Open Access Article
Open Access ArticleStructure revision and chemical synthesis of ligandrol's main bishydroxylated long-term metabolic marker†
Emmanuel N.
Pitsinos
 *a,
Yiannis S.
Angelis
*a,
Yiannis S.
Angelis
 *b and
Michael
Petrou
*b and
Michael
Petrou
 c
c
aNational Centre for Scientific Research “DEMOKRITOS”, Institute of Nanoscience and Nanotechnology, “Natural Products Synthesis and Bioorganic Chemistry” Laboratory, P.O. Box 60037, GR 153 10 Aghia Paraskevi, Athens, Greece. E-mail: e.pitsinos@inn.demokritos.gr
bNational Centre for Scientific Research “DEMOKRITOS”, Institute of Biosciences & Applications, Doping Control Laboratory of Athens, Neratziotissis & Amaryssias Artemidos Str, GR 151 23 Athens, Greece. E-mail: i.angelis@bio.demokritos.gr
cCyprus Anti-Doping Authority, Makarion Athletic Centre Avenue, Engomi, CY 2400 Nicosia, Cyprus
First published on 7th November 2022
Abstract
Although the main bishydroxylated long-term metabolite of the WADA-banned anabolic agent ligandrol (LGD-4033) is an important metabolic marker, it is not readily available in sufficient quantities to facilitate the development and validation of related analytical protocols or sensors. A chemically more robust structure was postulated as an alternative to the one previously established. The NMR spectra of the synthesized material and its LC–HRMS comparison with a relevant metabolic sample support the proposed structural revision.
Introduction
The nonsteroidal selective androgen receptor modulator (SARM) 4-{(2R)-2-[(1R)-2,2,2-trifluoro-1-hydroxyethyl]pyrroli-din-1-yl}-2-(trifluoromethyl)benzonitrile (1, Fig. 1) has not yet received official approval for clinical use.1 Nonetheless, it is commercially available as ligandrol or LGD-4033 (also known as anabolicum or VK5211) and is becoming increasingly popular among athletes2 due to its promising anabolic effects,3 despite the potential health risks associated with its use4–6 and the banning of such practices by the World Anti-Doping Agency (WADA).7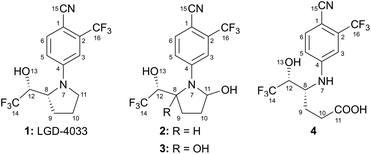 | ||
| Fig. 1 LGD-4033 (1), its mono- (2) and bishydroxylated (3) metabolites, and a constitutional isomer of the latter (4)‡. | ||
Several studies related to the detection of its illicit use by humans and on horses have been reported8–15 and have led to the identification of related metabolites in urine samples. A bishydroxylated metabolite is of particular interest for doping control purposes since it has been suggested as a preferred long-term marker for the detection of this SARM's illicit use.9,11 Interestingly, epimerization of the parent drug15 and isomeric metabolites with identical mass spectra11,15 have also been observed.
As an exquisite example of experimental prowess and a testimony to the power of modern analytical techniques, Thevis et al. have managed to isolate and characterize microgram quantities of a mono- and the main bis-hydroxylated metabolite through the in vitro metabolism of LGD-4033 with human liver microsomes.8 Based on the observed mass and NMR spectra, the structures 2 and 3 (Fig. 1) were assigned to them, respectively.8 It is important to note that the 1H NMR spectrum of the bishydroxylated metabolite was recorded in CD3OD and that the 13C NMR spectrum was not reported.
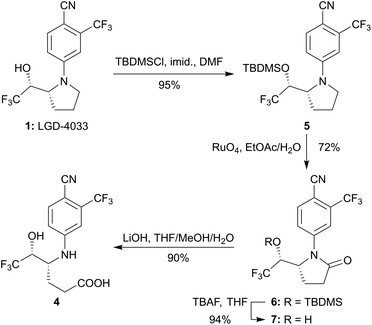
Chemical synthesis could secure the main bishydroxylated long-term metabolite of LGD-4033 in quantities sufficient not only to meet the needs of analytical methods development and validation (e.g., its semi-quantitative analysis for doping control purposes)15 but also to facilitate its full structural characterization. Hence, this important metabolic marker was pursued and we report herein the rational that prompted its structural revision and the synthesis, full characterization, and direct comparison of the revised structure with an authentic metabolic sample of LGD-4033.
