Open Access Article
Open Access ArticleUnravelling the one-pot conversion of biomass-derived furfural and levulinic acid to 1,4-pentanediol catalysed by supported RANEY® Ni–Sn alloy catalysts†
Rodiansono *ab,
Maria Dewi Astutia,
Kamilia Mustikasaria,
Sadang Husainc,
Fathur Razi Ansyahd,
Takayoshi Harae and
Shogo Shimazu
*ab,
Maria Dewi Astutia,
Kamilia Mustikasaria,
Sadang Husainc,
Fathur Razi Ansyahd,
Takayoshi Harae and
Shogo Shimazu e
e
aDepartment of Chemistry, Lambung Mangkurat University, Jl. A. Yani Km 36 Banjarbaru, Indonesia 70714. E-mail: rodiansono@ulm.ac.id; Fax: +62 5114773112; Tel: +62 5114773112
bCatalysis for Sustainable Energy and Environment (CATSuRe), Wetland-based Material Research Center, Lambung Mangkurat University, Indonesia
cDepartment of Physics, Lambung Mangkurat University, Jl. A. Yani Km 36, Banjarbaru, Indonesia 70714
dDepartment of Mechanical Engineering, Lambung Mangkurat University, Jl. A. Yani Km 35.5, Banjarbaru, Indonesia 70714
eGraduate School of Engineering, Chiba University, 1-33 Yayoi, Inage-ku, Chiba, Japan 263-8522
First published on 20th December 2021
Abstract
Bimetallic Ni–Sn alloys have been recognised as promising catalysts for the transformation of furanic compounds and their derivatives into valuable chemicals. Herein, we report the utilisation of a supported bimetallic RANEY® nickel–tin alloy supported on aluminium hydroxide (RNi–Sn(x)/AlOH; x is Ni/Sn molar ratio) catalysts for the one-pot conversion of biomass-derived furfural and levulinic acid to 1,4-pentanediol (1,4-PeD). The as prepared RNi–Sn(1.4)/AlOH catalyst exhibited the highest yield of 1,4-PeD (78%). The reduction of RNi–Sn(x)/AlOH with H2 at 673–873 K for 1.5 h resulted in the formation of Ni–Sn alloy phases (e.g., Ni3Sn and Ni3Sn2) and caused the transformation of aluminium hydroxide (AlOH) to amorphous alumina (AA). The RNi–Sn(1.4)/AA 673 K/H2 catalyst contained a Ni3Sn2 alloy as the major phase, which exhibited the best yield of 1,4-PeD from furfural (87%) at 433 K, H2 3.0 MPa for 12 h and from levulinic acid (up to 90%) at 503 K, H2 4.0 MPa, for 12 h. Supported RANEY® Ni–Sn(1.5)/AC and three types of supported Ni–Sn(1.5) alloy (e.g., Ni–Sn(1.5)/AC, Ni–Sn(1.5)/c-AlOH, and Ni–Sn(1.5)/γ-Al2O3) catalysts afforded high yields of 1,4-PeD (65–87%) both from furfural and levulinic acid under the optimised reaction conditions.
Introduction
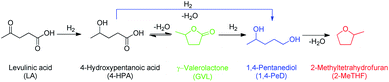
Nowadays, the development of innovative methods for the synthesis of building blocks of polymers from biomass-based materials to replace petroleum-based materials is an essential topic for many research groups.1 Furfural (FFald) and levulinic acid (LA), produced from lignocellulosic biomasses, are the most important renewable platform chemicals as they are a vital resource for the synthesis of biogenic C5 diols, such as 1,2-pentanediol (1,2-PeD), 1,4-pentanediol (1,4-PeD) or 1,5-pentanediol (1,5-PeD).2–4 In fact, 1,4-PeD can be used as a building block in the production of polyesters, thermo-plastic polyurethanes, plasticizers, and as a component of saturated and unsaturated polyester resins and alkyd resins.1,5,6 It can be also used as an intermediate for dyes and of pharmaceuticals and pesticides, and a precursor for the production of various heterocyclic compounds (e.g., 1-methylpiperidine).7–9Literatures show that 1,4-PeD can be synthesised from the catalytic hydrogenation of LA, ethyl levulinate (EL) or γ-valerolactone (GVL) both in liquid and vapor phases. Adkins and Folkers-types of Cu–CrO3 catalysts were mainly employed with moderate yield of 1,4-PeD (83%) even under harsh reaction conditions (523–546 K; ≥20 MPa).4,10 A number attempts have been devoted by using copper-based catalysts such as Cu/SiO2,11 Cu/ZnO,12 skeletal CuAlZn,13 and Cu–Ni–Zn/H-ZSM-5 (ref. 14) catalysts have been reported to produce high yield of 1,4-PeD (up to 93%). The main drawbacks associated with the Cu-based catalyst systems are due to possible leaching out metal active in aqueous phase system which may suffer the catalyst structure, stability, and its catalytic behaviors. On the other hand, heterogeneous noble metal-based catalysts such as Ru–Re/AC,15 Ru–MoOx/AC,16 Rh–MoOx/SiO2,17 Pt–Mo/HAP,18 and Au/TiO2 (ref. 19) showed great advantage to produce 1,4-PeD from moderate to good yields (70–95%). However, the use of noble metal-based catalysts and low substrate loading is not economic and less viability in the upgrading of biomass-derived platform industry.16,18
The synthesis route of 1,4-PeD from FFald was firstly proposed by Leuck et al.20 and followed by Schniepp et al.21–23 in the presence of nickel-based catalysts, in an 1,4-dioxane/H2O mixture solvent and the presence of a trace amounts of formic acid or acetic acid. The highest obtained yield of 1,4-PeD from this approached reaction pathway was reached up to 63%. Qiao et al. reported the synthesis of 1,4-PeD from FFald in the presence of noble metal Ru–6.3FeOx/AC catalyst combined with Amberlyst-15 and yielded 86% of 1,4-PeD.24 Schlaf and co-worker reported the catalytic conversion of FFalc and furfuryl acetate (FFace) to 1,4-PeD and cyclopentanol (CpOH)5 using homogeneous Ru-based catalysts. The highest yields of 1,4-PeD (43%) and CpOH (19%) were obtained in the presence of ruthenium–triphos complexes catalyst under severe reaction conditions (473 K and 5.5 MPa H2).25 The yields of 1,4-PeD and CpOH considerably increased to 68% and 35%, respectively, when FFace was as substrate using a ruthenium–phenanthroline complexes catalyst under the same reaction conditions.26 Cui et al. reported the conversion of FFald using a bifunctional ruthenium nanoparticles supported on supported on a sulfonated carbon layer coated SBA-15 catalyst and yielded 86% selectivity of 1,4-PeD.27 However, the presence of solid acid catalysts with high acid density led to the formation of undesired products (e.g., FFalc, LA or condensation products) and severely caused the leaching out of active metal catalyst.
