Open Access Article
Open Access ArticleCarboxylation of sodium arylsulfinates with CO2 over mesoporous K-Cu-20TiO2†
Yanjiao Chen,
Xuan Dai,
Wenwei Zhang,
Tao Wu,
Lei Chen and
Xinhua Peng *
*
School of Chemistry and Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China. E-mail: xhpeng@njust.edu.cn; xinhpeng@njust.edu.cn
First published on 24th December 2021
Abstract
A mesoporous ternary metal oxide (K-Cu-20TiO2) from a simple sol–gel method was prepared to catalyze heterogeneously the carboxylation reaction of various sodium arylsulfinates under atmospheric carbon dioxide. The catalyst showed excellent selectivity and good functional group tolerance to carboxylation recycle. The oxidation state of active copper(I) by characterization using FTIR, XRD, TG, XPS and TEM techniques proved to be efficacious to conduct atom economical reactions.
1. Introduction
In recent years, the term “green chemistry” is frequently mentioned by researchers.1 The emissions of carbon dioxide known as the “greenhouse gas” have far exceeded the level of pre-industry concentration.2,3 Moreover, the utilization of CO2 is rather attractive because of the abundant C1 source.4–7 In spite of its high thermodynamic and kinetic stabilities, CO2 has the characteristics of low electrophilicity.8–10 Therefore, it is important to activate CO2 with a Lewis acid to improve the electrophilic ability. For example, Olah et al. used the CO2–Al2Cl6/Al system to carboxylate ethylbenzene in 33% yield of p-ethyl benzoic acid under 5.7 MPa CO2 pressure.11,12On the other hand, a strong carbon nucleophile acts on CO2 to form valuable carboxylic acids and their derivatives.13,14 The chemical utilization of CO2 has made considerable progress.15,16 In particular, some carbon nucleophiles such as Grignard reagents were employed to attack CO2 in the syntheses of aliphatic carboxylic acids.17,18 The reaction is usually conducted under strict anhydrous and anaerobic conditions.19,20
Some mild reaction conditions are developed towards the CO2 carboxylation of organo-boron21,22 and zinc substrates23,24 in the presence of organometallic complexes. The reaction is accompanied by a cumbersome pre-functionalization process of the substrate and the requirements of sustainable chemistry are different.25,26 Fujihara et al.27–30 explored the carboxylation of aryl halides, styrene and propylene, etc. with CO2 by synergy between reductants such as Mn or Zn and some organometallic complexes. These typical synthetic methodologies have been summarized in Scheme 1.
Various transition metal catalysts can catalyze the coupling reaction of sodium sulfonates involving Heck desulfurization reactions,31 addition reactions of alkynes32 and cross-coupling reactions with polyfluoroaromatic hydrocarbons.33 Performing the carboxylation of sodium arylsulfinates with CO2 (ref. 34) brings similar advantages, due to the stability and the processability of aromatic sodium sulfinates as aryl sources.35,36
In addition, it has been reported that the homogeneous catalyst CuI was used to catalyze the carbonylation reaction of CO2 and sodium benzenesulfinate. However, CuI has the disadvantages of non-recoverability and easy loss of active sites. Recycling heterogeneous catalysts remains challenging. Here, the article describes a heterogeneous catalyst to promote the carboxylation of aromatic sodium arylsulfinates with CO2.
The heterogeneous catalyst, a mesoporous ternary metal oxide (K-Cu-20TiO2) is prepared using a sol–gel method by dissolving cheap polymers and metal alkoxides in a solution composed of glacial acetic acid, hydrochloric acid and ethanol (AcHE) (see ESI for details†).37–40 K-Cu-20TiO2 shows stable and excellent catalytic performances and promotes the high-selective substitution carboxylation of arylsulfinates with CO2 under mild conditions.