Results and discussion
The established structure for the main bishydroxylated derivative of ligandrol (3) is intriguing. Thus, contrary to the reported long-term persistence of this metabolite,9,11 a N-bridged bis-hemiaminal, such as 3, would be anticipated to be extremely labile, especially in aqueous media. Indeed, a literature survey revealed few examples of analogous compounds that, unless embedded in strategically functionalized16 or fused polycyclic structures,17–20 have been observed only as fleeting intermediates.21,22 Furthermore, it is interesting that the reported 1H NMR chemical shift for H-11‡ of compound 3 (δ = 4.00 ppm) is upfield from the corresponding proton of the closely related compound 2 (δ = 5.41 ppm).In this context, 4-arylamino-5-hydroxyhexanoic acid 4 (Fig. 1), a constitutional isomer of 3, emerged as a possible alternative structure for the main bishydroxylated long-term metabolite of ligandrol. This compound has exactly the same molar mass as 3, it should exhibit greater chemical stability, and its 1H NMR spectrum in CD3OD might be identical to the one reported for 3.8 Thus, in parallel to bis-hemiaminal 3, carboxylic acid 4 was synthetically pursued.
The chemical synthesis of the postulated alternative structure 4 starting from the parent SARM was straightforward via the four-step sequence summarized in Scheme 1. In particular, although rather forcing conditions were required (i.e., large excess of reagents, high concentration, long reaction time), protection of the secondary hydroxyl moiety of LGD-4033 (1, Scheme 1) as the corresponding silyl ether 5 was achieved employing TBDMSCl and imidazole in DMF. Subsequently, the use of RuO4, generated in situ from RuCl3 and 10% aqueous NaIO4, allowed the regioselective oxidation of the pyrrolidine ring under two-phase conditions23 (i.e., EtOAc/H2O) furnishing pyrrolidinone 6 in good yield (72%). It should be noted that the use of the same oxidant under single-phase conditions has been reported to favour the direct formation of 4-aminobutanoic acids from N-acyl-pyrrolidines.24 Interestingly however, in the case of pyrrolidine 5, pyrrolidinone 6 was isolated in similar yield under either two- or single-phase (i.e., tert-BuOH/H2O) conditions. This outcome could be an indication that the postulated oxidation intermediate,24 a pyrrolidin-2-ol, favours the aminal over the amino-aldehyde tautomeric form and bodes favourably for the eventual synthesis of the monohydroxylated metabolite 2 through reduction of pyrrolidinone 6. Focusing on the targeted carboxylic acid 4, TBAF-mediated cleavage of the silyl ether moiety of 6 provided pyrrolidinone 7, another postulated metabolite of LGD-4033.9,10 Finally, by employing aqueous basic conditions (LiOH in THF/MeOH/H2O), selective hydrolytic opening of the lactam ring in the presence of the arylnitrile moiety of 7 was achieved securing the desired hexanoic acid 4 in excellent yield (90%).
Gratifyingly, the 1H NMR spectrum of 4 (500 MHz, CD3OD; Fig. 2) was in agreement§ with the one reported by Thevis et al. for the main bishydroxylated long-term metabolite of LGD-4033.8 In particular, the recorded 1H NMR spectrum (Fig. 2) was in agreement with either the originally proposed structure (3) or the alternative one (4). However, it should be pointed out that, some of the observed signals had to be assigned differently for each structure (3 or 4). The most notable one was that the overlapping signals in the region δ = 4.10–4.04 ppm were assigned to H-12 & H-11 for structure 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 but had to be assigned to H-8 & H-12 for structure 4.
8 but had to be assigned to H-8 & H-12 for structure 4.