Recently, we reported the synthesis of 1,4-PeD from C5-furanic compounds (FFald, FFalc, and 2-methylfuran (2-MeF) using bulk structure Ni–Sn alloy catalysts in an ethanol/H2O solvent mixture. The highest yield of 1,4-PeD (92%) was obtained in the presence of bulk Ni–Sn(1.5) alloy at 433 K, 3.0 MPa H2, and 12 h.28,29 In the present report, we have extended the investigation of the selective synthesis of 1,4-PeD from biomass-derived furfural and levulinic acid using bimetallic RANEY® nickel–tin alloy supported on aluminium hydroxide (denoted as RNi–Sn(x)/AlOH; x is Ni/Sn molar ratio) instead of bulk Ni–Sn alloy catalysts. The as prepared RNi–Sn(1.4)/AlOH (Sn loading amount = 2.14 mmol) produced the highest yield of 1,4-PeD (78%) under the optimized conditions (433 K, H2 3.0 MPa for 12 h). The obtained yield of 1,4-PeD slightly increased to 87% when the H2-pre-reduced RNi–Sn(1.4)/AA 673 K/H2 (AA = amorphous alumina) catalyst was employed. Supported RANEY® Ni–Sn(1.5)/AC and three types of supported Ni–Sn(1.5) alloy catalysts (e.g., Ni–Sn(1.5)/AC), Ni–Sn(1.5)/c-AlOH, and Ni–Sn(1.5)/γ-Al2O3) also afforded high yield of 1,4-PeD (65–87%) under the optimized reaction conditions. The catalytic conversion of levulinic acid using the most active RNi–Sn(1.4)/AA 673 K/H2 catalyst resulted the maximum yield of 1,4-PeD (90%) at 513 K, H2 4.0 MPa, for 12 h (Scheme 1).
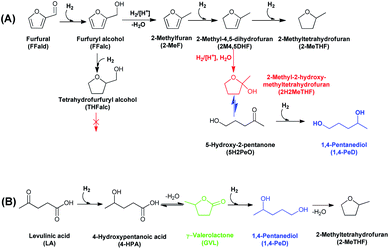 | ||
| Scheme 1 Conceivable routes for the synthesis of 1,4-PeD from (A) furfural and (B) levulinic acid using heterogeneous bimetallic Ni-based catalysts.21,29–31 | ||
Results and discussion
Catalyst characterisation
A series of RANEY® nickel–tin alloy supported on aluminium hydroxide (denoted as RNi–Sn(x)/AlOH, x = Ni/Sn molar ratio, ca. 14.7; 7.9; 3.7; 3.0; 1.4; and 1.0, which corresponding to Sn loading amounts of 0.26 mmol; 0.45 mmol; 0.76 mmol; 1.04 mmol; 2.14 mmol; and 3.96 mmol, respectively) were prepared via the hydrothermal treatment of a mixture of RANEY® nickel supported on aluminium hydroxide (RNi/AlOH)28,32,33 and a SnCl2·2H2O solution in an ethanol/H2O at 423 K for 2 h and produced the as-prepared RNi–Sn(x)/AlOH. After reduction of the as-prepared RNi–Sn(x)/AlOH with H2 gas at 673–873 K for 1.5 h, RNi–Sn(x) supported on amorphous alumina (RNi–Sn(x)/AA) catalysts were produced. The physico-chemical properties of RANEY® Ni, RANEY® Ni/AlOH, the as prepared RNi–Sn(x)/AlOH and H2-reduced RNi–Sn(x)/AA catalysts have been described in the previous reports.28,32,34,35 The detail experiment procedures and characterisation results (Tables S1, S2 and Fig. S1–S7†) are also provided in the ESI.†Selective synthesis of 1,4-PeD from furfural (FFald)
| Entry | Catalystb | Compositionb (mmol g−1) | Conv.c (%) | Yieldd/% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Al | Sn | 1,4-PeD | 1,2-PeD | 1,5-PeD | THFalc | 2H2MeTHF | Otherse | |||
a Reaction conditions: catalyst, 44 mg; substrate, 1.2 mmol; solvent, ethanol/H2O, 3.5 ml (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 volume ratio); initial H2 pressure, 3.0 MPa; 433 K, 12 h.b Values in the parentheses are the Sn loading amounts. The compositions were determined by the ICP-AES analysis.c Conversion of FFald was determined by GC analysis using an internal standard technique.d Yield of the product was determined by GC and GC-MS analyses using an internal standard technique.e Others include 2-methyltetrahydrofuran (2-MeTHF) and condensation product of FFalc, unless otherwise stated.f The catalyst was hydrothermally treated at 423 K for 2 h prior to catalytic reaction.g The catalyst was prepared by physical mixing of RNi/AlOH and SnCl2·2H2O or SnO (the loading amount of Sn was 2.35 mmol to keep the Ni/Sn molar ratio of approximately 1.5).28h The catalysts were obtained from the supported RANEY® Ni and ethanol solution of SnCl2·2H2O with Ni/Sn molar ratio = 1.5, hydrothermally treated at 423 K for 2 h, and reduced with H2 at 673 K for 1.5 h. FFald = furfuraldehyde. PeD = pentanediol. FFalc = furfuryl alcohol. THFalc = tetrahydrofurfuryl alcohol. 2H2MeTHF = 2-hydroxy-2-methyl tetrahydrofuran. BNT = bentonite. SMT = smectite. HT = hectorite. TN = taeniolite. 2.0 volume ratio); initial H2 pressure, 3.0 MPa; 433 K, 12 h.b Values in the parentheses are the Sn loading amounts. The compositions were determined by the ICP-AES analysis.c Conversion of FFald was determined by GC analysis using an internal standard technique.d Yield of the product was determined by GC and GC-MS analyses using an internal standard technique.e Others include 2-methyltetrahydrofuran (2-MeTHF) and condensation product of FFalc, unless otherwise stated.f The catalyst was hydrothermally treated at 423 K for 2 h prior to catalytic reaction.g The catalyst was prepared by physical mixing of RNi/AlOH and SnCl2·2H2O or SnO (the loading amount of Sn was 2.35 mmol to keep the Ni/Sn molar ratio of approximately 1.5).28h The catalysts were obtained from the supported RANEY® Ni and ethanol solution of SnCl2·2H2O with Ni/Sn molar ratio = 1.5, hydrothermally treated at 423 K for 2 h, and reduced with H2 at 673 K for 1.5 h. FFald = furfuraldehyde. PeD = pentanediol. FFalc = furfuryl alcohol. THFalc = tetrahydrofurfuryl alcohol. 2H2MeTHF = 2-hydroxy-2-methyl tetrahydrofuran. BNT = bentonite. SMT = smectite. HT = hectorite. TN = taeniolite. |
|||||||||||
| 1 | RANEY® Ni (in slurry) | 3.98 | 0.63 | — | >99 | 0 | 0 | 2 | 98 | 0 | 0 |
| 2 | RANEY® Ni/AC | 3.72 | 0.53 | — | >99 | 0 | 5 | 3 | 92 | 0 | 0 |
| 3 | RANEY® Ni/BNT | 3.87 | 0.60 | — | >99 | 0 | 3 | 7 | 90 | 0 | 0 |
| 4 | RANEY® Ni/SMT | 3.54 | 0.58 | — | >99 | 0 | 0 | 5 | 91 | 0 | 4 |
| 5 | RANEY® Ni/TN | 3.61 | 0.51 | — | >99 | 0 | 0 | 9 | 89 | 0 | 2 |
| 6 | RANEY® Ni/SiO2 | 3.58 | 0.47 | — | >99 | 0 | 0 | 0 | 96 | 0 | 4 |
| 7 | RANEY® Ni/Nb2O5 | 3.90 | 0.57 | — | >99 | 3 | 0 | 7 | 80 | 8 | 2 |
| 8 | RANEY® Ni/AlOH | 3.46 | 3.58 | — | >99 | 27 | 0 | 6 | 45 | 10 | 12 |
| 9f | RANEY® Ni/AlOH | 3.46 | 3.48 | — | >99 | 25 | 0 | 5 | 45 | 11 | 14 |
| 10 | RNi–Sn(0.26)/AlOH | 3.82 | 3.66 | 0.26 | >99 | 31 | 12 | 6 | 31 | 14 | 6 |
| 11g | RANEY® Ni/AlOH + SnCl2·2H2O | 3.46 | 3.80 | 2.35 | >99 | 23 | 0 | 0 | 44 | 23 | 10 |
| 12h | RANEY® Ni/AlOH + SnO | 3.46 | 3.80 | 2.32 | >99 | 15 | 0 | 0 | 59 | 17 | 9 |
| 13h | RANEY® Ni–Sn(1.5)/SiO2 | 3.12 | 0.27 | 2.12 | >99 | 13 | 0 | 0 | 77 | 2 | 8 |
| 14h | RANEY® Ni–Sn(1.5)/AC | 3.37 | 0.32 | 2.30 | >99 | 68 | 0 | 0 | 23 | 7 | 2 |
