5–10). Meanwhile, the solvents such as ethanol, CHCl3 and acetonitrile are appraised, indicating that methanol is satisfactory (Entries 11–13). So the most effective reaction condition is to use methanol as solvent under 50 °C within 40 minutes, with the suitable mole ratio of 1:1.5:0.6% for substrate, oxidate and catalyst, respectively.
The catalytic activities of 2 and 3, as well as raw materials molybdate and CrMo6 were further investigated under optimum condition, as shown in Table 3. When the complexes 2 and 3 are employed as catalysts, the excellent conversions and selectivity of 99.2% and 98.1% for 2, 99.0% and 97.3% for 3, can be obtained, which are comparable to the reported POM-based hybrids.46–48 However, the use of (NH4)6Mo7O24·4H2O and CrMo6 cause the lower conversions of 10.1% and 64.1%, despite the high selectivity of 90.9% and 91.4%. These results reveal that complexes 1–3 can show satisfying catalytic performances toward the oxidation of MPS to sulfoxide.
| Catalyst | Conv. (%) | Sel. (%) |
|---|---|---|
| 1 | 99.5 | 98.6 |
| 2 | 99.2 | 98.1 |
| 3 | 99.0 | 97.3 |
| (NH4)6Mo7O24·4H2O | 10.1 | 90.9 |
| CrMo6 | 64.1 | 91.4 |
Further, the catalytic ability of 1 as a representative was estimated by choosing the thioether derivatives including these containing different groups and alkyl thioethers under the optimum condition. The results are summarized in Table 4. It can be observed that the conversions and selectivity of 4-methoxythioanisole, 4-chlorothioanisole and diphenylthioether due to the larger steric resistance are lower than that of MPS (Entries 2–4), but the conversions and selectivity are satisfactory or higher than that of MPS when the alkyl thioethers such as dipropyl thioether and 2-chloroethyl ethyl thioether are chosen (Entries 5 and 6). These results manifest that the steric hindrance of substrate play key influences on the conversion of thioether to sulfoxide in the presence of 1 as catalyst, which is similar to those reports.49–51
Considering that the recyclability and stability are very important to evaluate the catalytic performance of catalyst, the 1 as representative was also investigated by utilizing the reaction of MPS to sulfoxide under optimum condition (Fig. 8).
The catalysts were filtered after catalytic reaction, and washed by using methanol, which were further reused to catalyze the oxidation of MPS to sulfoxide. After 3 runs, the catalytic ability of complex 1 has barely changed, and the conversion and selectivity remain still above 96%, indicating that the 1 as catalyst has good recyclability for the oxidation of MPS to sulfoxide. Furthermore, the stability of catalyst after catalytic reaction was assessed relying on PXRD (Fig. S5†), confirming the good stability of catalyst during the catalytic reaction.
Conclusions
In a word, three new POM-based complexes were prepared by using a bi-imidazole ligand containing carbazole connector. The metal centers and polyoxoanions play important roles in tuning the diverse architectures of the complexes. These complexes can be employed as the electrode materials for preparing the electrochemical sensors of BrO3− with lower LODs, likewise, as efficient catalysts for the oxidation of thioether to sulfoxide accompanying with satisfying recyclability and stability, which opens up a possible means for preparing novel POM-hybrids as electrochemical sensor and catalyst materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The National Natural Science Foundation of China (No. 21771025, 21971024, 21671025) and the General Program Fund for Education Department of Liaoning Province (LJ2019004) are gratefully acknowledged.Notes and references
- M. Sadakane and E. Steckhan, Chem. Rev., 1998, 98, 219 CrossRef CAS PubMed.
- D. Jana, H. K. Kolli, S. Sabnam and S. K. Das, Chem. Commun., 2021, 57, 9910 RSC.
- Y. Zou, H. Li, X. Zhao, J. Song, Y. Wang, P. Ma, J. Niu and J. Wang, Dalton Trans., 2021, 50, 12664 RSC.
- C. Lee, D. Jeon, J. Park, W. Lee, J. Park, S. J. Kang, Y. Kim and J. Ryu, ACS Appl. Mater. Interfaces, 2020, 12, 32689 CrossRef CAS PubMed.
- S. Herrmann, N. Aydemir, F. Nägele, D. Fantauzzi, T. Jacob, J. Travas-Sejdic and C. Streb, Adv. Funct. Mater., 2017, 27, 1700881 CrossRef.
- J.-J. Chen, M. D. Symes, S.-C. Fan, M.-S. Zheng, H. N. Miras, Q.-F. Dong and L. Cronin, Adv. Mater., 2015, 27, 4649 CrossRef CAS PubMed.