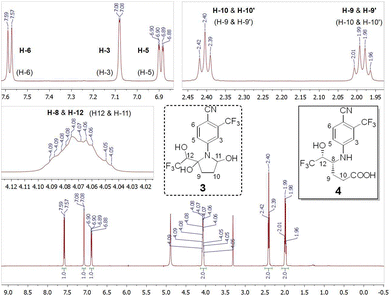 | ||
| Fig. 2 1H NMR (500 MHz, CD3OD) spectrum of 4 with regions of interest expanded. To aid comparisons, the structures of 3 and 4 are shown‡ along with the corresponding signal assignments (in parentheses for 3,8 in bold for 4). | ||
The COSY spectrum (see ESI†) was inconclusive, being consistent with either structure. However, the 13C, HSQC, and HMBC NMR spectra (see ESI†) did not support structure 3 but were consistent with the synthesized 4-arylamino-5-hydroxy-hexanoic acid 4. In particular, the presence of a signal in the 13C NMR spectrum (125 MHz, CD3OD) at δ = 176.8 ppm could be assigned to the carboxylic acid moiety (C-11) of 4 but is not expected for bis-hemiaminal 3. In addition, the signal observed at δ = 52.0 ppm, based on the HSQC spectrum, was assigned to C-8 of hexanoic acid 4. For the bis-hemiaminal structure (3) this signal would have to be assigned to C-11, significantly upfield from the expected region for a carbon substituted with two heteroatoms.¶
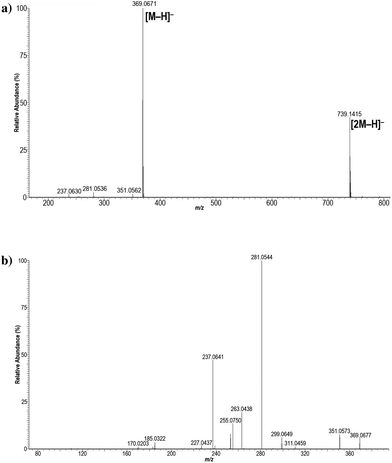
Regarding the mass spectrum obtained, the observed anion at m/z 369.0671 (Fig. 3a) was the one expected for the formula C14H11F6N2O3−, shared by the deprotonated bis-hemiaminal 3 and hexanoic acid 4. Importantly, the product ion mass spectrum of the deprotonated molecular ion [M − H]− with m/z 369 (Fig. 3b) was identical with the one observed (see ESI†) for ligandrol's main bishydroxylated metabolite, which was extracted from a human urine sample obtained in the frame of a previous related excretion study,11 as well as the one previously reported for this metabolite.8
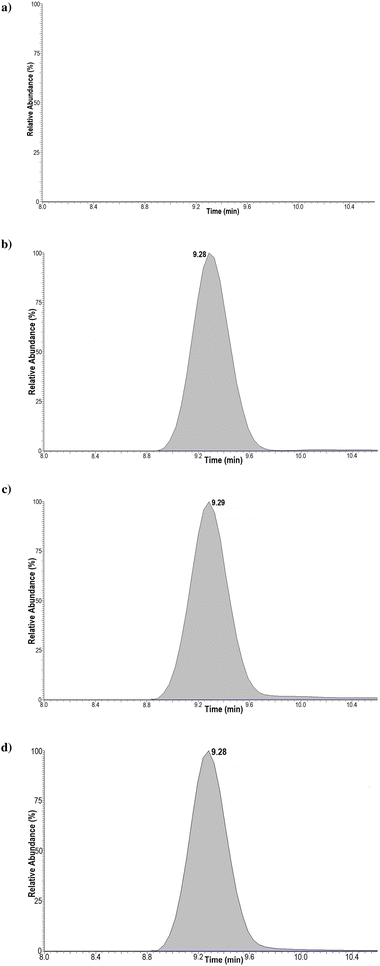
Furthermore, the retention time data (Fig. 4) obtained from the LC-HRMS analysis of substance 4 against the LGD 4033 main bishydroxylated long-term metabolite deemed the two analytes identical based on WADA's rules for confirmation.25
The above spectroscopic and analytical data support the hypothesis that 4-arylamino-5-hydroxyhexanoic acid 4 is indeed the main “bishydroxylated” long-term metabolite of LGD-4033.