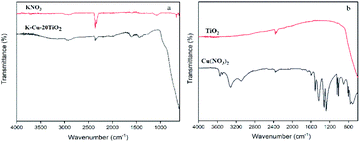
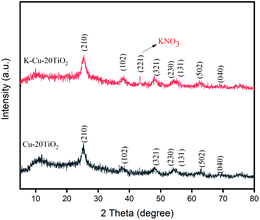
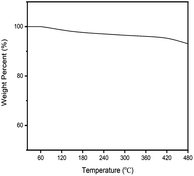
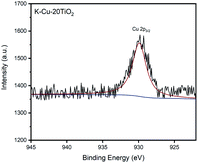
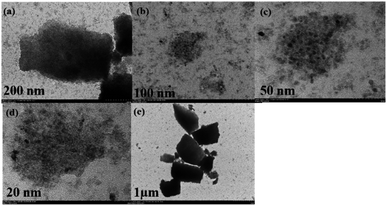
As shown in Fig. 1, 2 and 4, K-Cu-20TiO2 from a uniform combination of potassium and copper components in tetra-butyl titanate stabilizes the oxidation state of active copper(I) based on the characterization of FTIR, XRD and XPS characterizations. In Fig. 3, the TG curve displays its good thermal stability up to 480 °C. In addition, it exhibits a high degree of uniformity with dense accumulation and mesoporous channels in the TEM image (Fig. 5).
2. Results and discussion
2.1. Characterizations of the catalyst
FTIR spectra of KNO3, Cu(NO3)2, TiO2 and K-Cu-20TiO2 are depicted in Fig. 1. K-Cu-20TiO2 exhibits the characteristic peaks of the stretching vibrations of KNO3 at 2819–3006 cm−1 and 2360 cm−1 in Fig. 1(a). Similarly, the characteristic peak of TiO2 stretching vibration at 3541–3868 cm−1 (Fig. 1(b)) exists in the spectrum of catalytic material, which shows the importance of the TiO2 support.XRD patterns of Cu-20TiO2 and K-Cu-20TiO2 are shown in Fig. 2. There is a strong 2θ peak at 25.3° corresponding to the (210) plane, which is attributed to TiO2. The peaks at 36.2°, 48.0°, 54.3°, 54.5°, 62.0° and 68.9° are in good agreement with (102), (321), (230), (131), (502) and (040) planes of TiO2 (PDF#72-0100), respectively. Peaks at 42.9° are consistent with the (221) plane of KNO3 (PDF#81-0070).
The recorded TG curve of K-Cu-20TiO2 is shown in Fig. 3. When 7.084 mg K-Cu-20TiO2 is heated to 350 °C and 480 °C, the total mass loss is 0.275 and 0.492 mg, respectively. This shows that the catalyst demonstrates excellent thermal stability under reaction conditions.
XPS is used to characterize the Cu oxidation state. The existence of Cu(I) in fresh K-Cu-20TiO2 is observed with the major peak at 929.9 eV (Fig. 4), which has no shake-up satellites in the range of 931–933 eV. It is demonstrated that Cu(I) ions are homogeneously incorporated into the TiO2 matrix in mesoporous K-Cu-20TiO2 materials.
TEM images in Fig. 5 are employed to analyze the morphology features of K-Cu-20TiO2. From Fig. 5(a) to (e), the morphology of K-Cu-20TiO2 is a tightly packed cubic structure with sizes in the range 2–50 nm, in which there are some pores on the surface of K-Cu-20TiO2 as seen in Fig. 5(a). This proves that TiO2 is closely packed as a framework to form the mesoporous structure, while Cu and K are evenly dispersed and embedded in the crystal.