Interestingly, after a 0.26 mmol Sn was introduced to RANEY® Ni/AlOH to form RNi–Sn(14.7)/AlOH, the yield of 1,4-PeD slightly increased to 31%, whereas the yield of THFalc significantly decreased to 31% (entry 10). This result suggests that the presence of Sn in RNi–Sn(14.7)/AlOH enhanced the dual hydrolysis-hydrogenation reaction of FFald to produce 1,4-PeD, whereas the hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in FFald to produce THFalc diminished simultaneously.28,30 Further discussion to get the insight into the effect of Sn loading amounts on the yield of 1,4-PeD will be discussed later in this paper. Furthermore, to confirm the role of Sn addition, physical mixing of RANEY® Ni/AlOH and SnCl2·2H2O or SnO2 catalysts (the loading amount of Sn was 2.35 mmol to keep the Ni/Sn molar ratio of approximately 1.5) were prepared and also used for the reaction. Over RANEY® Ni/AlOH + SnCl2·2H2O, the conversion of FFald was >99% and the products were distributed to 1,4-PeD (23%), THFalc (44%), 2H2MeTHF (23%), and others (10%) (entry 11). These results suggest that SnCl2·2H2O synergistically promoted the dual hydrolysis-hydrogenation reaction of FFald to form 1,4-PeD and 2H2MeTHF. The RANEY® Ni/AlOH + SnO2 was also active for the conversion of FFald (>99% conversion) and the products were distributed to 1,4-PeD (15%), THFalc (59%), 2H2MeTHF (17%), and others (9%) (entry 12). The yields of 2H2MeTHF and others (containing 2-MeTHF and the condensation product of FFalc, unless otherwise stated) obtained over this catalyst were smaller than that of the RANEY® Ni/AlOH + SnCl2·2H2O catalyst system. These results suggested that the presence of both Sn2+ and SnO2 showed notable promotion effect on the 1,4-PeD formation, which are laterally different between with and without the addition of SnCl2·2H2O or SnO powder. We have described the plausible reaction pathways for the formation of 1,4-PeD from FFald-derived molecules in our recently published works.29,30 Note that the presence of Sn or oxidic tin (Sn2+)37,38 in bimetallic Ni–Sn alloy and autoprotolysis of ethanol/H2O solvent39,40 played prominent role during the direct conversion of FFald, thus leading to high yield of 1,4-PeD. The effect of the second metals rather than tin (Sn) such as iron (Fe), cobalt (Co), indium (In), silver (Ag), zirconium (Zr), zirconium (Zr), vanadium (V), gallium (Ga), and niobium (Nb) (denoted as the as-prepared RNi–M/AlOH (M = Fe, Co, In, Ag, Ga, Zr, V, and Nb)) on the yield of 1,4-PeD was also evaluated. However, the results of catalytic reaction are unsatisfied and no 1,4-PeD yield was obtained at full conversion of FFald (Table S3, in the ESI†). In addition, only the RNi–In(x)/AlOH and RNi–Ga(x)/AlOH catalysts obviously afforded high chemoselectivity toward FFalc (up to 99%) and it was already described in the previous report.41
C bond in FFald to produce THFalc diminished simultaneously.28,30 Further discussion to get the insight into the effect of Sn loading amounts on the yield of 1,4-PeD will be discussed later in this paper. Furthermore, to confirm the role of Sn addition, physical mixing of RANEY® Ni/AlOH and SnCl2·2H2O or SnO2 catalysts (the loading amount of Sn was 2.35 mmol to keep the Ni/Sn molar ratio of approximately 1.5) were prepared and also used for the reaction. Over RANEY® Ni/AlOH + SnCl2·2H2O, the conversion of FFald was >99% and the products were distributed to 1,4-PeD (23%), THFalc (44%), 2H2MeTHF (23%), and others (10%) (entry 11). These results suggest that SnCl2·2H2O synergistically promoted the dual hydrolysis-hydrogenation reaction of FFald to form 1,4-PeD and 2H2MeTHF. The RANEY® Ni/AlOH + SnO2 was also active for the conversion of FFald (>99% conversion) and the products were distributed to 1,4-PeD (15%), THFalc (59%), 2H2MeTHF (17%), and others (9%) (entry 12). The yields of 2H2MeTHF and others (containing 2-MeTHF and the condensation product of FFalc, unless otherwise stated) obtained over this catalyst were smaller than that of the RANEY® Ni/AlOH + SnCl2·2H2O catalyst system. These results suggested that the presence of both Sn2+ and SnO2 showed notable promotion effect on the 1,4-PeD formation, which are laterally different between with and without the addition of SnCl2·2H2O or SnO powder. We have described the plausible reaction pathways for the formation of 1,4-PeD from FFald-derived molecules in our recently published works.29,30 Note that the presence of Sn or oxidic tin (Sn2+)37,38 in bimetallic Ni–Sn alloy and autoprotolysis of ethanol/H2O solvent39,40 played prominent role during the direct conversion of FFald, thus leading to high yield of 1,4-PeD. The effect of the second metals rather than tin (Sn) such as iron (Fe), cobalt (Co), indium (In), silver (Ag), zirconium (Zr), zirconium (Zr), vanadium (V), gallium (Ga), and niobium (Nb) (denoted as the as-prepared RNi–M/AlOH (M = Fe, Co, In, Ag, Ga, Zr, V, and Nb)) on the yield of 1,4-PeD was also evaluated. However, the results of catalytic reaction are unsatisfied and no 1,4-PeD yield was obtained at full conversion of FFald (Table S3, in the ESI†). In addition, only the RNi–In(x)/AlOH and RNi–Ga(x)/AlOH catalysts obviously afforded high chemoselectivity toward FFalc (up to 99%) and it was already described in the previous report.41
By using the as prepared RNi–Sn(14.7)/AlOH catalyst (Sn loading amount = 0.26 mmol), 31% yield of 1,4-PeD was obtained and accompanied by 12% 1,2-PeD, 6% 1,5-PeD, 31% THFalc, 14% 2H2MeTHF and 6% others (mainly consist of 2-MeTHF and condensation product of FFalc, unless otherwise stated) (entry 11, Table 1). The yields of 1,4-PeD significantly increased to 58% using RNi–Sn(7.9)/AlOH catalyst, whereas THFalc yield reduced to 23%. When the loading amount of Sn was increased to 0.76 mmol (Ni/Sn = 3.7) and 1.04 mmol (Ni/Sn = 3.0), the yield of 1,4-PeD remarkably increased to 63% and 66%, respectively, and this high yield was kept (78%) when the loading amount of Sn was furtherly increased to 2.14 mmol (Ni/Sn = 1.4). The highest yield of 1,4-PeD was obviously obtained when the increase in loading amount of Sn reaches to Ni/Sn molar ratio of 3.0 and 1.4, which are very good consistent with our previous results on bulk Ni–Sn(3.0) and Ni–Sn(1.5) catalysts.29 The catalytic activity of metallic Ni in RNi–Sn(x)/AlOH towards hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond rather than C
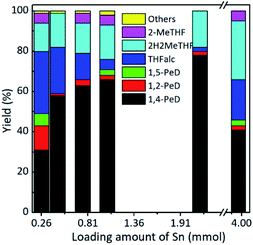
O bond rather than C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in FFald was drastically suppressed by the addition of Sn.28 As the results, low yield of THFalc (5%) and (2%) was obtained as the loading amount was increased to 1.04 mmol and 2.14 mmol respectively, then dramatically increased to 20% at Sn loading amount of 3.96 mmol (Ni/Sn ratio = 1.0). The dependence of 1,4-PeD yield on the Sn loading amounts was clearly observed in Fig. 1, which seems to be a volcano-like plot with maximum obtained 1,4-PeD yield at Sn loading amount of 2.14 mmol (Ni/Sn = 1.4). Moreover, RNi–Sn(1.0)/AlOH (Sn loading amount = 3.96 mmol) catalyst produced 41% 1,4-PeD accompanied by 2% 1,2-PeD, 3% 1,5-PeD, 20% THFalc, 29% 2H2MeTHF, and 5% others.