- D. Pakulski, A. Gorczyński, W. Czepa, Z. Liu, L. Ortolani, V. Morandi, V. Patroniak, A. Ciesielski and P. Samorì, Energy Storage Mater., 2019, 17, 186 CrossRef.
- S. G. Mitchell, C. Streb, H. N. Miras, T. Boyd, D.-L. Long and L. Cronin, Nat. Chem., 2010, 2, 308 CrossRef CAS PubMed.
- J. W. Jordan, G. A. Lowe, R. L. McSweeney, C. T. Stoppiello, R. W. Lodge, S. T. Skowron, J. Biskupek, G. A. Rance, U. Kaiser, D. A. Walsh, G. N. Newton and A. N. Khlobystov, Adv. Mater., 2019, 31, 1904182 CrossRef CAS PubMed.
- S. Maity, M. Je, B. R. Biradar, P. R. Chandewar, D. Shee, P. P. Das and S. S. Mal, Energy Fuels, 2021, 35, 18824 CrossRef CAS.
- P. Mialane, C. Mellot-Draznieks, P. Gairola, M. Duguet, Y. Benseghir, O. Oms and A. Dolbecq, Chem. Soc. Rev., 2021, 50, 6152 RSC.
- R. Yu, X.-F. Kuang, X.-Y. Wu, C.-Z. Lu and J. P. Donahue, Coord. Chem. Rev., 2009, 253, 2872 CrossRef CAS.
- C.-Y. Sun, S.-X. Liu, D.-D. Liang, K.-Z. Shao, Y.-H. Ren and Z.-M. Su, J. Am. Chem. Soc., 2009, 131, 1883 CrossRef CAS PubMed.
- X.-J. Yu, H. Zhong, Y.-M. Xian, Z.-P. Wang, S. Schneider, J. Scherr, T. Abu-Husein, Z. Zhang and A. Terfort, Dalton Trans., 2020, 49, 16627 RSC.
- C. Zou, Z. J. Zhang, X. Xu, Q. H. Gong, J. Li and C. D. Wu, J. Am. Chem. Soc., 2011, 134, 87 CrossRef PubMed.
- Y.-R. Wang, Q. Huang, C.-T. He, Y. Chen, J. Liu, F.-C. Shen and Y.-Q. Lan, Nat. Commun., 2018, 9, 4466 CrossRef PubMed.
- Q. Huang, J. Liu, L. Feng, Q. Wang, W. Guan, L.-Z. Dong, L. Zhang, L.-K. Yan, Y.-Q. Lan and H.-C. Zhou, Natl. Sci. Rev., 2019, 7, 53 CrossRef PubMed.
- C. Li, N. Mizuno, K. Yamaguchi and K. Suzuki, J. Am. Chem. Soc., 2019, 141, 7687 CrossRef CAS PubMed.
- A. Al-Yasari, P. Spence, H. El Moll, N. Van Steerteghem, P. N. Horton, B. S. Brunschwig, K. Clays and J. Fielden, Dalton Trans., 2018, 47, 10415 RSC.
- V. S. V. Satyanarayana, P. G. Reddy and C. P. Pradeep, RSC Adv., 2015, 5, 59609 RSC.
- L. Marleny Rodriguez-Albelo, A. R. Ruiz-Salvador, A. Sampieri, D. W. Lewis, A. Gómez, B. Nohra, P. Mialane, J. Marrot, F. Sécheresse, C. Mellot-Draznieks, R. Ngo Biboum, B. Keita, L. Nadjo and A. Dolbecq, J. Am. Chem. Soc., 2009, 131, 16078 CrossRef CAS PubMed.
- Y.-G. Lee, H. J. Lee and A. Jang, Sens. Actuators, B, 2017, 244, 157 CrossRef CAS.
- H. B. Teh and S. F. Y. Li, J. Chromatogr. A, 2015, 1383, 112 CrossRef CAS PubMed.
- M. R. Khan, S. M. Wabaidur, Z. A. Alothman, R. Busquets and M. Naushad, Talanta, 2016, 152, 513 CrossRef CAS PubMed.
- H. M. Al-Saidi and M. S. El-Shahawi, Spectrochim. Acta, Part A, 2015, 138, 736 CrossRef CAS PubMed.
- S. Li, B. Lu, J. Xin, L. Zhang, J. Pan, Y. Chen and X. Tan, J. Solid State Chem., 2019, 278, 120905 CrossRef CAS.
- B. R. Lu, S. B. Li, X. Z. Zhang, D. Q. Zhang, L. L. Fan, E. Y. Yan, Y. J. Zhang and L. Yu, New J. Chem., 2019, 43, 15804 RSC.