2.2. Optimization studies
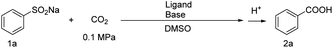
The reaction of sodium benzenesulfinate with atmospheric CO2 over the K-Cu-20TiO2 catalyst turns out to be efficacious for the carboxylation with excellent selectivity. Control experiments were conducted with various bases, ligands and DMSO medium (Table 1). The reaction becomes rather weak in the absence of the catalyst as compared to that without the ligand or base (entries 1–3 in Table 1). Ligands and bases promote the reaction by synergy with the catalyst. Of the tested ligands, o-phenanthroline is the most effective as compared with other ligands such as pyridine, 2,2′-bipyridine, 4,4′-bipyridine and 2,2′,6′,2′′-terpyridine in the presence of KOtBu (entries 4–8 in Table 1). Of the tested bases, Cs2CO3 proves to be quite efficient when compared with its counterparts, such as K2CO3, KOH, NaOC2H5, LiOtBu and KOtBu, in the presence of o-phenanthroline (entries 4, 9–13 in Table 1). An optimum combination of the substrate (0.10 mmol), K-Cu-20TiO2 (20.9 mg), o-phenanthroline (30 mol%) and Cs2CO3 (3.0 equiv.) in DMSO (2.5 mL) produced 85% conversion of sodium benzenesulfinate with 99.8% selectivity of benzoic acid during carboxylation (entry 10 in Table 1).| Entry | Catalyst | Ligand | Base | Conv.b (%) | Sel.c (%) |
|---|---|---|---|---|---|
| a The mixture of sodium benzenesulfinate (0.10 mmol), CO2 (0.1 MPa), K-Cu-20TiO2 (20.9 mg), ligand (0.03 mmol, phen: o-phenanthroline, py: pyridine, bipy: 2,2′-bipyridine, 4,4′-bipy: 4,4′-bipyridine, terpy: 2,2′,6′,2′′-terpyridine, same as below), base (0.30 mmol) and DMSO (2.5 mL) was reacted at 120 °C for 16 h in a sealed Schlenk tube.b Conversion, determined by product yield and selectivity from GC and GC-MS analyses using 2-methylimidazole as internal standard.c Selectivity, mass percentage of benzoic acid in the product mixtures from GC and GC-MS analyses using 2-methylimidazole as internal standard. | |||||
| 1 | K-Cu-20TiO2 | — | KOtBu | 33 | >99.2 |
| 2 | K-Cu-20TiO2 | Phen | — | 15 | >99.3 |
| 3 | — | Phen | KOtBu | Trace | — |
| 4 | K-Cu-20TiO2 | Phen | KOtBu | 77 | >99.5 |
| 5 | K-Cu-20TiO2 | Py | KOtBu | 70 | >99.5 |
| 6 | K-Cu-20TiO2 | Bipy | KOtBu | 70 | >99.4 |
| 7 | K-Cu-20TiO2 | 4,4′-Bipy | KOtBu | 60 | >99.3 |
| 8 | K-Cu-20TiO2 | Terpy | KOtBu | 50 | >99.3 |
| 9 | K-Cu-20TiO2 | Phen | K2CO3 | 80 | >99.5 |
| 10 | K-Cu-20TiO2 | Phen | Cs2CO3 | 85 | >99.8 |
| 11 | K-Cu-20TiO2 | Phen | KOH | 73 | >99.7 |
| 12 | K-Cu-20TiO2 | Phen | NaOC2H5 | 55 | >99.4 |
| 13 | K-Cu-20TiO2 | Phen | LiOtBu | 50 | >99.5 |
Moreover, the amounts of catalyst, ligand and base are able to affect the product yield (Table 2). The increase in the amount of the catalyst (entries 1–4 in Table 2), ligands (entries 5–7 in Table 2) and base (entries 8–10 in Table 2) can improve substrate conversion and carboxylation yield.
| Entry | K-Cu-20TiO2 (mg) | Phen (mmol) | Cs2CO3 (mmol) | Conv.b (%) |
|---|---|---|---|---|
| a The mixture of sodium benzenesulfinate (0.10 mmol), CO2 (0.1 MPa), K-Cu-20TiO2, o-phenanthroline, Cs2CO3 and DMSO (2.5 mL) was reacted for 16 h at 120 °C in a sealed Schlenk tube.b Conversion, determined by product yield and selectivity from GC and GC-MS analyses using 2-methylimidazole as internal standard. | ||||
| 1 | 62.9 | 0.03 | 0.30 | 87 |
| 2 | 41.8 | 0.03 | 0.30 | 86 |
| 3 | 20.9 | 0.03 | 0.30 | 85 |
| 4 | 10.5 | 0.03 | 0.30 | 41 |
| 5 | 20.9 | 0.04 | 0.30 | 86 |
| 6 | 20.9 | 0.02 | 0.30 | 75 |
| 7 | 20.9 | 0.01 | 0.30 | 35 |
| 8 | 20.9 | 0.03 | 0.40 | 87 |
| 9 | 20.9 | 0.03 | 0.20 | 72 |
| 10 | 20.9 | 0.03 | 0.10 | 55 |
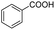
The carboxylation performance of various aromatic sodium arylsulfinates is inspected in optimum reaction conditions. As shown in Table 3, the reactions of arylsulfinates with CO2 form corresponding carboxylated products smoothly with moderate substrate conversion and high product selectivity. These substrates with functional groups involving methyl, ethyl, methoxy, chloro, iodo and phenyl have no intimate relevance to the electron and conjugate effects by the electron-donating and electron-withdrawing groups on the aromatic ring.