C bond in FFald was drastically suppressed by the addition of Sn.28 As the results, low yield of THFalc (5%) and (2%) was obtained as the loading amount was increased to 1.04 mmol and 2.14 mmol respectively, then dramatically increased to 20% at Sn loading amount of 3.96 mmol (Ni/Sn ratio = 1.0). The dependence of 1,4-PeD yield on the Sn loading amounts was clearly observed in Fig. 1, which seems to be a volcano-like plot with maximum obtained 1,4-PeD yield at Sn loading amount of 2.14 mmol (Ni/Sn = 1.4). Moreover, RNi–Sn(1.0)/AlOH (Sn loading amount = 3.96 mmol) catalyst produced 41% 1,4-PeD accompanied by 2% 1,2-PeD, 3% 1,5-PeD, 20% THFalc, 29% 2H2MeTHF, and 5% others.
The differences in product distribution (yield) obtained from the catalytic conversion of FFald using the as prepared RANEY® Ni–Sn(x)/AlOH with different Sn loading amounts compared with the supported RANEY® Ni or RANEY® Ni/AlOH catalysts were clearly observed (Table 1 and Fig. 1). These can be roughly attributed to the surface modified-Ni due to the presence of Sn or the formation Ni–Sn alloy during the hydrothermal synthesis. The XRD patterns of the as prepared RNi–Sn(x)/AlOH catalysts with different Sn loading amounts showed the broadened diffraction peaks at 2θ = 44.44° compared with the conventional RANEY® Ni or RANEY® Ni/AlOH. The broadened peaks at 2θ = 44.44° can be attributed to the formation of Ni–Sn alloys, i.e., Ni3Sn and Ni3Sn2 (Fig. S3, in the ESI†).28,42–44
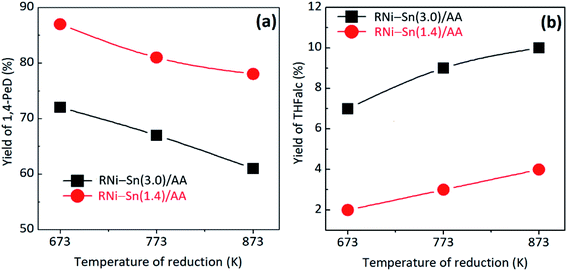 | ||
| Fig. 2 Yields of (a) 1,4-PeD and (b) THFalc obtained from selective conversion of FFald over RNi–Sn(3.0)/AA and RNi–Sn(1.4)/AA catalysts after reduction with H2 at 673–873 K for 1.5 h. Reaction conditions refer to the Fig. 1. | ||
Fig. 2 shows the profiles of 1,4-PeD and THFalc yields obtaining from the catalytic conversion of FFald using RNi–Sn(x)/AA (x = 3.0 and 1.4) catalysts after reduction with H2 at 673–873 K for 1.5 h. The yields of 1,4-PeD over pre-reduced RNi–Sn(3.0)/AA and RNi–Sn(1.4)/AA catalysts at 673 K were 72% and 87%, respectively, which are higher than that of the as prepared RNi–Sn(3.0)/AlOH and RNi–Sn(1.4)/AlOH catalysts. However, the yield of 1,4-PeD slightly decreased to 67% and 81%, respectively when the temperature of reduction was increased to 773 K and the 1,4-PeD yield reached to 61–78% after temperature reduction was 873 K (Fig. 2(a)). The yield of THFalc increased smoothly over both RNi–Sn(3.0)/AA and RNi–Sn(1.4)/AA catalysts after reduction with H2 at 673–873 K for 1.5 h, whereas the yield of THFalc over RNi–Sn(3.0)/AA is higher than that of RNi–Sn(1.4)/AA catalyst (Fig. 2(b)). The differences in the hydrogenation rate of THFalc formation between RNi–Sn(3.0)/AA and RNi–Sn(1.4)/AA systems can be attributed to the amount remained metallic nickel (Ni0) (or Ni/Sn molar ratio) and major alloy phases (Fig. S5 and S6, in the ESI†).45 A great portion of Ni3Sn2 alloy phases and less extent of metallic nickel (Ni0) in the bimetallic Ni–Sn alloy catalyst has been proven by theoretical studies or catalytic reactions to play a pivotal role for the high chemoselectivity in the hydrogenation of unsaturated carbonyl compound,41,46 substituted aromatic nitro compounds,47 or direct synthesis of hydroxy-ketones from cellulose.48 These results also can be rationalised to the fact that RNi–Sn(3.0)/AA has higher Ni/Sn molar ratio than that of RNi–Sn(1.4)/AA, which means RNi–Sn(3.0)/AA has higher metallic Ni (Ni0) concentration on the outer surface, higher activity to hydrogenate than to hydrolyze-hydrogenate the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of FFalc.45 Consequently, hydrogenation of C
C bond of FFalc.45 Consequently, hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of FFalc will take place rapidly, leading to relatively high yield of THFalc, on the other hand yield of 1,4-PeD decreased oppositely (Fig. 2(a)).
C bond of FFalc will take place rapidly, leading to relatively high yield of THFalc, on the other hand yield of 1,4-PeD decreased oppositely (Fig. 2(a)).