Conclusions
The chemical synthesis of hexanoic acid 4 has been accomplished by a short and efficient sequence starting from LGD-4033 (1). The synthetic material (4) and an authentic metabolic sample demonstrated identical characteristics by LC–HRMS analysis. Furthermore, the 1H NMR spectrum (500 MHz, CD3OD) of 4 was in accordance§ with the one reported for the main “bishydroxylated” metabolite of LGD-4033 and assigned to bis-hemiaminal 3.8 However, the recorded 13C, HSQC, and HMBC NMR spectra, although consistent with the synthesized 4-arylamino-hexanoic acid structure 4, did not support the previously established one (i.e., 3) for the main “bishydroxylated” metabolite of LGD-4033.Thus, the molecular structure of this important metabolic marker should be revised from 4-[2,5-dihydroxy-2-(2,2,2-trifluoro-1-hydroxyethyl)pyrrolidin-1-yl]-2-(trifluoromethyl)-benzonitrile (3) to (4R,5R)-4-{[4-cyano-3-(trifluoromethyl)-phenyl]amino}-6,6,6-trifluoro-5-hydroxyhexanoic acid (4).||
The availability of hexanoic acid 4 and its synthetic precursor pyrrolidinone 7 should facilitate further studies on the metabolism and doping control of this SARM, including the development of related sensors.26 Furthermore, the synthetic sequence employed can be readily adapted for the synthesis of additional related metabolites8–15 (e.g., the mono- and trishydroxylated ones or the postulated 12-epi-4). Efforts towards these goals are currently underway.
Author contributions
E. N. P.: conceptualization, methodology, investigation, data curation, validation, visualization, funding acquisition, project administration, and writing – original draft, review/editing. Y. S. A.: investigation, formal analysis, data curation, validation, visualization, funding acquisition, and writing – original draft, review/editing. M. P.: resources and writing – review/editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has been carried out with the support of the World Anti-Doping Agency (grant number: 21A17EP).References
- G. W. P. D. Fonseca, E. Dworatzek, N. Ebner and S. Von Haehling, Expert Opin. Invest. Drugs, 2020, 29, 881–891 CrossRef CAS PubMed.
- U.S. Anti-Doping Agency (USADA), Sanctions – Anti-Doping Violations, https://www.usada.org/news/sanctions/ (accessed 15/10/2022).
- S. Basaria, L. Collins, E. L. Dillon, K. Orwoll, T. W. Storer, R. Miciek, J. Ulloor, A. Zhang, R. Eder, H. Zientek, G. Gordon, S. Kazmi, M. Sheffield-Moore and S. Bhasin, J. Gerontol., Ser. A, 2013, 68, 87–95 CrossRef CAS PubMed.
- M. Barbara, S. Dhingra and A. L. Mindikoglu, ACG Case Rep. J., 2020, 7, e00370 CrossRef PubMed.
- S. B. Machek, T. D. Cardaci, D. T. Wilburn and D. S. Willoughby, Steroids, 2020, 164, 108753 CrossRef CAS PubMed.
- J. E. Flores, S. Chitturi and S. Walker, Hepatol. Commun., 2020, 4, 450–452 CrossRef PubMed.
- WADA, World Anti-Doping Code, International Standard Prohibited List 2022, https://www.wada-ama.org/en/resources/world-anti-doping-program/prohibited-list (accessed 15/10/2022).
- M. Thevis, A. Lagojda, D. Kuehne, A. Thomas, J. Dib, A. Hansson, M. Hedeland, U. Bondesson, T. Wigger, U. Karst and W. Schänzer, Rapid Commun. Mass Spectrom., 2015, 29, 991–999 CrossRef CAS PubMed.
- H. D. Cox and D. Eichner, Drug Test. Anal., 2017, 9, 127–134 CrossRef CAS PubMed.