- B. X. Dong, F. Y. Bu, Y. C. Wu, J. Zhao, Y. L. Teng, W. L. Liu and Z. W. Li, Cryst. Growth Des., 2017, 17, 5309 CrossRef CAS.
- X. L. Wang, Z. W. Cui, H. Y. Lin and Z. H. Chang, CrystEngComm, 2021, 23, 2113 RSC.
- L. Li, X. Wang, N. Xu, Z. H. Chang, G. C. Liu, H. Y. Lin and X. L. Wang, CrystEngComm, 2020, 22, 8322 RSC.
- L. Zhang, L. Chen, S. X. Liu, J. Gong, Q. Tang and Z. M. Su, Dalton Trans., 2018, 47, 105 RSC.
- Y. N. Zhang, Y. Zhang, L. Li, J. L. Chen, P. Z. Li and W. H. Huang, J. Electroanal. Chem., 2020, 861, 113939 CrossRef CAS.
- G. G. Papagianni, D. V. Stergiou, G. S. Armatas, M. G. Kanatzidis and M. I. Prodromidis, Sens. Actuators, B, 2012, 173, 346 CrossRef CAS.
- X. Wang, H. Li, J. Lin, C. Wang and X.-L. Wang, Inorg. Chem., 2021, 60, 19287 CrossRef CAS PubMed.
- G. M. Sheldrick, SHELXS-2014, Program for Structure Solution, University of Göottingen, Germany, 2014 Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339 CrossRef CAS.
- I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B: Struct. Sci., 1985, 41, 244 CrossRef.
- X. Pan, X. L. Wang, X. Wang, Y. Li, G. C. Liu and H. Y. Lin, CrystEngComm, 2019, 21, 6472 RSC.
- M. L. Yang, S. Rong, X. M. Wang, H. Y. Ma, H. J. Pang, L. C. Tan, Y. X. Jiang and K. Q. Gao, ChemNanoMat, 2021, 7, 299 CrossRef CAS.
- Y. L. Wang, Y. Y. Ma, Q. Zhao, L. Hou and Z. G. Han, Sens. Actuators, B, 2020, 305, 127469 CrossRef CAS.
- B.-X. Dong, L. Chen, S. Y. Zhang, J. Ge, L. Song, H. Tian, Y. L. Teng and W. L. Liu, Dalton Trans., 2015, 44, 1435 RSC.
- L. Chen, L. Tian, L. Liu, X. F. Tian, W. B. Song, H. D. Xu and X. H. Wang, Sens. Actuators, B, 2005, 110, 271 CrossRef CAS.
- Y. C. Li, W. F. Bu, L. X. Wu and C. Q. Sun, Sens. Actuators, B, 2005, 107, 921 CrossRef CAS.
- K. Liu, Z. X. Yao and Y.-F. Song, Ind. Eng. Chem. Res., 2015, 54, 9133 CrossRef CAS.
- X. R. Sun, J. Dong, Z. Li, H. F. Liu, X. T. Jing, Y. N. Chi and C. W. Hu, Dalton Trans., 2019, 48, 5285 RSC.
- Y. Zhang, W.-D. Yu, B. Li, Z.-F. Chen and J. Yan, Inorg. Chem., 2019, 58, 14876 CrossRef CAS PubMed.
- Q. F. Xu, X. P. Sun, F. Hu, R. Wan, V. Singh, P. T. Ma, J. Y. Niu and J. P. Wang, Materials, 2017, 10, 1173 CrossRef PubMed.
- C. F. Pereira, F. Figueira, R. F. Mendes, J. Rocha, J. T. Hupp, O. K. Farha, M. M. Q. Simões, J. P. C. Tomé and F. A. A. Paz, Inorg. Chem., 2018, 57, 3855 CrossRef CAS PubMed.
- X.-S. Wang, Y.-B. Huang, Z.-J. Lin and R. Cao, Dalton Trans., 2014, 43, 11950 RSC.
- D. J. Thompson, Y. Zhang and T. Ren, J. Mol. Catal. A: Chem., 2014, 392, 188 CrossRef CAS.
- J.-K. Li, J. Dong, C.-P. Wei, S. Yang, Y.-N. Chi, Y.-Q. Xu and C.-W. Hu, Inorg. Chem., 2017, 56, 5748 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Table of bond lengths and angles, PXRD, FTIR, EIS spectra, SEM and Table summarizing sensors of Cr(VI). CCDC [2123433–2123435]. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra08861k |
| This journal is © The Royal Society of Chemistry 2022 |