To confirm the important of the formation of bimetallic Ni3Sn2 alloy structure has significantly influenced on the high yield of 1,4-PeD, two types supported RANEY® Ni–Sn(1.5)/SiO2 and RANEY® Ni–Sn(1.5)/AC catalysts were prepared (the XRD patterns are shown in Fig. S8, in the ESI†). RANEY® Ni–Sn(1.5)/SiO2 catalyst gave only 13% yield of 1,4-PeD (entry 13, Table 1), whereas RANEY® Ni–Sn(1.5)/AC afforded 68% yield of 1,4-PeD (entry 14, Table 1) which are comparable with the bulk Ni3Sn2 alloy catalysts as described in previous report.29 The influence of the supports used for the Ni–Sn alloy catalysts on the yield of 1,4-PeD are subsequently discussed in this paper. Moreover, the XRD patterns of RNi–Sn(3.0)/AA confirmed the presence of metallic Ni as Ni(111) or Ni(200) as shown in Fig. S5, in the ESI.† The results of catalytic reaction using RNi–Sn(x)/AA (x = Ni/Sn molar ratio; 7.9; 3.7; and 1.0) catalysts after reduction with H2 at 673–873 K for 1.5 h are summarised in Table S4, in the ESI.† Over RNi–Sn(7.9)/AA 673 K/H2 catalyst, 26% yield of 1,4-PeD and 67% yield of THFalc were obtained, while 2H2MeTHF was only 7% (entry 1). The yield of 1,4-PeD slightly increased to 29% and 39% when H2-reduced RNi–Sn(7,9)/AA at 773 K and 873 K catalysts were employed (Fig. S4, in the ESI†), while the yield of THFalc remained unchanged in high yield (entries 1–3). In the case of RNi–Sn(3.7)/AA catalysts, the yield of 1,4-PeD decreased to (32–55%) as the temperature of reduction was increased (entries 4–6). Moreover, pre reduced RNi–Sn(1.0)/AA at 673 K, 773 K, and 873 K catalysts (Fig. S7, in the ESI†) gave only 42%, 46%, and 49% yield of 1,4-PeD, respectively. The obtained THFalc yield was around 22–25%, while the yield of 2H2MeTHF was 23–26% (entries 7–9).
| Entry | Catalystb | Conversionc (%) | Yieldd (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1,4-PeD | 1,2-PeD | 1,5-PeD | FFalc | THFalc | 2H2MeTHF | 2-MeTHF | Otherse | |||
a Reaction conditions: catalyst, 44 mg; substrate, 1.2 mmol; solvent, ethanol/H2O, 3.5 ml (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 volume ratio); initial H2 pressure, 3.0 MPa, 433 K, 12 h.b Values in the parenthesis are Ni/Sn molar ratio, determined by using ICP-OES.c Conversion of FFald was determined by GC analysis using an internal standard technique.d Yield of product was determined by GC and GC-MS analyses using an internal standard technique.e Unknown product may be the condensation product of FFald or FFalc according to GC and GC-MS data.f Commercial aluminium hydroxide (consist of bayerite and gibbsite structures). PeD = pentanediol. FFalc = furfuryl alcohol. THFalc = tetrahydrofurfuryl alcohol. 2H2MeTHF = 2-hydroxy-2-methyl tetrahydrofuran. 2-MeTHF = 2-methyl tetrahydrofuran. 2.0 volume ratio); initial H2 pressure, 3.0 MPa, 433 K, 12 h.b Values in the parenthesis are Ni/Sn molar ratio, determined by using ICP-OES.c Conversion of FFald was determined by GC analysis using an internal standard technique.d Yield of product was determined by GC and GC-MS analyses using an internal standard technique.e Unknown product may be the condensation product of FFald or FFalc according to GC and GC-MS data.f Commercial aluminium hydroxide (consist of bayerite and gibbsite structures). PeD = pentanediol. FFalc = furfuryl alcohol. THFalc = tetrahydrofurfuryl alcohol. 2H2MeTHF = 2-hydroxy-2-methyl tetrahydrofuran. 2-MeTHF = 2-methyl tetrahydrofuran. |
||||||||||
| 1 | Ni–Sn(1.5)/TiO2 | 100 | 0 | 0 | 0 | 87 | 12 | 0 | 1 | 0 |
| 2 | Ni–Sn(1.5)/ZnO | 100 | 0 | 0 | 0 | 91 | 9 | 0 | 0 | 0 |
| 3 | Ni–Sn(1.5)/c-AlOHf | 100 | 87 | 3 | 0 | 0 | 2 | 8 | 0 | 0 |
| 4 | Ni–Sn(1.5)/γ-Al2O3 | 100 | 83 | 0 | 0 | 0 | 0 | 13 | 4 | 0 |
| 5 | Ni–Sn(1.5)/AC | 100 | 65 | 0 | 0 | 0 | 0 | 16 | 14 | 5 |
| 6 | Ni–Sn(1.5)/MgO | 90 | 0 | 10 | 5 | 0 | 58 | 0 | 17 | 0 |
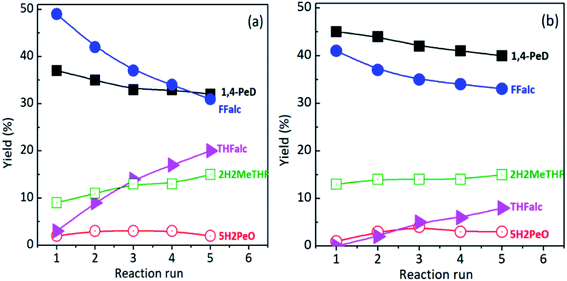
Fig. 3(a) shows the product distribution obtained from the reusability tests of RNi–Sn(3.0)/AlOH catalyst. The yields of 1,4-PeD and FFalc decreased smoothly after the five times of recycle runs without any changes of FFald conversion even after the fifth reaction run. In contrast, the yield of THFalc increased linearly with the number of recyclability tests, suggesting the hydrogenation rate of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of FFalc correspond to THFalc catalyzed by metallic Ni species in RNi–Sn(3.0)/AlOH system increased during the recyclability tests. Moreover, the yield of 2H2MeTHF increased slowly during the recyclability test but the number are lower than that of THFalc, suggesting the rate of hydrogenation reaction of FFalc to THFalc higher than that of the hydrolysis reaction as indicated by the significant increase of THFalc yield (Fig. 3(a)).
C bond of FFalc correspond to THFalc catalyzed by metallic Ni species in RNi–Sn(3.0)/AlOH system increased during the recyclability tests. Moreover, the yield of 2H2MeTHF increased slowly during the recyclability test but the number are lower than that of THFalc, suggesting the rate of hydrogenation reaction of FFalc to THFalc higher than that of the hydrolysis reaction as indicated by the significant increase of THFalc yield (Fig. 3(a)).
To complete the investigation and for a comparison, the reusability tests of RNi–Sn(1.4)/AlOH catalyst were also performed and the results are shown in Fig. 3(b). The yields of 1,4-PeD and FFalc decreased as the increase of number of recycle runs at full conversion of FFald. The reaction rate of hydrogenation of FFalc over RNi–Sn(3.0)/AlOH catalyst (Fig. 3(a)) higher than that of RNi–Sn(1.4)/AlOH catalyst (Fig. 3(b)), thus higher yield of THFalc is obtained over RNi–Sn(3.0)/AlOH catalyst. This is very consistent with the yield of THFalc obtained over RNi–Sn(x)/AA catalysts as shown in Fig. 2 and Table S4, in the ESI.† The amounts of metal leaching into the reaction solution were not analysed due to the technical issues. The XRD patterns of recovered RNi–Sn(3.0)/AlOH and RNi–Sn(1.4)/AlOH catalysts confirmed that there no significant change in the catalyst structures even after the fifth reaction run (Fig. S11, in the ESI†).