| Entry | Substrate | Product | Conv.b (%) | Sel.c (%) |
|---|---|---|---|---|
| a The mixture of substrate (0.10 mmol), CO2 (0.1 MPa), K-Cu-20TiO2 (20.9 mg), phen (0.03 mmol), Cs2CO3 (0.30 mmol) and DMSO (2.5 mL) was reacted for 16 h at 120 °C in a sealed Schlenk tube.b Conversion, determined by product yield and selectivity from GC and GC-MS analyses using 2-methylimidazole as internal standard.c Selectivity, mass percentage of benzoic acid in the product mixtures from GC and GC-MS analyses using 2-methylimidazole as internal standard. | ||||
| 1 | 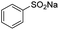 |
 |
85 | >99.8 |
| 2 | 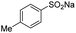 |
 |
80 | >99.6 |
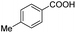
| 3 | 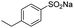 |
 |
75 | >99.3 |
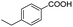
| 4 | 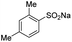 |
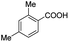 |
78 | >99.6 |
| 5 | 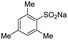 |
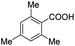 |
70 | >99.3 |
| 6 | 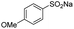 |
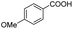 |
23 | >99.1 |
| 7 | 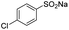 |
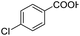 |
83 | >99.3 |
| 8 | 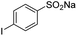 |
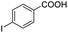 |
77 | >99.3 |
| 9 | 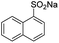 |
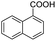 |
45 | >99.4 |
| 10 | 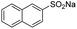 |
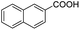 |
66 | >99.5 |
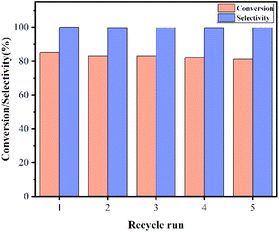
The reusability of K-Cu-20TiO2 for the carboxylation of sodium benzenesulfinate was investigated. The results under optimal conditions are shown in Fig. 6. It is clear that there are only insignificant decreases in conversion and selectivity after five cycles; the conversion and selectivity were about 83% and 99.8%, respectively. The catalyst is regenerated by the usual process involving centrifugation separation, washing with ethanol and deionized water, followed by drying at 70 °C prior to reuse.
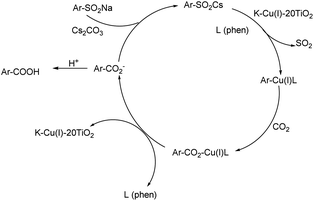
The catalytic properties are implied by the mechanism presented in Scheme 2. Sodium benzenesulfinate in stronger alkaline conditions can favor metathesis from active Cu(I) of K-Cu-20TiO2 based on the XPS analysis. The Cu(I) complex on solid surface is further coordinated with ligands to form an intermediate Ar–CuL (aromatic copper complex), accompanied by the release of molecular sulfur dioxide from the substrate. Subsequently, CO2 is inserted into Ar–CuL to form a carbon–carbon coupling product of benzoate by the electrophilic substitution effect. This method based on the catalytic cycle can readily synthesize aromatic carboxylic acid by mild desulfination.