- L. Geldof, O. J. Pozo, L. Lootens, W. Morthier, P. Van Eenoo and K. Deventer, Drug Test. Anal., 2017, 9, 168–178 CrossRef CAS PubMed.
- A. G. Fragkaki, P. Sakellariou, P. Kiousi, N. Kioukia-Fougia, M. Tsivou, M. Petrou and Y. Angelis, Drug Test. Anal., 2018, 10, 1635–1645 CrossRef CAS PubMed.
- A. Hansson, H. Knych, S. Stanley, E. Berndtson, L. Jackson, U. Bondesson, M. Thevis and M. Hedeland, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2018, 1074–1075, 91–98 CrossRef CAS PubMed.
- P. Kintz, A. Ameline, L. Gheddar and J. S. Raul, Toxicol. Anal. et Clin., 2019, 31, 56–63 Search PubMed.
- C. Cutler, M. Viljanto, P. Hincks, J. Habershon-Butcher, T. Muir and S. Biddle, Drug Test. Anal., 2020, 12, 247–260 CrossRef CAS PubMed.
- F. Wagener, S. Guddat, C. Görgens, Y. S. Angelis, M. Petrou, A. Lagojda, D. Kühne and M. Thevis, Anal. Bioanal. Chem., 2022, 414, 1151–1162 CrossRef CAS PubMed.
- F. Sajjad, A. G. K. Reddy, J. Che, W. Hu and D. Xing, Org. Lett., 2020, 22, 3094–3098 CrossRef CAS PubMed.
- T. Kametani, T. Ohsawa, M. Ihara and K. Fukumoto, J. Chem. Soc., Perkin trans. 1, 1978, 460–464 RSC.
- J. W. Benbow, K. F. McClure and S. J. Danishefsky, J. Am. Chem. Soc., 1993, 115, 12305–12314 CrossRef CAS.
- S. Saito, T. Kubota and J. I. Kobayashi, Tetrahedron Lett., 2007, 48, 3809–3812 CrossRef CAS.
- B.-J. Zhang, C. Liu, M.-F. Bao, X.-H. Zhong, L. Ni, J. Wu and X.-H. Cai, Tetrahedron, 2017, 73, 5821–5826 CrossRef CAS.
- R. M. Williams, M. R. Sabol, H. D. Kim and A. Kwast, J. Am. Chem. Soc., 1991, 113, 6621–6633 CrossRef CAS.
- L. Guan and M. M. Greenberg, J. Am. Chem. Soc., 2009, 131, 15225–15231 CrossRef CAS PubMed.
- K.-I. Tanaka, S. Yoshifuji and Y. Nitta, Chem. Pharm. Bull., 1986, 34, 3879–3884 CrossRef CAS.
- M. Kaname, S. Yoshifuji and H. Sashida, Tetrahedron Lett., 2008, 49, 2786–2788 CrossRef CAS.
- WADA, Technical Document – TD2021IDCR, https://www.wada-ama.org/en/resources/lab-documents/td2021idcr (accessed 15/10/2022).
- A. Henderson, M. V. Sullivan, R. A. Hand and N. W. Turner, J. Mater. Chem. B, 2022, 10, 6792–6799 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and copies of NMR spectra of all new compounds. See DOI: https://doi.org/10.1039/d2ob01907h |
| ‡ To facilitate NMR spectra comparison, the established LGD-4033 skeleton numbering8 has been used for all compounds when assigning signals. |
| § A detailed comparison between the observed 1H NMR data for 4 and those previously reported8 for the bishydroxylated metabolite is presented in the ESI.† |
| ¶ For example, a signal at δ = 86.6 ppm has been assigned to C-11 of aminal 2.8 |
| || The stereochemical integrity of pre-existing stereocenters is assumed based on: (i) the mild and chemoselective transformations employed, (ii) the high chemical yields observed, and (iii) the chromatographic and spectroscopic homogeneity of synthetic intermediates and the final product (see ESI†). |
| This journal is © The Royal Society of Chemistry 2022 |