| Entry | Substrate | Product | Conv.b (%) | Yieldc (%) |
|---|---|---|---|---|
a Reaction conditions: catalyst, 44 mg; substrate, 1.2 mmol; solvent, ethanol/H2O, 3.5 ml (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 volume ratio), 433 K, 12 h.b Conversion and yield of the product were determined by GC analysis using an internal standard technique.c Yield of 1,4-PeD.d Yield of butanol. 2.0 volume ratio), 433 K, 12 h.b Conversion and yield of the product were determined by GC analysis using an internal standard technique.c Yield of 1,4-PeD.d Yield of butanol. |
||||
| 1 | 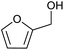 |
 |
100 | 71 |
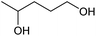
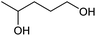
| 2 | 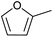 |
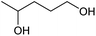 |
71 | 42 |
| 3 | 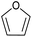 |
 |
67 | 24d |
| 4 | 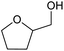 |
No product was observed | 0 | 0 |
Selective synthesis of 1,4-PeD from levulinic acid (LA)
The synthesis of GVL and 1,4-PeD from levulinic acid (LA) in the presence of the most effective RNi–Sn(1.4)/AA 673 K/H2 catalyst was examined at temperature range of 473–523 K. We have reported that the hydrogenation of LA to GVL effectively occurred in the presence of Ni–Sn alloy catalysts at temperature 393–453 K (100% conversion and >99% yield of GVL) without the formation of 1,4-PeD even after the reaction time was extended to 12 h (Table 3, entry 1).34,53 We then performed the catalytic hydrogenation of LA at temperature range of 473–523 K, H2 4.0 MPa, for 6 h to evaluate the effect of reaction temperature on the formation of 1,4-PeD and the results are shown in Fig. 4. At 473 K, LA was converted completely to GVL and no 1,4-PeD was observed. When the reaction temperature increased to 483 K, the major product was GVL (96% yield) and accompanied by a small amount of 1,4-PeD (4%). The yield of 1,4-PeD remarkably increased to 36% at the reaction temperature of 493 K then gradually increased and reached the maximum yield of 71% as reaction temperature was increased up to 513–523 K. The increase of reaction temperature not only increased the yield of 1,4-PeD but also promoted the further reaction of GVL to form 2-MeTHF, thus the obtained yield of 2-MeTHF was 20% at 523 K. The high stability of ring structure in GVL indeed inhibits some hydrogenation catalysts;54 therefore, the yield of 1,4-PeD depends on the activity of catalysts as well as some strict reaction conditions. Previous reports have indicated that the hydrogenations of levulinic acid, ethyl levulinate and GVL using typical Cu-based catalysts required high reaction temperature (523–546 K) and high H2 pressure (20 MPa) to produce 1,4-PeD (up to 83%).4,10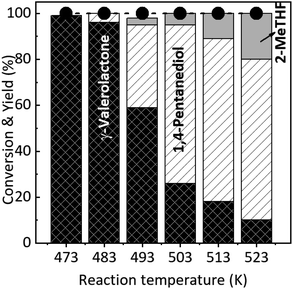 | ||
| Fig. 4 Results of LA hydrogenation to GVL, 1,4-PeD, and 2-MeTHF using RNi–Sn(1.4)/AA 673 K/H2 catalyst. Reaction conditions: catalyst, 44 mg; substrate, 1.2 mmol; solvent, H2O, H2 4.0 MPa, 6 h. | ||
The catalytic reactions of levulinic acid in the presence of various bimetallic RANEY® Ni-based catalysts were carried out at 503 K, H2 4.0 MPa for 3 h and the results are summarized in Table 4. By using RANEY® Ni–Sn(1.4)/AA, supported Ni–Sn(1.5)/γ-Al2O3 and RANEY® Ni–Sn(1.4)/AC catalysts, the yields of 1,4-PeD were 90%, 82% and 68% at >99% conversion of LA after 12 h, respectively (entries 1–3). In contrast to the bimetallic Ni–Sn catalysts, various second metals such as titanium (Ti), zinc (Zn), cobalt (Co), iron (Fe), copper (Cu), and indium (In) were also prepared and employed for the reaction and the results are also summarized in Table 3, entries 4–9. However, only RANEY® Ni–Zn(1.5)/AA, RANEY® Ni–Fe(1.5)/AA, RANEY® Ni–Cu(1.5)/AA catalyst gave 1,4-PeD with yield of 12%, 11%, and 9%, respectively (entries 3–6). On the other hand, RANEY® Ni–Ti(1.5)/AA, RANEY® Ni–Co(1.5)/AA, RANEY® Ni–In(1.5)/AA catalysts gave only GVL under the same reaction conditions.
| Entry | Catalyst | Conv.b (%) | Yieldb (%) | ||
|---|---|---|---|---|---|
| 1,4-PeD | GVL | Othersc | |||
| a Reaction conditions: catalyst, 44 mg; substrate, 1.2 mmol; solvent, H2O, H2 4.0 MPa, 503 K, 3 h.b Conversion and yield of the product were determined by GC analysis using an internal standard technique.c Others are included 2-methyl tetrahydrofuran (2-MeTHF) and 2-pentanol (2-PeOH) according to GC-MS data.d The reaction time was 12 h. | |||||
| 1d | RANEY® Ni–Sn(1.4)/AA | >99 | 90 | 4 | 6 |
| 2d | Ni–Sn(1.5)/γ-Al2O3 | >99 | 82 | 18 | 0 |
| 3 | RANEY® Ni–Sn(1.4)/AC | >99 | 68 | 32 | 0 |
| 4 | RANEY® Ni–Zn(1.5)/AA | >99 | 12 | 83 | 5 |
| 5 | RANEY® Ni–Fe(1.5)/AA | >99 | 11 | 80 | 9 |
| 6 | RANEY® Ni–Cu(1.5)/AA | >99 | 9 | 88 | 3 |
| 7 | RANEY® Ni–Ti(1.5)/AA | 99 | Trace | 96 | 3 |
| 8 | RANEY® Ni–Co(1.5)/AA | >99 | Trace | 99 | 0 |
| 9 | RANEY® Ni–In(1.5)/AA | >99 | Trace | 98 | 2 |
Furthermore, the catalytic performance of RNi–Sn(1.4)/AA 673 K/H2 catalyst on the conversion LA, ethyl levulinate (EL) and GVL was evaluated at short reaction time (1 h) and the results are summarized in Table 5.
| Entry | Substrate | Product | Conv.b (%) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: catalyst, 44 mg; substrate, 1.2 mmol; solvent, H2O, H2 4.0 MPa, 503 K, 1 h.b Conversion and yield of the product were determined by GC analysis using an internal standard technique.c The value in the parenthesis is the yield of GVL.d The value in the parenthesis is the yield of 2-MeTHF. | ||||
| 1 | 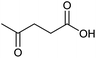 |
 |
>99 | 9(90)c |
| 2 | 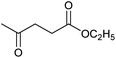 |
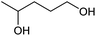 |
98 | 27(71)c |
| 3 | 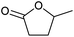 |
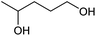 |
29 | 22(7)d |
At a >99% conversion of LA, 9% yield 1,4-PeD and 90% yield GVL were obtained at a reaction time of 1 h (entry 1). Ethyl levulinate (EL) afforded a notable yield of 1,4-PeD (27%) under the same reaction conditions (entry 2). The rate reaction of 1,4-PeD formation from levulinic acid and ethyl levulinate changed in the following order: ethyl levulinate (EL) > levulinic acid (LA). In addition, the conversion of GVL was only 29% to give 22% yield of 1,4-PeD under the same reaction conditions (entry 3), indicating that the ring opening reaction of GVL occurred slowly under current reaction conditions. Alternatively, it was suggested that the partial hydrogenation of LA easily occurred on the surface of metal catalyst in aqueous media under hydrogen atmosphere and produced 4-hydroxypentanoic acid (4-HPA), which can be quickly equilibrated to form GVL,55 then transformed into 1,4-PeD.56 Therefore, the formation of 1,4-PeD might be thoroughly generated from 4-HPA or GVL. Bert F. Sels and co-workers have reported that the ring opening of GVL is thermodynamically disfavored under hydrothermal conditions at 543 K using Pt catalyst.57
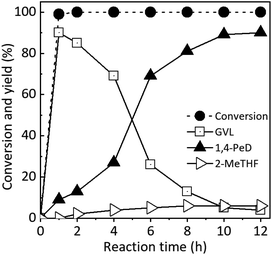
To evaluate the possible reaction pathways of LA transformation to GVL and 1,4-PeD, the kinetic reactions were performed at 503 K, H2 4.0 MPa for 1–12 h using RNi–Sn(1.4)/AA 673 K/H2 catalyst and the results are shown in Fig. 5. As expected, the hydrogenation of LA to GVL took place rapidly at early reaction time (1 h) with >99% conversion and gave 90% yield of GVL and small amount of 1,4-PeD (9%). These results are very good agreement with previously reported work that the first step reaction is lactonization of LA to GVL in the presence of Ni–Sn alloy catalyst in water.34 After reaction time of 2 h, the yield of 1,4-PeD slightly increased to 13%, whereas the yield of GVL went to 85%. Further extent of reaction time up to 12 h, the yield of 1,4-PeD increased smoothly to reach the maximum yield of 90%, while the yield of GVL decreased to reach nearly zero at the extent of reaction time of 12 h. On the other hand, the yield of 2-MeTHF obviously remained unchanged, suggesting that the hydrodeoxygenation reaction of GVL is inhibited using RNi–Sn(1.4)/AA 673 K/H2 catalyst under these reaction conditions (entry 3, Table 5 and Fig. 4 and 5). These results suggest that the formation of GVL and 1,4-PeD from LA may thoroughly follow the proposed reaction pathways (Scheme 2).