3. Experimental
3.1. Preparation and regeneration for catalyst
A mixture of 10 mmol Ti(OBu)4, 40 mmol HOAc (99.5%), 12 mmol HCl (36–38%), 0.50 mmol Cu(NO3)2·3H2O and 0.50 mmol KNO3 was dissolved in 2 mL deionized water, and 1.6 g F68 (polyethylene-polypropylene glycol) in 30 mL ethanol was stirred vigorously for 1 h at room temperature. Then the mixture was further concentrated to form a yellow transparent film by rotary evaporation at 50 °C under reduced pressure. The film was aged for 24 h at 70 °C and then calcined for 5 h at 350 °C in nitrogen atmosphere. The film material was ground into a fine powder before use.The solid catalyst separated from the reaction mixture by the centrifugation process was stirred for 1 h in 5.0 mL ethanol and then in 5.0 mL deionized water. After being aged for 24 h at 70 °C and then calcined for 5 h at 350 °C in nitrogen atmosphere, the solid was reused in the next run.
3.2. Procedure for carboxylation reaction
A 10 mL sealed Schlenk tube was ventilated with CO2 added substrate (0.10 mmol), K-Cu-20TiO2 (20.9 mg), ligand (0.03 mmol), base (0.30 mmol) and DMSO (2.5 mL). The mixture was stirred for 16 h at 120 °C under 0.1 MPa carbon dioxide atmosphere. After the mixture was cooled to room temperature, 1 mol L−1 HCl (1.5 mL) was added to the mixture. The catalyst was separated by centrifugation, and the product was extracted with EtOAc (5 mL × 3) and washed with saturated NaCl aqueous solution (5 mL × 3). The organic phase was dried by Na2SO4, and then concentrated by distillation and purified by TLC.4. Conclusions
In summary, an excellent K-Cu-20TiO2 catalyst is used to promote the atom economic carboxylation of CO2 as the C1 source. Carboxylation of arylsulfinates does not need the usual pre-activation on the substrate in the presence of the solid catalyst. In addition, the reaction is heterogeneously performed in mild conditions on the solid surface with high product selectivity. Therefore, this methodology expands CO2 fixation utilization.Conflicts of interest
There are no conflicts to declare.References
- S. Fenner and L. Ackermann, Green Chem., 2016, 18, 3804–3807 RSC.
- R. Zevenhoven, S. Eloneva and S. Teir, Catal. Today, 2006, 115, 73–79 CrossRef CAS.
- S. N. Riduan and Y. Zhang, Dalton Trans., 2010, 39, 3347–3357 RSC.
- T. Sakakura, J. C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365–2387 CrossRef CAS PubMed.
- X. Gao, Z. Zhang, X. Wang, J. Tian, S. Xie, F. Zhou and J. Zhou, Chem. Sci., 2020, 11, 10414–10420 RSC.
- A. Tortajada, F. Juliá-Hernández, M. Börjesson, T. Moragas and R. Martin, Angew. Chem., Int. Ed., 2018, 130, 16178–16214 CrossRef.
- J. Louie, Curr. Org. Chem., 2005, 9, 605–623 CrossRef CAS.
- T. Ohishi, M. Nishiura and Z. Hou, Angew. Chem., Int. Ed., 2008, 47, 5792–5795 CrossRef CAS PubMed.
- J. Luo and I. Larrosa, ChemSusChem, 2017, 10, 3317–3332 CrossRef CAS PubMed.
- M. Aresta, Focus on Catalysts, 2010, 4, 1351–4180 Search PubMed.
- G. A. Olah, B. Torok, J. P. Joschek, I. Bucsi, P. M. Esteves, G. Rasul and G. K. Surya Prakash, J. Am. Chem. Soc., 2002, 124, 11379–11391 CrossRef CAS PubMed.
- Y. Suzuki, T. Hattori, T. Okuzawa and S. Miyano, Chem. Lett., 2002, 31, 102–103 CrossRef.
- Q. Liu, L. Wu, R. Jackstell and M. Beller, Nat. Commun., 2015, 6, 5933 CrossRef PubMed.
- T. E. Muller and W. Leitner, Beilstein J. Org. Chem., 2015, 11, 675–677 CrossRef PubMed.
- Y. Kuwahara and H. Yamashita, J. CO2 Util., 2013, 1, 50–59 CrossRef CAS.
- J. Rintjema, L. P. Carrodeguas, V. Laserna, S. Sopena and A. W. Kleij, Top. Organomet. Chem., 2016, 53, 39–71 CrossRef CAS.
- A. Correa and R. Martin, Angew. Chem., Int. Ed., 2009, 48, 6201–6204 CrossRef CAS PubMed.
- O. Vechorkin, N. Hirt and X. Hu, Org. Lett., 2010, 12, 3567–3569 CrossRef CAS PubMed.
- A. Nagaki, Y. Takahashi and J. Yoshida, Chem.–Eur. J., 2014, 20, 7931–7934 CrossRef CAS PubMed.
- A. Polyzos, M. O'Brien, T. P. Petersen, I. R. Baxendale and S. V. Ley, Angew. Chem., Int. Ed., 2011, 123, 1222–1225 CrossRef.
- J. Takaya, S. Tadami, K. Ukai and N. Iwasawa, Org. Lett., 2008, 10, 2697–2700 CrossRef CAS PubMed.
- K. Ukai, M. Aoki, J. Takaya and N. Iwasawa, J. Am. Chem. Soc., 2006, 128, 8706–8707 CrossRef CAS PubMed.
- C. S. Yeung and V. M. Dong, J. Am. Chem. Soc., 2008, 130, 7826–7827 CrossRef CAS PubMed.
- H. Ochiai, M. Jang, K. Hirano, H. Yorimitsu and K. Oshima, Org. Lett., 2008, 10, 2681–2683 CrossRef CAS PubMed.
- K. Shimomaki, K. Murata, R. Martin and N. Iwasawa, J. Am. Chem. Soc., 2017, 139, 9467–9470 CrossRef CAS PubMed.
- X. Zhang, W. Z. Zhang, L. L. Shi, C. X. Guo, L. L. Zhang and X. B. Lu, Chem. Commun., 2012, 48, 6292–6294 RSC.
- Y. Sato, M. Mori, M. Takimoto and M. Kawamura, Synlett, 2005, 134, 2019–2022 Search PubMed.
- J. Takaya and N. Iwasawa, J. Am. Chem. Soc., 2008, 130, 15254–15255 CrossRef CAS PubMed.
- T. Fujihara, K. Nogi, T. Xu, J. Terao and Y. Tsuji, J. Am. Chem. Soc., 2012, 134, 9106–9109 CrossRef CAS PubMed.
- T. Moragas, M. Gaydou and R. Martin, Angew. Chem., Int. Ed., 2016, 55, 5053–5057 CrossRef CAS PubMed.
- G. W. Wang and T. Miao, Chemistry, 2011, 17, 5787–5790 CrossRef CAS PubMed.
- S. Liu, Y. Bai, X. Cao, F. Xiao and G. Deng, Chem. Commun., 2013, 49, 7501–7503 RSC.
- T. Miao and L. Wang, Adv. Synth. Catal., 2014, 356, 429–436 CrossRef CAS.
- S. Sun, J. Yu, Y. Jiang and J. Cheng, Adv. Synth. Catal., 2015, 357, 2022–2026 CrossRef CAS.
- W. Chen, P. Li, T. Miao, L. G. Meng and L. Wang, Org. Biomol. Chem., 2013, 11, 420–424 RSC.
- M. Wang, D. Li, W. Zhou and L. Wang, Tetrahedron, 2012, 68, 1926–1930 CrossRef CAS.
- J. Fan, S. W. Boettcher and G. D. Stucky, Chem. Mater., 2006, 18, 6391–6396 CrossRef CAS.
- P. Yang, D. Zhao, D. I. Margolese, B. F. Chmelka and G. D. Stucky, Chem. Mater., 1999, 11, 2813–2826 CrossRef CAS.
- D. Grosso, F. Cagnol, G. J. d. A. A. Soler-Illia, E. L. Crepaldi, H. Amenitsch, A. Brunet-Bruneau, A. Bourgeois and C. Sanchez, Adv. Funct. Mater., 2004, 14, 309–322 CrossRef CAS.
- J. Fan, Y. Dai, Y. Li, N. Zheng, J. Guo, X. Yan and G. D. Stucky, J. Am. Chem. Soc., 2009, 131, 15568–15569 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra05228d |
| This journal is © The Royal Society of Chemistry 2022 |