Conclusions
We described the selective synthesis of 1,4-PeD from biomass-derived furfural and levulinic acid using bimetallic RANEY® nickel–tin alloy supported on aluminium hydroxide (RNi–Sn(x)/AlOH; x = Ni/Sn molar ratio; 14.7; 7.9; 3.7; 3.0; 1.4; and 1.0) catalysts. The as prepared RNi–Sn(1.4)/AlOH catalysts gave the highest yield of 1,4-PeD (78%). After reduction with H2 at 673–873 K for 1.5 h, RNi–Sn(1.4)/AA 673 K/H2 catalysts gave a significant enhancement of 1,4-PeD yield (up to 87%) at 433 K, H2 3.0 MPa for 12 h. Supported RANEY® Ni–Sn(1.5)/AC, Ni–Sn(1.5)/AC, Ni–Sn(1.5)/c-AlOH, and Ni–Sn(1.5)/γ-Al2O3 catalysts also gave yield of 1,4-PeD (65–87%) under the optimised reaction conditions. The high yield of 1,4-PeD over RNi–Sn(1.4)/AA catalyst can be rationalised due to the well-dispersed Ni3Sn2 species on amorphous alumina (AA), relatively low extent of metallic nickel (Ni0). These properties preferentially catalysed the dual hydrolysis-hydrogenation of furfural or the selective transformation of levulinic acid into 1,4-PeD. In addition, the as prepared RNi–Sn(3.0)/AlOH and RNi–Sn(1.4)/AlOH catalysts were reusable and stable for at least five consecutive reaction runs in the catalytic conversion of FFald to 1,4-PeD. The catalytic conversion of levulinic acid using the best catalysts (e.g., RNi–Sn(1.4)/AA 673 K/H2, Ni–Sn(1.5)/γ-Al2O3, and RANEY® Ni–Sn(1.4)/AC) resulted the maximum yield of 1,4-PeD (up to 90%) at 503 K, H2 4.0 MPa, for 12 h.Author contributions
Rodiansono: conceptualization, methodology, investigation, writing – original draft, writing – review & editing. Maria Dewi Astuti, Kamilia Mustikasari: formal analysis, investigation. Sadang Husain and Pathur Razi Ansyah: visualization. Takayoshi Hara and Shogo Shimadzu: resources, writing – review & editing, supervision.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors acknowledge the RISET DASAR FY 2018–2020, and the RISET DASAR FY 2019–2021 (DIPA-042.06-1.401516/2020), RISET DASAR FY 2021–2023 (119/E4.1/AK.04.PT/2021) from the Ministry of Education, Culture, Research and Technology, Indonesian Government for financially support this work.References
- B. M. Stadler, A. Brandt, A. Kux, H. Beck and J. G. de Vries, ChemSusChem, 2020, 13, 556–563 CrossRef CAS PubMed.
- X. Li, P. Jia and T. Wang, ACS Catal., 2016, 6, 7621–7640 CrossRef CAS.
- D. Sun, S. Sato, W. Ueda, A. Primo, H. Garcia and A. Corma, Green Chem., 2016, 18, 2579–2597 RSC.
- R. V. Christian, H. D. Brown and R. M. Hixon, J. Am. Chem. Soc., 1947, 69, 1961–1963 CrossRef CAS.
- J. Heller, J. Barr, S. Ng, H. R. Shen, R. Gurny, K. Schwach-Abdelaoui, A. Rothen-Weinhold and M. Van de Weert, J. Controlled Release, 2002, 78, 133–141 CrossRef CAS PubMed.
- A. Yamaguchi, N. Hiyoshi, O. Sato, K. K. Bando and M. Shirai, Green Chem., 2009, 11, 48–52 RSC.
- P. Werle, M. Morawietz, S. Lundmark, K. Sörensen, E. Karvinen and J. Lehtonen, in Ullmann's Encyclopedia of Industrial Chemistry, 2008, pp. 263–281 Search PubMed.
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Chem. Soc. Rev., 2012, 41, 8075–8098 RSC.
- O. G. Ellert, M. V Tsodikov, S. A. Nikolaev and V. M. Novotortsev, Russ. Chem. Rev., 2014, 83, 718–732 CrossRef.
- K. Folkers and H. Adkins, J. Am. Chem. Soc., 1932, 54, 1145–1154 CrossRef CAS.
- I. Simakova, Y. Demidova, M. Simonov, S. Prikhod’ko, P. Niphadkar, V. Bokade, P. Dhepe and D. Y. Murzin, Reactions, 2020, 1, 54–71 CrossRef.
- D. Sun, T. Saito, Y. Yamada, X. Chen and S. Sato, Appl. Catal., A, 2017, 542, 289–295 CrossRef CAS.
- D. Ren, X. Wan, F. Jin, Z. Song, Y. Liu and Z. Huo, Green Chem., 2016, 18, 5999–6003 RSC.
- N. Karanwal, M. G. Sibi, M. K. Khan, A. A. Myint, B. Chan Ryu, J. W. Kang and J. Kim, ACS Catal., 2021, 11, 2846–2864 CrossRef CAS.
- L. Corbel-Demailly, B. K. Ly, D. P. Minh, B. Tapin, C. Especel, F. Epron, A. Cabiac, E. Guillon, M. Besson and C. Pinel, ChemSusChem, 2013, 6, 2388–2395 CrossRef CAS PubMed.
- J. Cui, J. Tan, Y. Zhu and F. Cheng, ChemSusChem, 2018, 11, 1316–1320 CrossRef CAS PubMed.
- M. Li, G. Li, N. Li, A. Wang, W. Dong, X. Wang and Y. Cong, Chem. Commun., 2014, 50, 1414–1416 RSC.
- T. Mizugaki, Y. Nagatsu, K. Togo, Z. Maeno, T. Mitsudome, K. Jitsukawa and K. Kaneda, Green Chem., 2015, 17, 5136–5139 RSC.
- F. Bucciol, S. Tabasso, G. Grillo, F. Menegazzo, M. Signoretto, M. Manzoli and G. Cravotto, J. Catal., 2019, 380, 267–277 CrossRef CAS.
- G. J. Leuck, J. P. Evanston and F. N. Peters Jr, US Pat., 2,097,493, 1937 Search PubMed.
- L. E. Schniepp, H. H. Geller and R. W. von Korff, J. Am. Chem. Soc., 1947, 69, 672–674 CrossRef CAS.
- N. S. Zolotarev, P. P. Latvis, A. A. Buimov, V. I. Sirotenko, I. M. Lisnyanskii, K. E. Novikova, Y. V. Bogatyrev and E. S. Zhdanovich, Pharm. Chem. J., 1972, 6, 184–187 CrossRef.
- M. S. Perchenok, V. S. Shevchenko, V. M. Komarov and D. Z. Zavel'skii, Pharm. Chem. J., 1976, 10, 222–226 CrossRef.
- L. Lin, A. M. Sheveleva, I. da Silva, C. M. A. Parlett, Z. Tang, Y. Liu, M. Fan, X. Han, J. H. Carter, F. Tuna, E. J. L. McInnes, Y. Cheng, L. L. Daemen, S. Rudić, A. J. Ramirez-Cuesta, C. C. Tang and S. Yang, Nat. Mater., 2020, 19, 86–93 CrossRef CAS PubMed.
- E. M. J. Banz Chung, M. K. Stones, E. Latifi, C. Moore, A. D. Sutton, G. Umphrey, D. Soldatov and M. Schlaf, Can. J. Chem., 2021, 99, 113–126 CrossRef CAS.
- M. K. Stones, E. M. J. Banz Chung, I. T. Da Cunha, R. J. Sullivan, P. Soltanipanah, M. Magee, G. J. Umphrey, C. M. Moore, A. D. Sutton and M. Schlaf, ACS Catal., 2020, 10, 2667–2683 CrossRef CAS.
- K. Cui, W. Qian, Z. Shao, X. Zhao, H. Gong, X. Wei, J. Wang, M. Chen, X. Cao and Z. Hou, Catal. Lett., 2021, 151, 2513–2526 CrossRef CAS.
- R. Rodiansono, T. Hara, N. Ichikuni and S. Shimazu, Chem. Lett., 2012, 41, 769–771 CrossRef.
- R. Rodiansono, M. Dewi Astuti, T. Hara, N. Ichikuni and S. Shimazu, Green Chem., 2019, 21, 2307–2315 RSC.
- R. Rodiansono, M. D. Astuti, S. Husain, A. Nugroho and S. Sutomo, Bull. Chem. React. Eng. Catal., 2019, 14, 529–541 CrossRef CAS.
- R. Rodiansono, S. Khairi, T. Hara, N. Ichikuni and S. Shimazu, Catal. Sci. Technol., 2012, 2, 2139–2145 RSC.
- R. Rodiansono and S. Shimazu, Bull. Chem. React. Eng. Catal., 2013, 8, 40–46 CAS.
- J. Petró, A. Bóta, K. László, H. Beyer, E. Kálmán and I. Dódony, Appl. Catal., A, 2000, 190, 73–86 CrossRef.
- R. Rodiansono, M. D. Astuti, T. Hara, N. Ichikuni and S. Shimazu, Catal. Sci. Technol., 2016, 6, 2955–2961 RSC.
- R. Rodiansono, T. Hara, N. Ichikuni and S. Shimazu, Bull. Chem. React. Eng. Catal., 2014, 9, 53–59 Search PubMed.
- A. Gandini, Polym. Chem., 2010, 1, 245–251 RSC.
- L. Sordelli, R. Psaro, G. Vlaic, A. Cepparo, S. Recchia, C. Dossi, A. Fusi and R. Zanoni, J. Catal., 1999, 182, 186–198 CrossRef CAS.
- J. L. Margitfalvi, G. Vankó, I. Borbáth, A. Tompos and A. Vértes, J. Catal., 2000, 190, 474–477 CrossRef CAS.
- L. Wang, J. Zhang, X. Wang, B. Zhang, W. Ji, X. Meng, J. Li, D. S. Su, X. Bao and F. S. Xiao, J. Mater. Chem. A, 2014, 2, 3725–3729 RSC.
- E. Kiliç and N. Aslan, Microchim. Acta, 2005, 151, 89–92 CrossRef.
- R. Rodiansono, M. D. Astuti, D. R. Mujiyanti, U. T. Santoso and S. Shimazu, Mol. Catal., 2018, 445, 52–60 CrossRef.
- JCPDS-ICDD, Powder diffraction files, JCPDS-International center for diffraction data (JCPDS-ICDD), 1991 Search PubMed.
- H. E. Swift and J. E. Bozik, J. Catal., 1968, 12, 5–14 CrossRef CAS.
- H. Wang, H. Wang, X. Li and C. Li, Appl. Surf. Sci., 2017, 407, 456–462 CrossRef CAS.
- R. Rodiansono, M. D. Astuti, K. Mustikasari, S. Husain and Sutomo, Catal. Sci. Technol., 2020, 10, 7768–7778 RSC.
- Y. Yang, L. Chen, Y. Chen, W. Liu, H. Feng, B. Wang, X. Zhang and M. Wei, Green Chem., 2019, 21, 5352–5362 RSC.
- N. Yamanaka, T. Hara, N. Ichikuni and S. Shimazu, Chem. Lett., 2018, 47, 971–974 CrossRef CAS.
- H. Wang, C. Zhu, Q. Liu, J. Tan, C. Wang, Z. Liang and L. Ma, ChemSusChem, 2019, 12, 2154–2160 CrossRef CAS PubMed.
- R. Rodiansono, M. D. Astuti, S. Khairi and S. Shimazu, Bull. Chem. React. Eng. Catal., 2016, 11, 1–9 CrossRef CAS.
- H. Liu, Z. Huang, F. Zhao, F. Cui, X. Li, C. Xia and J. Chen, Catal. Sci. Technol., 2016, 6, 668–671 RSC.
- H. Liu, Z. Huang, H. Kang, C. Xia and J. Chen, Chin. J. Catal., 2016, 37, 700–710 CrossRef CAS.
- S. L. Scott, ACS Catal., 2018, 8, 8597–8599 CrossRef CAS.
- R. Rodiansono, M. D. Astuti, A. Ghofur and K. C. Sembiring, Bull. Chem. React. Eng. Catal., 2015, 10, 192–200 CAS.
- J. Q. Bond, D. Martin Alonso, R. M. West and J. A. Dumesic, Langmuir, 2010, 26, 16291–16298 CrossRef CAS PubMed.
- C. Y. Y. Wong, A. W. T. Choi, M. Y. Lui, B. Fridrich, A. K. Horváth, L. T. Mika and I. T. Horváth, Struct. Chem., 2017, 28, 423–429 CrossRef CAS.
- A. Piskun, J. G. M. Winkelman, Z. Tang and H. J. Heeres, Catalysts, 2016, 6, 131 CrossRef.
- M. Al-Naji, J. Van Aelst, Y. Liao, M. D'Hullian, Z. Tian, C. Wang, R. Gläser and B. F. Sels, Green Chem., 2020, 22, 1171–1181 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental sections (catalyst preparation, characterisation, and procedure of catalytic activity tests), XRD patterns of the as prepared RNi–Sn(x)/AlOH and RNi–Sn(x)/AA, various supported RANEY® Ni–Sn, and supported Ni–Sn(1.5) catalysts. See DOI: 10.1039/d1ra06135f |
| This journal is © The Royal Society of Chemistry 2022 |